Abstract
The Nrf2-Keap1 pathway regulates transcription of a wide array of antioxidant and cytoprotective genes and offers critical protection against oxidative stress. This pathway has demonstrated benefit for a variety of retinal conditions. Retinal ischemia plays a pivotal role in many vision threatening diseases. Retinal vascular endothelial cells are an important participant in ischemic injury. In this setting, Nrf2 provides a protective pathway via amelioration of oxidative stress and inflammation. In this study, we investigated a potent small molecule inhibitor of the Nrf2-Keap1 protein-protein interaction (PPI), CPUY192018, for its therapeutic potential in retinal cells and retinal ischemia-reperfusion injury. In human retinal endothelial cells (HREC), treatment with CPUY192018 increased Nrf2 protein levels and nuclear translocation, stimulated Nrf2-ARE-induced transcriptional capacity, and induced Nrf2 target gene expression. Furthermore, CPUY192018 protected HREC against oxidative stress and inflammatory activation. CPUY192018 also activated Nrf2 and suppressed inflammatory response in macrophages. In the retinal ischemia-reperfusion (I/R) model, administration of CPUY192018 induced Nrf2 target gene activation in the retina. Both systemic and topical treatment with CPUY192018 rescued visual function after ischemia-reperfusion injury. Taken together, these findings indicate that small molecule Keap1-Nrf2 PPI inhibitors can activate the Nrf2 pathway in the retina and provide protection against retinal ischemic and inflammatory injury, suggesting Keap1-Nrf2 PPI inhibition in the treatment of retinal conditions.
Keywords: Ischemia, Nrf2, Inflammation, Oxidative stress, Retina, Endothelial cell
1. Introduction
Retinal ischemia is a major cause of visual loss and is implicated in the pathogenesis of multiple eye diseases including acute angle-closure glaucoma, retinal vascular occlusions, and diabetic retinopathy [1]. An important facet of retinal ischemia is ischemia-reperfusion (I/R), which occurs after restoration of blood supply to a tissue bed following an episode of ischemia. Induction of oxidative stress and inflammation play a major pathogenic role in the subsequent tissue injury, including neuroretinal dysfunction and death. Significant insights in therapy for retinal ischemia have arisen through the investigation of rodent models of ischemia-reperfusion (I/R). This ischemia-reperfusion leads to oxidative stress with production of reactive oxygen species (ROS) and inflammation [2-4] that result in retinal damage, including vascular injury [5,6] and neuronal dysfunction and/or neurodegeneration. Currently, there are no therapies for retinal ischemia.
Nrf2 (NF-E2-related factor 2) is a master regulator of the antioxidant response in multiple tissues and plays a major role in cytoprotection from endogenous and exogenous stresses [7,8]. Nrf2 serves as one of the most important cellular pathways in protecting against oxidative stress and also has significant anti-inflammatory effects. This transcription factor acts largely through binding to the antioxidant response element (ARE) to activate expression of a wide array of antioxidant and cytoprotective genes. Largely through investigations in experimental animal models, Nrf2 has been implicated as an important protective regulator in multiple retinal conditions, including diabetic retinopathy [9-13], age-related macular degeneration [14-17], uveitis [18], retinopathy of prematurity [19,20], and retinal ischemia-reperfusion [21-23]. Pharmacologic activation of Nrf2 has therefore emerged as an attractive strategy for multiple retinal diseases [24].
Kelch-like ECH-associated protein 1 (Keap1) is known to be the major inhibitory regulator of Nrf2. Under physiologic unstressed conditions, Keap1 binds to Nrf2 and directs it toward proteosomal degradation [25,26]. Inhibition of Keap1 by exogenous and endogenous molecules including reactive oxygen species can prevent this proteosomal degradation, resulting in nuclear translocation and accumulation of Nrf2 and its transcriptional activation of an array of cytoprotective and antioxidant genes [7,8].
Pharmacologic activation of Nrf2 has essentially focused on inhibition of Keap1 [27]. The vast majority of Keap1 inhibitors are compounds that covalently modify cysteine residues in Keap1, including naturally occurring compounds such as sulforaphane and triterpenoids such as bardoxolone methyl (CDDO-Me) [28]. Modification of these cysteine residues which act as redox sensors leads to conformational changes in Keap1, resulting in release of Nrf2 and inhibition of Nrf2 ubiquitination. These inhibitors have received great attention as therapeutic candidates for Nrf2 activation, including bardoxolone methyl, which has been investigated extensively in clinical trials for kidney disease [29]. Regrettably, a large trial investigating bardoxolone methyl for chronic kidney disease was terminated due to safety concerns [30].
Although Keap1 inhibition via covalent modification of cysteine residues is an effective approach for activating Nrf2, there are lingering concerns relating to target specificity and safety, given the pervasiveness of cysteine residues in cells [25]. Recently, attention has been directed toward a novel class of Nrf2 activators, namely Keap1-Nrf2 protein-protein interaction (PPI) inhibitors, whose development has been facilitated by the availability of the crystal structure of the Keap1 Kelch domain [25]. This approach which relies on non-electrophilic, non-covalent compounds offers an attractive alternative for Keap1 inhibition that does not depend on covalent modification. One of the initial compounds that was reported in this class of PPI inhibitors was CPUY192018 [31]. Indeed, the effectiveness of CPUY192018 as an Nrf2 activator has been demonstrated both in vitro and in vivo, providing cytoprotection for colonic cells and therapeutic benefits in an experimental model of ulcerative colitis [32]. CPUY192018, via Nrf2 activation, was similarly found to be cytoprotective for renal proximal tubular epithelial cells and therapeutic in a model of renal inflammation [33]. Keap1-Nrf2 PPI inhibition therefore represents a novel and promising approach for diseases characterized by excessive oxidative stress and inflammation.
Notably, Keap1-Nrf2 PPI inhibitors have predominantly been evaluated for peripheral inflammatory conditions [25]. Much less attention has been devoted to exploring the potential of this class of Nrf2 activator in diseases of the central nervous system and of the retina, an extension of the CNS. This is significant because many conditions of the retina and CNS require chronic administration of Nrf2 activating drug, necessitating a favorable safety profile. In addition, these central conditions pose additional challenges, including drug delivery and bioavailability due to the blood-retinal barrier and blood-brain barrier, respectively.
