Abstract
Background
In heart failure with reduced ejection fraction (HFrEF), elevated soluble neprilysin (sNEP) levels are associated with an increased risk of cardiovascular death, and its inhibition with sacubitril/valsartan has improved survival.
Objectives
This study sought to determine the relevance of sNEP as a biomarker in heart failure with preserved ejection fraction (HFpEF) and to compare circulating sNEP levels in HFpEF patients with normal controls.
Methods
A case-control study was performed in 242 symptomatic HFpEF patients previously enrolled in the RELAX and NEAT-HFpEF clinical trials, and 891 asymptomatic subjects without HF or diastolic dysfunction (confirmed by NT-proBNP levels <200 pg/ml and echocardiography), who were enrolled in the Prevalence of Asymptomatic Left Ventricular Dysfunction study. sNEP was measured using a sandwich ELISA assay in all subjects.
Results
Overall, sNEP levels were lower in HFpEF compared to controls (3.5 ng/ml [CI 2.5, 4.8] vs 8.5 ng/ml [CI 7.2, 10.0], p<0.001). After adjusting for age, gender, BMI and smoking history, mean sNEP levels were also lower in HFpEF compared with controls (4.0 ng/ml [CI 2.7,5.4] vs 8.2 ng/ml [CI 6.8, 9.7], p 0.002). The cohorts were propensity matched based on age, BMI, diabetes, hypertension, smoking history and renal function, and sNEP levels remained lower in HFpEF compared with controls (median 2.4 ng/ml [IQR 0.6, 27.7] vs 4.9 ng/ml [IQR 1.2, 42.2], p=0.02).
Conclusions
HFpEF patients on average have significantly lower circulating sNEP levels compared to controls. These findings challenge our current understanding of the complex biology of circulating sNEP in HFpEF.
Keywords: neprilysin, diastolic dysfunction, heart failure
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) can result from abnormalities in the process of both active relaxation and passive stiffness of the heart (1). Atrial natriuretic peptide (ANP) activates phospholipase C-b resulting in intracellular calcium mobilization and active relaxation of the cardiomyocyte. The natriuretic peptides (NPs) via cyclic guanosine monophosphate (cGMP) inhibit smooth muscle and fibroblast proliferation and collagen deposition that can affect passive stiffness (2–4). NPs directly reduce peripheral vascular resistance (5), and affect natriuresis (6–9), therefore playing an important role in maintaining homeostasis in the HFpEF population (10–12).
Neprilysin (NEP) is a cell membrane bound metalloendopeptidase that mediates the anti-fibrotic, anti-proliferative, myocardial relaxation, vasodilator and diuretic properties of NPs by being responsible for removal of at least 50% of circulating NPs (13), and its inhibition therefore results in significant increases in plasma ANP, urinary cGMP and urinary sodium, and reduction in intra-cardiac filling pressures (14,15). Sacubitril/valsartan when administered to patients with HFpEF in a phase 2 study resulted in significantly reduced NT-proBNP levels, a surrogate marker of LV wall stress, as compared to being treated with valsartan alone (16). Sacubitril/valsartan therefore is being tested as treatment for HFpEF in a Phase 3 trial, PARAGON-HF, evaluating its efficacy in reducing cardiovascular (CV) death and heart failure (HF) hospitalizations as compared to valsartan.
NEP can be released from the cell surface, and in a recent study, circulating soluble neprilysin (sNEP) level in a linear relationship was significantly associated with CV death or HF hospitalization in patients with heart failure with reduced ejection fraction (HFrEF) (17), supporting the hypothesis that its inhibition may be beneficial in HFrEF. sNEP levels in HFpEF were evaluated in 144 patients and not found to be associated with NYHA Class, 6-minute walk test or cardiac hospitalization and death (18). However, to understand its role as a biomarker, sNEP levels should be measured in and compared with control subjects without diastolic dysfunction or HF, a study that has not been previously performed.
The aim of our study was to determine the role of sNEP levels as a biomarker in HFpEF and its concentration in patients with HFpEF as compared with normal controls.
METHODS
Study population
HFpEF subjects
This case-control study included 242 patients with definite clinical HFpEF, previously enrolled in the National Institutes of Health funded multicenter RELAX and NEAT-HFpEF clinical trials for whom blood samples were available for testing (19,20). The RELAX study was a multicenter, randomized clinical trial of 216 stable outpatients with HFpEF (19). Patients with normal (≥50%) EF and symptomatic HF (NYHA class II-IV) with objective exercise limitation (peak oxygen consumption<60% predicted) were recruited. Definite clinical HFpEF was required as defined by previous HF hospitalization, chronic loop diuretic therapy with left atrial enlargement, invasively documented elevation in left sided filling pressures. In addition, study entry also required an elevated (≥400 pg/mL) NT-proBNP level, or invasive confirmation if NTproBNP level was less than 400 pg/mL further increasing the rigor of HFpEF diagnosis.
NEAT-HFpEF was a 110 subject multicenter, randomized study evaluating the effect of oral nitrate therapy on daily physical activity in outpatients with HFpEF (20). Similar to RELAX, patients were required to have an EF≥50% or more and objective evidence of heart failure exacerbation by either previous HF hospitalization, elevated invasive filling pressures, elevated level of NT-proBNP (>400 pg per mL), or Doppler echocardiographic evidence of elevated filling pressures.
Control group
The control group comprised 891 asymptomatic subjects without HF or diastolic dysfunction and with NT-proBNP <200 pg/ml who were previously enrolled in the Prevalence of Asymptomatic Left Ventricular Dysfunction (PAVD) study from Olmsted County (21,22). Diastolic dysfunction was rigorously defined by comprehensive Doppler techniques and established criteria, as previously described (22). These subjects underwent a physical examination, echocardiogram, and phlebotomy for biorepository samples, and a follow-up visit that included echocardiography four years after the initial evaluation to determine the effects of aging on the development of diastolic dysfunction and HFpEF and were followed for more than 10 years after initial evaluation (21,22). We have previously used the PAVD population in multiple studies to interpret the distribution of BNP concentration and other biomarkers in a normal subset of subjects (23).
