Abstract
Precision medicine requires materials and devices that can sense and adapt to dynamic physiological and pathological conditions. This motivates the design and manufacture of biohybrid materials that mimic the responsive behaviors demonstrated by natural biological systems. This report presents two parallel approaches to biohybrid design, biomimetics and biointegration. Biohybrid hydrogels that mimic the form and function of natural materials, or that integrate living cells or bioactive moieties, can respond to a range of environmental stimuli in parallel, including heat, light, pH, hydration, enzymes, and electric, mechanical, and magnetic forces. It presents a range of examples that illustrate the tremendous potential of this nascent discipline, and outline ongoing technical challenges related to manufacturing, storage, transport, and external non-invasive control of these materials that will need to be overcome in the coming years. The report also discusses the ethical, educational, and regulatory challenges that will govern translation of biohybrid design into medical applications. Personalized medical therapies that target the precise needs of a patients are a critically needed and expanding market. Biohybrid design offers the unique ability to manufacture materials and devices that match the dynamic and patient-specific in vivo environment, promising to generate more effective and safe therapies that enable personalized care.
Keywords: biohybrid, bioinspiration, biomimetics, hydrogels, implantable devices
Biohybrid

Implantable devices function in dynamic environments, but have largely been manufactured using synthetic materials alone. Recent advances in integrating responsive biological materials or bioinspired properties into such devices promise to harness the adaptive functional behavior of organic materials into engineered systems. This report highlights significant advances in biohybrid design, specifically the development of new responsive materials for precision medicine.
1. Introduction
Biological materials sense and respond to dynamic environments, resulting in complex adaptive behaviors that far surpass the functional capabilities of synthetic materials.[1–3] Implantable therapeutic devices function in dynamic environments and at physiological conditions, but have largely been manufactured using synthetic materials alone. Recent advances in integrating biological materials or bioinspired properties into such devices promise to harness the responsive functional behavior of organic materials into engineered systems. This form of dynamic adaptation will enable safer and more efficacious therapeutic response that is specific to the in vivo environment of a particular patient. This report aims to highlight some of the most interesting and significant advances in the field of biohybrid design, specifically the development of new responsive materials for precision medicine.
We will discuss two parallel approaches to biohybrid design: biomimetics and biointegration. The first term, biomimetics, pertains to the nature-inspired design of new synthetic materials, often accomplished by copying the microstructure-driven macro-function of natural materials. Commonly cited examples include gecko feet-inspired adhesives and butterfly wing-inspired solar panels. The second, biointegration, pertains to the incorporation of natural organic materials into synthetic materials and systems. These include systems that integrate bio-active components, such as proteins or living cells, into synthetic devices, such as probiotic ingestibles and growth-factor releasing bandages. Both biomimetic and biointegrated approaches have demonstrated significant advances in the past few decades, and are rapidly growing and changing. We will track the progress of biohybrid design as it pertains to advances in personalized medical therapies, and map out ongoing challenges and future goals for the field.
The focus of this report will be on the class of materials that offers a greatest diversity of examples of both biomimetic and biointegrated design: hydrogels. This class of materials is, moreover, very compelling for use inside the body, as the highly tunable, soft, wet, and stretchy material properties of hydrogels make them particularly well suited for use in vivo.[4–8] We will first explore the development of biomimetic hydrogels responsive to a range of environmental cues, such as heat, pH, light, pressure, and hydration before exploring their potential for medical use in vitro and in vivo. We will then discuss biointegrated hydrogels that incorporate active biomolecules and living cells, and elaborate on how this enables, for the first time, integration of patient-specific biomaterials into therapeutic treatment strategies and implantable devices.
Biohybrid design is a nascent discipline with tremendous potential and, as such, faces several ongoing challenges. The technologies required for large-scale design and production of biomimetic and biointegrated materials are being developed concurrently, and face challenges of their own regarding multi-material and high-resolution manufacturing. Moreover, control systems for manipulating biohybrid systems in vivo are in very early stages of development, and need to achieve high levels of predictability and functionality before these technologies can be integrated into therapeutics for human patients. Accompanying the technical challenges facing biohybrid design are the societal challenges of integrating new materials into the toolbox of every innovator. It would be helpful to develop new pedagogical techniques for effectively disseminating the principles of biohybrid design, and also need to develop ethical rules and governmental regulations around the manufacture of responsive semi-organic systems capable of non-natural or hyper-natural functional behaviors.
Personalized medical therapies that target the precise needs of a particular patient are a critically needed and expanding market. Biohybrid design offers the unique ability to manufacture materials and devices that match the dynamic and patient-specific in vivo environment, promising to generate more effective and safe therapies that enable personalized care. This review aims to highlight current progress and future directions for the development of biohybrid technologies tackle ongoing public health challenges.
2. Responsive Biomimetic Hydrogels
Biomimicry involves imitating the function of materials and systems in nature, and has been applied towards a range of human challenges ranging from healthcare to sustainability. Dynamically adaptive hydrogels that mimic some of the responsive properties of natural materials have seen tremendous progress in recent years. Similar to natural materials, they demonstrate tunable response to a range of environmental stimuli such as heat, pressure, hydration, light, pH, magnetic, and chemical triggers. These triggers, in addition to providing a range of functional behaviors activated by unique environmental cues, do not rely on tethered wires or batteries to power implantable devices. They thus offer a significant advantage over the current state-of-the-art in electronically-powered synthetic material actuation in terms of both safety, sustainability, and compatibility with standard clinical imaging modalities such as MRI. In this section we highlight a range of studies that develop and implement biomimetic hydrogels, and discuss their potential use in precision medicine.
2.1. Heat-Responsive Biomimetic Hydrogels
Thermal triggers are of particular interest in biomedical applications, as the body’s precisely regulated temperature provides a carefully controlled ambient environment for the deployment of heat-responsive materials. Thermo-responsive biomimetic hydrogels include natural polymers (gelatin, chitosan, etc.), PEG-based polyester copolymers, poly (organophosphazenes) and 2-(dimethylamino) ethyl methacrylate, poly (ethylene oxide)-b-poly (propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO), and poly (N-isorpopylacrylamide) (PNIPAAm).[9,10]
Hydrogels based on PEO-PPO-PEO triblock copolymers have seen significant use as heat-responsive materials for biomedical applications. These materials, commonly referred to as Pluronic®, demonstrate low mechanical strength and are thus often used in conjunction with other polymer backbones. For example, Lee et al developed an injectable hydrogel formulation of Pluronic and hyaluronic acid that polymerized from a liquid state into a gelled solid state when transferred from room temperature to body temperature.[11] This can be particularly useful for noninvasive delivery of therapeutic drug-loaded hydrogels to in vivo cavities. Soo et al reported that the gelation temperature of Pluronic-based hydrogels can be tuned by regulating the percentage content of copolymers such as chitosan.[12] They demonstrated that such modifications enabled precisely tunable release profiles for embedded biomolecules, such as human growth hormone. While Pluronic-based and other materials capable of thermo-sensitive gellation are interesting, PNIPAAm-based hydrogels form the basis of many thermo-responsive biomimetic hydrogels reported in the literature due to their ability to accomplish reversible heat-triggered shape-change. Below physiological temperature, the polymer is highly hydrophilic and absorbs water to form a swollen 3D hydrogel network. Above physiological temperature, however, PNIPAAm becomes hydrophobic, resulting in shrinkage of the material corresponding to expelling water from the 3D network. This switchable swelling behavior is reversible, providing a unique and repeatable environmental trigger for shape-changing functionality.
Breger et al have demonstrated self-folding of stiff micro-grippers, formed from the non-swellable polymer polypropylene fumarate (PPF), actuated by a layer of PNIPAAm that is photo-patterned onto the surface of the PPF.[13] The grippers take the form of a multi-armed hinged star, and the hinges are actuated by the temperature-controlled swelling or de-swelling of the PNIPAAm gel. Opening and closing of the gripper is thus tightly regulated by environmental temperature, and the researchers showed that this behavior is reversible and that the extent of folding is dependent on the environmental temperature, the dimensions of the stiff substrate material and the flexible PNIPAAm gel, and the swelling function (the ratio of hydrogel weight in the dry and swollen states) of the thermo-responsive PNIPAAm. Since the materials used in this gripper have been established to be biocompatible, the authors tested and successfully demonstrated the ability of the microgrippers to excise live cells from a clump of cultured fibroblasts in vitro. They anticipate that efforts to improve external control and manipulation of these grippers would enable their use in vivo, and tested the effect of magnetic control by incorporating iron oxide nanoparticles into the PPF layer of the device. A potential medical use case could be injection of these microgrippers, in a cold saline solution, via a catheter into a desired in vivo location for a microscopic tissue biopsy. The grippers would excise tissue in the desired location after heat-induced actuation, triggered by physiological temperature, and could be retrieved using a magnet. This micro-invasive procedure would enable patient-specific diagnosis and treatment planning, and could even be operated in reverse to deliver boluses of drugs entrapped within closed grippers that open at physiological temperature.
Zhang et al demonstrated that heating of thermo-responsive gels need not be regulated by ambient environmental temperature, but can also be controlled with high spatiotemporal precision by heating up gold nanorods embedded within the hydrogel using near-infrared light.[14] They developed a triblock copolymer with a polydimethylacrylamide (PDMA) central group with a copolymer of acrylamide (AAM) and acrylonitrile (AN) on each end: P(AAm-co-AN)-b-PDMA-b-P(AAM-co-AN). These hydrogels were loaded with gold nanorods, and remained stably loaded within micelle cores that constitute the hydrogel’s 3D structure. These hydrophobic micelle cores dissolve when light-induced heating of the gold nanorods triggers increased water solubility of the thermo-responsive polymer. The researchers investigated transition temperatures for their heat-triggerable hydrogels that were above physiological temperature, providing a unique responsive functionality that cannot be activated merely by injecting the material into the body, as in the case of PNIPAAm gels. Rather, the material must be heated above body temperature to be activated, and this could prove useful in in vivo applications where immediate actuation of the material is not desirable. For example, injected hydrogels could be selectively triggered using a fiber optic device or LED to release embedded cargo stored within the hydrophobic micelle structures. This could take the form of biochemical tags to enable in situ visualization of patient-specific morphologies or biomarker expression. It could also be used to release drugs at a rate that is dynamically triggerable and matched to observed patient response to treatment. Providing the transition temperature of the material is not high enough to cause lasting damage to the surrounding in vivo microenvironment, these hydrogels could provide significant and interesting heat-responsive functionality that can be exploited for a range of in vivo diagnostics and therapeutics.
