Abstract
Objective:
Sending blood cultures in children at low risk of bacteremia can contribute to a cascade of unnecessary antibiotic exposure, adverse effects, and increased costs. We aimed to describe practice variation, clinician beliefs, and attitudes about blood culture testing in critically ill children.
Design:
Cross-sectional electronic survey
Setting:
15 pediatric intensive care units (PICUs) enrolled in the Bright Star collaborative, an investigation of blood culture use in critically ill children in the United States
Subjects:
PICU (bedside nurses, resident physicians, fellow physicians, nurse practitioners, physician assistants, and attending physicians)
Interventions:
None
Measurement and Main Results:
Survey items explored typical blood culture practices, attitudes and beliefs about cultures, and potential barriers to changing culture use in a PICU setting. 15/15 sites participated, with 347 total responses, 15-45 responses per site, and an overall median response rate of 57%. We summarized median proportions and interquartile ranges of respondents who reported certain practices or beliefs: 86% (73%-91%) report that cultures are ordered reflexively; 71% (61%-77%) do not examine patients before ordering cultures; 90% (86%-94%) obtain cultures for any new fever in PICU patients; 33% (19%-61%) do not obtain peripheral cultures when an indwelling catheter is in place, and 64% (36%-81%) sample multiple (vs single) lumens of central venous catheters for new fever. When asked about barriers to reducing unnecessary cultures, 80% (73%- 90%) noted fear of missing sepsis. Certain practices (culture source and indication) varied by clinician type. Obtaining surveillance cultures and routinely culturing all possible sources (each lumen of indwelling catheters and peripheral specimens) are positively correlated with baseline blood culture rates.
Conclusions:
There is variation in blood culture practices in the PICU. Fear and reflexive habits are common drivers of cultures. These practices may contribute to over-testing for bacteremia. Further investigation of how to optimize blood culture use is warranted.
Keywords: blood culture, bacteremia, sepsis, clinical decision-making, infection, quality improvement
Introduction
In hospitalized children, severe sepsis, as defined by the 2005 International Pediatric Sepsis Consensus Conference criteria, is common (8.2% prevalence) and deadly (25% mortality rate) [1, 2]. Delayed initiation of broad-spectrum antibiotics increases morbidity and mortality [3]. Accordingly, pediatric hospitals now prioritize early sepsis recognition and rapid antibiotic administration as key performance metrics, and sepsis is a never-miss diagnosis [4]. Rapid diagnosis of bacterial sepsis is clearly beneficial, but in practice, this process can be challenging and subjective. In children, clinical symptoms like fever or leukocytosis are neither sensitive nor specific for infection, and no single biomarker or decision rule can perfectly identify patients likely to have bacteremia [5]. The diagnostic uncertainty that is pervasive in sepsis can lead to under treatment if the disease is not recognized, as well as harmful overtreatment in the form of adverse events from unnecessary antibiotics, antibiotic resistance, and strain on health care resources [6].
Blood cultures are the gold standard for diagnosing bacteremia, an important cause of sepsis. Blood cultures are typically coupled with empiric antibiotics, and results guide subsequent therapy. Blood cultures, however, have an overall yield of only a 5-15% and up to a 50% false positive rate [7,8, 9]. Indiscriminate use of blood cultures leads to avoidable false positive results, unnecessary antibiotics, increased lengths of stay, and increased costs [10]. Diagnostic stewardship, defined as refining the use of diagnostic tools in order to improve treatment decisions, is thus critically needed for blood cultures, specifically as it relates to the decision to obtain or not obtain blood cultures [11]. Preliminary work has shown that reduction in unnecessary blood cultures in pediatric intensive care unit (PICU) patients is feasible and safe [12, 13, 14]. Presently, however, no widely accepted guideline for the optimal use of blood cultures in PICU patients exists, and little work has systematically explored how practice patterns for blood cultures vary across institutions or by PICU clinician type and experience level.