Assessment of Ventricular Structure and Function
The echocardiographic methods have been extensively described previously (22,23). Ejection fraction was measured by a semi-quantitative method, integrating 2-D quantitative measurements and visual assessment. Left ventricular mass and left atrial volume were calculated from M-mode and 2D measurements (22). Diastolic dysfunction was recorded as indeterminate, mild (grade 1), moderate (grade II), or severe (grade III/IV) based on the analysis of trans-mitral inflow velocities at rest and with Valsalva maneuver, mitral annular tissue velocity, and pulmonary venous inflow patterns (22). Pulmonary artery systolic pressure was estimated using the modified Bernoulli equation, 4 * (peak tricuspid regurgitation velocity) + 5 mmHg (24).
Soluble Neprilysin assay
A commercially available sandwich ELISA assay for sNEP (RnD Systems, MN) was used to measure circulating NEP levels in serum. ELISA plates were coated with NEP capture antibody overnight, and then plates were washed per manufacturer’s instructions and used for analysis of the samples. The samples were incubated in the antibody coated plate for 2 hours at room temperature. At the end of the incubation, the plates were washed four times and incubated with the NEP detection antibody for another 2 hours. The plates were then washed and incubated with streptavidin-HRP conjugate for 20 minutes and washed before incubating for another 20 minutes with the substrate. At the end of the incubation period, the reaction was halted by adding a stop solution and the plate was corrected for background interference at 570 nm read at 450 nm. SoftmaxPro 7.2 software was used to extrapolate sNEP concentrations of samples. The standard curve range was from 0.12 ng/mL to 8 ng/mL. The intra assay and inter assay variability was 7.5% and 9.5% respectively. Based on initial sNEP measurement, any sample exceeding the standard curve range was diluted appropriately and re-assayed for accurate measurement. All the measurements were done in duplicate. A freeze-thaw analysis was performed on other control samples and found that sNEP levels were stable for up to two freeze thaw cycles. In addition, NEP was obtained from RnD System, USA and was dissolved in PBS and diluted to the varying concentrations as described in Supplemental Table 1. Subsequently, NEP concentration and activity in buffer solution was measured as described above and by using a fluorimetric assay, with 5-FAM/QXL® 520 Neprilysin substrate (SensoLyte Neprilysin Elisa Kit) respectively.
All the samples from the control and test cohort were analyzed using the same lot of Neprilysin Kit. The analyses of calibration controls from randomly selected plates are shown in Supplemental Table 2. The overall percentage co-efficient of variation for the different calibration controls was less than 10%.
Statistical Analysis
Subjects with HFpEF from both RELAX and NEAT-HFpEF were compared with PAVD subjects without evidence of HF or diastolic dysfunction. Continuous variables were reported as mean (standard deviation) or median (interquartile range) depending on data distribution, and categorical data were reported as frequency and percentage. sNEP was transformed using the natural logarithm prior to analyses to approximate a normal distribution. Linear regression was used to analyze log transformed sNEP with and without adjustment for comorbidities. Results of these analyses are presented using the adjusted mean sNEP and associated 95% confidence interval after back transformation to original scale. A secondary analysis was performed after propensity matching PAVD subjects to HFpEF subjects on age, BMI, DM, HTN, eGFR, and smoking history. Due to large differences between groups on these important characteristics, only a subset of HFpEF subjects were able to be matched.
To examine the associations between sNEP and baseline characteristics, sNEP was grouped into tertiles, and associations were tested as a trend across tertiles after adjusting for age, gender, body mass index, and smoking history. All tests were 2-sided, with a p value < 0.05 considered significant. All analyses were performed using SAS version 9.4 (SAS institute, Cary, NC).
RESULTS
Study population
The final study cohort included 242 HFpEF patients from RELAX/NEAT (n= 165 and n=77) respectively, and 891 from PAVD who had sNEP levels measured and were without HF or diastolic dysfunction at baseline (Table 1). Clinical characteristics were, in general, comparable between the HFpEF patients from RELAX and NEAT trials with similar sNEP levels and so were combined to form the overall HFpEF cohort (Supplemental Table 3).
Table 1.
Baseline Characteristics of HFpEF Patients Compared to Community Based Controls without HFpEF
| Controls (N=891) | HFpEF (N=242) | P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age | 58± 8 | 69± 10 | <.001 |
| Male, n (%) | 418(47%) | 117(48%) | 0.69 |
| Body mass Index, kg/m2I | 27.9± 4.9 | 34.2± 7.3 | <.001 |
| sNEP level (ng/mL), median (Q1, Q3) | 4.80(1.0, 43.9) | 1.55 (0.5, 25.0) | <.001 |
| Current/Former Smoker, n (%) | 453 (51%) | 144 (60%) | 0.011 |
| Prior MI history, n (%) | 15 (2%) | 25 (10%) | <.001 |
| Prior percutaneous coronary intervention, n (%) | 25 (3%) | 37 (15%) | <.001 |
| Prior coronary artery bypass graft history, n (%) | 13 (1%) | 38 (16%) | <.001 |
| Hypertension, n (%) | 157 (18%) | 207 (86%) | <.001 |
| Stroke, n (%) | 4 (0%) | 16 (7%) | <.001 |
| Diabetes, n (%) | 43 (5%) | 97 (40%) | <.001 |
| Hyperlipidemia, n (%) | 99 (12%) | 174 (72%) | <.001 |
| Calculated GFR (MDRD), median (Q1, Q3) | 85 (74,90) | 56 (43, 73) | <.001 |
| NT pro BNP (pg/mL), median (Q1, Q3) | 48 (20,87) | 497 (149,1075) | <.001 |
| heart rate (bpm) | 70±10 | 70± 12 | 0.45 |
| systolic blood pressure (mmHg) | 127±18 | 128±17 | 0.16 |
| diastolic blood pressure (mmHg) | 73±10 | 70±10 | 0.003 |
| Medication use | |||
| ACE inhibitor use, n (%) | 42 (5%) | 97 (40%) | <.001 |
| angiotensin receptor blocker use, n (%) | 7 (1%) | 68 (28%) | <.001 |
| beta blocker use, n (%) | 79 (10%) | 176 (73%) | <.001 |
| calcium channel blocker use, n (%) | 36 (4%) | 69 (29%) | <.001 |
| statin use, n (%) | 99 (12%) | 147 (61%) | <.001 |
| furosemide equivalent use, n (%) | 86 (11%) | 173 (71%) | <.001 |
| Echocardiography | |||
| LV diastolic dimension (cm) | 4.9±0.4 | 4.7±0.6 | <.001 |
| LV systolic dimension (cm) | 2.9±0.4 | 3.0±0.6 | 0.18 |
| PW diastolic thickness (cm) | 0.9±0.1 | 1.0±0.2 | 0.12 |
| MV inflow: E velocity at leaf tip (m/sec) | 0.7±0.1 | 1.0±0.3 | <.001 |
| E/A ratio | 1.2±0.3 | 1.6±1.0 | <.001 |
| Ejection fraction (%) | 64±5 | 64±9 | 0.81 |
| pulmonary artery systolic pressure (mmHg) | 22±4 | 41±13 | <.001 |
| LV mass index (gm/m2) | 92±19 | 81±27 | <.001 |
| E/e′ (m/sec) | 8±2 | 17±10 | <.001 |
NEP- neprilysin, MI- myocardial infarction, ACE- angiotensin converting enzyme, GFR- glomerular filtration rate, LV- left ventricular, PW- posterior wall, MV-mitral valve
HFpEF characteristics
The HFpEF patients represented a typical HFpEF demographic and were elderly (mean age 69 ± 10 years), obese (mean BMI 34 ± 7 kg/m2), with a high prevalence of hypertension (86%) and diabetes (40%). More than half of this population (n=144, 60%) were prior or active smokers. At baseline, the median calculated glomerular filtration rate was decreased at 56 ml/min/1.73m2 (IQR 43 – 73) and median baseline NT proBNP was elevated at 497 pg/mL (IQR 149 – 1075). Baseline ejection fraction was preserved at 64 ± 9 %, with an elevated mean E/e’ ratio of 18 ± 10 m/sec and pulmonary artery systolic pressure of 41 ± 13 mmHg.