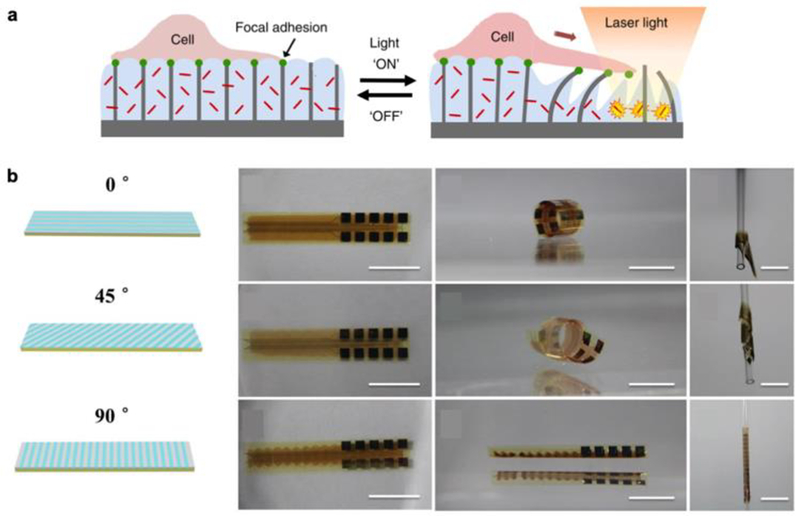
Sutton et al followed a similar approach to develop a composite PNIPAAm-gold nanorod hydrogel material, which they patterned around a series of micro-scale pillars coating a flat substrate designed for cell culture.[15] Since the pillars were embedded within the thermo-responsive gel, the rate and degree of gel swelling regulated the deflection of the micropillars, yielding microscale deformation of the cell culture substrate. This allows for precise and controllable micro-manipulation of cells seeded on the responsive hydrogel with high spatiotemporal resolution (Figure 1a). The researchers demonstrate this ability to manipulate live cell adhesion and migration through light-targeted actuation of the heat-responsive gel, setting the stage for investigating the biomechanics of single cells and cell sheets in both physiological and pathological states. In the context of personalized medicine, it is particularly interesting to envision this platform as a method for studying the biomechanics of cells extracted from patient samples. Understanding the mechanisms that underlie cell adhesion, motility, and force transmission could help understand the onset and progression of cancerous tumors, and lend insight into the biological impact of new untested therapeutic drugs.
Figure 1.
a) Schematic of light-triggered actuation of heat-responsive gel that serves as a cell culture substrate. Reproduced with permission.[15] 2017, Springer Nature. b) Bending and twisting of heat-responsive hydrogel is regulated by periodic gel stripes of varying crosslinking density oriented at different angles, enabling conforming around curved substrates, such as a hollow cylinder. Scale bar: 10 mm. Reproduced with permission.[16] 2017, John Wiley and Sons.
Du et al have also investigated the use of PNIPAAm-gold nanorod composite hydrogels, but have focused on macro-scale actuation of these materials, rather than microscopic manipulation as discussed in the study by Sutton et al.[16] By regulating the crosslinking density of these hydrogels in a set of periodic stripes on a substrate, the researchers demonstrated that self-rolling of the substrate could be triggered by light-activated heating of the material. Regulating the angle of the stripes could lead to more complex actuation behaviors such as twisting (Figure 1b). Moreover, this shape change was proven to be reversible, and could trigger bending and twisting in millimeter-scale structures within seconds. The triggerable conformation changes of the biomimetic gels developed in this study enabled behaviors such as clasping and conforming to curved or irregular structures. This is of especial interest in the context of implanted biomedical devices, as they must often conform and adhere to soft, curved, and irregular tissues and organs in vivo. The researchers demonstrated actuation of the material to conform around an artificial eye ball, and even showed that the material could be combined with other synthetic materials to enable multi-functional devices. For example, by integrating their thermo-responsive gels with flexible microelectrode arrays, they anticipate being able to measure and modulate electrical signals within the structure around which the material conforms. This opens up very interesting avenues for quantitative in vivo biosensing and patient need-derived therapeutic electrical and biochemical stimulation, while maintaining the material properties and biocompatibility required to safely interact with soft tissues and organs in a dynamic bodily environment.
2.2. Mechano-Responsive Biomimetic Hydrogels
A range of hydrogels that elicit a functional response to mechanical forces have been investigated for medical applications, providing an interesting range of cues that are compatible with in vivo use. These mechano-responsive materials have been developed and demonstrated from a range of baseline polymers, and derive their dynamically adaptive functionalities from different underlying mechanisms, ranging from micro-structural morphology to chemical signaling.
Si et al have developed a biomimetic pressure-responsive hydrogel by imitating the hierarchical cellular structure observed in spider webs or honeycombs.[17] The researchers created a 3D micro-structure composed of stacked cylinders formed from flexible silicon dioxide nanofibers. An alginate hydrogel is cast around this structure and, when stress is applied to the resultant material, bending and buckling of the deformable nanofibrous cylinder walls results in a drastic reduction in the macro-scale gel size and a corresponding increase in the electrical conductivity of the gel. They demonstrated that their gels could detect a wide range of dynamic pressures with high sensitivity, and that this mechano-responsive behavior could be replicated across hundreds of cycles. The bio-inspired hierarchical design of these mateirals motivates their use in a range of medical applications, such as scaffolds for tissue engineering that demonstrate different functional behaviors in response to repeated mechanical stress, induced either by a bioreactor in vitro or through targeted use in vivo. It is well established that the mechanical microenvironment differs significantly between people, and it is likely that implanted engineered tissues will need to adapt their morphology and function to the precise needs of the patient in whom they are implanted.
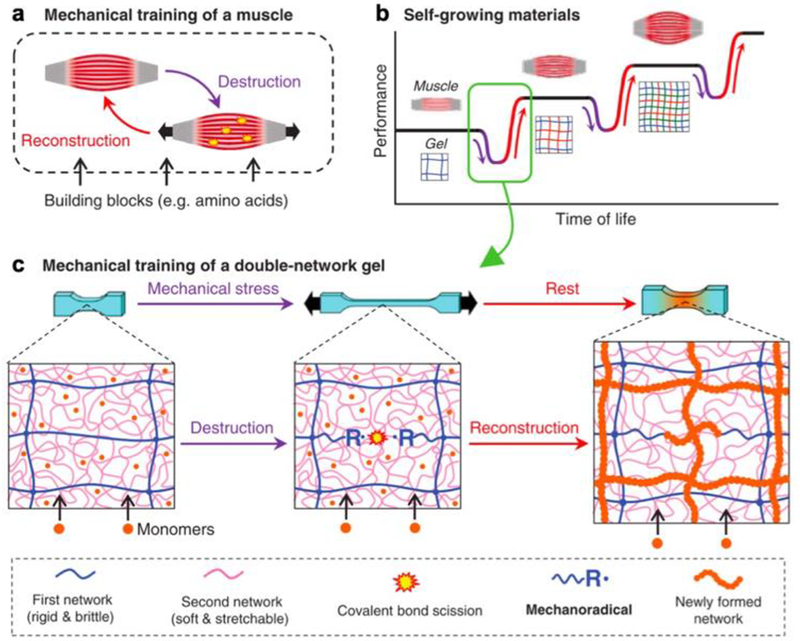
Matsuda et al have shown that mechano-responsive functionality can also lead to behavioral changes beyond tunable size, shape, and conductivity.[18] They have developed a double network hydrogel that grows larger and stronger in response to externally imposed mechanical forces through a deconstruction-reconstruction procedure (Figure 2). The researchers used as their substrate hydrogel an interpenetrating double network of poly(2-acrylamido-2-methylpropanesulfonic acid) sodium salt (PNaAMPS) and poly(acrylamide) (PAAM). The first network is brittle, the second network is stretchy, and both are covalently crosslinked. This enables the formation of tough, robust, deformable gels with superior mechanical properties and motivate their in vivo use. When mechanical stress is imposed on these gels, the brittle network breaks, while the stretchable network remains intact and preserves the macroscale form of the gel. If the gel is immersed in a solution of monomers, the monomers react with the broken ends of the brittle network to form a new cross-linked network that stiffens the gel. This results in an increase in both the size and strength of the material, and repetition of this breaking-reforming cycle results in progressive increases of both properties. By mimicking the self-strengthening behavior observed in biological tissues such as muscle, which uses a similar destruction-reconstruction process to boost performance, the researchers are able to extract a compelling responsive functionality in their bioinspired hydrogel. The use of biocompatible hydrogels in this study sets the stage for the use of such materials in biomedical applications. Self-strengthening materials can be used in vitro to study the response of cells seeded on the hydrogel substrate to changing mechanical cues, leading to improved fundamental understanding of the biomechanical behavior of tissues in both physiological and pathological states. If the unreacted monomer solution can be kept in a separate container, or exoskeleton, one can also imagine that self-strengthening implants that grow more robust as needed for an in vivo application can be used inside the body. By enabling dynamic adaptation to unpredictable mechanical environments, without precisely programming the material to withstand a specific magnitude or type of force, this type of material gives greater flexibility to engineers and scientists designing devices for medical use.
Figure 2.
a) Skeletal muscle undergoes a mechanical stress-induced growth and strengthening process. b) Mimicking a similar process in polymers could lead to materials that self-grow and self-strengthen. c) Schematic of mechanical stress-induced self-growing and self-strengthening in double network hydrogels. Reproduced with permission.[18] 2019, The American Association for the Advancement of Science.
2.3. Hydro-Responsive Biomimetic Hydrogels
Hydrogels are defined, to a great extent, by their highly absorbent properties. While all hydrogels demonstrate swelling in response to immersion in a hydrated environment, some types of hydrogels are capable of remarkable size ratios between their dried and swollen states. Exploiting this dramatic hydro-responsive actuation can lead to a range of interesting properties that motivate their therapeutic use. Perhaps the most widely used hydrogels that match this functionality are those based on a poly (acrylic acid) (PAA) network. It is a super-absorbent polymer with carboxylic acid groups that rapidly ionize in water, resulting in significant expansion of volume, as can be readily visualized in some of the commercial uses of this product such as in fillers for disposable diapers.
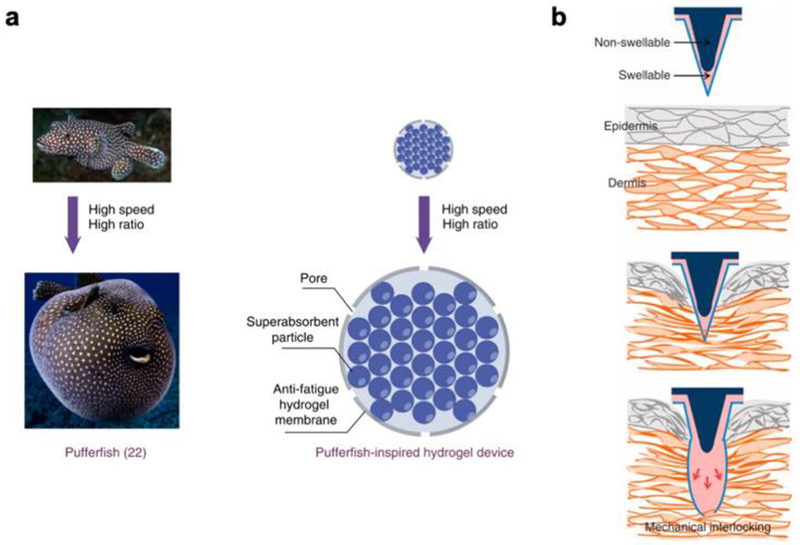
Liu et al, inspired by the defensive response of pufferfish, used PAA as the basis for a fast-swelling gastro-retentive implantable device.[19] Pufferfish stomachs are highly elastic and, when the fish are confronted by predators, can be rapidly filled with water and inflated to a spherical form that proves dangerous and hard to digest. The researchers placed a large number of absorbent PAA particles inside a stretchable and porous polyvinyl alcohol membrane and showed that immersion of the resultant device in water resulted in a rapid expansion in the volume of the device (Figure 3a). The mechanical robustness of the device, which could withstand compressive forces well above that demonstrated in in vivo environments such as the gastrointestinal tract, motivated testing their applicability in vivo. The devices were inserted into the stomachs of Yorkshire pigs, whose gastrointestinal anatomical dimensions mimic those of humans, and quickly swelled to a size larger than the pylorus, the opening that connects the stomach to the small intestines. As a consequence, the PAA-based devices demonstrated gastric retention over the period of several days. In the most simple form of the device, the rapid expansion of the gels provides a functional response on its own, as space-occupying balloon-like systems are currently used in clinical settings for bariatric applications. By reducing the volume of food intake for a patient, such expandable gastro-retentive structures can help promote sustainable weight loss. This study investigated further functionalization of their PAA devices by incorporating temperature sensors into the biomimetic hydrogel system, showing that such platforms could also be used for applications such as long-term physiological monitoring. By providing patient-specific readouts of a range of internal signals, this could have a significant impact on applications in personalized medicine.