A 15-site quality improvement collaborative called Bright Star, (Blood Culture Improvement Guidelines and Diagnostic Stewardship for Antibiotic Reduction in Critically Ill Children), was created to optimize blood culture use in the PICU setting. The primary objectives of Bright Star are to determine if reducing blood cultures is associated with a decrease in broad-spectrum antibiotic use and to evaluate the impact of such an approach on a variety of patient outcomes. An important early phase of this project was to more broadly explore provider-focused elements of current blood culture practices across a large and diverse group of PICUs. We presently have a limited understanding of how PICU clinicians think about, use, or perceive this diagnostic test. Such an understanding must precede and inform planned wider-scale dissemination of efforts to safely reduce unnecessary use of this diagnostic test. Therefore, this investigation focuses on a survey done during the early phase of Bright Star, which had three objectives: 1) to describe the degree of practice variation across sites and clinician type in typical use and decision making around blood cultures in critically ill children, 2) explore clinician perceptions and attitudes about current use and overuse of blood cultures in critically ill children, and 3) identify potential barriers to altering blood culture use within the 15 PICUs enrolled in the Bright Star project. Bright Star sites kicked off in 2019, so outcome data such as antibiotic use and blood culture results are not yet available.
Material and Methods
Participants and regulatory approval
We performed a cross-sectional multipurpose electronic survey of pediatric critical care clinicians (bedside registered nurses [RN], trainee physicians, nurse practitioners [NP], physician assistants [PA], and attending physicians) at 15 PICUs in the Bright Star collaborative (Qualtrics, Provo, UT). The enrolled PICUs were ICUs that care primarily for critically ill children between birth and 21 years, though the upper limit of patient age did vary by institution. In general, while these PICUs had institutional policies to guide blood culture volume and blood culture collection technique, they did not, at the time of enrollment, have standardized protocols about when clinicians should or should not order blood cultures (the focus of our survey). Some units did have limited protocols in place about ordering cultures for specific patient populations (such as oncology patients), but did not track compliance with these protocols. The Johns Hopkins University Institutional Review Board approved this study as the coordinating center for all sites in the collaborative.
Survey development
The survey was developed based on our preceding qualitative “work system assessment,” in which we used the Systems Engineering Initiative for Patient Safety (SEIPS) 2.0 model and conducted interviews to identify the work system factors (people, tasks, tools/technologies, organizational conditions, physical environment, and external environment) that influence blood culture decision making and ordering [13, 15]. The survey included 50 questions focused on three main content areas: current blood culture practices (such as triggers to obtain a culture and sources of culture), current perceptions and beliefs about blood culture practices, and potential barriers to reducing unnecessary blood cultures in the PICU setting (Supplement 1). It was devised by a multi-disciplinary team with expertise in pediatric infectious diseases, critical care, quality improvement, and human factors engineering. Items were piloted by local pediatric critical care physicians, and then modified based on feedback.
Survey administration
The final survey was distributed to the two physician project leads at each of the 15 Bright Star PICUs by email in the form of an electronic link. The two physician leads shared this link with PICU clinicians within their own institution. A minimum of 15 surveys were requested from each site. We collected information about clinician type, years of experience, and associated institution. Survey distribution and data collection occurred between March and July 2018.
Statistical Analysis
Data were analyzed in two ways. We first used descriptive statistics to summarize, in general, how respondents answered the survey questions. We then explored if and how responses to certain questions varied by clinician type, experience, and sites’ baseline blood culture rates. Data analysis overall focused on three primary content areas: perceptions and attitudes about blood cultures, current blood culture practices, and potential barriers to changing blood culture use in the PICU. Other survey items (for example, questions about the process of peripheral blood culture collection) were asked for the larger purposes of the Bright Star project but were not included for primary analysis here.
For each site, we first summarized the proportion of respondents who reported certain practices, perceptions/attitudes, and barriers. Questions with 5-point Likert scales were categorized as positive responses by combining “agree,” and “strongly agree,” or “somewhat likely” and “extremely likely,” and as negative responses by combining “neutral”, “disagree” and “strongly disagree” or “neutral,” “somewhat unlikely” and “extremely unlikely” [16]. Results are presented as medians and interquartile ranges (IQR) of the proportion of positive or negative responses across all study sites (n = 15).