Control characteristics
The control subjects from PAVD (n= 891) were middle aged (mean age 58 ± 8) with a mean BMI in the overweight range (28 ± 5 kg/m2). Approximately half of the population (n= 453, 51%) were prior or active smokers and subjects also had hypertension (18%) or diabetes (5%). The median calculated GFR was preserved at 85 (IQR 74 – 90), median NT proBNP was normal 48 (IQR 20 – 87) as was the ejection fraction (64 ± 5%), and mean E/e’ ratio was 8 ± 2 m/sec. As expected, clinical characteristics and echocardiographic parameters were significantly different between HFpEF subjects and controls (Table 1).
Tertiles of sNEP across control and HFpEF subjects
The non-HF PAVD control group was split into tertiles of sNEP (Table 2) to determine variables associated with sNEP in a large community-based cohort. In this group, there was a clear association between smoking and lower levels of sNEP (p <.001). There was no association between sNEP tertiles and age, gender, BMI, diabetes, hypertension, NT proBNP levels or cardiac structure and function determined by echocardiography in this cohort.
Table 2.
Tertiles of Circulating Soluble Neprilysin in Controls
| Tertile 1: 0.98–1.61 (N= 297) | Tertile 2: 1.62–25.5 (N= 297) | Tertile 3: 25.6 – 8427 (N=297) | Age/Gender/Smoking/adjusted P value (age/gender adjusted only) | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 58 ± 8 | 58 ± 8 | 58 ± 8 | 0.82 (0.87) |
| Male, n (%) | 136 (46%) | 134 (45%) | 148 (50%) | 0.10 (0.33) |
| Body Mass Index, kg/m2I | 28 ± 5 | 28 ± 5 | 28 ± 5 | 0.76 (0.62) |
| sNEP level (ng/mL), median (Q1, Q3) | 0.7 (0.4, 1.0) | 4.8 (2.5, 11.2) | 122.7 (43.9, 627.5) | |
| Current/Former Smoker, n (%) | 169 (57%) | 159 (54%) | 125 (42%) | < .001 |
| Prior MI history, n (%) | 5 (2%) | 4 (1%) | 6 (2%) | 0.8 (0.83) |
| Prior percutaneous coronary intervention, n (%) | 5 (2%) | 10 (3%) | 10 (3%) | 0.23 (0.26) |
| Prior coronary artery bypass graft history, n (%) | 2 (1%) | 3 (1%) | 8 (3%) | 0.06 (0.06) |
| Hypertension, n (%) | 57 (19%) | 51 (17%) | 49 (16%) | 0.40 (0.38) |
| Stroke, n (%) | 1 (0%) | 1 (0%) | 2 (1%) | 0.46 (0.55) |
| Atrial Fib/Flutter, n (%) | 9 (3%) | 5 (2%) | 5 (2%) | 0.20 (0.22) |
| Diabetes, n (%) | 16 (5%) | 15 (5%) | 12 (4%) | 0.47 (0.40) |
| Hyperlipidemia, n (%) | 31 (12%) | 32 (12%) | 36 (13%) | 0.65 (0.67) |
| Calculated GFR (MDRD), median (Q1, Q3) | 85 (74, 95) | 85 (74, 90) | 84 (74, 90) | 0.73 (0.51) |
| NT pro BNP (pg/mL), median (Q1, Q3) | 55 (21, 89) | 43 (18, 88) | 47 (22, 85) | 0.94 (0.81) |
| Heart rate (bpm) | 70 ± 10 | 70 ± 10 | 70 ± 10 | 0.51 (0.58) |
| Systolic blood pressure (mmHg) | 127 ± 18 | 126 ± 18 | 126 ± 17 | 0.38 (0.34) |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 72 ± 10 | 73 ± 9 | 0.60 (0.55) |
| Medication use | ||||
| ACE inhibitor use, n (%) | 16 (6%) | 12 (4%) | 14 (5%) | 0.54 (0.56) |
| Angiotensin receptor blocker use, n (%) | 2 (1%) | 3 (1%) | 2 (1%) | 0.93 (0.96) |
| Beta blocker use, n (%) | 39 (15%) | 23 (8%) | 17 (6%) | 0.001 (<.001) |
| Calcium channel blocker use, n (%) | 8 (3%) | 11 (4%) | 17 (6%) | 0.06 (0.07) |
| Statin use, n (%) | 31 (12%) | 32 (12%) | 36 (13%) | 0.65 (0.67) |
| Furosemide equivalent use, n (%) | 29 (11%) | 31 (11%) | 26 (10%) | 0.58 (0.73) |
| Echocardiography | ||||
| LV diastolic dimension (cm) | 4.9 ± 0.5 | 4.9 ± 0.4 | 4.9 ± 0.4 | 0.45 (0.36) |
| LV systolic dimension (cm) | 2.9 ± 0.4 | 2.9 ± 0.4 | 2.9 ± 0.4 | 0.42 (0.37) |
| PW diastolic thickness (cm) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.80 (0.80) |
| MV inflow: E velocity at leaf tip (m/sec) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.76 (0.95) |
| MV inflow: A velocity at leaf tip (m/sec) | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.87 (0.90) |
| E/A ratio | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.86 (0.93) |
| Ejection fraction (%) | 64 ± 5 | 64 ± 5 | 63 ± 5 | 0.54 (0.61) |
| Pulmonary artery systolic pressure (mmHg) | 22 ± 4 | 22 ± 4 | 22 ± 4 | 0.70 (0.69) |