Figure 3.
a) Pufferfish-inspired design of a rapidly inflating hydrogel device designed for gastric residence. Reproduced with permission.[19] Adapted with permission: http://creativecommons.org/licenses/by/4.0/. 2019, Springer Nature. b) Schematic of bioinspired microneedle that demonstrates hydrogel swelling-driven mechanical interlocking with tissue. Reproduced with permission.[20] 2013, Springer Nature.
Yang et al utilized the rapid expansion of a modified PAA-based hydrogel to develop a microneedle patch that formed a quick and tough mechanical interlock with biological tissue without requiring any chemical adhesives that could generate an inflammatory response.[20] To accomplish this goal, they patterned a swellable layer of polystyrene-block-poly(acrylic acid) (PS-b-PAA) onto a non-swellable array of micro-scale needles, manufactured via standard photolithographic and injection molding techniques. Upon insertion into any hydrated substrate, such as a hydrogel or biological tissue, the PS-b-PAA layer demonstrated rapid expansion, forming a micro-scale mechanical interlock with the substrate into which the microneedle array was inserted (Figure 3b). The researchers found that this hydro-responsive shape change provided robust adhesion to skin over long periods of time and a range of mechanical loads. Moreover, the shape change was reversible, and microneedle patches could be reused in biological tissues following sterilization. As a demonstration of the potential for such materials in vivo, the researchers demonstrated the advantage of using their adhesive over traditional surgical fixatives, such as staples, by showing a reduction in bacterial infiltration into skin grafts. They also demonstrated the use of these microneedle arrays on other tissues, such as the tissue lining intestinal surfaces, to motivate their use throughout the body in any location where adhesion and sealing, or localized drug delivery, is required. By providing a hydration-mediated and rapidly activated adhesive material, that can be readily modified to delivery bioactive molecules in a range of in vivo settings, this biomimetic hydrogel shows significant promise for medical applications.
Gulyuz et al also investigated a modified PAA-based hydrogel system, targeting replication of the self-healing and shape memory behaviors demonstrated by a range of materials in nature.[21] They developed a PAA hydrogel hydrophobically modified with cetyltrimethylammonium (CTA) counterions. The induced hydrophobicity causes the gels to collapse and become opaque when immersed in water, and reswell into larger transparent structures in acidic solutions (pH < 2). As a result, the mechanical stiffness of the gels is significantly enhanced when immersed in water, providing an interesting and unique property that differentiates it from other hydrogels. The researchers were also able to demonstrate self-healing functionality in these hydrogels by heating the cut ends of a hydrogel, with enhanced healing efficiency demonstrated with surfaces that had been pre-treated with acid or surfactant solutions. While in vivo use of these materials was not explored in this study, it is readily conceivable that the hydro-responsive shrinkage of the material could be utilized to manufacture a range of implantable devices that demonstrate unique functional behaviors when placed in contact with biological tissues and organs, similar to the studies by Liu et al and Yang et al described above.
2.4. pH-Responsive Biomimetic Hydrogels
Hydrogels demonstrate different swelling behaviors when immersed in solutions of differing pH, as described in some of the case studies explored in the previous sections. pH-responsive behaviors in these hydrogels are largely mediated by carboxyl groups, which are deprotonated in neutral of alkaline solutions, resulting in the rapid elimination of intermolecular hydrogen bonds. As the in vivo environment has several regions of widely varying and tightly regulated pH, this provides a unique environmental trigger for actuation of pH-responsive biomimetic hydrogels in the body. Moreover, many such materials have already been approved for in vivo use, enhancing the ease and rapidity of their transition into a range of clinical applications.
Zhang et al developed a supramolecular polymer gel composed of poly(acryloyl 6-aminocaproic acid) (PA6ACA) and poly(methacrylic acid-co-ethyl acrylate) (EUDRAGIT L 100–55).[22] The resulting hydrogel is flexible, as a result of the loosely crosslinked nature of the supramolecular gel network, and contains pH-responsive carboxyl groups that lead to disintegration of the material in non-acidic environments. The researchers used this gel as a bonding material to link together beads composed of polycaprolactone (PCL), a biocompatible material that is commonly used for passive release of therapeutic drugs in vivo. A ring like structure composed of these two materials demonstrated gastric retention, proving the integrity of the pH-responsive gel in acidic environments. When the device passed through the pylorus into the intestines, the change in pH triggered dissolution of the supramolecular gel linker, resulting in a breakdown of the device that enabled safe passage. Loading the PCL beads with therapeutic drugs offers a readily approachable use case for these devices in vivo, as prolonged drug delivery from a single ingestion event can be achieved, and the precise combination and release rates for the drugs can be tuned to match the needs of a specific patient.
Bellinger et al actually demonstrated one such clinical use of this type of pH-responsive material by designing a gastric-retentive device for safe and sustained delivery of therapeutic drugs.[23] The researchers designed a device that, when folded, took the form of an ingestible pill and unfolded into a large star in the stomach with a form factor that promoted gastric retention. The arms of the star-like device, connected by a pH-responsive linker (based on EUDRAGIT L 100–55, as above), were composed of PCL loaded with ivermectin. This well-established drug has been proven to be efficacious against a variety of infectious diseases, ranging from treating river blindness to preventing the transmission of malaria. The researchers were able to demonstrate that their device could produce sustained serum concentrations of ivermectin for at least one week post administration in a Yorkshire pig model. Following passage into the intestine, the pH-responsive linkers disintegrated and the drug-eluting device was safely passed from the body. In addition to serving as platforms for releasing patient-specific amounts and combinations of bioactive molecules, devices such as the one described in this study could also serve as platforms for ingestible electronics. Gastric resident electronic sensors that monitor an individual’s physiological signals over a clinically relevant period of time could provide precise real-time information about a patient that help clinicians guide their care.
2.5. Light-Responsive Biomimetic Hydrogels
A variety of natural materials and systems demonstrate optically responsive behaviors, and efforts to incorporate such light-tunable functionalities into biomimetic hydrogels have met with significant success in recent years. Hydrogels that demonstrate light-responsive stiffening, as well as those that demonstrate light-responsive degradation, have been employed for a range of biomedical applications in in vitro and, to a lesser extent, in vivo settings. While the chemical moieties that govern photo-conjugation versus photo-cleavage are different, the methodologies used to test and modulate their function, and the in vivo control systems that must be developed to employ them in therapeutic applications, are broadly similar.
Kloxin et al demonstrated early advances in developing materials with light-tunable mechanical properties by incorporating photo-cleavable moieties into poly (ethylene glycol) (PEG)-based hydrogels.[24] Functionalizing a PEG backbone with a photolabile nitrobenzyl ether-derived acrylated functional group enabled the researchers to create a 3D hydrogel network with light-degradable cross-linkers. They showed that this functionalization enabled light-responsive reduction in mechanical properties with high spatiotemporal precision, and were able to generate 3D features on the micron scale using a laser. While prior demonstrations of light-responsive cross-linking dynamics in hydrogels had focused primarily on light-initiated radical polymerization, this study set a new precedent for light-driven patterning and functionalization by including light-initiated cleavage into the toolbox of polymer engineering. Control over light wavelength and intensity offered the researchers the ability to finely tune light absorbance within the gel and subsequent efficiency of linker cleavage.
DeForest and Anseth built on this work by incorporating both photo-conjugable and photo-cleavable moieties into click chemistry-based hydrogels, generating multiple light-responsive functionalities from a single material, with function dependent on light wavelength.[25] The photo-reactive functionable groups were activated by visible light, and the photo-degradable groups were activated by ultraviolet light, enabling orthogonal control of gel chemical patterning and erosion through selective activation of light-responsive crosslinkers. Recognizing that this platform could serve as an interesting platform for studying cell cultures, the researchers used these gels as substrates for culturing human mesenchymal stem cells and used light to selectively pattern spatiotemporally varying biochemical and biophysical cues to guide cell adherence and motility. They hypothesized that this platform could be used to test hypotheses around cell-material interactions, as it provides a non-invasive and biocompatible mechanism for dynamically tuning hydrogel properties during in vitro culture. This could serve as a particularly interesting tool for studying both physiological and pathological biomechanics of engineered tissues in standard in vitro culture or in organ-on-a-chip platforms. Cells extracted from animal models of human disease, or from human subjects themselves, could be studied in the context of a synthetic and externally regulated mimic of the in vivo microenvironment. This tool could enable studying personalized medical therapies that suit the needs of an individual patient.
2.6. Multi Stimuli-Responsive Biomimetic Hydrogels
Integrating a variety of bioinspired stimuli-responsive behaviors into hydrogels in parallel has offered even further control over the morphology and function of these materials in recent years. Orthogonal control over different aspects of functionality offers the ability to engineer more complex behaviors than possible with single cues alone. In the biomedical context, this offers the flexibility to study cells and tissues in complex engineered microenvironments in vitro, while also providing robust and environmentally responsive materials for implant design and manufacture. Assuming that implanted devices function in dynamic environments that vary widely between individuals, this flexibility in tunable response is of particular interest in the context of personalized medical care.
Xiao et al investigated the ability to integrate light-, pH-, and thermo-responsive behaviors into a single hydrogel network, and demonstrated that such hydrogels demonstrated a triple shape memory effect.[26] Using a baseline poly(acrylamide) polymer, the researchers incorporated both light-sensitive and pH-sensitive chemical moieties into the polymer backbone. The resultant material demonstrated dual shape memory in response to either light or pH, and triple shape memory in response to thermal stimuli, which occurred when pH and light stimuli were applied sequentially. As a result, the polymers they developed were able to adopt one of three shapes based on exposure to environmental stimuli, a capability that has significant potential impact on the design and manufacture of biomedical devices. Such devices must often be inserted into the body through minimally invasive procedures, necessitating a slim-profile design, while also requiring complex 3D shapes once implanted. An environmentally triggered approach towards transforming the configuration of a slim-profile material into a complex shape would therefore provide significant advantages in the biomedical realm.