We then examined if answers to select questions 1) varied by 3 categories of respondent type (bedside registered nurse (RN), trainee physician/nurse practitioner/physician assistant, and attending physician) or years of experience (< 5 years, ≥ 5 years), or 2) were correlated with 24 months of baseline, pre-enrollment blood culture rate data for each site. We grouped trainee physician/nurse practitioner/physician assistants together to reflect that these are all clinicians who provide front-line care for patients (such as physical exams, order entry, and certain procedures) under the supervision of an attending physician. We chose the <5 year and >5 year cutoff for clinician experience based on a consensus definition within the institutions of the study team members, as well as existing literature, that “junior” pediatric critical care clinicians are those with 1–5 years of experience [17, 18]. The selected questions were chosen based on preliminary data indicating that these specific attitudes and practices are particularly important for blood culture practice change, and are annotated on Supplement 1 [12]. Logistic regression models were used to quantify the association between responding positively to the select questions and clinician type and experience level; the logistic regression models included a random intercept to link responses within the same site. The relationship between the proportion of positive answers and the mean baseline blood culture rates (transformed to the log-base 2 scale) was explored by calculating the Pearson correlation coefficient for the 14 sites that were able to provide baseline blood culture rate data. P-values less than 0.05 were considered significant. Statistical analysis was performed using Stata/IC, version 13.1.
Results
All sites responded to the survey and provided at least the minimum requested 15 survey respondents (median number of respondents per site: 21, minimum: 15, maximum: 45). There were 347 total responses: 114 (33%) attending physicians, 50 (14%) fellow physicians, 57 (16%) resident/nurse practitioner (NP)/physician assistants (PA), 114 (33%) bedside registered nurses, and 12 (4%) other. Fourteen sites tracked the number of clinicians that received the survey, and among these, the median response rate overall was 57%. Characteristics of participating sites are displayed in Table 1. Additional detail about each site is available in Supplement 2.
Table 1.
Characteristics of pediatric intensive care units participating in survey
| Characteristic | Median, interquartile range |
|---|---|
| Annual number of admissions | 6250 (2000-9700) |
| Unit size (number of beds) | 36 (25-40) |
| Baseline blood culture rate (total cultures per 100 patient days) in preceding 24 months | 15.9 (11-18.6) a |
| Survey responses received | 22 (18-25) |
| Characteristic | Number of sites (%) |
| Hematopoietic stem cell transplants | 13 (87) |
| Solid organ transplants | 13 (87) |
| Cardiac surgical patients | 6 (40) |
| ECMO | 14 (93) |
| Pediatric critical care fellowship program | 15 (100) |
| Advanced practice nurses | 9 (60) |
| Bedside nurses perform peripheral venipuncture | 11 (73) |
| Phlebotomy team performs peripheral venipuncture | 13 (87) |
ECMO = extracorporeal membrane oxygenation
Two sites were noted to be outliers for baseline blood culture rate, with rates of 38.68 and 48.05, but were not excluded from analysis
Descriptive results
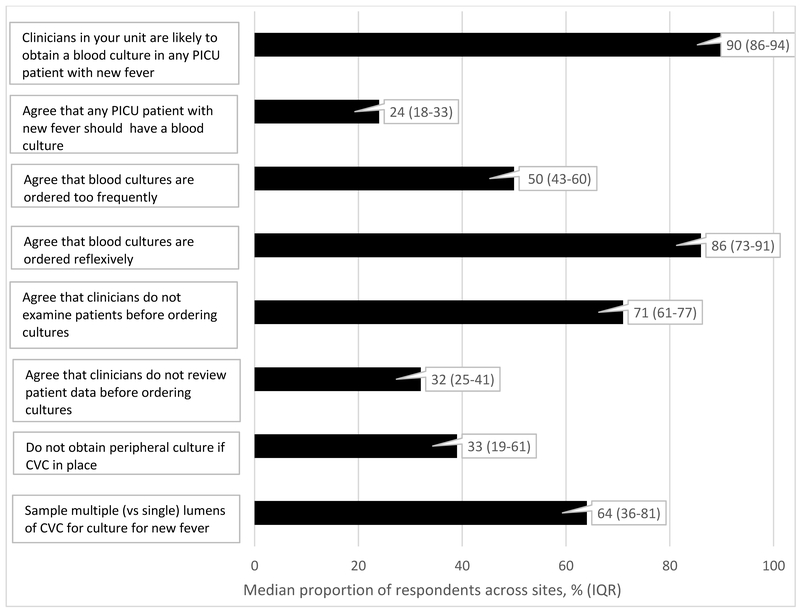