| E/e’ (m/sec) | 8 ± 2 | 8 ± 2 | 8 ± 1 | 0.65 (0.63) |
| LV mass index (gm/m2) | 93 ± 19 | 91 ± 17 | 93 ± 18 | 0.92 (0.87) |
To further investigate non-linear relationships between low and high sNEP, the HFpEF group was also split into tertiles of sNEP levels (Table 3). The lowest tertile group (n=85), had sNEP levels <0.6 ng/ml, the highest tertile group (n=80) had circulating levels of NEP above 9.4 ng/ml, with the intermediate group (n=77) had levels in between. After adjustment for age, gender, and smoking history, there was a paradoxical association of low sNEP with hyperlipidemia (p= 0.005) and statin use (p=0.03) in the lowest tertile group compared to the mid and the highest tertile group. There also was a trend towards an association with a higher prevalence of hypertension (p=0.09) and ACE-I use (p=0.01) in the lowest tertile group. In contrast, there was a higher prevalence of baseline NYHA Class III/IV symptoms in HFpEF patients in the highest tertile (51%) versus the lowest tertile (35%), p=0.08. However, there did not appear to be an association with sNEP tertiles and NT proBNP levels, echocardiographie measurements, or prior HF hospitalization.
Table 3.
Tertiles of Circulating Soluble Neprilysin (ng/mL) in HFpEF
| Tertile 1: 0.1 –0.6 (N= 85) | Tertile 2: 0.7 – 9.3 (N= 77) | Tertile 3: 9.4 – 2222 (N=80) | Age/Gender/Smoking/adjusted P value (age/gender adjusted) | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Study, n (%) | 0.11 | |||
| -RELAX | 51 (60%) | 58 (75%) | 56 (70%) | (0.15) |
| -NEAT | 34 (40%) | 19 (25%) | 24 (30%) | |
| Age | 69 ± 9 | 69 ± 10 | 68 ± 11 | 0.9 (0.82) |
| Male, n (%) | 45 (53%) | 38 (49%) | 34 (43%) | 0.36 (0.17) |
| Minority race, n (%) | 12 (14%) | 3 (4%) | 6 (8%) | 0.14 (0.12) |
| Body Mass Index, kg/m2I | 34 ± 7 | 34 ± 7 | 34 ± 8 | 0.64 (0.82) |
| Cigarette Smoking, n (%) | 0.19 | |||
| -Current | 8 (10%) | 10 (13%) | 7 (9%) | |
| -Quit < 6 mo | 1 (1%) | 3 (4%) | 0 (0%) | |
| -Quit > 6 mo | 47 (56%) | 32 (42%) | 36 (46%) | |
| -Never | 28 (33%) | 31 (41%) | 36 (46%) | |
| Current/Former Smoker, n (%) | 56 (67%) | 45 (59%) | 43 (54%) | 0.14 |
| Prior MI history, n (%) | 8 (7%) | 9 (8%) | 0.80 | |
| Prior percutaneous coronary intervention, n (%) | 15 (18%) | 8 (10%) | 14 (18%) | 0.62 (0.86) |
| Prior coronary artery bypass graft history, n (%) | 14 (16%) | 11 (14%) | 13 (16%) | 0.73 (0.77) |
| Hypertension, n (%) | 76 (89%) | 68 (88%) | 63 (79%) | 0.09 (0.09) |
| Transient ischemic attack history, n (%) | 7 (8%) | 4 (5%) | 6 (8%) | 0.89 (0.77) |
| Stroke, n (%) | 5 (6%) | 5 (6%) | 6 (8%) | 0.59 (0.61) |
| Arrhythmia history, n (%) | 44 (52%) | 31 (40%) | 40 (50%) | 0.84 (0.94) |
| Diabetes, n (%) | 41 (48%) | 23 (30%) | 33 (41%) | 0.48 (0.41) |
| Hyperlipidemia, n (%) | 69 (81%) | 56 (73%) | 49 (61%) | 0.005 (0.006) |
| Chronic obstructive pulmonary disease, n (%) | 18 (21%) | 13 (17%) | 12 (15%) | 0.57 (0.35) |
| Baseline peripheral edema, n (%) | 0.48 (0.53) | |||
| -None | 38 (45%) | 34 (44%) | 31 (39%) | |
| -Trace | 32 (38%) | 29 (38%) | 36 (45%) | |
| -Moderate | 13 (15%) | 14 (18%) | 13 (16%) | |
| -Severe | 1 (1%) | 0 (0%) | 0 (0%) | |
| Baseline NYHA heart failure class, n (%) | 0.08 (0.05) | |||
| -1 | 0 (0%) | 0 (0%) | 1 (1%) | |
| -2 | 55 (65%) | 34 (44%) | 38 (48%) | |
| -3 | 30 (35%) | 43 (56%) | 40 (50%) | |
| -4 | 0 (0%) | 0 (0%) | 1 (1%) | |
| Baseline NYHA class 3/4, n (%) | 30 (35%) | 43 (56%) | 41 (51%) | 0.08 (0.05) |
| Creatinine (mg/dL) median (Q1, Q3) | 1.10 (0.9, 1.5) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.3) | 0.89 (0.92) |
| NT pro BNP (pg/mL), median (Q1, Q3) | 480 (140, 1252) | 481 (144, 861) | 522 (188, 1133) | 0.95 (0.98) |
| Heart rate (bpm) | 69 ± 13 | 70 ± 10 | 70 ± 14 | 0.97 (0.79) |
| Systolic blood pressure (mmHg) | 129 ± 16 | 127 ± 16 | 129 ± 17 | 0.92 (0.82) |
| Diastolic blood pressure (mmHg) | 70 ± 10 | 71 ± 10 | 70 ± 11 | 0.98 (0.89) |