In this study, we investigated the therapeutic potential of CPUY192018, investigating this prototypical Keap1-Nrf2 PPI inhibitor on retinal endothelial cells and macrophages as well as evaluating this compound in vivo in a retinal ischemia-reperfusion injury model. We especially focused on retinal endothelial cells, given the important role of vascular endothelial cells in ischemic injury. We find that CPUY192018 can activate the Nrf2 pathway in human retinal vascular endothelial cells and stimulate Nrf2-ARE-induced transcriptional capacity, leading to induction of Nrf2 target genes. Additionally, CPUY192018 treatment has a cytoprotective effect on HREC against oxidative stress and inflammatory change. In vivo, both topical and systemic treatment with CPUY192018 rescued visual function after ischemia-reperfusion injury, with associated activation of Nrf2 target genes. These findings establish Keap1-Nrf2 PPI inhibitors as a viable approach for protecting against retinal ischemic and inflammatory injury. These PPI inhibitors could serve as treatments for retinal conditions.
2. Materials and methods
2.1. Cell culture
Human retinal endothelial cells (HRECs) (Cell Systems, Kirkland, WA), human monocytic cell line (THP-1 cells) (ATCC, Manassas, VA), and mouse macrophage cell line (RAW264.7 cells) (ATCC, Manassas, VA) were used for these studies. HRECs were cultured in dishes coated with fibronectin (Invitrogen, Carlsbad, CA). HRECs, THP-1, and RAW264.7 were grown in EGM2-MV medium, RPMI-1640 supplemented with 10% FBS and DMEM+ (Invitrogen) with 10% FCS respectively. Cells were maintained in a humidified 5% CO2 incubator at 37 °C throughout the entire study. Media were changed every 2–3 days.
2.2. ARE-luciferase activity assay in HRECs
HRECs were seeded into 12 well plates at a density of 8 × 104 cells per well. The next day, cells were transfected with phQR41-ARE-Luc and pRLSV40 using lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The next day, the medium was replaced with fresh EGM2MV. 48 h after transfection, the cells were treated with different doses of CPUY192018 for 16 h. ARE luciferase activity was measured using Promega's Dual-luciferase reporter assay system. Briefly, the cells were washed with PBS once and lysed in 100 μL 1x reporter lysis buffer by scraping, followed by freeze/thawing. 20 μL of the cell lysate was used to determine the luciferase activity and the luminescence was measured using GloMax 20/20 luminometer according to the manufacturer's protocol (Promega, Rosemont, WI).
2.3. Western blotting
Nuclear and cytoplasmic proteins were extracted using the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Waltham, US) according to the manufacturer's protocol. The proteins collected from fresh cells samples were then stored at −80 °C until use. The protein concentration was determined by Bio-Rad DC protein assay kit. Equal amounts of protein were applied to 10% SDS-PAGE and transferred to Hybond ECL nitrocellulose membrane (GE Healthcare). The membranes were incubated with anti-Nrf2 (Abcam, Cambridge, MA), monoclonal anti-histone (Cell Signaling Technology, Danvers, MA) and anti-β-tubulin (Sigma, St Louis, MO). Secondary antibodies were detected by the SuperSignal West Pico. The total Nrf2 protein was isolated from HRECs and RAW cells after indicated treatments using Laemmli sample buffer (BioRad Laboratories, Hercules, CA). The membranes respectively were incubated with anti-Nrf2 (Abcam) and anti-Nrf2 (Proteintech, Rosemont, IL). β-actin (Cell Signaling Technology) was used as the loading control. All band intensities were quantitated by ImageJ.
2.4. Immunofluorescence staining of Nrf2 in HRECs
Treated cells attached onto the fibronectin-coated coverglass were fixed in 4% paraformaldehyde (w/v) for 5 min at room temperature. After washing with PBS, cells were permeabilized in 0.5% Triton X-100 in PBS, then incubated with Nrf2 antibody (1:200) in PBS plus 4% BSA and 0.05% Tween 20 (PBST) overnight at 4 °C. Cells were then incubated with anti-rabbit IgG conjugated with Alexa flour 594 (Invitrogen) for 1 h at room temperature. Cell nuclei were stained with DAPI. Fluorescence was detected by laser scanning confocal microscopy (Zeiss, Wetzlar, Germany).
2.5. Dichlorofluorescein assay in HRECs
ROS production in HRECs were quantified by the dichlorofluorescein (DCF) assay. Sixteen hours after 50 μM CPUY192018 administration, HRECs were treated with or without tert-butyl hydroperoxide (TBH) (Sigma, St Louis, MO) for 1 h. CM-H2DCFDA (10 μmol/l, Invitrogen) was added, and the cells were incubated for 30 min. DCF fluorescence intensity was measured with a spectrophotometer (BMG Labtech, Durham, NC).
2.6. Rat model of retinal ischemia-reperfusion and treatment
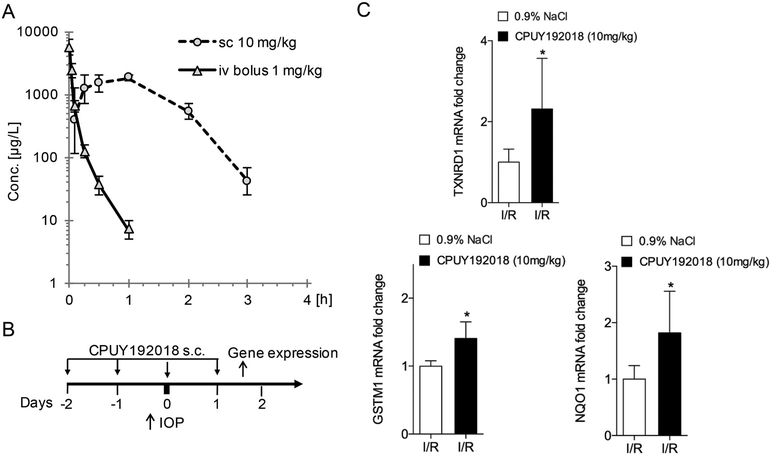
All animal studies were performed according to guidelines of the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine and/or according to the current law for protection of animals (German protection of animals act (2006/05/18 and EU directives 63/2010)) and by the institutional animal care and use committee of Bayer AG. All study procedures involving rats were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Animals were treated with 0.9% NaCl and 10 mg/kg CPUY192018 by subcutaneous injection or 10 μl topical CPUY192018 in aqueous vehicle 1 mg/ml, BID daily starting 2 days before IOP elevation until 2 days after IOP elevation. For the ischemia/reperfusion animal model, male Wistar rats were anesthetized with Rompun (Bayer, PZN-1320422) and Ketavet (Bayer, PZN-3151811) by intraperitoneal injection. The anterior chamber was then punctured with a 30 G needle. 0.9% NaCl solution was infused through a tube into the anterior chamber with a pressure 120 mmHg. The intraocular pressure (IOP) was elevated for 20 min. After 20 min, the needle was removed. OKT was performed at 5 days after I/R to allow adequate time to develop I/R-induced damage. Nrf2 targeted-gene expression was measured at 3–4 h after treatment day 1 following elevation of I/R (Fig. 5B).