Figure 1 demonstrates the median proportion and interquartile range (IQR) of respondents across sites that report certain perceptions, attitudes, and practices specific to blood cultures in the pediatric intensive care unit. Notable findings about current practices include: the majority of respondents (90%, IQR 86%-94%) report that current practice in their unit is to obtain a blood culture in any PICU patient with a new fever (fever after being afebrile for preceding 48 hours), though only 24% (IQR 18%-33%) agreed that blood cultures should always be obtained for new fever. Notable findings about perceptions and attitudes include: half of respondents agree blood cultures are ordered too frequently (50%, IQR 43–60%); a majority of respondents (86%, IQR 73–91%) report that blood cultures are ordered reflexively in critically ill children; and a majority of respondents (71%, IQR 61–77%) report that clinicians do not examine patients before ordering blood cultures.
Figure 1.
Perception, attitudes and typical practices for blood cultures in the pediatric intensive care unit (median proportion of respondents across sites, and interquartile ranges)
CVC = central venous catheter
When asked about likely barriers to reducing unnecessary blood culture use in PICU patients, 80% of respondents (IQR 73%- 90%) noted fear of missing bacterial sepsis as a primary barrier, and 61% (IQR 56%-73%) reported that it would likely be difficult to achieve standardization of approach among different clinicians.
Results of analysis by respondent type and experience level
When examining the relationship between respondent type or experience level and reported blood culture practices/beliefs, we found the following results, with the reported odds ratios [ORs] reflecting odds of agreeing with or selecting the stated practice or belief. There was no difference in blood culture source chosen for workup of new fever when answers were analyzed by respondent type or years of experience. Of note, overall, 55.3% of our sample (56% of bedside RNs, 61% of trainee physicians/NPs/PAs, and 48% of attending physicians) reported that they routinely sample a dialysis catheter for a blood culture to work up a new fever (in addition to other sources); while 39.2% (48% of bedside RNs, 31% of trainee physicians/NPs/Pas, and 38% of attending physicians) reported that they routinely sample an arterial catheter for a blood culture for new fever (in addition to other sources). For workup of persistent fever, there was no difference in blood culture sources chosen when answers were analyzed by respondent type or years of experience.
There was disagreement across respondent type on whether certain types of patients should always have a blood culture. Compared to bedside nurses, both attending physicians and those in the trainee physician/NP/PA group were less likely to agree that all PICU patients with new fever should have a blood culture obtained (41% agreement vs 17% and 23% agreement, respectively; OR 0.48, 95% CI 0.26–0.9 and OR 0.30, 95% CI 0.15–0.59, respectively). Furthermore, when asked about PICU patients with new fever and a central venous catheter, the trainee physician/NP/PA clinician group was more likely to agree that all of these patients should have a blood culture obtained (89% agreement, OR 2.47, 95% CI 1.08–5.64) compared to attending physicians (77% agreement), though a majority of all clinician types agreed with this statement.
In terms of the practice of obtaining adequate knowledge of the patient prior to ordering a blood culture, the trainee physician/NP/PA group was more likely to agree that clinicians do specifically review vital signs and clinical data before ordering a blood culture (57% agreement, OR 2.45, 95% CI 1.45–4.16) than bedside RNs (35% agreement) or attending physicians (28% agreement). The trainee physician/NP/PA group was also more likely than nurses or attending physicians to agree that clinicians do perform a physical exam before ordering a blood culture (20% agreement, OR 3.5, 95% CI 1.40–8.85), though agreement overall with this statement was low (bedside RNs, 6% agreement and attending physicians, 9% agreement).