| Number of HF hospitalization within 12 months | 0.85 (0.93) | |||
| -0 | 62 (73%) | 58 (75%) | 59 (74%) | |
| -1 | 13 (15%) | 16 (21%) | 15 (19%) | |
| -2 | 6 (7%) | 1 (1%) | 2 (3%) | |
| -3 | 1 (1%) | 1 (1%) | 1 (1%) | |
| -4 | 2 (2%) | 0 (0%) | 1 (1%) | |
| -5 | 1 (1%) | 0 (0%) | 0 (0%) | |
| -6 | 0 (0%) | 0 (0%) | 1 (1%) | |
| -7 | 0 (0%) | 0 (0%) | 1 (1%) | |
| -8 | 0 (0%) | 1 (1%) | 0 (0%) | |
| Medication use | ||||
| ACE inhibitor use, n (%) | 43 (51%) | 29 (38%) | 25 (31%) | 0.01 (0.01) |
| Angiotensin receptor blocker use, n (%) | 23 (27%) | 19 (25%) | 26 (33%) | 0.63 (0.47) |
| Beta blocker use, n (%) | 66 (78%) | 50 (65%) | 60 (75%) | 0.87 (0.83) |
| Aldosterone antagonist use, n (%) | 12 (14%) | 11 (14%) | 9 (11%) | 0.80 (0.64) |
| Calcium channel blocker use, n (%) | 22 (26%) | 23 (30%) | 24 (30%) | 0.50 (0.55) |
| Statin use, n (%) | 59 (69%) | 46 (60%) | 42 (53%) | 0.03 (0.03) |
| Furosemide equivalent use, n (%) | 66 (78%) | 49 (64%) | 58 (73%) | 0.52 (0.44) |
| Metolazone use, n (%) | 5 (6%) | 3 (4%) | 4 (5%) | 0.89 (0.85) |
| Echocardiography | ||||
| LV diastolic dimension (cm) | 4.8 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.6 | 0.70 (0.73) |
| LV systolic dimension (cm) | 3.0 ± 0.7 | 2.9 ± 0.5 | 3.0 ± 0.6 | 0.88 (0.63) |
| PW diastolic thickness (cm) | 1.0 ± 0.23 | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.55 (0.51) |
| MV inflow: E velocity at leaf tip (m/sec) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.78 (0.78) |
| MV inflow: A velocity at leaf tip (m/sec) | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.42 (0.46) |
| Left atrial 4ch area (cm) | 25 ± 7 | 26 ± 7 | 26 ± 7 | 0.09 (0.07) |
| Ejection fraction (%) | 64 ± 8 | 65 ± 9 | 63 ± 10 | 0.92 (0.75) |
| Pulmonary artery systolic pressure (mmHg) | 41 ± 12 | 38 ± 12 | 42 ± 14 | 0.91 (0.91) |
| Filling pressure septal (medial) E/e’ (m/sec) | 17 ± 10 | 16 ± 8 | 19 ± 10 | 0.59 (0.57) |
| LV mass index (gm/m2) | 84 ± 23 | 82 ± 34 | 78 ± 22 | 0.49 (0.48) |
Comparison of sNEP levels between HFpEF and controls
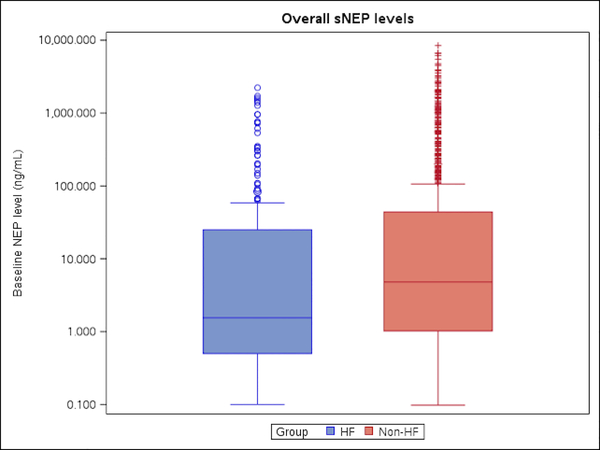
Despite age and comorbidity burden being significantly higher in patients with HFpEF, median sNEP levels were lower in these patients (3.5 ng/ml; CI 2.5, 4.8 compared with controls (8.5 ng/ml; CI 7.2,10.0) p< 0.001 (Central Illustration). After adjusting for age, gender, BMI, and smoking history, sNEP levels remained lower in the HFpEF subjects (4.0 ng/ml; CI 2.6, 5.0) compared with controls (8.2 ng/ml; CI 6.8, 9.7), p =0.002 (Table 4a). With further adjustment for hypertension as well, this association remained significant with sNEP being lower in HFpEF (4.2 ng/ml; CI 2.8, 6.4) compared with controls (8.0 ng/ml; CI 6.7, 9.6), p=0.01. Despite the subset of HFpEF patients with Class III/IV symptoms having higher sNEP levels compared to Class I/II patients, this sub-group had sNEP levels that were lower than controls on average (similar to the overall HFpEF group), p = 0.01 (Table 4b).
Central Illustration. Circulating Soluble Neprilysin Levels.
This figure depicts median circulating sNEP in patients with heart failure with preserved ejection fraction (HFpEF) compared to community based controls without HFpEF or diastolic dysfunction, and illustrates that sNEP levels are lower in HFpEF compared with normal controls.
Table 4a.
Soluble Neprilysin (ng/mL) Levels in HFpEF Patients Compared to Community Based Controls without HFpEF
| Adjustment | HF adj mean (CI) N=242 | Non-HF adj mean (CI) N=891 | p-value |
|---|---|---|---|
| None | 3.5 (2.5, 4.8) | 8.5 (7.2, 10.0) | <0.001 |
| Smoking hx | 3.6 (2.6, 5.0) | 8.4 (7.1, 9.8) | <0.001 |
| Age, Gender, BMI, Smoking hx | 4.0 (2.7, 5.9) | 8.2 (6.8, 9.7) | 0.002 |
| Age, Gender, BMI, Smoking hx, Ace-I | 4.3 (2.8, 6.4) | 8.1 (6.7, 9.8) | 0.009 |
| Age, Gender, BMI, Smoking hx, Ace-I, Hyperlipidemia | 4.5 (2.9, 7.0) | 8.0 (6.6, 9.7) | 0.04 |
| Age, Gender, BMI, Smoking hx, HTN | 4.2 (2.8, 6.4) | 8.0 (6.7, 9.6) | 0.01 |
Table 4b.