Fig. 5.
Systemic administration of CPUY192018 activates retinal Nrf2 target gene expression in ischemia-reperfusion injury. (A) Plasma exposure of CPUY192019 after i.v. and s.c. administration in rats. The values shown are the geometric means with error bars indicating geometric SD (n = 3). (B) Schematic diagram of CPUY192018 administration and mRNA isolation for gene expression studies relative to the ischemia-reperfusion injury. (C) Subcutaneous administration of CPUY192018 results in induction of Nrf2 target genes TXNRD1, GSTM1 and NQO1 in rat retinas following I/R injury. Values shown are mean ± SD (n = 6). *, p < 0.05.
2.7. Evaluation of pharmacokinetics
To evaluate the pharmacokinetics of CPUY192018 in vivo, the compound was dissolved in appropriate formulation vehicles (rat plasma or 0.9% NaCl, respectively). The test substance CPUY192018 were then administered to rats intravenously or subcutaneously. The intravenous application was performed as bolus injection with dose of 1 mg/kg. For subcutaneous administration a dose of 10 mg/kg was chosen, as this represented a dose to be used in pharmacodynamic experiments. Blood samples were retrieved via a catheter and plasma was generated from the blood via centrifugation. Blood samples were taken over an appropriate time interval, usually lasting up to 24 h after administration. If possible, time points were chosen so that the initial absorption phase, the maximum plasma concentration (Cmax), and the elimination phase could be well described. The quantitative measurement of the test substance in the samples was performed using calibration curves in the respective matrices. The protein content of the samples was precipitated using acetonitrile or methanol. Thereafter, the samples were separated using HPLC in combination with reversed phase chromatography columns. The HPLC system was coupled to a triple quadrupole mass spectrometer via an electrospray interface. The evaluation of the plasma concentration/time profiles are afterwards evaluated via non-compartmental analysis (NCA) using a validated pharmacokinetics evaluation program.
2.8. Real-time PCR
Total RNA was extracted from treated cells or retinas of Wistar Rats with RNeasy-Kit (Qiagen, Valencia, MD). First stranded cDNA was synthesized from 1000 ng total RNA using an oligo (dT) 18-mer as primer and the MMLV Reverse Transcriptase (Invitrogen, Carlsbad, CA) in a final reaction volume of 20 ml. Real-time PCR was performed with the SYBR Green PCR Kit (Thermo) and run on standard Real-time PCR System (Applied Biosystem, Waltham, US). Each amplification reaction was done in duplicates. The following primers were used: HO-1 sense primer AACTTTCAGAAGGGCCAGGT and antisense primer GTAGACA GGGGCGAAGACTG; TNF-α sense primer CTGCTGCACTTTGGAGTGAT and antisense primer GCCAGAGGGCTGATTAGAGA; AMWAP sense primer TGTTGATCAATGCTCAGGAGATG and antisense primer CAGA CATGACCACAGCTATTGCT; NQO-1 sense primer CGCAGACCTTGTGA TATTCCAG and antisense primer CGTTTCTTCCATCCTTCCAGG); GCLM sense primer TTGGA GTTGCACAGCTGGATTC and antisense primer TGGTTTTACCTGTGCCCA; VCAM1 sense primer AAAAGCGGAGACAGGAGACA and antisense primer AGCACGAGAAGCTCAGGAGA; β-actin sense primer AATGTGGCCGAGGACTTTGATTGC and antisense primer AGGATGGCAAGGGACTTCCTGTAA. PCR was performed using a LightCycler (Roche) with an annealing temperature of 60 °C with 40 cycles. Data analysis was done using Microsoft Excel and GraphPad Prism. To calculate fold change induction of gene expression, we used the 2−ΔΔCt method as described by Schmittgen et al., 2008.
2.9. Behavioral assessment of visual thresholds in retinal ischemia-reperfusion rats
A virtual optomotor system (OptomotryTM; Cerebral Mechanics, White Plains, NY) was used to evaluate visual function in I/R rats with or without CPUY192018 treatment. The equipment has four inward-facing computer monitors arranged in a square around an elevated testing platform. A moving vertical sine wave grating is displayed on the monitors, creating an illusion of a virtual cylinder rotating at 12°/s around the testing platform. A video camera mounted to the top lid of the apparatus reports to a computer program, enabling the experimenter to score the test animal's optokinetic reflex response to the moving grating. The spatial frequency (SF) threshold was assessed in awake and freely moving rats. Rats were judged able to visualize the moving grating target by their characteristic head movement tracking the cylinder rotation. SF (cycles/degree) was always measured at maximum contrast (100%) by using a staircase method beginning with a minimum preset frequency of 0.072 cycles/degree. The SF threshold was identified as the highest values that still elicited a response in the rats. Twelve rats were used for visual threshold testing.
