Abstract
Neuroanatomical correlates of apathy and disinhibition, behavioural abnormalities in behavioral variant Frontotemporal dementia (bvFTD) remains unclear. In this study 45 participants (25 bvFTD patients and 20 controls) provided data on clinical, neuropsychological, behavioural (on Frontal Systems Behaviour (FrSBe) Scale), cortical volume (on voxel-based morphometry (VBM)) and tract based spatial fractional anisotropy ((FA) on magnetic resonance imaging (MRI), allowing examination of the neural correlates of apathy and disinhibition. The patients with bvFTD had predominant grey matter loss and corresponding white matter fractional anisotropy reductions in the frontal and temporal lobe compared to the controls. Grey matter loss in frontal, temporal and limbic structures correlated with apathy and degeneration in temporal limbic brain areas correlated with disinhibition. FA changes in inferior fronto-occipital fasciculus and forceps minor correlated with apathy and fibre integrity changes in the superior longitudinal fasciculus correlated with disinhibition. The current study suggests that apathy and disinhibition arises due to changes in the frontal, temporal and limbic brain cortices in bvFTD.
Keywords: behavioral variant FTD, fractional anisotropy, Inferior fronto-occipital fasciculus, Frontal System Behavioural Scale
Introduction
Frontotemporal Dementia (FTD) is characterized by early changes in personality and social conduct (Neary, Snowden, & Mann, 2005). Even though behavioural abnormalities are common in all FTD syndromes, predominant changes occur in frontal or behavioural variant FTD (bvFTD) (McKhann et al., 2001). Insidious onset personality changes, behavioral abnormalities, and poor insight are seen in patients with bvFTD. Previous studies have suggested that volume loss in particular brain regions leads to specific behavioural abnormalities (Whitwell et al., 2007; Williams, Nestor, & Hodges, 2005). The bvFTD is characterized by the presence of behavioural manifestations of apathy, disinhibition and executive dysfunction. Patients with apathy have lack of initiative, interest and emotional concern, and patients with disinhibition present with production of socially inappropriate comments and/or actions (Robert et al., 2002). In the early stages of the disease, the distinction between the behavioural profiles is evident, but a significant overlap may occur in the later stages (Snowden et al., 2001). Structural and functional neuroimaging studies have explored the neural correlates of the behavioural underpinnings in bvFTD. Among these, a few have focused on the gray matter correlates of apathy and disinhibition (Franceschi et al., 2005; Le Ber et al., 2006; Peters et al., 2006) in bvFTD. A previous finding in FTD revealed that the severity of apathy correlated with atrophy in the right dorsolateral prefrontal cortex (DLPFC) and the severity of disinhibition correlated with atrophy in the right nucleus accumbens, right superior temporal sulcus, and medio-temporal limbic structures (Zamboni, Huey, Krueger, Nichelli, & Grafman, 2008). Some studies have attempted to characterize the white matter associations with apathy and disinhibition (Borroni et al., 2007; Hornberger, Geng, & Hodges, 2011; Powers et al., 2014) in bvFTD and revealed inconsistent results. Borroni and colleagues have demonstrated a relationship between fractional anisotropy (FA) in superior longitudinal fasciculus (SLF) and disinhibition (Borroni et al., 2007). Hornberger et al. suggested an association between FA in uncinate fasciculus (UF), forceps minor, and cingulum with disinhibition measured by Haling test (Hornberger et al., 2011). Recently, Powers et al., have shown that FA in UF correlated with apathy and in right corona radiata with disinhibition (Powers et al., 2014). But a combined analysis of grey and white matter contribution to apathy and disinhibition in bvFTD is not yet reported previously, and our study was set up to precisely do that.
The present study used a voxel-based analysis to examine the grey and white matter changes in bvFTD and correlated these changes with measures of apathy and disinhibition. We hypothesized that apathy and disinhibition would be associated with distinct regions of gray matter atrophy and white matter integrity loss in bvFTD and that brain areas would be responsible for the production of these behavioural traits in humans.