Soluble Neprilysin Levels (ng/mL) in HFpEF NYHA Class III/IV Subset Compared to Community Based Controls without HFpEF
| Adjustment | HF adj mean (CI) N=114 | Non-HF adj mean (CI) N=891 | p-value |
|---|---|---|---|
| None | 4.5 (2.8, 7.2) | 8.5 (7.2, 10.0) | 0.01 |
| Smoking hx | 4.5 (2.8, 7.1) | 8.4 (7.1, 9.9) | 0.01 |
| Age, Gender, BMI, Smoking hx | 5.5 (3.1, 9.5) | 8.2 (7.0, 9.7) | 0.18 |
| Age, Gender, BMI, Smoking hx, Ace-I | 5.5 (3.1, 9.9) | 8.3 (7.0, 10.0) | 0.20 |
| Age, Gender, BMI, Smoking hx, Ace-I, Hyperlipidemia | 5.4 (2.9, 9.9) | 8.3 (7.0, 10.0) | 0.19 |
| Age, Gender, BMI, Smoking hx, HTN | 5.4 (3.0, 9.8) | 8.2 (6.9, 9.8) | 0.18 |
Propensity matching
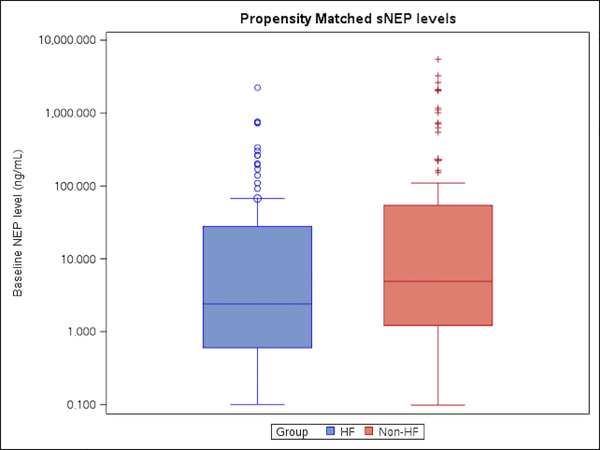
As described above, there was no association of co-morbidities with sNEP levels in the large control group other than smoking. However, to more comprehensively adjust for imbalances between comorbidities, the two groups were propensity matched by age, BMI, diabetes, hypertension, smoking history, and renal function (Table 5). In this subgroup of 111 subjects from each cohort, median sNEP levels remained lower in the HFpEF group 2.4 ng/ml (IQR 0.6, 27.7) compared with controls 4.9 ng/ml (IQR 1.2, 54.2) (p= 0.02) (Figure 1).
Table 5.
Propensity Matched Baseline Characteristics of HFpEF Patients Compared to Community Based Controls without HFpEF
| Non-Heart Failure (N=111) | HF (Heart Failure) (N=111) | P value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age | 65.7 ± 9.2 | 66.3 ± 10.8 | 0.65 |
| Male, n (%) | 53 (48%) | 46 (41%) | 0.34 |
| Body mass Index, kg/m2I | 30.8 ± 5.2 | 31.9 ± 6.4 | 0.19 |
| sNEP level (ng/mL), median (Q1, Q3) | 4.9 (1.2, 54.2) | 2.4 (0.6, 27.7) | 0.02 |
| Current/Former Smoker, n (%) | 57 (51%) | 59 (53%) | 0.79 |
| Prior MI history, n (%) | 8 (7%) | 9 (8%) | 0.80 |
| Prior percutaneous coronary intervention, n (%) | 13 (12%) | 9 (8%) | 0.37 |
| Prior coronary artery bypass graft history, n (%) | 4 (4%) | 18 (16%) | 0.002 |
| Hypertension, n (%) | 81 (73%) | 79 (71%) | 0.76 |
| Stroke, n (%) | 1 (1%) | 8 (7%) | 0.02 |
| Arrhythmia history, n (%) | 5 (5%) | 30 (27%) | < .001 |
| Diabetes, n (%) | 20 (18%) | 25 (23%) | 0.40 |
| Hyperlipidemia, n (%) | 20 (19%) | 76 (68%) | <.001 |
| Calculated GFR (MDRD), median (Q1, Q3) | 71 (62, 77) | 69 (54, 82) | 0.28 |
| NT pro BNP (pg/mL), median (Q1, Q3) | 79 (44, 126) | 360 (111, 861) | <.001 |
| Heart rate (bpm) | 70 ± 12 | 71 ± 11 | 0.48 |
| Systolic blood pressure (mmHg) | 136 ± 17 | 126 ± 16 | <.001 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 72 ± 10 | 0.77 |
| Medication use | |||
| ACE inhibitor use, n (%) | 15 (14%) | 38 (34%) | <.001 |
| Angiotensin receptor blocker use, n (%) | 5 (5%) | 30 (27%) | <.001 |
| Beta blocker use, n (%) | 31 (29%) | 73 (66%) | <.001 |
| Calcium channel blocker use, n (%) | 19 (18%0 | 31 (28%) | 0.07 |
| Statin use, n (%) | 20 (19%) | 57 (51%) | <.001 |
| Furosemide equivalent use, n (%) | 44 (41%) | 70 (63%) | <.001 |
| Echocardiography | |||
| LV diastolic dimension (cm) | 5.0 ± 0.4 | 4.7 ± 0.6 | <.001 |
| LV systolic dimension (cm) | 2.9 ± 0.4 | 3.0 ± 0.6 | 0.53 |
| PW diastolic thickness (cm) | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.01 |
| MV inflow: E velocity at leaf tip (m/sec) | 0.7 ± 0.1 | 1.0 ± 0.3 | <.001 |
| MV inflow: A velocity at leaf tip (m/sec) | 0.7 ± 0.2 | 0.7 ± 0.3 | 0.99 |
| Ejection fraction (%) | 65 ± 5 | 64 ± 9 | 0.74 |
| Pulmonary artery systolic pressure (mmHg) | 23 ± 5 | 41 ± 14 | <.001 |
| LV mass index (gm/m2) | 102 ± 20 | 76 ± 24 | <.001 |
Figure 1. Circulating Soluble Neprilysin Levels in Propensity Matched Cohorts.