2.10. Statistical analysis
Data were presented as mean ± SD. The Student t-test and one-way ANOVA were used to perform statistical analysis. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. CPUY192018 activates Nrf2 and enhances Nrf2 nuclear translocation
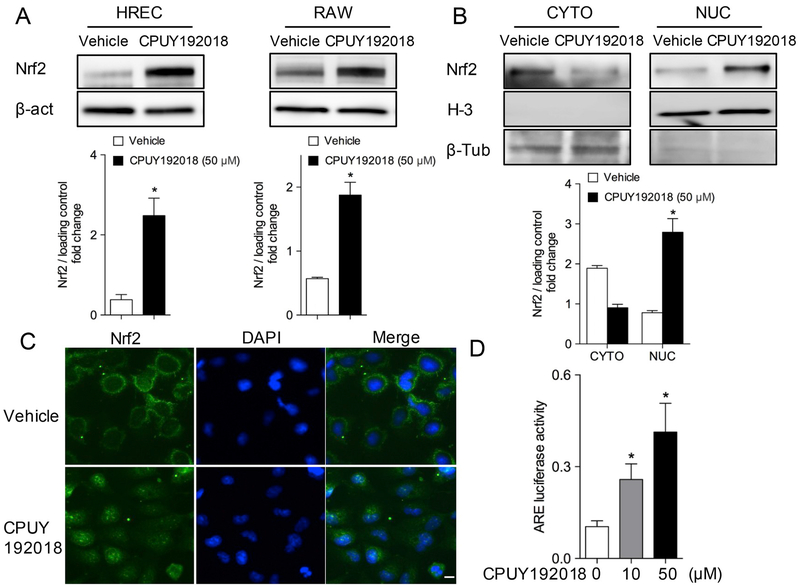
Previous studies of the CPUY192018 protein-protein interaction inhibitor demonstrated activation of Nrf2 in colonic and renal cells. We were interested in assessing this compound in human retinal endothelial cells (HRECs) and RAW264.7 macrophages, which are important cell types involved in ischemic retinal diseases. Vascular endothelial cells in particular are an important participant in ischemic injury, and endothelial cells in general have not received as much attention with regard to pharmacologic targeting of Nrf2. As shown in Fig. 1A, treatment with 50 μM CPUY192018 for 6 h resulted in an increase of Nrf2 protein levels in both HRECs and RAW264.7 macrophage. In addition, western blotting results show that CPUY192018 significantly increased nuclear translocation of Nrf2 in HRECs, as indicated by western blotting of nuclear and cytosolic fractions (Fig. 1B). Immunofluorescence staining was further used to assess the cellular localization of Nrf2 in HRECs under basal conditions and in response to CPUY192018. Consistent with western blotting results, cells exposed to CPUY192018 (50 μM) for 6 h showed increased Nrf2 protein staining, particularly in the nucleus, as compared to untreated control cells (Fig. 1C). To determine whether CPUY192018 could affect the transcriptional activity of Nrf2 via the conserved antioxidant responsive element (ARE), HRECs were transiently transfected with pARE-Luciferase reporter and treated with different doses of CPUY192018. As shown in Fig. 1D, CPUY192018 induced ARE activity in a dose-dependent fashion.
Fig. 1.
CPUY192018 activates Nrf2 and enhances Nrf2 nuclear translocation in HRECs. (A) Nrf2 protein levels in HRECs and RAW264.7 macrophage increased following treatment with 50 μM CPUY192018 for 6 h. (B) Treatment with CPUY192018 for 6 h induced Nrf2 nuclear translocation and accumulation. Data are presented in bar-graphs as mean ± SD (n = 3). *, p < 0.05 versus DMSO vehicle. (C) Immunofluorescence staining indicates increased expression and nuclear localization of Nrf2 in response to CPUY192018 treatment (50 μM) for 6 h. (D) CPUY192018 induces ARE-mediated luciferase gene expression in HRECs. Cells were exposed to drug or DMSO vehicle for 16 h. The activities are shown as the ratio to the vehicle control. The values shown are the means ± SD (n = 6 independent observations).
3.2. CPUY192018 inhibits Nrf2-regulated gene expression in HRECs
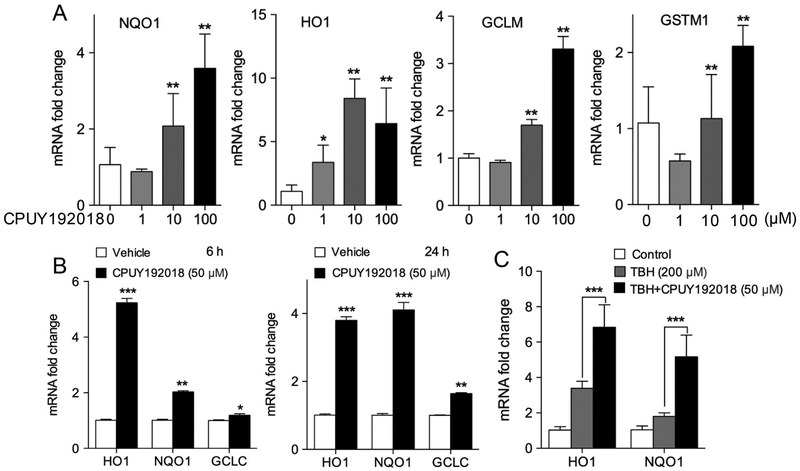
Given that the Keap1-Nrf2 PPI inhibitor CPUY192018 robustly activated Nrf2 in HRECs, we next examined induction of Nrf2 target gene expression by CPUY192018. Treatment of HRECs with CPUY192018 for 24 h significantly upregulated mRNA levels of NQO1, HO1, GCLM, and GSTM1 in a dose-dependent fashion (Fig. 2A). HO-1 expression was significantly induced as early as 6 h after addition of CPUY192018 (50 μM), while NQO1 and GCLC expression especially increased 24 h after addition of CPUY192018 (Fig. 2B). We also investigated CPUY192018 induction of Nrf2 target gene expression under conditions of oxidative stress. Exposure of HRECs to the oxidant tert-butylhydroperoxide (TBH) induced expression of the Nrf2 target genes HO1 and NQO1, consistent with the known effect of reactive oxygen species to activate Nrf2 via covalent modification of Keap1. Pre-treatment of HRECs with CPUY192018 significantly increased expression of both HO1 and NQO1, as compared to TBH exposure alone (Fig. 2C).
Fig. 2.
CPUY192018 induces Nrf2 target gene expression in HRECs. (A) mRNA levels of Nrf2 target genes NQO1, HO1, GCLM and GSTM1 were increased in a dose-dependent fashion in HRECs treated with CPUY192018 for 24 h. (B) mRNA levels of NQO1, HO1 and GCLC were increased in HRECs at both 6 h and 24 h after treatment with 50 μM CPUY192018. (C) mRNA levels of NQO1 and HO1 in HRECs were increased by exposure to 200 μM TBH oxidant for 1 h and further increased by pre-treatment with 50 μM CPUY192018 treatment for 16 h. Vehicle control for the above experiments is 0.1% DMSO. The values shown are mean ± SD (n = 3). *, p < 0.05. **, p < 0.01 versus vehicle group.