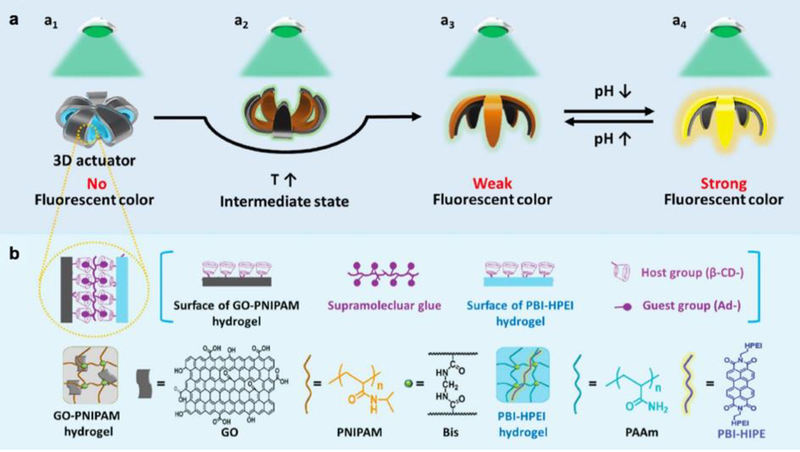
Ma et al also investigated the intersection of light-, pH-, and thermo-responsive behaviors in the context of a bilayer hydrogel that acted as both an actuator and a fluorescent indicator.[27] In their study, the researchers layered a heat-responsive graphene oxide-PNIPAAm hydrogel on top of a sheet of perylene bisimide-functionalized hyperbranched polyethylenimine (PBI-HPEI) that demonstrated pH-responsive fluorescence. Actuation of their bilayer flower-like design from a closed state to an open state was driven by an increase in temperature, following which a reduction in pH triggered the generation of a fluorescent signal upon irradiation of the device with green light (Figure 4). In the context of the implantable biosensors, which would likely travel through several bodily environments with spatially varying stimuli, it is interesting to imagine a sensor that actuates and fluoresces only under certain environmental conditions. These types of triggers can enable an implanted hydrogel to act as a sensor that can be read externally to convey important information regarding the in vivo microenvironment.
Figure 4.
a) Schematic of bilayer hydrogel device that demonstrates temperature-triggered actuation and pH-responsive fluorescence. b) Host-guest interaction drives adhesion between the thermo-responsive hydrogel layer and the pH-responsive hydrogel layer. Reproduced with permission.[27] 2017, John Wiley and Sons.
2.7. Biomimetic Hydrogels Responsive to Other Stimuli
We have devoted this section of this report to an overview of some key studies investigating the bioinspired design and manufacture of stimuli-responsive hydrogels. This is not, however, an exhaustive list of the many varied studies that investigate engineering adaptive and responsive behaviors into synthetic hydrogel polymer systems. In addition to the stimuli discussed above as potential environmental triggers of predefined functions, a range of other stimuli, such as magnetic fields, electrical fields, and ultrasound waves, have also been investigated as potential mechanisms for evoking hydrogel response.[28] For example, a recent study by Chin et al demonstrated that a PEG-based implantable device doped with iron oxide nanoparticles could be controlled externally via a magnet to release drug payloads on demand.[29] Similarly, we have demonstrated that encapsulating superparamagnetic iron oxide nanoparticles (SPIONs) in a PEG-based gel can enhance magnetic resonance contrast.[30] By segregating SPIONs and encapsulated bioactive moieties in separate compartments of a “Janus” PEG microparticle, we were able to demonstrate responsive hydrogels that were applicable towards both diagnostic imaging and targeted drug delivery in vivo. Other triggers, such as electrical triggers, have also been demonstrated by researchers such as Bassetti et al. They have investigated electrical triggers that control hydrogel swelling and integrated them into microfluidic platforms, providing an interesting potential organ-on-a-chip platform for studying cell mechanics and electrical activity.[31] A combination of the approaches and external trigger stimuli presented in this section will likely be required to design the next generation of adaptive materials. Potential applications in biomedical engineering, and specifically precision medicine, include investigating the behavior of physiological and pathological cells and tissues in vitro and the design and manufacture of responsive therapeutic implants in vivo.
3.0. Responsive Biointegrated Hydrogels
Biointegration involves incorporating biological materials into engineered synthetic machines and systems. Biointegrated materials do not need to mimic the responsive behavior of natural materials through the incorporation of triggerable chemical moieties or microscale physical features. Rather, they simply utilize the innate adaptive functionalities of the biological components they contain. The protocols to manufacture and robustly integrate active natural materials, such as biomolecules and living cells, into hybrid materials can involve more complex assembly techniques, or methodologies with stricter environmental constraints. However, the resultant functional properties of these biointegrated polymers have the potential to exceed those demonstrated by biomimetic hydrogels that are purely synthetic. Complex and multi-step adaptive behaviors such as self-healing or self-assembly, driven by the biological components that make up the organic-hybrid hydrogels, could make such biointegrated materials particularly interesting in the context of biomedical applications. Precision medicine necessitates materials and devices that are responsive to unpredictable environmental cues, rather than simply generating engineered responses to stimuli known a priori. This particular ability to respond to a range of unknown and unpredictable stimuli may be unique to biointegrated materials and systems, and hold tremendous promise for personalized therapies.
3.1. Biomolecule-Integrated Responsive Hydrogels
A significant body of the literature surrounding the design and engineering of biointegrated hydrogels has developed around incorporating organic compounds, such as polysaccharides and proteins, into polymeric networks.[32] These networks often display unique and interesting responsive functionalities, while also serving as bioactive materials that have the potential to generate therapeutic responses when implanted in a site of disease or damage in vivo. They could also serve as smart tools or sensors for continuous monitoring of physiological signals, helping guide precision medicine by providing clinicians with crucial information about the personalized baseline for each individual patient.
Liu et al developed a triggerable tough hydrogel formed from a synthetic PAAM network with an interpenetrating network of the polysaccharide alginate.[33] They made use of the fact that the stimuli-responsive calcium ionic and disulfide bonds that hold these double network hybrid gels together could be dissolved with a biocompatible chelator and reducing agent. The researchers showed that the material they had developed was robust in response to mechanical deformation such as stretching, twisting, and slicing. They then demonstrated that immersion of the tough hydrogels in ethylenediaminetetraacetic acid (EDTA) and glutathione (GSH) resulted in dissolution of the polymers. Interestingly, this study further investigated the ability of these responsive materials to be delivered and triggered in an in vivo setting. They formed rectangular strips of their material and introduced them endoscopically into the stomachs of Yorkshire pigs, and demonstrated that introducing the chemical trigger into the swine stomachs drove dissolution of the hydrogels into a viscous solution. This type of environmentally responsive behavior could have a range of applications in personalized medicine, especially in the context of gastric-resident structures for prolonged drug delivery. Individuals with such devices in their stomachs could experience adverse side effects to the delivered drugs, necessitating the need for quick removal of the devices via an external trigger. If such a trigger can be non-invasively delivered via ingestion of a pill, rather than an invasive endoscopic or other surgical procedure, this could greatly enhance the safety and efficacy of personalized therapies for a range of human diseases.
Haider et al also investigated interpenetrating double network hybrid hydrogels formed from alginate and PAAM by developing a technique to render these robust materials magnetic.[34] The researchers dispersed alginate-coated and magnetic iron oxide nanoparticles throughout the double network gel structure. They were able to show that in addition to maintaining the positive mechanical attributes of tough hydrogels, such as stretchability, resistance to large loads, and notch insensitivity, they also responded strong attraction to a magnet. The researchers used their newly developed material to develop a cantilever beam-like structure whose tip displacement was governed by proximity to an external magnetic guide. They were also able to show that such a beam could be navigated, while untethered, through a tube in response to a moving magnet held at a distance from the tube and the hydrogel. They anticipate that such dexterity and externally controlled motility could extend the use of tough hydrogels towards clinical applications such as invasive surgical interventions. Since morphology of structures within the body can vary widely between individuals, it is important to be able to control the movement of soft but tough devices, such as drug delivery catheters engineered from responsive hydrogels, to a region of interest deep within the body.
Lei et al have explored other uses of alginate-based polymers by studying them in the context of self-healing ionic skins capable of pressure sensing.[35] They physically crosslinked calcium carbonate nanoparticles within networks of polyacrylic acid (PAA) and alginate, producing a flexible ionic gel that readily conforms to nonlinear substrates. They then placed this supramolecular mineral hydrogel onto a finger and were able to detect finger bending via a change in the real time capacitance signal of the material. Other behaviors, such as speaking, laughing, and changes in blood pressure, also resulted in detectable changes in capacitance. An interesting property of this hybrid organic-inorganic material was the ability to autonomously heal when two fractured pieces of gel were brought into contact, recovering its mechanical and electrical properties. A biointegrated pressure and strain sensor, such as the one these researchers developed, has tremendous potential in the context of personalized wearables for continuous measurement of baseline physiological signals. Soft, conformable, and functional materials such as this ionic skin could readily integrate with existing wearable devices or clothing to enable precise and personalized healthcare monitoring.
Meng et al also reported a modified alginate-based self-healable hydrogel with shape memory functionality and pH-responsive properties.[36] The researchers developed a supramolecular hydrogel composed of phenylboronic acid (PBA) grafted alginate and poly (vinyl alcohol) (PVA). The PBA-diol ester bonds, which crosslinked the hydrogel network, were pH responsive and could be formed or dissolved by changing the pH to alkaline to acidic, respectively. The dynamic nature of these bonds also led to autonomous self-healing, with healed hydrogels demonstrating similar mechanical properties as compared to undamaged control hydrogels. Shape memory was imparted to the hydrogels by immersing them in a solution of calcium chloride, where the interactions between calcium ions and alginate generated temporary crosslinks. This technique was used to fix the hydrogels in a temporary shape that could easily be erased by transferring the gel to a solution of either sodium carbonate or EDTA. The multi-stimuli responsive functionality of these biointegrated hydrogels offers many interesting and advantageous properties to consider in biomedical applications. The authors propose the material as a guide wire for catheters, a suture material, or even a substrate for a polymeric stent graft, as any mechanical damage or laceration that occurs during insertion of these devices into the body could be quickly healed. Moreover, a temporary shape used during a minimally invasive insertion procedure could be transformed into a different shape that conforms to the in vivo environment, utilizing the shape memory properties of this biointegrated material.