To more comprehensively adjust for imbalances between comorbidities, the normal control group was propensity matched to the HFpEF group based on age, body mass index, diabetes, hypertension, renal dysfunction, and smoking history. This figure depicts that after propensity matching, sNEP levels remained lower in the HFpEF group compared to normal controls.
DISCUSSION
This is the first study to directly compare circulating NEP levels between rigorously defined HFpEF subjects and controls that had no evidence of diastolic dysfunction or HF at the time of evaluation or long–term follow up. We have previously used the PAVD population to interpret the determinants and concentration of biomarkers in a normal subset of subjects (26). There were lower sNEP levels in HFpEF patients compared with controls despite worse cardiac remodeling, elevated filling pressures and comorbidity burden in HFpEF. These results remained robust despite both rigorous multivariable adjustment and propensity matching. sNEP levels also failed to correlate with NP levels, cardiovascular remodeling, or HF hospitalization. These data fundamentally question our simplistic assumptions of sNEP as a potential biomarker in HFpEF, contrary to its recent importance in HFrEF.
Previous literature has focused on the importance of NEP as a biomarker in HFrEF given its significant association with morbidity and mortality, and the decrease in mortality associated with NEP inhibition (15). A linear correlation between NEP levels and adverse outcomes in HFrEF has been demonstrated in a prior study, but sNEP levels in ambulatory HFrEF patients have not been compared with normal controls (17); therefore inferences regarding sNEP levels in HFrEF as compared with controls cannot be made.
It is important to emphasize the complexity of the NEP - natriuretic peptide pathway. NEP is very non-specific in terms of substrate selection, and breaks down both beneficial peptides (25–27) in addition to harmful substrates. Therefore, the pathophysiological role and levels of sNEP may differ depending on the complex interplay of these various substrates in the pathophysiology of the HF syndrome. HFpEF remains an incompletely understood disease process with systemic inflammation believed to drive cardiac remodeling. In addition, natriuretic peptide elevation is not universal in HFpEF given many patients have normal BNP levels despite elevated filling pressures (28), and on average BNP levels are lower in HFpEF compared to HFrEF (29). Taken together our findings of lower sNEP compared with controls without HFpEF question whether sNEP concentration is a reliable theranostic biomarker in HFpEF or whether NEP plays an important role in the pathophysiology of HFpEF.
We recently investigated sNEP levels to determine clinical correlates and relationship to the onset of cardiovascular disease in the general population (n=1536) (30). In this large community based cohort of participants without HF, there was no relationship between sNEP and circulating ANP, BNP, or CNP, a finding which highlights the complexity of the role and contribution of sNEP in the proteolysis of natriuretic peptides. This study importantly also found that lower sNEP levels were associated with diastolic dysfunction, dyslipidemia and hypertension similar to our current findings in HFpEF. Overall, our study extends the concept that lower sNEP levels are associated with not only diastolic dysfunction in the community as observed in PAVD, but also with clinical HFpEF.
Goliasch et al (18) measured sNEP in a well phenotyped single center HFpEF cohort and similar to our findings found its association with smoking, but did not find any association with functional or cardiac parameters including symptoms, 6 minute walk test, invasive hemodynamics, left ventricular fibrosis by biopsy or cardiac MRI. Neprilysin levels also failed to correlate with clinical outcomes (17). The sNEP levels in our HFpEF cohort (3.5 ng/ml; CI 2.5, 4.8) were also comparable to that found in Goliasch et al’s cohort of 144 HFpEF patients (2.9 ng/mL) therefore demonstrating the validity and reproducibility of our sNEP assay (22). Our study builds upon the findings by Goliasch et al with a larger, multicenter HFpEF cohort, and for the first time compares these patients with controls, clarifying its role as a biomarker in HFpEF.
Limitations of this study include the possibility that circulating sNEP levels may not correlate with biologically active tissue levels, which is a common limitation amongst all serologic biomarker research. However, prior investigations have demonstrated low lung tissue NEP expression (both at a protein and transcript level) in smokers (31,32) which is concordant with our independent finding in a prior study of low circulating NEP levels in smokers when analyzed in a large community cohort (33). This trend of lower circulating NEP levels in smokers was also observed in Goliasch et al’s smaller cohort of HFpEF patients (71% smokers in the lowest sNEP tertile versus 52% in the highest tertile) (18).The lower circulating levels in smokers may be related to increased urinary excretion of NEP as compared with non-smokers (34). The significant association between lower levels of circulating sNEP and smoking in the general community and HFpEF patients, and its independent corroboration in prior observations of lower NEP protein expression in smokers’ lung tissue and higher NEP excretion in smokers, strengthens the validity of our results and affirms the reliability of our assay.
We did not measure sNEP activity and assess its relationship to HFpEF as compared to controls. A previous study in 639 patients by Vodovar et al did not demonstrate a correlation between sNEP levels and NEP activity (35). Also, Nougué et al demonstrated that switching 73 HFrEF patients from an ACEi or ARB to sacubitril/valsartan was associated with a dose-dependent decrease in sNEP activity, while sNEP levels remained unchanged, which highlights that sNEP levels may not correlate with enzymatic activity (36). These findings are not surprising because enzymatic activity in the blood can vary based on the concentration and potential interference of circulating factors in the blood that may serve as substrates of NEP. We demonstrate this concept by comparing NEP levels with NEP activity in buffer solution, and unlike the findings in plasma as described by Vodovar et al, we illustrate a direct linear correlation between NEP concentration and activity (R2=0.99) (Supplemental Figure 1). We have also previously shown a similar linear correlation between NEP cellular expression and enzymatic activity (37). Finally, given the lack of ethnic diversity in the PAVD control group, generalizability to other racial populations may not be valid
CONCLUSIONS
Our study shows for the first time that patients with HFpEF have lower levels of sNEP when compared with controls without diastolic dysfunction or HF. In addition, there was no association in HFpEF between baseline sNEP with baseline NPs, cardiac structure or function. These findings may have important clinical implications, on the role of sNEP as a biomarker and its potential importance in predicting clinical efficacy with NEP inhibition in HFpEF. Further investigation is needed to elucidate the complex relationship of sNEP concentration, sNEP activity, and NPs and its feedback loops in subjects with and without HFpEF.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
Circulating sNEP levels have not been measured in HFpEF patients and compared to well-defined controls without diastolic dysfunction or HFpEF. After multivariable adjustment including age and gender and propensity matching including multiple comorbidities such as diabetes and hypertension sNEP levels were lower in HFpEF compared with controls.