3.3. CPUY192018 protects HREC from oxidative and inflammatory injury
In addition to the effects of CPUY192018 on Nrf2 activation and target gene expression, we explored the enhancement of antioxidant capacity of HRECs against TBH-induced cellular oxidative stress. Intracellular ROS levels were measured by detecting the fluorescence intensity of the oxidative products of the exogenously administered H2DCF-DA. Exposure of HRECs to tert-butylhydroperoxide at a concentration of 200 μM for 1 h resulted in high levels of ROS. Pre-treatment of HRECs with CPUY192018 (50 μM) sharply inhibited ROS levels, confirming that CPUY192018 can enhance antioxidant capacity in HRECs under conditions of oxidative stress (Fig. 3A). In addition to its antioxidant effects, we also investigated anti-inflammatory effects of CPUY192018. LPS is known to promote an inflammatory phenotype in vascular endothelial cells, reflected by induction of pro-inflammatory genes including the adhesion molecule VCAM1. Exposure of HRECs to LPS induced expression of VCAM1, as expected. However, pre-treatment of HRECs with CPUY192018 significantly abrogated this LPS effect. (Fig. 3B).
Fig. 3.
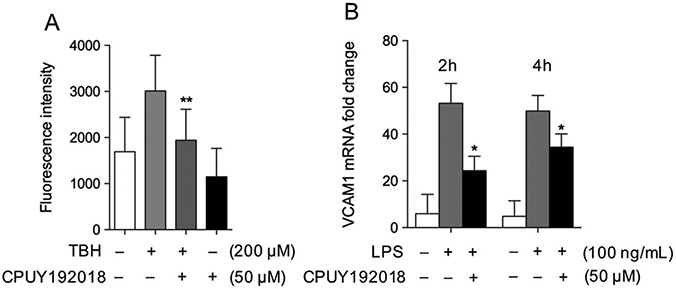
CPUY192018 protects HREC from oxidative stress and inflammatory change in HRECs. (A) The TBH-induced increase in levels of reactive oxygen species in HRECs were abrogated by pre-treatment with CPUY192018 (50 μM) for 16 h. (B) LPS induction of mRNA levels of the pro-inflammatory adhesion gene VCAM1 in HRECs were reduced by pre-treatment with CPUY192018 (50 μM) for 16 h. The values shown are mean ± SD (n = 3). *, p < 0.05. **, p < 0.01 versus TBH or LPS group.
3.4. CPUY192018 inhibits inflammatory gene expression in macrophages
In addition to retinal endothelial cells, we also investigated the effects of CPUY192018 on macrophages, which are known to be important in promoting inflammation in ischemic retinal conditions. We examined the regulation of inflammatory gene expression by CPUY192018 in both THP-1 cells and RAW264.7 macrophages against LPS-induced stress. Exposure to THP-1 and RAW264.7 to LPS (100 ng/mL) resulted in upregulation of the inflammatory regulators TNF-α and AMWAP, respectively. Pretreatment with CPUY192018 significantly inhibited the expression of these genes (Fig. 4A-B), indicating the regulation of inflammatory gene expression by CPUY192018 in LPS-treated macrophages. CPUY192018 treatment did not exhibit cytotoxicity in either THP-1 or RAW264.7 cells (Suppl. Fig. 1).
Fig. 4.
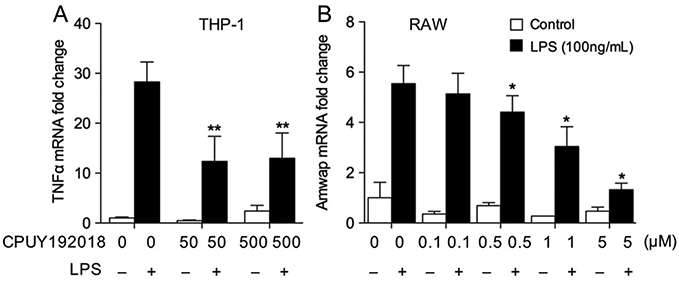
CPUY192018 inhibits inflammatory gene expression in macrophages. (A) LPS-induced expression of TNF-α in THP-1 cells was reduced by pre-treatment with CPUY192018. (C) LPS-induced expression of the inflammatory regulator AMWAP in RAW264.7 macrophages was significantly reduced by pre-treatment with CPUY192018. Values shown are mean ± SD (n = 5). *, p < 0.05 versus LPS group.
3.5. Systemic administration of CPUY192018 activates retinal Nrf2 target gene expression in ischemia-reperfusion injury
In preparation for in vivo experiments, we performed pharmacokinetic studies comparing intravenous and subcutaneous administration of CPUY192108. As shown in Fig. 5A, CPUY192018 is rapidly cleared after intravenous administration in rats. The compound demonstrates a high blood clearance and a short half-life (see Suppl. Table 1 for details). In addition, CPUY192018 displayed a very low transcellular permeability (data not shown). For this reason, intravenous administration was deemed to be suboptimal to achieve an exposure necessary for pharmacological efficacy. The subcutaneous route of administration, which provided complete bioavailability, was therefore chosen as the preferred option for systemic administration.
Using the subcutaneous approach, we first evaluated whether systemic administration of CPUY192018 could activate Nrf2 target genes in the retina in the setting of ischemia-reperfusion injury (Fig. 5B). As shown in Fig. 5C, systemic treatment with CPUY192018 significantly increased expression of the Nrf2 downstream target genes GSTM1, TXNRD1 and NQO1 in ischemia-reperfusion in rats, as compared to the control group treated with 0.9% NaCl. This confirmed the ability systemically administered CPUY192018 to activate the Nrf2 antioxidant system in the retina.
3.6. Systemic administration CPUY192018 confers retinal neuroprotection in ischemia-reperfusion injury
In addition to the effect of systemically administered CPUY192018 on Nrf2 activation and target gene induction in the retina, we were interested in the potential therapeutic benefit in retinal ischemia-reperfusion. Retinal I/R is known to have adverse effects on neuronal function in the retina, including compromise of visual function. In rodent models, evaluation of the optomotor response using a virtual optomotor system is a well-accepted, reliable method for quantitative assessment of spatial vision [34,35]. We investigated the effect of systemically administered CPUY192018 on visual function at 5 days after I/R injury (Fig. 6A), using the virtual optomotor system that allows precise quantification of spatial frequency thresholds. As shown in Fig. 6B, subcutaneous administration of CPUY192018 strongly improved visual function following I/R injury as compared with 0.9% NaCl control. This improvement from CPUY192018 treatment was similar to the improvement resulting from treatment with CDDO-Me, a potent triterpenoid known to activate Nrf2 via covalent modification of Keap1 (Fig. 6C).