In terms of barriers to reducing unnecessary blood cultures, both the trainee physician/NP/PA group (70%, OR 2.39, 95% CI 1.37–4.18) and the attending physician group (71%, OR 2.59, 95% CI 1.45–4.62) were more likely to agree that achieving standardization of practice would be a barrier to reducing unnecessary use of blood cultures compared to bedside RN’s (50%). More experienced clinicians were also more likely to identify difficulty with standardization as a barrier (71%, OR 1.79, 95% CI 1.13–2.83) compared to less experienced clinicians of all types.
Finally, a majority of all groups surveyed indicated that fear of missing bacterial sepsis would be a likely barrier to reducing unnecessary use of blood cultures, with the trainee MD/NP/PA group the most likely to identify this as a barrier (88%, OR 3.37, 95% CI 1.67–6.77) compared to the attending MD (88%) and bedside RNs (68%).
Results of analysis by sites’ baseline blood culture rates
Table 2 displays how a site's baseline blood culture rate was correlated to the results of the survey. Answers to three items had a strong positive correlation with a site’s baseline blood culture rate: reporting that it is “very likely” or “likely” to use surveillance blood cultures in the unit (screening in asymptomatic high-risk patient groups) (r = 0.63 and p = .02); preferring to culture all sources for workup of new fever (each lumen of central venous catheter [CVC], arterial catheter, dialysis catheter, and peripheral specimen) versus only some of these sources (r = 0.64 and p = .01); and agreeing that all PICU patients with new fever and a CVC should have a blood culture drawn (r = 0.61 and p = .02).
Table 2.
Correlation between sites’ responses and their baseline blood culture ratesa
| Survey items – perceptions of blood culture practices in the PICUb | Correlation coefficient (r) |
p-value |
|---|---|---|
| Clinicians in your unit are likely to obtain a blood culture for new fever | 0.47 | 0.09 |
| Clinicians in your unit are likely to obtain a blood culture for persistent fever | 0.33 | 0.25 |
| Clinicians in your unit are likely to obtain a blood culture for any fever in a patient with a CVC | 0.4 | 0.16 |
| Clinicians in your unit are likely to obtain surveillance blood cultures (e.g. screening culture in asymptomatic high-risk patients) | 0.63 | 0.02 |
| In your unit, typical source(s) for blood culture in a patient with new fever includes all of the following if present: each lumen of CVC, arterial catheter, dialysis catheter, and peripheral specimen | 0.64 | 0.01 |
| In your unit, typical source(s) for blood culture in a patient with persistent fever includes all of the following if present: each lumen of CVC, arterial catheter, dialysis catheter, and peripheral specimen | −0.15 | 0.61 |
| Survey items – attitudes regarding blood culture use in the PICUc | Correlation coefficient (r) |
p-value |
| All PICU patients with fever should have a blood culture drawn | 0.43 | 0.12 |
| All PICU patients with new fever and a CVC in place should have a blood culture drawn | 0.61 | 0.02 |
| Blood cultures are ordered too frequently in our unit | −0.31 | 0.27 |
| In your unit, the decision to order blood cultures is often made by attending physicians | 0.2 | 0.49 |
| In your unit, clinicians currently order blood cultures reflexively | −0.23 | 0.42 |
CVC = central venous catheter
Calculated for the 14 sites who could provide baseline culture rate data
Responses included in this analysis reflect combinations of “extremely likely” and “somewhat likely”
Responses included in this analysis reflect combinations of “strongly agree” and “agree”
Discussion
Excess use of blood cultures in critically ill children is being increasingly recognized as a cause of unintended negative consequences both for the patient (false positive tests, repeated lab draws, longer hospital stays) and our health care system (increased costs, antimicrobial resistance). The Bright Star Collaborative is a multi-institutional effort currently underway to address this problem. This work system assessment survey took a key first step in implementing the collaborative’s new clinical approach to blood cultures by seeking a better understanding of current national practice patterns, attitudes, and perceptions about blood cultures. Four key points have emerged from our survey results.