Translational Outlook
Higher sNEP levels were associated with increased morbidity and mortality and inhibition of NEP with sacubitril/valsartan improved survival in HFrEF. Whether sNEP levels can predict efficacy of sacubitril/valsartan in HFpEF remains to be proven.
Acknowledgments
Funding/Disclosures:
-Dr. Naveen Pereira is supported by National Institute on Aging Grant R21AG53512
-Dr. Michael Felker has received research grants from NHLBI, American Heart Association, Amgen, Merck, Cytokinetics, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, BMS, Medtronic, Cardionomic, Relypsa, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, and SC Pharma
-The remaining authors have nothing to disclose
Abbreviations List
- HFrEF
heart failure with reduced ejection fraction
- sNEP
soluble neprilysin
- HFpEF
heart failure with preserved ejection fraction
- BMI
body mass index
- NYHA
New York Heart Association
- NP
natriuretic peptides
- HF
heart failure
- EF
ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387–93. [DOI] [PubMed] [Google Scholar]
- 2.Huntley BK, Sandberg SM, Noser JA et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol 2006;209:943–9. [DOI] [PubMed] [Google Scholar]
- 3.Itoh H, Pratt RE, Dzau VJ. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest 1990;86:1690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangaralingham SJ, Huntley BK, Martin FL et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension 2011;57:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen WM, de Jong PE, van der Hem GK, de Zeeuw D. Effect of human atrial natriuretic peptide on blood pressure after sodium depletion in essential hypertension. Br Med J (Clin Res Ed) 1986;293:351–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillingham MA, Anderson RJ. Inhibition of vasopressin action by atrial natriuretic factor. Science 1986;231:1572–3. [DOI] [PubMed] [Google Scholar]
- 7.Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 1987;326:697–8. [DOI] [PubMed] [Google Scholar]
- 8.Steele MK, Gardner DG, Xie PL, Schultz HD. Interactions between ANP and ANG II in regulating blood pressure and sympathetic outflow. Am J Physiol 1991;260:R1145–51. [DOI] [PubMed] [Google Scholar]
- 9.Yang RH, Jin HK, Wyss JM, Chen YF, Oparil S. Pressor effect of blocking atrial natriuretic peptide in nucleus tractus solitarii. Hypertension 1992;19:198–205. [DOI] [PubMed] [Google Scholar]
- 10.Marin-Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 1986;324:473–6. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg H, Honrath U, Chong CK, Wilson DR. Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. Am J Physiol 1986;250:F963–6. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 13.Charles CJ, Espiner EA, Nicholls MG et al. Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Physiol 1996;271:R373–80. [DOI] [PubMed] [Google Scholar]
- 14.Chen HH, Lainchbury JG, Harty GJ, Burnett JC Jr. Maximizing the natriuretic peptide system in experimental heart failure: subcutaneous brain natriuretic peptide and acute vasopeptidase inhibition. Circulation 2002;105:999–1003. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SD, Zile M, Pieske B et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. [DOI] [PubMed] [Google Scholar]
- 17.Bayes-Genis A, Barallat J, Galan A et al. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol 2015;65:657–65. [DOI] [PubMed] [Google Scholar]
- 18.Goliasch G, Pavo N, Zotter-Tufaro C et al. Soluble neprilysin does not correlate with outcome in heart failure with preserved ejection fraction. Eur J Heart Fail 2016;18:89–93. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Chen HH, Borlaug BA et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakeri R, Levine JA, Koepp GA et al. Nitrate’s effect on activity tolerance in heart failure with preserved ejection fraction trial: rationale and design. Circ Heart Fail 2015;8:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane GC, Karon BL, Mahoney DW et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82. [DOI] [PubMed] [Google Scholar]
- 24.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009;119:2663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florkowski CM, Richards AM, Espiner EA, Yandle TG, Sybertz E, Frampton CM. Low-dose brain natriuretic peptide infusion in normal men and the influence of endopeptidase inhibition. Clin Sci (Lond) 1997;92:255–60. [DOI] [PubMed] [Google Scholar]
- 26.Richards AM, Crozier IG, Espiner EA, Yandle TG, Nicholls MG. Plasma brain natriuretic peptide and endopeptidase 24.11 inhibition in hypertension. Hypertension 1993;22:231–6. [DOI] [PubMed] [Google Scholar]
- 27.Richards AM, Wittert GA, Crozier IG et al. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J Hypertens 1993;11:407–16. [DOI] [PubMed] [Google Scholar]
- 28.Anjan VY, Loftus TM, Burke MA et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 2012;110:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwanaga Y, Nishi I, Furuichi S et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006;47:742–8. [DOI] [PubMed] [Google Scholar]
- 30.Reddy Y, Iyer S, Scott CG et al. Serum Neprilysin and Its Relationship to Cardiovascular Disease in the General Population. Circulation, 2018. [Google Scholar]
- 31.Wick MJ, Buesing EJ, Wehling CA et al. Decreased neprilysin and pulmonary vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AR, Ashton J, Schulz WW, Erdos EG. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis 1985;132:564–8. [DOI] [PubMed] [Google Scholar]
- 33.Bayes-Genis A, Barallat J, Richards AM. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J Am Coll Cardiol 2016;68:639–653. [DOI] [PubMed] [Google Scholar]
- 34.Nortier J, Bernard A, Roels H, Deschodt-Lanckman M, Gueuning C, Lauwerys R. Urinary neutral endopeptidase in workers exposed to cadmium: interaction with cigarette smoking. Occup Environ Med 1997;54:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodovar N, Seronde MF, Laribi S et al. Elevated Plasma B-Type Natriuretic Peptide Concentrations Directly Inhibit Circulating Neprilysin Activity in Heart Failure. JACC Heart Fail 2015;3:629–36. [DOI] [PubMed] [Google Scholar]
- 36.Nougue H, Pezel T, Picard F et al. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail 2018. [DOI] [PubMed] [Google Scholar]
- 37.Pereira NL, Aksoy P, Moon I et al. Natriuretic peptide pharmacogenetics: membrane metallo-endopeptidase (MME): common gene sequence variation, functional characterization and degradation. J Mol Cell Cardiol 2010;49:864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.