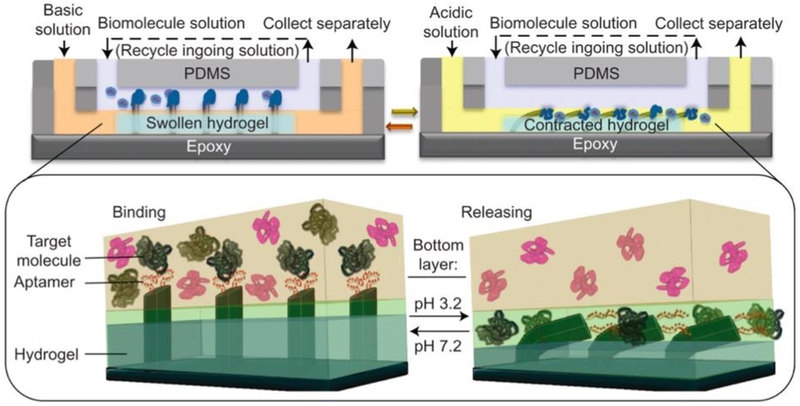
Shastri et al have shown that, beyond simply integrating a natural hydrogel backbone with a synthetic polymer backbone, modifications to synthetic hydrogels with other biomolecules can also lead to interesting adaptive functional behaviors.[37] The researchers designed an in vitro biomolecule catch-and-release system modulated by chemomechanical signals by combining a thrombin-specific aptamer with pH-dependent binding strength with a pH-responsive hydrogel, poly (acrylamide-co-acrylic acid) (P(AAC-co-AAM)). This hydrogel contracts at pH < 4.5 and swells at pH > 4.5. When the hydrogel is in its swollen state, microfins embedded within protrude out of the gel and interface with liquid solutions above. When the hydrogel contracts, the embedded microfins bend and do not interact with the liquid solution above the gel. By functionalizing these microfins with a thrombin-specific aptamer, the researchers were able to “catch” thrombin in liquid solutions placed above the hydrogel when in its swollen state, and “release” thrombin from the aptamer-functionalized microfins when the hydrogel contracted in an acidic environment (Figure 5). This type of biomolecule-integrated material system has significant potential applications in biomedical diagnostics. Samples extracted from individual patients can be readily analyzed in a microfluidic device containing such a catch-and-release system, enabling ready quantification of the presence and concentration of a variety of biomarkers of interest present within the sample. As rapid and personalized diagnoses of human disease will be a necessary precursor to developing targeted individualized therapies, this and other similar tools will be critical in the field of precision medicine.
Figure 5.
Schematic of aptamer-enabled biomolecule catch-and-release microfluidic system, driven by pH-responsive gel. Reproduced with permission.[37] 2015, Springer Nature.
Ehrbar et al have also investigated integrating responsive biomolecules into hydrogels for triggering environmental stimuli-controlled action.[38] They coupled a genetically engineered enzyme, bacterial gyrase subunit B, with a poly(acrylamide) backbone and showed that the addition of an antibiotic, novobiocin, dissociated the enzyme subunits and resulted in hydrogel degradation. Triggerable and dose-dependent release of entrapped proteins within these drug-sensing hydrogels served as a demonstration that these materials have significant potential for in vivo applications. If implanted in vivo, these materials could enable precisely triggerable delivery of active biomolecules in response to an ingested pharmaceutical. The timeline of release could be precisely tuned to the needs of an individual patient by adjusting the dose of pharmaceutical delivered to the patient.
Maitz et al have presented another interesting example of biomolecule-integrated responsive hydrogels in the form of a PEG-based hydrogel integrated with heparin and triggered by environmental thrombin.[39] Interestingly, they showed that such responsive hydrogels were capable of feedback control, as the heparin release downregulated the coagulation trigger. The material they developed could have significant potential in regulating a patient’s response to a blood-contacting implantable device with precise individualized control over anticoagulant administration. This provides a powerful example of a feedback-controlled approach towards designing a stimuli-responsive material.
3.2. Biomolecule-Encapsulating Responsive Hydrogels
Biomolecules integrated within the backbones of synthetic hydrogels offer several unique and responsive behaviors, as outlined above, but are not the only potential use for biomolecules in this context. Early studies investigated hydrogels as passive delivery vehicles for encapsulated biomolecules. However, recent advances in developing stimuli-responsive hydrogels have significantly improved our ability to tune the timing and rate of biomolecule release in response to specific environmental cues. This is of particular interest in personalized medicine, as one can readily imagine that an implanted gel releasing bioactive therapeutic moieties should include the ability to change the rate of biochemical release based on the observed patient outcome. Developing such tunable and controllable biointegrated hydrogels will thus be critical towards translating this technology into real world clinical applications.
Griffin et al demonstrated early advances in generating multi-stage release profiles for active biomolecules encapsulated within a stimulus-responsive hydrogel.[40] The researchers functionalized a PEG hydrogel with photodegradable ortho-nitrobenzyl (oNB) group, and conjugated each oNB group with a fluorophore designed to mimic different therapeutic agents. The three fluorophores they used (fluorescein, rhodamine, and aminomethylcoumarin acetate (AMCA)) were conjugated such that activating the light-sensitive linker resulted in direct release of the pure fluorophore without attached fragments of the oNB moiety. Furthermore, the linkers were designed to bias release of conjugated fluorophores at three different wavelengths (365, 405, and 436 nm), yielding the ability to predictably and independently release model therapeutic molecules separately and together. This technique enables complex spatiotemporal control of a hydrogel microenvironment, which could offer personalized biological signal presentation in both in vitro organ-on-a-chip and tissue engineering applications as well as in in vivo settings.
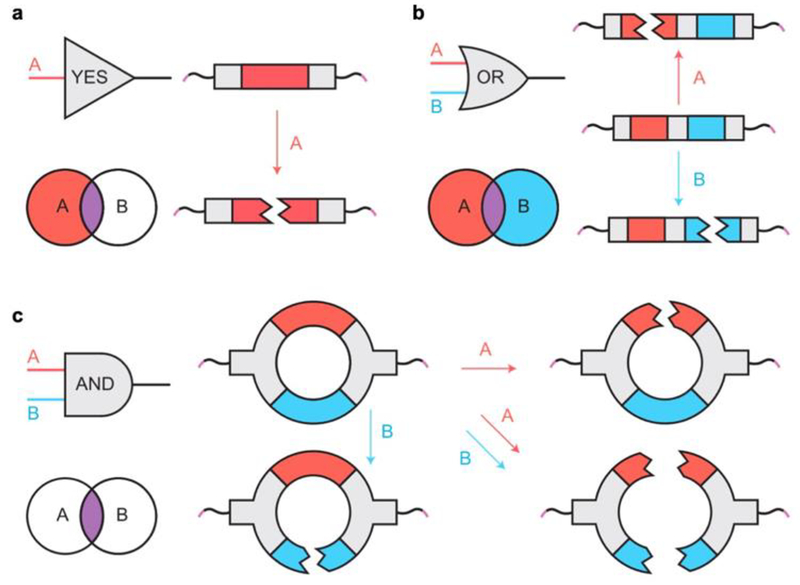
Badeau et al also investigated the development of stimuli-driven payload release from biointegrated hydrogels, but expanded the range of potential input stimuli to include enzymes and reductants in addition to light.[41] The researchers engineered modular “logic gates” that generated varying responsive release behaviors based on whether different environmental cues were presented sequentially or in parallel (Figure 6). This logic-gated approach generated a range of programmable degradation responses, which they exploited to study the release of a chemotherapeutic drug, doxorubicin, to generate apoptotic death of cervical cancer-derived HeLa cells. Their approach enabled gel degradation-triggered therapeutic release only in response to specific cancer microenvironment cues: reducing conditions and matrix metalloproteinase (MMP). This dual input approach resulted in complete cell eradication, offering a truly compelling case for tunable and logic-driven release of small-molecule therapeutics in response to a range of pathological signals. Extending this protocol towards other diseases and drugs could yield significant advances in the use of stimuli-responsive biointegrated hydrogels for precision medicine.
Figure 6.
a) Schematic of YES-gate crosslinker with a single stimuli-responsive chemical moiety. b) Schematic of OR-gate crosslinker with two stimuli-responsive chemical moieties connected in series. c) Schematic of AND-gate crosslinker with two stimuli-responsive chemical moieties connected in parallel. Reproduced with permission.[41] 2018, Springer Nature.
Lai et al demonstrated another approach towards protein release that relied on an aptamer-functionalized hydrogel.[42] The researchers utilized a multi-step approach to program biomolecule release, wherein a light stimulus freed an internal molecular signal which then induced the release of a protein via a hybridization reaction. This offered a method for externally controlling protein release that did not change the elastic modulus of the gel, as the crosslinks that determined the mechanical properties of the 3D polymer network were not affected by protein release. Protein release could be increased by increasing irradiation time, while preserving the mechanical integrity of the hydrogel system. By mimicking the signal transduction cascades observed in living systems in a biointegrated material, the researchers were able to offer the unique functionality of triggerable delivery of bioactive compounds. Allowing orthogonal control over biomolecule release and material stiffness could offer a better platform for investigating cellular response to both mechanical and biochemical signals in vitro. Moreover, if implanted as a drug delivery system, the implanted device would maintain its structural integrity in the dynamic in vivo microenvironment while still retaining programmable release functionality.
Lee et al have shown that biomolecules integrated within a hydrogel network can have display functional outcomes beyond the release of therapeutic drugs.[43] They developed a PEG-based hydrogel that, when triggered with an external stimulus, in this case blue light, presented cell adhesive RGD peptides on the surface of the gel. This enabled on-demand triggering of cell adhesion on regions of the gel that were triggered to present the caged peptides. The researchers demonstrated that this trigger could be activated transdermally when hydrogels were implanted in vivo in mice. In addition to modulating cell adhesion on the implanted biomaterial, this also triggered cell invasion and vascularization, providing an interesting method for externally tuning in vivo response to implantable devices and tissues. In the context of precision medicine, this spatiotemporal tuning of host-implant interactions could aid precisely tuning the presentation of different bioactive cues to regulate inflammatory response and long-term integration with the surrounding microenvironment.
3.3. Cell-Laden Responsive Hydrogels
Beyond integration of active biomolecules into hydrogels, more complex responsive and bioactive behaviors can be triggered by incorporating living cells within biocompatible polymer matrices. This has a range of applications including in vitro studies of 3D cell cultures and in vivo implants for cell therapy and regenerative medicine. Incorporating cells that demonstrate pathological morphology or function similar to that demonstrated by a patient, or incorporating cells derived from the patient being study, into engineered hydrogels could have tremendous potential impact on precision medicine. Studying tissue behavior in response to precisely regulated environmental cues, within the 3D context that most closely mimics the in vivo microenvironment, will provide unprecedented insight into the cascade of multi-cellular interactions that drive complex functional behaviors in biological systems. In this section, we will explore some salient examples of cell-laden responsive hydrogels, and consider each study presented within the context of its potential impact on patient-specific therapeutic care.
Jamal et al investigated the use of β-TC-6 cells encapsulated within hydrogels as biological factories to produce insulin.[44] Prior to cellular encapsulation, the researchers developed protocols to pattern PEG hydrogel bilayers with varying molecular weight PEG in each layer. Due to differential swelling in each layer, as determined by the molecular weights of the polymer used, the bilayers self-folded in water. By generating controllable micro-scale folds through photopatterning, complex shapes such as spherical capsules, helices, cylinders, and cylinders with micro-posts or micro-holes could be readily fabricated from a 2D structure. Encapsulating cells within these PEG bilayers resulted in 2D designs that could self-fold into complex 3D shapes and structures in a process termed “bio-origami”. Different populations of cells encapsulated within different layers also generated the ability to generate spatially controllable distribution of biological functionality (Figure 7). This is of functional interest, as cell-laden bilayers could be micro-invasively inserted into an in vivo environment prior to letting hydration drive the hydrogel’s self-assembly into a more complex 3D design. Interestingly, the hydration-driven folding resulted in an additional functional advantage by generating increased secretion of insulin from encapsulated β-TC-6 cells when stimulated with glucose. The researchers hypothesize that this is likely due to changes in the porosity and strain in the hydrogel matrix as a result of the macro-scale curvature induced by folding. The platform they developed could thus serve as an interesting model to explore the effect of 3D microenvironment on cell morphology and function, leading to a closer mimic of the in vivo microenvironment. It could also be used to develop multi-cellular devices that can be implanted to serve as biological factories and produce a range of bioactive and therapeutic molecules in response to environmental cues.