Fig. 6.
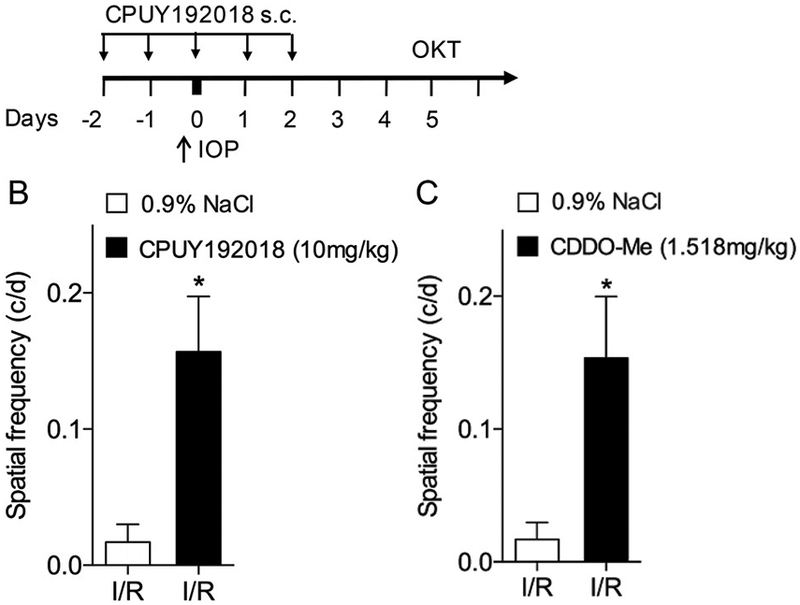
Systemic administration of CPUY192018 confers retinal neuroprotection in ischemia-reperfusion injury. (A) Schematic diagram of CPUY192018 administration and OKT assay in rats subjected to ischemia-reperfusion injury. (B) Spatial frequency measured by OKT testing following ischemia-reperfusion injury was improved by systemic administration of CPUY192018 (10 mg/kg) through systemic administration. (C) Spatial frequency was similarly improved by treatment with the triterpenoid Nrf2 activator CDDO-Me (1.5 mg/kg), used as a positive control. Values shown are mean ± SD (n = 6). *, p < 0.05.
3.7. Topical administration of CPUY192018 also confers retinal neuroprotection in ischemia-reperfusion injury
Given the significant neuroprotection against retinal ischemia-reperfusion injury conferred by systemic administration of CPUY192018, we considered another important route of administration for eye diseases, namely local administration via the topical route (Fig. 7A). Following retinal I/R injury, rats with topical 0.9% NaCl treatment exhibited a significantly lower spatial frequency threshold during optokinetic testing compared with No I/R injury controls, as expected. In contrast, topical treatment with CPUY192018 significantly rescued visual function following I/R injury, with markedly increased SF threshold (Fig. 7B).
Fig. 7.
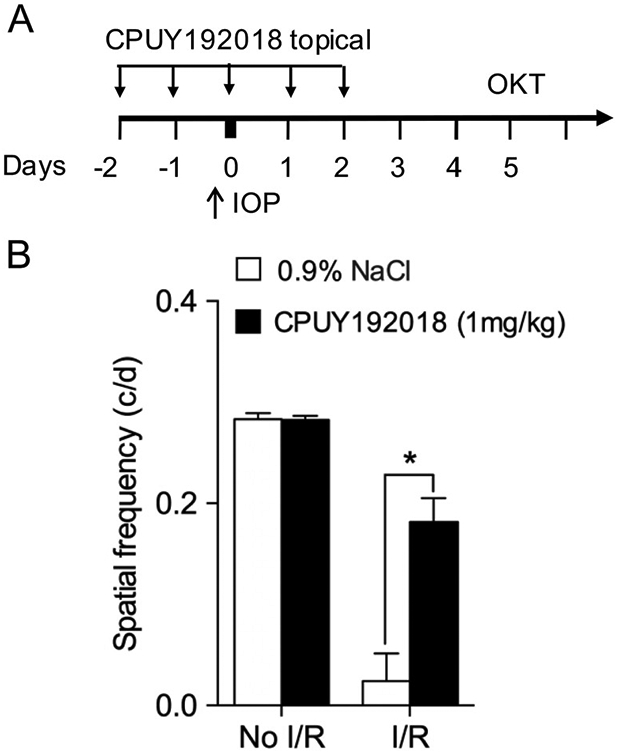
Topical administration of CPUY192018 confers retinal neuroprotection in ischemia-reperfusion injury. (A) Schematic diagram of topical CPUY192018 administration and OKT assay in rats subjected to ischemia-reperfusion injury. (B) Spatial frequency measured by OKT testing follow in ischemia-reperfusion injury was improved by topical administration of CPUY192018 (1 mg/kg). Values shown are mean ± SD (n = 6). *, p < 0.05.
4. Discussion
Ischemic retinal diseases are major causes of blindness worldwide, and there are currently no available treatments. Oxidative stress and pro-inflammatory processes are drivers of disease progression and damage in these conditions. Our lab and others have found Nrf2 to be an important protective modulator of retinal disease processes. Therapeutic targeting of the Keap1-Nrf2 pathway is a promising strategy for mitigating oxidative stress and inflammation. The great majority of drug candidates act by covalent modification of cysteine residues in Keap1, including compounds of demonstrated benefit in multiple experimental models of retinal disease [15,18,20,21,23]. These compounds include the potent triterpenoids CDDO-Me (bardoxolone) and CDDO-Im. More recently, Keap1-Nrf2 protein-protein interaction (PPI) inhibitors, including CPUY192018 [31], have emerged as a novel alternative for Nrf2 activation that might reduce concerns relating to target specificity [25]. CPUY192018 has been shown to be effective as an Nrf2 activator with cytoprotective effects in both colon mucosal epithelial cells and renal proximal tubular epithelial cells [32,33]. Additionally, this drug was beneficial in ameliorating damage in experimental models of colitis and renal inflammation [32,33], strongly supporting a possible therapeutic role for Keap1-Nrf2 PPI inhibition in systemic inflammatory conditions. In the current study, our objective was to gain insights into the potential utility of Keap1-Nrf2 PPI inhibition, specifically with CPUY192018, in retinal disease. We had a particular interest in studying the effect of CPUY192018 in human retinal endothelial cells, both with respect to CPUY192018 and to Nrf2 activation in general. Together, our current results indicate Nrf2 activation as an important strategy for vasculoprotection in the retina and Keap1-Nrf2 PPI inhibition as a promising approach for retinal diseases.