First, while there appears to be agreement about certain current blood culture practices, such as obtaining a culture for new fever in a patient with a central venous catheter; several aspects of blood culture decisions vary across sites and across clinician demographics. Specifically, we noted that answers about preferred culture source (if clinicians obtain cultures from arterial catheters or dialysis catheters; if they sample multiple vs single lumens of a central venous catheter for a culture; and if they include a peripheral venipuncture sample for a patient with an existing central vascular catheter in place) were not uniform among our respondents. Evidence about the yield, contamination, and colonization rates of specimens from peripheral venipuncture and various types of vascular catheters can conflict, and perhaps this is driving some of these inconsistent practices [19, 20, 21, 22]. It may also be that less experienced or trainee clinicians simply take their cues from senior practitioners in their environment, or from historical precedent (“this is how we do it here”). One of the drivers to create the Bright Star collaborative was a strong suspicion that the PICU community has not achieved widespread consensus on the optimal use of blood cultures. Acknowledging the limitations of our sample size, this survey now explicitly demonstrates some of this practice variation. Recognizing variation in blood culture practices is a necessary prerequisite before successfully improving the use of cultures in diverse settings.
Second, PICU clinicians appear to acknowledge that there are potential deficiencies in some of their current blood culture practices. Only a small minority of respondents agreed that the “usual” approach of culturing all newly febrile PICU patients represents best practice. The majority of respondents agreed that blood cultures are ordered reflexively and usually without a preceding physical exam (and sometimes without review of any patient data at all). Perhaps clinicians are using the type 1, “fast” thinking process described by Daniel Kahneman – an automatic, quick clinical response to a question or patient finding (such as fever); versus the type 2, “slow” thinking that requires gathering further clinical data before making a clinical diagnosis [23]. It may also be that time constraints of a busy PICU environment limit clinicians’ perceptions of how feasible it is to evaluate patients and patient data before ordering a blood culture; though that was not specifically assessed in this survey. Interestingly, the trainee physician/NP/PA group was more likely to report that physical exams are done before ordering blood cultures, while very few attending physicians or bedside nurses agreed with this statement. There may be two possible explanations for this discrepancy: 1) trainees or frontline clinicians are actually performing pre-culture physical exams, but attending physicians are not always aware of those exams; or 2) trainees or frontline clinicians may unconsciously over-report how frequently they perform pre-culture physical exams, while bedside nurses, who spend the most time with the patients, report a different perspective.
Third, our sample had relatively consistent answers about likely barriers to blood culture reduction in the PICU: fear of missing sepsis due to bacteremia and anticipated difficulty with standardizing clinician behavior. Fear of sepsis is particularly important when considering how to safely optimize the use of blood cultures in the PICU. While rapid initiation of broad-spectrum antibiotics and other supportive therapies is undoubtedly key to improving sepsis outcomes, clinical presentations of sepsis in pediatrics can be non-specific and overlap with many other physiologic processes (including non-infectious diseases like tissue injury). Sepsis can also be caused by non-bacterial infections. We must acknowledge how much diagnostic uncertainty clinicians regularly encounter regarding this disease, and that over-diagnosis of sepsis is common [6]. Ensuring that blood cultures continue to be used by clinicians when signs or symptoms of sepsis are present is central to this collaborative’s mission, even as we attempt to define clinical scenarios in which cultures can be safely deferred [12]. Our survey findings emphasize the importance to PICU clinicians of achieving this balance between reducing harmful over-testing while maintaining appropriate vigilance for bacterial sepsis. Specific exploration of fear of missed diagnoses as a driver of diagnostic testing and over-testing in the critical care setting may be warranted. Furthermore, such fear of missed diagnoses likely has multiple antecedents that should be explored across a spectrum from fear of patient harm to fear of malpractice claims. Unpacking such drivers may illuminate alternative opportunities to better refine diagnostic testing.