Figure 7.
a) Schematic of bilayer self-folding hydrogel with different cell populations encapsulated in different layers. b) Fluorescent image of self-folding hydrogel with an inner layer encapsulating blue Hoeschst-stained fibroblasts and with an outer layer encapsulating green calcein AM-stained fibroblasts. Length of hydrogel = 4 mm. Reproduced with permission.[44] 2014, John Wiley and Sons.
Kapyla et al also investigated encapsulating cells within 2D hydrogel bilayers that undergo a shape change to form a 3D structure.[45] Their study, however, relied on an external light stimulus rather than a hydration stimulus to generate this shape change. C2C12 mouse myoblasts encapsulated within a light-degradable PEG thin film were irradiated with ultraviolet light. As the light was attenuated throughout the thickness of the gel, the portion of the gel closest to the light source demonstrated greater degradation, and hence increased swelling when hydrated. This resulted in self-folding through a similar mechanism as that described above. The researchers were able to demonstrate that ultraviolet light irradiation did not adversely impact the viability of encapsulated cells. This provided the ability to precisely tune the time at which self-folding could be triggered, offering a greater degree of temporal control than offered by purely hydration-driven folding approaches. This ability to accomplish a precisely timed shape-change offers interesting advantages both in the context of in vitro studies of cellular biomechanics in physiological and pathological conditions and in vivo implants for therapeutic monitoring or drug release.
Griffin et al have also investigated encapsulating cells within light-degradable PEG thin films, but focused on the platform as a mechanism for therapeutic cell delivery rather than shape change.[46] They demonstrated that sustained exposure to ultraviolet light could accomplish spatially controlled release of cells via gel erosion, and that different cells encapsulated within different regions of the gel could be released separately. To accomplish this goal, they investigated a range of different compositions for the oNB linkers previously developed by their group, choosing optimal linker compositions that degraded quickly while allowing for bulk erosion throughout the thickness of the gel. This has very interesting potential applications in the development of new cell therapies, such as implantable platforms for the delivery of stem cells to a site of disease or damage. Timing the release rate of cells from the encapsulating gel could help improve engraftment rates and allow tuning therapeutic cell delivery to the needs and microenvironment of individual patients.
Liu et al have looked beyond stimuli-driven behavior that results in a permanent shape change or degradation of a cell-laden hydrogel by developing materials that are capable of cyclic control of polymer stiffness over time.[47] The researchers developed protein-polymer hybrid hydrogels that could undergo reversible stiffening in response to environmental cues by engineering protein cross-linkers that exhibit different conformations and end-to-end lengths in response to an external stimulus. As the length of the polymer chains that connect 3D hydrogel networks determine the resultant mechanical properties of the macroscale material, this ability to tune the length of the crosslinker in a reversible fashion enabled cyclic control over polymer stiffness. As expected, the gel demonstrated greater changes in stiffness with greater concentrations of protein crosslinker binding the matrix, and the researchers explored a range of external stimuli for eliciting stiffness change including calcium and light. The choice of stimulus used is dependent on the real-world application, as light offers greater spatiotemporal resolution but that is not always required. The researchers explored an interesting potential use for their material in vitro by studying the effect of cyclic mechanical loading on cells encapsulated within the gels. This is just one of many examples of this material technology on mechanobiology, organ-on-a-chip devices, and tissue engineering. Orthogonal control over cellular extracellular matrix mimics that is reversible could provide advantages over other stimuli-dependent hydrogels that are not capable of cyclic tuning.
Yeh et al investigated cell-laden hydrogels as micro-scale building blocks for tissue engineered structures, in parallel with several other researchers in the field.[48] Their technique for manufacturing such structures relied on patterning micro-scale cell-laden gels using a poly (dimethyl siloxane) (PDMA) stamp and standard photolithographic techniques. Cells were suspended in either a poly (ethylene glycol) diacrylate (PEGDA) or methacrylated hyaluronic acid (MeHA) prepolymer solution prior to ultraviolet light-initiated cross-linking. The resultant micro-molded gels contained evenly distributed cells and could be assembled into 3D structures that mimic in vivo architecture. The researchers in this study showed that different cell types could be encapsulated within different gel blocks, and that the blocks could be assembled to engineer a complex multi-cellular structure with programmable shape and biochemical composition. This ability to recreate cell-cell and cell-matrix interactions has applications both as disease models and in regenerative medicine.
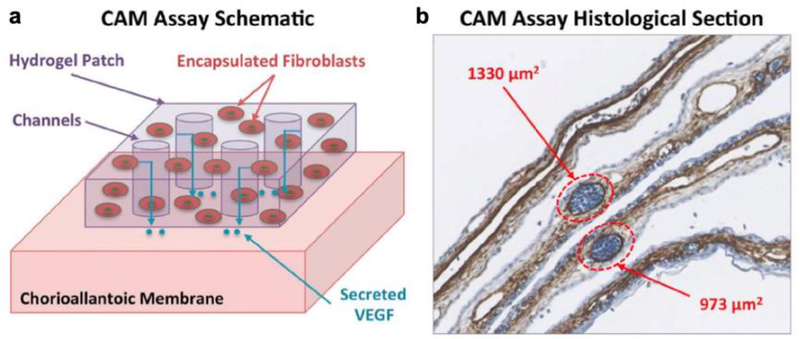
Several researchers have built on these early studies of cell-laden hydrogels by leveraging advanced 3D bio-printing technologies as precise and high-throughput manufacturing tools. For example, we have printed a range of murine cell lines (fibroblasts, myoblasts, endothelial cells, and bone marrow stromal cells) encapsulated within PEGDA matrices using a custom-built 3D printer and shown that the cells remain viable up to two weeks post-fabrication.[49] The use of 3D printing technologies, such as the projection stereolithographic apparatus (SLA) designed in this study, has a range of advantageous properties including rapid and high-resolution patterning of complex designs. The apparatus we built enabled spatial patterning of multiple cell types with feature resolution < 5 μm, and also was able to pattern smooth curved features from a single layer by spatially tuning the intensity of projected light. Fibroblasts encapsulated within PEGDA hydrogels were stimulated to secrete VEGF through the addition of tetradecanoylphorbol 13-acetate (TPA). Cell-laden hydrogels patterned with microscale channels secreted greater concentrations of VEGF as this design increased the gel surface area/volume ratio and resultant diffusive flux. The angiogenic functionality of these printed cell-laden constructs was assessed via a chick chorioallantoic membrane assay, demonstrating that VEGF-secreting patches resulted in enhanced angiogenesis (Figure 8). Targeted stimulation of the growth of new blood vessels has significant potential applications in active wound healing. Incorporating other types of cells and cell-secreted factors into these hydrogels extends the potential range of applications further. Providing a method for rapid manufacturing of multicellular constructs with tunable mechanical and biochemical environments offers significant advantages over other approaches for manufacturing complex 3D cell culture environments.
Figure 8.
a) Schematic of chick chorioallantoic membrane (CAM) assay used to assess the functionality of 3D printed micropatterned cell-encapsulating hydrogels. b) Histological section of CAM assay showing cell nuclei (blue) and alpha-smooth actin (brown). Two blood vessels are highlighted to show how vessels were identify and measured. Reproduced with permission.[49] 2015, John Wiley and Sons.
3.4. Tissue-Integrated Responsive Hydrogels
Some of the most complex examples of cell-laden hydrogels are tissue engineered constructs intended to mimic the morphology and reverse engineer the function of native tissue. These structures are, of course, primarily intended for applications in regenerative medicine, as they are intended to replace diseased or damaged tissue and organs in vivo. Tissue engineered constructs using a variety of cell types have been presented in several other in-depth reviews, and are beyond the scope of this report. It is mainly important to recognize that replacing the cell types used in these constructs with patient-derived cells has significant implications for personalized medicine, and that ongoing advances in multi-cellular manufacturing of large vascularized constructs will be critical towards driving advances in this field.
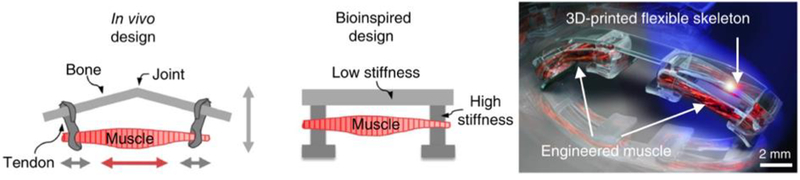
A subset of tissue-integrated hydrogels that have not been discussed in the context of patient-specific therapy are those that have been forward engineered to accomplish non-natural or even hyper-natural functional behaviors. Efforts in this field have primarily focused on tissue engineered skeletal muscle as a replacement for synthetic actuators in machines such as locomotive robots and pumps. Incorporating soft biological functional components in such engineered systems has the potential to improve the safety and ease of integration of these machines in in vivo settings. Moreover, using biohybrid actuators as replacements for synthetic electronics reduces the risks associated with implanted batteries and diagnostic imaging techniques that are incompatible with metals. There is thus a significant unmet need for biohybrid design in the context of active implantable therapeutic devices.
Early advances in the field of biohybrid actuators focused primarily on bilayer approaches, such as cultures of cardiac muscle sheets on flexible PDMS thin film substrates. Feinberg et al reported that synchronous contraction of engineered cardiac muscle could generate large deformations in an underlying flexible substrate, and used this insight to engineer cardiac muscle-powered robots that could accomplish functional behaviors such as walking, swimming, pumping, and gripping.[50] Nawroth et al built on this work through biomimicry by engineering a cardiac muscle-powered swimming robot that imitated the form and function of jellyfish.[51] This biomimetic approach could also be applied to reverse engineer the function of other systems, as demonstrated by Park et al in their demonstration of a cardiac muscle-powered string ray.[52] The researchers demonstrated that using optogenetic cells to engineer the muscle enabled phototactic guidance of their machines, enabling external noninvasive control over the actuation of a biohybrid system. Williams et al drastically reduced the size scale of these engineered machines by developing single millimeter-scale sperm-mimicking swimming robots actuated by the contraction of a few cardiomyocytes.[53] Chan et al moved towards integrating cardiac muscle sheets with hydrogels by engineering a 3D printed PEGDA walking robot.[54] These and other studies set the stage for engineering biohybrid systems that utilized the interesting functional properties of biological materials, but came with several limitations including the inability to engineer on/off control over the contraction of cardiac muscle and the requirement of using primary cardiac muscle cells with unpredictable performance characteristics.[55,56] Similar concerns related to using primary cells or excised tissue are also present with the use of muscle derived from insects.