An important aspect of our current study was the investigation of CPUY192018-mediated Nrf2 activation in human retinal endothelial cells. This was predicated on the central involvement of vascular endothelial cells in ischemia-reperfusion injury. Although I/R injury has obvious consequences for the tissue bed affected by the involved blood supply, it is evident that the microvasculature itself and particularly the vascular endothelial cells are significantly impaired by I/R injury [36]. Indeed this microvascular injury strongly influences the severity of damage to the involved tissue parenchyma. As with other tissue beds, the retinal microvasculature is significantly compromised during I/R, with sequelae including capillary degeneration [5] and blood-retinal barrier dysfunction [6]. The formation of reactive oxygen species, both by vascular cells and by associated cells including immune cells, is an important instigator of microvascular damage [36], suggesting an important role for Nrf2 in vasculoprotection. Indeed, Nrf2 deficiency exacerbates vascular injury in retinal I/R [22].
The relevance of Nrf2 for vascular endothelial cells has been appreciated, especially in the context of cardiovascular disease processes [37] and diabetes, a condition in which endothelial dysfunction is well-recognized [38]. Nevertheless, the therapeutic effect of Nrf2 activators on vascular endothelial cells remains relatively under-studied. We were therefore particularly interested in investigation CPUY192018 on human retinal endothelial cells (HREC).
Our studies indicate that this drug induces a robust activation of the Nrf2 pathway, including increase in levels and translocation of Nrf2 protein, ARE-induced transcription, and Nrf2 target gene expression. Importantly, CPUY192018 conferred protection in HREC against both oxidative stress and inflammatory activation. This indicates that Nrf2 activators including Keap1-Nrf2 PPI inhibitors represent a viable strategy for safeguarding retinal and other vascular endothelial cells against oxidative and inflammatory injury.
We next broadened our studies to investigate the effect of CPUY192018 treatment in vivo in an experimental model of retinal ischemia-reperfusion, in which the intraocular pressure is raised beyond systolic pressure for a defined duration followed by reperfusion [39]. We found that systemic administration of CPUY2018 achieved Nrf2 activation in the retina, as indicated by Nrf2 target gene activation, similar to administration of the potent triterpenoid Nrf2 activator CDDO-Me (Bardoxolone). In order to determine the therapeutic benefit of CPUY2018, we investigated the optokinetic response, a means for measuring visual function in rodents [34] that is compromised in retinal I/R [40]. Systemic administration of CPUY2018 dramatically rescued visual function after I/R-injury in a similar fashion to treatment with the triterpenoid CDDO-Me. In addition to systemic treatment, topical administration of CPUY2018 also rescued visual function in a similar fashion to topical administration of brimonidine, which is known to have neuroprotective effects. Together these results indicate both systemic and topical administration as viable therapeutic approaches for attaining retinal activation of Nrf2.
Our results demonstrating the therapeutic benefit of systemically administered CPUY192018 on retinal I/R injury also suggests that this drug could be useful not only in other retinal conditions, but potentially in diseases of the central nervous system as well. The bioactivity of CPUY192018 for retina may have implications for the CNS: the blood-retinal barrier and blood-brain barrier, respectively, pose similar issues relating to bioavailability of systemically administered compounds. Taken together, our study indicates that Keap1-Nrf2 protein-protein interaction inhibition, specifically with CPUY192018 and related compounds, could be a promising treatment strategy for ischemic retinopathies and other retinal disease conditions.
5. Conclusion
In this study, the Keap1-Nrf2 protein-protein interaction (PPI) inhibitor, CPUY1920018, was investigated, both for Nrf2 activation in human retinal endothelial cells (HREC) and for treatment of retinal ischemia-reperfusion (I/R) injury. This compound was found to be a highly effective Nrf2 activator in HREC that confers protection against both oxidative stress and inflammatory stimuli. In addition, both systemic and topical administration of CPUY192018 activated Nrf2 in the retina and rescued visual function from I/R injury. Together, these findings indicate that CPUY192018 and other PPI inhibitors are promising activators of the Nrf2 pathway that provide protection against retinal ischemic and inflammatory injury, suggesting Keap1-Nrf2 PPI inhibition in the treatment of retinal conditions.
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institutes of Health (EY022383 and EY022683; to E.J.D.) and CORE Grant P30EY001765, Imaging and Microscopy Core Module, and was part of a research collaboration with Bayer AG.
Footnotes
Declaration of competing interest
The authors declare no relevant competing financial interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2019.10.414.