Fourth, some components of a PICU’s culture or practice environment itself may drive current use (including potential overuse) of blood cultures. Acknowledging that presently, the ideal or “correct” rate of blood cultures for a given PICU is not known, we did note that increased reporting of the use of surveillance blood cultures, the use of all possible sources for cultures during new fever, and the belief that any new fever in a patient with a CVC should have a blood culture obtained was associated with higher baseline rates of blood cultures. This finding suggests that these practices may be driving up culture use in certain sites. Considering the potential harm of false-positive culture results and iatrogenic infection from repeated entry into CVCs, it is unclear how much value these practices are adding. They may represent important targets for improvement work to optimize how blood cultures are used in the PICU setting, and further investigation is needed.
Finally, our findings also prompt additional questions about blood culture practices that we did not explore in this survey, but may have important implications. For example, we did not ask sites how strictly they adhere to institutional policies in place for blood volume collection for cultures, or if blood volume is ever adjusted for single versus multiple simultaneous cultures. Blood volume, however, is clearly related to overall culture yield, so more specific investigation about this component of blood culture use could prove useful to efforts to optimize practices.
This investigation has several limitations. Our data reflect self-reported practices owing to the survey methodology, without secondary validation of responses. While we did achieve our target number of responses from each site (15), the numbers of responses and response rates did vary across the sites. This variation may have introduced some bias into the data. We attempted to account for this by reporting median proportions and IQRs of responses across all sites rather than aggregate summary statistics, but bias may remain. The sites enrolled in Bright Star may represent a sample that is more interested in blood culture improvement than the overall PICU community, which could limit generalizability. Our response rate of 57% was fairly robust, but may not be entirely representative of PICU clinicians in general [24]. Our analysis of the relationship between sites’ baseline blood culture rates and responses to certain questions was a) not completed for one site, because that site could not provide us with baseline blood culture rate data and b) not adjusted for clinical details such as proportion of high-risk groups in each unit (such immunocompromised or oncology patients), or illness severity. It may be that, in some sites, increased clinician belief in the need to obtain cultures for any new fever in a patient with a CVC reflects a higher proportion of high-risk patients who could reasonably need to be evaluated for bacteremia. Patient severity of illness may influence clinician decision making about blood cultures, but we were not able to stratify sites by illness severity (i.e., Pediatric Index of Mortality or Pediatric Risk of Mortality scores), because such data was not uniformly available.
Conclusion
This multi-institutional survey of PICU clinicians reveals several important insights into the use of blood cultures in critically ill children. Variation in practices appears common, and in our sample, clinicians report using blood cultures reflexively or automatically in response to a trigger such as a fever, without consistently first evaluating patient data or performing a physical exam. Fear of missing sepsis emerged as an important potential barrier to work aimed at reducing unnecessary testing for bacteremia. Varied practice and reflexive decision making may represent particularly important drivers of over-testing for bacteremia in this clinical setting, and further investigation of how to target these behaviors to optimize blood culture use is warranted.
Additional work currently underway includes examination of how each site used their work system assessment results to develop and implement site-specific new approaches to blood culture use, and how these adaptations relate to overall primary outcomes (blood culture rates, proportion of positive vs negative blood cultures, antibiotic use, and others) of the larger collaborative. Together, this work strives to be part of a greater effort to determine the ideal use of blood cultures for critically ill children and to achieve greater standardization of practice, with the ultimate goal of improving patient outcomes and reducing unintended harm.
Supplementary Material
Acknowledgments
We thank the clinicians and project teams at each site for their assistance with this survey and for their participation in the Bright Star collaborative.
Financial support: The study was funded by the Agency for Healthcare Research and Quality (grant 1R18HS025642). Dr. Woods-Hill also receives support from a National Institute of Health Ruth L. Kirschstein National Research Service Award (T32HL098054-55).
Footnotes
The coordinating center for the study was the Johns Hopkins Children’s Center in Baltimore, Maryland.
Copyright form disclosure: Drs. Woods-Hill and Colantuoni’s institution received fundingfrom the National Institutes of Health (NIH) and the Agency for Healthcare Research and Quality (AHRQ). Dr. Woods-Hill received honorarium for giving grand rounds at St Jude Children's Research Hospital, which was a discussion about the Bright Star project. Drs. Woods-Hill and Miller received support for article research from the NIH. Drs. Koontz, Voskertchian,and Milstone received support for article research from the AHRQ. Drs. King, Milstone, and Xie’s institutions received funding from the AHRQ. The remaining authors have disclosed thatthey do not have any potential conflicts of interest.