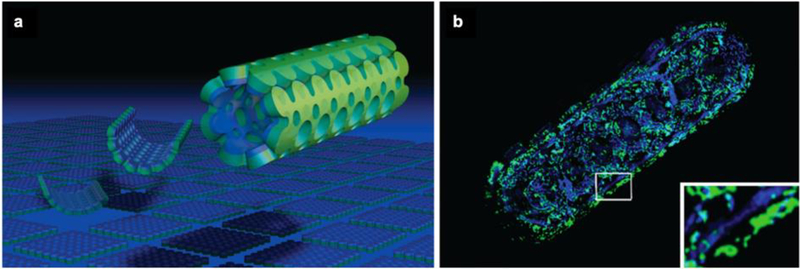
We have developed a method for engineering tissue-integrated responsive hydrogel actuators from a skeletal muscle cell line, and shown that such biohybrid materials are contractile in response to both electrical and optical stimulation.[57–59] In our studies, we mixed C2C12 murine myoblast cells within a solution composed of naturally-derived hydrogels, fibrinogen and Matrigel™, prior to polymerization of the mock extracellular matrix through the addition of thrombin. The cells proliferated and compacted the surrounding natural hydrogel matrix into a dense bio-integrated material composed of mature contractile myotubes. By tethering this tissue-integrated hydrogel with a synthetic hydrogel skeleton, designed to mimic the tethering of skeletal muscle to articulating joints in vivo, we induced tensile strain in the tissue that aligned the embedded myotubes. This resulted in controllable directional macroscale contraction that could be used to drive directional locomotion and rotation in response to a non-invasive blue light stimulus (Figure 9).
Figure 9.
a) Schematic of bioinspired design of skeletal muscle-powered machines. b) Light stimulation of optogenetically modified cells drives contraction of biohybrid actuators and directional locomotion of hydrogel skeleton. Reproduced with permission.[57] 2014, National Academy of Sciences.
As compared to traditional synthetic actuators, these bioactuators can adapt their form and function to changing environmental stimuli, such as by increasing force production in response to external biochemical factors, like human insulin-like growth factor, and exercise, in the form of passive mechanical stretch and light-induced cyclic contraction. To test the hypothesis that biointegrated materials can mimic the complex functional behaviors observed in natural biological systems, we studied the effect of induced damage to engineered muscle and developed a protocol to completely heal the tissue within two days. By mimicking the wound healing process observed in vivo, we showed that a combination of undifferentiated myoblasts, localized secretion of growth factors, and exercise-driven remodeling could yield recovery of bioactuator form and function, a capability that had not previously been demonstrated in any synthetic actuator.[60]
Biohybrid actuators are the first step towards developing tissue-integrated synthetic hydrogels for other responsive behaviors, such as sensing and processing, that can be applied towards engineering organic-inorganic hybrid machines.[61,62] Others have built upon the protocol we have developed to integrate neurons and neuromuscular junctions with skeletal muscle actuators,[63] and future studies surrounding the integration of vascular networks or chemical sensors could add further functionality to these machines. In the context of personalized medicine, these tissue-integrated responsive hydrogels offer an interesting platform to study mesoscale tissue function in physiological and pathological conditions. They could also be integrated into implantable devices for sensing and drug delivery to enable dynamic modulation of device function over time. The responsive biomimetic and biointegrated hydrogels described in previous sections could be combined with these tissue-integrated active hydrogel components to generate a range of complex functionalities that mimic or even surpass the functionality of natural systems, offering a unique advantage over synthetic inorganic materials. Incorporating biohybrid materials towards implants for patient-specific therapy promises to be a rapidly growing and compelling discipline with significant potential impact on the standards and efficacy of clinical care.
4.0. Ongoing Challenges and Future Directions for Biohybrid Design
Both biomimicry and biointegration, the parallel approaches that form the foundation of biohybrid design, offer promising advantages for the design and manufacture of next-generation devices that diagnose and treat diseases. There remain, however several unanswered questions and remaining technical challenges that must be addressed prior to wide-scale implementation of these technologies in real-world precision medicine applications.
Patterning millimeter to centimeter scale materials and devices, or potentially even larger devices, with micro-scale mechanical features and spatially distributed biochemical moieties, presents a significant technical hurdle. Early efforts in this field focused on adapting manufacturing technologies such as injection molding or photolithography to pattern hydrogel pre-polymer solutions into solid structures.[48,64] More recent advances in biofabrication technologies, such as 3D printing through stereolithographic or fused deposition modeling, have generated a significant body of knowledge around the design and manufacture of hydrogels and have been documented in several extensive papers and reviews.[65–69] Ongoing challenges in the field include enabling rapid multi-material fabrication of large hydrogel structures with micron-scale feature sizes. Building with living cells and proteins and stimuli-responsive synthetic materials places further stringent constraints on the ambient environment required during manufacture. The temperature, humidity, biochemical composition, and other environmental stimuli must be precisely controlled to preserve the function and prevent the death or degradation of embedded material components. It is likely, however, that combining insights from different biofabrication technologies, and choosing fabrication methodologies that suit the specific needs of a potential application, will lead to the implementation of these responsive hybrid materials in real-world applications.
Advancing bio-manufacturing technologies must be coupled with concurrent advances in protocols for long-term storage of biofabricated materials, to enable off-site manufacture and shipping of biohybrid machines and systems. We have done some preliminary work in this field by proving that engineered skeletal muscle tissue-integrated hydrogels can be frozen, stored for months, and revived to completely recover contractile function without a loss in cell viability.[70] Interestingly, this process even introduces micro-scale pores into the natural hydrogel extracellular matrix of the tissue, improving myotube alignment and generating larger forces in response to external stimulation. Similar studies of the long-term storage and transport of biomimetic and biointegrated materials must be performed to ensure their viability as tools for engineering the next generation of responsive implantable devices. This is particularly important in the context of biohybrid hydrogels, the case study explored in this report, as dehydration can drastically affect the form and function of these materials, and must be thoroughly characterized prior to use.
Beyond manufacturing, storage, and transport, biohybrid design faces significant technical challenges in the context of implantable devices for precision medicine. Ideally, an implanted biohybrid therapeutic device would offer real-time monitoring and personalized patient-specific therapy, as guided by the microenvironmental cues presented to the device in vivo. However, this response behavior is not always autonomous, and indeed may be required not to be, as physicians often must make care decisions based on a range of complex factors beyond mere quantitative measures of physiological signals. We thus require concurrent advances in control systems for tuning the action of biohybrid systems when implanted in vivo, and these may have to incorporate a range of cues such as the ones described throughout this report. Mechanical, electrical, biochemical, magnetic, pH, temperature, and light-based stimuli may need to operate in parallel to control a multi-functional biohybrid machine. Furthermore, they need to interact with implanted devices in a non-invasive and biocompatible manner that takes into consideration the specific environmental conditions of the body cavity into which the device is implanted. This will pose a significant design and manufacturing challenge in the coming years, but is likely to advance concurrently with the implementation of newly developed biomimetic and biointegrated materials into in vivo models.
Technical challenges are not the only hurdles facing the implementation of biohybrid design in personalized care. Building with responsive materials requires a significant shift in the design rules and principles engineers are traditionally taught to manufacture devices. Environmental constraints during manufacture, as well as during use, must be taken into consideration, and tools and techniques for selectively activating a range of environmental cues must be incorporated into educational curricula. Broadening the use of responsive materials in real-world applications thus requires new pedagogical techniques that more effectively disseminate the rules governing biohybrid design. We have investigated the effect of a project-based hypothesis-guided approach towards teaching the principles of biofabrication in an undergraduate classroom setting.[71] This has proved to be an effective methodology for translating lab protocols into robust implementable techniques, setting the stage for classrooms of the future that include biomimetic and biointegrated materials into the standard innovator’s toolbox.
Pedagogical approaches must include active discussions of the ethics of building responsive semi-organic systems, as these discussions will shape the regulatory guidelines that govern safe integration of this new discipline into our existing industrial framework. Biohybrid machines may utilize responsive components or living cells or tissues, and are designed to adapt to changing environmental conditions, but their autonomy must be governed by a range of engineering controls. Complex behaviors such as self assembly, self repair, self maintenance, and self replication are only desirable if they can be precisely controlled. This is of critical importance in precision medicine applications, as the safety profile and controllability of these machines must be fully proven prior to implementation inside humans. The ethics of designing semi-autonomous adaptive machines, and the responsibilities involved therein, such as the development of external controls over device function and lifetime, must thus be incorporated into educational curricula, discussed at research conferences, and used to guide conversations with relevant stakeholders in industry and government science policy.
This report presents a range of examples of biohybrid hydrogels, developed via either biomimetic or biointegration approaches, and outlines the technical and societal challenges that must be overcome prior to large-scale implementation of these materials in personalized medicine. While hydrogels offer a compelling and diverse set of case studies with which to examine this topic, biohybrid design approaches that incorporate other classes of materials are also of interest to many researchers in this field, and are worth considering when designing systems for patient-specific therapy. Individualized therapies that respond to the needs of particular patients promise to drastically improve the current clinical standard-of-care. Responsive biohybrid materials have the potential to better mimic the in vivo environment, producing more effective tools for studying biological systems in in vitro settings. Moreover, the improved ability of biohybrid materials to integrate with and adapt to the dynamic in vivo environment motivate their use as responsive functional implants in the coming years. Continuing advances in this field promise to tackle a wide variety of growing public health challenges, with significant potential impact on human health, wellbeing, and quality of life.
Acknowledgements
The authors acknolwedge support from the National Institutes of Health (Grant 5RO1EB000244) and the Koch Institute Support (core) grant P30-CA14051 from the National Cancer Institute. R.R. was funded by the AAAS L’Oréal USA For Women in Science Fellowship.
Biography
Ritu Raman is a postdoctoral fellow at MIT, supported by a range of awards and recognitions, including the L’Oreal USA For Women in Science Fellowship and the NASEM Ford Foundation Fellowship. Her PhD research as an NSF Graduate Research Fellow helped shape the field of skeletal muscle-powered robotics, and has been recognized by several prestigious prizes. Her research interests focus on developing biohybrid materials and machines for applications in translational medicine. Dr. Raman received her Bachelors Degree from Cornell University in 2012 and her Ph.D. from the University of Illinois at Urbana-Champaign in 2016, both in Mechanical Engineering.

Robert Langer is one of ten Institute Professors at MIT and an expert in tissue engineering and drug delivery. He has written over 1300 articles, has over 1100 issued and pending patents worldwide, and is the most cited engineer in history. He is one of four living indivdiuals to receive both the National Medal of Science and the National Medal of Technology and Innovation, and the youngest person to be elected to all three United States National Academies. Prof. Langer received his Bachelors Degree from Cornell University in 1970 and his Sc.D. from MIT in 1974, both in Chemical Engineering.

Contributor Information
Ritu Raman, Dr. Ritu Raman, 500 Main St., Cambridge, MA, USA 02142.
Robert Langer, Prof. Robert Langer, 500 Main St., Cambridge, MA, USA 02142.