References
- [1].Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J, Retinal ischemia: mechanisms of damage and potential therapeutic strategies, Prog. Retin. Eye Res 23 (1) (2004) 91–147. [DOI] [PubMed] [Google Scholar]
- [2].Pellegrini-Giampietro DE, Cherici G, Alesiani M, Carla V, Moroni F, Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage, J. Neurosci. : Off. J. Soc. Neurosci 10 (3) (1990) 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McCord JM, Oxygen-derived free radicals in postischemic tissue injury, N. Engl. J. Med 312 (3) (1985) 159–163. [DOI] [PubMed] [Google Scholar]
- [4].Korthuis RJ, Granger DN, Reactive oxygen metabolites, neutrophils, and the pathogenesis of ischemic-tissue/reperfusion, Clin. Cardiol 16 (4 Suppl 1) (1993) I19–I26. [DOI] [PubMed] [Google Scholar]
- [5].Zheng L, Gong B, Hatala DA, Kern TS, Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes, Investig. Ophthalmol. Vis. Sci 48 (1) (2007) 361–367. [DOI] [PubMed] [Google Scholar]
- [6].Abcouwer SF, Lin CM, Wolpert EB, Shanmugam S, Schaefer EW, Freeman WM, Barber AJ, Antonetti DA, Vascular permeability and apoptosis are separable processes in retinal ischemia-reperfusion injury: effects of ischemic preconditioning, bevacizumab and etanercept, Investig. Ophthalmol. Vis. Sci 51 (11) (2010) 5920–5933. [DOI] [PubMed] [Google Scholar]
- [7].Kensler TW, Wakabayashi N, Biswal S, Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway, Annu. Rev. Pharmacol. Toxicol 47 (2007) 89–116. [DOI] [PubMed] [Google Scholar]
- [8].Yamamoto M, Kensler TW, Motohashi H, The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis, Physiol. Rev 98 (3) (2018) 1169–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, Du Y, Kern TS, Thimmulappa R, Barber AJ, Biswal S, Duh EJ, NRF2 plays a protective role in diabetic retinopathy in mice, Diabetologia 57 (1) (2014) 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhong Q, Mishra M, Kowluru RA, Transcription factor NF-E2-Related factor 2 (Nrf2)-Mediated antioxidant defense system in the development of diabetic retinopathy, Investig. Ophthalmol. Vis. Sci 54 (6) (2013) 3941–3948, 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kowluru RA, Mishra M, Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2, Free Radical Biol. Med 103 (2017) 155–164, 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mishra M, Zhong Q, Kowluru RA, Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy, Investig. Ophthalmol. Vis. Sci 55 (11) (2014) 7256–7265, 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mishra M, Zhong Q, Kowluru RA, Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression, Free Radical Biol. Med 75 (2014) 129–139, 10.1016/j.freeradbiomed.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT, Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degeneration, Vis. Res 50 (7) (2010) 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang L, Kondo N, Cano M, Ebrahimi K, Yoshida T, Barnett BP, Biswal S, Handa JT, Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells, Free Radical Biol. Med 70 (2014) 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lambros ML, Plafker SM, Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration, Adv. Exp. Med. Biol 854 (2016) 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J, Age-related retinopathy in NRF2-deficient mice, PLoS One 6 (4) (2011) e19456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nagai N, Thimmulappa RK, Cano M, Fujihara M, Izumi-Nagai K, Kong X, Sporn MB, Kensler TW, Biswal S, Handa JT, Nrf2 is a critical modulator of the innate immune response in a model of uveitis, Free Radical Biol. Med 47 (3) (2009) 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei Y, Gong J, Xu Z, Duh EJ, Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase-2 in oxygen-induced retinopathy, Free Radical Biol. Med 99 (2016) 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wei Y, Gong J, Xu Z, Thimmulappa RK, Mitchell KL, Welsbie DS, Biswal S, Duh EJ, Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A, Proc. Natl. Acad. Sci. U.S.A 112 (50) (2015) E6927–E6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cho H, Hartsock MJ, Xu Z, He M, Duh EJ, Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion, J. Neuroinflammation 12 (1) (2015) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ, Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury, Free Radical Biol. Med 51 (1) (2011) 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu Z, Cho H, Hartsock M, Mitchell KL, Gong J, Wu L, Wei Y, Wang S, Thimmulappa RK, Sporn MB, Biswal S, Welsbie DS, Duh EJ, Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion, J. Neurochem 133 (2) (2015) 233–241, 10.1111/jnc.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nakagami Y, Nrf2 is an attractive therapeutic target for retinal diseases, Oxid Med Cell Longev 2016 (2016) 7469326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT, Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases, Nat. Rev. Drug Discov 18 (4) (2019) 295–317. [DOI] [PubMed] [Google Scholar]
- [26].Canning P, Sorrell FJ, Bullock AN, Structural basis of Keap1 interactions with Nrf2, Free Radical Biol. Med 88 (Pt B) (2015) 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Robledinos-Anton N, Fernandez-Gines R, Manda G, Cuadrado A, Activators and inhibitors of NRF2: a review of their potential for clinical development, Oxid Med Cell Longev 2019 (2019) 9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liby KT, Yore MM, Sporn MB, Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer, Nat. Rev. Cancer 7 (5) (2007) 357–369. [DOI] [PubMed] [Google Scholar]
- [29].Ruiz S, Pergola PE, Zager RA, Vaziri ND, Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease, Kidney Int. 83 (6) (2013) 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, Investigators BT, Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease, N. Engl. J. Med 369 (26) (2013) 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang ZY, Xu LL, Lu MC, Chen ZY, Yuan ZW, Xu XL, Guo XK, Zhang XJ, Sun HP, You QD, Structure-activity and structure-property relationship and exploratory in vivo evaluation of the nanomolar keap1-Nrf2 protein-protein interaction inhibitor, J. Med. Chem 58 (16) (2015) 6410–6421. [DOI] [PubMed] [Google Scholar]
- [32].Lu MC, Ji JA, Jiang YL, Chen ZY, Yuan ZW, You QD, Jiang ZY, An inhibitor of the Keap1-Nrf2 protein-protein interaction protects NCM460 colonic cells and alleviates experimental colitis, Sci. Rep 6 (2016) 26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu MC, Zhao J, Liu YT, Liu T, Tao MM, You QD, Jiang ZY, CPUY192018, a potent inhibitor of the Keap1-Nrf2 protein-protein interaction, alleviates renal inflammation in mice by restricting oxidative stress and NF-kappaB activation, Redox Biol. 26 (2019) 101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Prusky GT, Alam NM, Beekman S, Douglas RM, Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system, Investig. Ophthalmol. Vis. Sci 45 (12) (2004) 4611–4616. [DOI] [PubMed] [Google Scholar]
- [35].Prusky GT, Silver BD, Tschetter WW, Alam NM, Douglas RM, Experience-dependent plasticity from eye opening enables lasting, visual cortex-dependent enhancement of motion vision, J. Neurosci. : Off. J. Soc. Neurosci 28 (39) (2008) 9817–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yu H, Kalogeris T, Korthuis RJ, Reactive species-induced microvascular dysfunction in ischemia/reperfusion, Free Radical Biol. Med 135 (2019) 182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen B, Lu Y, Chen Y, Cheng J, The role of Nrf2 in oxidative stress-induced endothelial injuries, J. Endocrinol 225 (3) (2015) R83–R99. [DOI] [PubMed] [Google Scholar]
- [38].Cheng X, Siow RC, Mann GE, Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway, Antioxidants Redox Signal. 14 (3) (2011) 469–487. [DOI] [PubMed] [Google Scholar]
- [39].Hartsock MJ, Cho H, Wu L, Chen WJ, Gong J, Duh EJ, A mouse model of retinal ischemia-reperfusion injury through elevation of intraocular pressure, J. Vis. Exp. : J. Vis. Exp 113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Joly S, Guzik-Kornacka A, Schwab ME, Pernet V, New mouse retinal stroke model reveals direction-selective circuit damage linked to permanent optokinetic response loss, Investig. Ophthalmol. Vis. Sci 55 (7) (2014) 4476–4489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.