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015;191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14(7):686–693. [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42(11):2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Children’s Hospital Association. Improving Pediatric Sepsis Outcomes (IPSO) is successfully challenging sepsis. Available at: https://www.childrenshospitals.org/sepsiscollaborative. Accessed June 11, 2019.
- 5.Hsiao A, Baker M. Fever in the new millennium: a review of recent studies of markers of serious bacterial infection in febrile children. Current Opin Pediatr 2005; 17(1), 56–61 [DOI] [PubMed] [Google Scholar]
- 6.Klompas M, Calandra T, Singer M. Antibiotics for Sepsis—Finding the Equilibrium. JAMA 2018;320(14):1433–1434. [DOI] [PubMed] [Google Scholar]
- 7.Bone RC, Fisher CJ Jr., Clemmer TP, et al. Sepsis syndrome: a valid clinical entity. Crit Care Med 1989;17(5):389–393. [PubMed] [Google Scholar]
- 8.Lamy B, Dargere S, Arendrup MC, et al. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front Microbiol 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafazand S, Weinacker AB. Blood cultures in the critical care unit: improving utilization and yield. Chest 2002;122(5):1727–1736. [DOI] [PubMed] [Google Scholar]
- 10.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 2011;77(3):233–236. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship-leveraging the laboratory to improve antimicrobial use. JAMA 2017;318(7):607–608. [DOI] [PubMed] [Google Scholar]
- 12.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a Clinical Practice Guideline With Blood Culture Use in Critically Ill Children. JAMA Pediatr 2017;171(2):157–164. [DOI] [PubMed] [Google Scholar]
- 13.Xie A, Woods-Hill CZ, King AF, et al. Work system assessment to facilitate the dissemination of a quality improvement program for optimizing blood culture use: a case study using a human factors engineering approach. J Pediatric Infect Dis Soc 2017. Epub ahead of print. doi: 10.1093/jpids/pix097. [DOI] [PubMed] [Google Scholar]
- 14.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a novel framework to improve blood culture use in three pediatric intensive care units. Pediatr Qual Saf 2018;3:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden RJ, Carayon P, Gurses AP, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics 2013; (56):1669–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen E, Seaman CA (2007). Likert Scales and Data Analyses. Quality Progress 2007:40, 64–65 [Google Scholar]
- 17.Buchanan George. Academic promotion and tenure: a user’s guide for junior faculty members. ASH Education Book; vol. 2009. no. 1, 736–741. [DOI] [PubMed] [Google Scholar]
- 18.Kohan DE. Moving from trainee to junior: faculty: a brief guide. Physiologist 2014;57(1):3–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson S Blood culture contaminants. J Hosp Infect 2014: 87 (1) 1–10. [DOI] [PubMed] [Google Scholar]
- 20.Martinez JA, DesJardin JA, Aronoff M, et al. Clinical utility of blood cultures drawn from central venous or arterial catheters in critically ill surgical patients. Crit Care Med 2002. 30(1):7–13. [DOI] [PubMed] [Google Scholar]
- 21.Berger I, Gil Margolis M, Nahum E, et al. Blood Cultures Drawn From Arterial Catheters Are Reliable for the Detection of Bloodstream Infection in Critically Ill Children. Pediatr Crit Care Med 2018. May;19(5):e213–e218. [DOI] [PubMed] [Google Scholar]
- 22.Handrup MM, Møller JK, Rutkjaer C, et al. Importance of blood cultures from peripheral veins in pediatric patients with cancer and a central venous line. Pediatr Blood Cancer 2015. 62(1):99–102. [DOI] [PubMed] [Google Scholar]
- 23.Kahneman D Thinking Fast and Slow. New York, Farrar, Strauss and Giroux; 2011. [Google Scholar]
- 24.Schutt RK. Investigating the social world: The process and practice of research. Second Edition. Thousand Oaks, Pine Forge Press, 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.