References
- [1].Raman R, Bashir R, Adv. Healthc. Mater 2017, 1700496, 1. [DOI] [PubMed] [Google Scholar]
- [2].Kamm RD, Bashir R, Ann. Biomed. Eng 2014, 42, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kamm RD, Bashir R, Arora N, Dar RD, Gillette MU, Griffith LG, Kemp ML, Kinlaw K, Levin M, Martin AC, McDevitt TC, Nerem RM, Powers MJ, Saif TA, Sharpe J, Takayama S, Takeuchi S, Weiss R, Ye K, Yevick HG, Zaman MH, APL Bioeng. 2018, 2, 040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomatsu I, Peng K, Kros A, Adv. Drug Deliv. Rev 2011, 63, 1257. [DOI] [PubMed] [Google Scholar]
- [5].Zhang YS, Khademhosseini A, Science (80-. ). 2017, 356, DOI 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, 18, 1345. [Google Scholar]
- [7].Anseth KS, Bowman CN, Brannon-Peppas L, Biomaterials 1996, 17, 1647. [DOI] [PubMed] [Google Scholar]
- [8].Oyen ML, Int. Mater. Rev 2014, 59, 44. [Google Scholar]
- [9].Klouda L, Mikos AG, Eur. J. Pharm. Biopharm 2008, 68, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klouda L, Eur. J. Pharm. Biopharm 2015, 97, 338. [DOI] [PubMed] [Google Scholar]
- [11].Lee Y, Chung HJ, Yeo S, Ahn CH, Lee H, Messersmith PB, Park TG, Soft Matter 2010, 6, 977. [Google Scholar]
- [12].Yoo HS, J. Biomater. Sci. Polym. Ed 2007, 18, 1429. [DOI] [PubMed] [Google Scholar]
- [13].Breger JC, Yoon C, Xiao R, Kwag HR, Wang MO, Fisher JP, Nguyen TD, Gracias DH, ACS Appl. Mater. Interfaces 2015, 7, 3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang H, Guo S, Fu S, Zhao Y, Polymers (Basel). 2017, 9, DOI 10.3390/polym9060238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sutton A, Shirman T, Timonen JVI, England GT, Kim P, Kolle M, Ferrante T, Zarzar LD, Strong E, Aizenberg J, Nat. Commun 2017, 8, 14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Du X, Cui H, Sun B, Wang J, Zhao Q, Xia K, Wu T, Humayun MS, Adv. Mater. Technol 2017, 1700120, 1. [Google Scholar]
- [17].Si Y, Wang L, Wang X, Tang N, Yu J, Ding B, Adv. Mater 2017, 1700339, 1. [DOI] [PubMed] [Google Scholar]
- [18].Matsuda T, Kawakami R, Namba R, Nakajima T, Gong JP, Science (80-. ). 2019, 508, 504. [DOI] [PubMed] [Google Scholar]
- [19].Liu X, Steiger C, Lin S, Parada GA, Liu J, Chan HF, Yuk H, Phan NV, Collins J, Tamang S, Traverso G, Zhao X, Nat. Commun 2019, 10, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang SY, O’Cearbhaill ED, Sisk GC, Park KM, Cho WK, Villiger M, Bouma BE, Pomahac B, Karp JM, Nat. Commun 2013, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gulyuz U, Okay O, Macromolecules 2014, 47, 6889. [Google Scholar]
- [22].Zhang S, Bellinger AM, Glettig DL, Barman R, Lee Y-ALA, Zhu J, Cleveland C, Montgomery VA, Gu L, Nash LD, Maitland DJ, Langer R, Traverso G, Nat. Mater 2015, 14, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee Y-AL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T, Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G, Sci. Transl. Med 2016, 8, DOI 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Science (80-. ). 2009, 324, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DeForest CA, Anseth KS, Nat. Chem 2011, 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiao Y-Y, Gong X-L, Kang Y, Jiang Z-C, Zhang S, Li B-J, Chem. Commun 2016, 52, 10609. [DOI] [PubMed] [Google Scholar]
- [27].Ma C, Lu W, Yang X, He J, Le X, Wang L, Zhang J, Serpe MJ, Huang Y, Chen T, Adv. Funct. Mater 2017, 1704568, 1704568. [Google Scholar]
- [28].Stular D, Simoncic B, Tomsic B, Tekstilec 2017, 60, 76. [Google Scholar]
- [29].Chin SY, Poh YC, Kohler A-C, Compton JT, Hsu LL, Lau KM, Kim S, Lee BW, Lee FY, Sia SK, Sci. Robot 2017, 2, DOI 10.1126/science.1252826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Raman R, Clay NE, Sen S, Melhem M, Qin E, Kong H, Bashir R, Biomed. Microdevices 2016, 18, 49. [DOI] [PubMed] [Google Scholar]
- [31].Bassetti MJ, Moore JS, Beebe DJ Annu. Int. IEEE-EMBS Spec. Top. Conf. Microtechnologies Med. Biol. - Proc. 2002, 410. [Google Scholar]
- [32].Freudenberg U, Liang Y, Kiick KL, Werner C, Adv. Mater 2016, 28, 8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu J, Pang Y, Zhang S, Cleveland C, Yin X, Booth L, Lin J, Lucy Lee YA, Mazdiyasni H, Saxton S, Kirtane AR, Von Erlach T, Rogner J, Langer R, Traverso G, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Haider H, Yang CH, Zheng WJ, Yang JH, Wang MX, Yang S, Zrínyi M, Osada Y, Suo Z, Zhang Q, Zhou J, Chen YM, Soft Matter 2015, 11, 8253. [DOI] [PubMed] [Google Scholar]
- [35].Lei Z, Wang Q, Sun S, Zhu W, Wu P, Adv. Mater 2017, DOI 10.1002/adma.201700321. [DOI] [PubMed] [Google Scholar]
- [36].Meng H, Xiao P, Gu J, Wen X, Xu J, Zhao C, Zhang J, Chen T, Chem. Commun. (Camb). 2014, 50, 12277. [DOI] [PubMed] [Google Scholar]
- [37].Shastri A, McGregor LM, Liu Y, Harris V, Nan H, Mujica M, Vasquez Y, Bhattacharya A, Ma Y, Aizenberg M, Kuksenok O, Balazs AC, Aizenberg J, He X, Nat. Chem 2015, 7, 447. [DOI] [PubMed] [Google Scholar]
- [38].Ehrbar M, Schoenmakers R, Christen EH, Fussenegger M, Weber W, Nat. Mater 2008, 7, 800. [DOI] [PubMed] [Google Scholar]
- [39].Maitz MF, Freudenberg U, Tsurkan MV, Fischer M, Beyrich T, Werner C, Nat. Commun 2013, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Griffin DR, Kasko AM, ACS Macro Lett. 2012, 1, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA, Nat. Chem 2018, DOI 10.1038/nchem.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lai J, Jiang P, Gaddes ER, Zhao N, Abune L, Wang Y, Chem. Mater 2017, DOI 10.1021/acs.chemmater.7b00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, García AJ, Nat. Mater 2015, 14, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jamal M, Kadam SS, Xiao R, Jivan F, Onn TM, Fernandes R, Nguyen TD, Gracias DH, Adv. Healthc. Mater 2013, 2, 1142. [DOI] [PubMed] [Google Scholar]
- [45].Kapyla E, Delgado SM, Kasko AM, ACS Appl. Mater. Interfaces 2016, 8, 17885. [DOI] [PubMed] [Google Scholar]
- [46].Griffin DR, Kasko AM, J. Am. Chem. Soc 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liu L, Shadish JA, Arakawa CK, Shi K, Davis J, DeForest CA, Adv. Biosyst 2018, 1800240, 1800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Langer R, Khademhosseini A, Biomaterials 2006, 27, 5391. [DOI] [PubMed] [Google Scholar]
- [49].Raman R, Bhaduri B, Mir M, Shkumatov A, Lee MK, Popescu G, Kong H, Bashir R, Adv. Healthc. Mater 2015, 1. [DOI] [PubMed] [Google Scholar]
- [50].Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK, Science (80-. ). 2007, 317, 1366. [DOI] [PubMed] [Google Scholar]
- [51].Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, Dabiri JO, Parker KK, Nat. Biotechnol 2012, 30, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Park S-J, Gazzola M, Park KS, Park S, Di Santo V, Blevins EL, Lind JU, Campbell PH, Dauth S, Capulli AK, Pasqualini FS, Ahn S, Cho A, Yuan H, Maoz BM, Vijaykumar R, Choi J-W, Deisseroth K, Lauder GV, Mahadevan L, Parker KK, Science (80-. ). 2016, 353, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Williams BJ, Anand SV, Rajagopalan J, Saif M. T. a., Nat. Commun 2014, 5, 1. [DOI] [PubMed] [Google Scholar]
- [54].Chan V, Park K, Collens MB, Kong H, a Saif T, Bashir R, Sci. Rep 2012, 2, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dorvel B, Damhorst G, Chan V, Shim J, Banerjee S, Cvetkovic C, Raman R, Bashir R, Nanomedicine 2013, 8, 13. [Google Scholar]
- [56].Chan V, Raman R, Cvetkovic C, Bashir R, ACS Nano 2013, 7, 1830. [DOI] [PubMed] [Google Scholar]
- [57].Cvetkovic C, Raman R, Chan V, Williams BJ, Tolish M, Bajaj P, Sakar MS, Asada HH, Saif MTA, Bashir R, Proc. Natl. Acad. Sci. U. S. A 2014, 111, 10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Raman R, Cvetkovic C, Uzel SGM, Platt RJ, Sengupta P, Kamm RD, Proc. Natl. Acad. Sci 2016, DOI 10.1073/pnas.1516139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Raman R, Cvetkovic C, Bashir R, Nat. Protoc 2017, 12, 519. [DOI] [PubMed] [Google Scholar]
- [60].Raman R, Grant L, Seo Y, Cvetkovic C, Gapinske M, Palasz A, Dabbous H, Kong H, Pinera PP, Bashir R, Adv. Healthc. Mater 2017, 1700030, 1700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Raman R, Science (80-. ). 2019, 363. [DOI] [PubMed] [Google Scholar]
- [62].Ricotti L, Trimmer B, Feinberg AW, Raman R, Parker KK, Bashir R, Sitti M, Martel S, Dario P, Menciassi A, Sci. Robot 2017, 1. [DOI] [PubMed] [Google Scholar]
- [63].Cvetkovic C, Rich MH, Raman R, Kong H, Bashir R, Microsystems Nanoeng. 2017, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Puetzer JL, Bonassar LJ, Tissue Eng. Part A 2016, 22, 1. [DOI] [PubMed] [Google Scholar]
- [65].Raman R, Bashir R, Stereolithographic 3D Bioprinting for Biomedical Applications, Elsevier Inc., 2015.
- [66].Neiman JAS, Raman R, Chan V, Rhoads MG, Raredon MSB, Velazquez JJ, Dyer RL, Bashir R, Hammond PT, Griffith LG, Biotechnol. Bioeng 2015, 112, 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].V Murphy S, Atala A, Nat. Biotechnol 2014, 32, 773. [DOI] [PubMed] [Google Scholar]
- [68].Cohen DL, Malone E, Lipson H, Bonassar LJ, Tissue Eng. 2006, 12, 1325. [DOI] [PubMed] [Google Scholar]
- [69].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H, Ramadan MH, Hudson AR, Feinberg AW, Sci. Adv 2015, 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Grant L, Raman R, Cvetkovic C, Ferrall-Fairbanks MC, Pagan-Diaz GJ, Hadley P, Ko E, Platt MO, Bashir R, Tissue Eng. Part A 2018, 00, ten. TEA 20180202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Raman R, Mitchell M, Perez-Pinera P, Bashir R, DeStefano L, J. Biol. Eng 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]