Materials and Methods
Subjects
Twenty-five patients were recruited from the Dementia Clinic of our hospital, and evaluated by an experienced cognitive neurologist (P.S.M) using the recently published consensus criterion for probable bvFTD by Rascovsky and colleagues (Rascovsky et al., 2011). We excluded the patients with a history of central nervous system disease other than FTD, alcohol abuse, head injury, other major medical illness or a past history of depression or psychiatric illness. Twenty healthy volunteers with no history of any medical, neurological or psychiatric illness and demographically comparable to the bvFTD participants were selected from the panel of control subjects at the SCTIMST dementia clinic. The demographic and clinical characteristics of the participants are reported in Table 1. All participants and caregivers gave written informed consent approved by the Institutional Ethical Committee of the institute. The assessment of cognitive impairment and disease severity was by the Addenbrooke’s Cognitive Examination (ACE) (Mathuranath et al.,2004), Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) and clinical dementia rating (CDR) (Hughes et al., 1982) and of behavioural changes was by the Frontal System Behavioural Scale (FrSBe). FrSBe is a questionnaire designed to provide measures of frequencies and characteristics of behavioral disturbances (Malloy, Tremont, Grace, & Frakey, 2007). It is completed by the patient in self-assessment and by the caregiver. It includes the rating of the frequency of 46 different behaviors on a 5-point Likert scale. The 46 items are framed in three theoretically derived (Malloy et al., 2007) subscales which measure three frontal features: apathy, disinhibition, and Executive Dysfunction.
Table 1:
Demographic and clinical characteristics of patients with behavioural variant frontotemporal dementia and healthy controls.
| Group | Controls(n=20) | bvFth(n=25) |
|---|---|---|
| Age(Years) | 62.8±4.9 | 63.68±9.22 |
| Education(Years) | 15.63±1.3 | 17.03±3.1 |
| Disease duration(Years) | NA | 2.98±1.71 |
| Gender(M/F) | 12/8 | 15/10 |
| MMSE | 28.96±1.5 | 20.9±7.71* |
| ACE | 94.0 ± 4.01 | 51.5 ± 26.64* |
| CDR | 0±0 | 1.4±0.65* |
NC normal control, bvFTD behavioral variant Frontotemporal dementia, MMSE Mini Mental State Examination, ACE Addenbrooke’s Cognitive Examination, CDR, Clinical Dementia Rating
, represents a significant p-value of p<0.001.
Image Acquisition
MRI scanning was performed on a 1.5 Tesla Siemens Magnetom – Avanto, SQ MRI scanner. In all subjects, structural MR images of the entire brain was obtained using a high resolution 3-dimensional, Flash Spoiled Gradient echo Sequence with the standard parameters (TR= 11ms; TE =4.95 ms; flip angle = 150; slices =176; slice thickness = 1 mm; matrix size = 256× 256). A 30 direction diffusion tensor imaging (DTI) was performed using a single-shot spin echo echo-planar sequence (TR=5400 ms, TE=88 ms, matrix=112 × 108, field of view= 220 mm, slice thickness = 3mm with 1.5 mm gap averaged twice and with b values of 0 and 1000 s/mm2).
Greymatter Image Analysis
The grey matter structural data were analysed using Voxel based Morphometry tool box (VBM8; version 435; University of Jena, Department of Psychiatry) in Statistical Parametric Mapping (SPM8; Functional Imaging Laboratory, University College London, London, UK) implemented in MATLAB 7.4 (Mathworks Inc., Sherborn, Mass., USA). Initially, the images were normalized to MNI space and segmented into gray matter, white matter, and cerebrospinal fluid (CSF). The segmented and modulated normalized gray matter images were smoothed with a Gaussian kernel of 12 mm full-width half-maximum (FWHM) filter. Whole brain statistical comparisons between groups were made to localize the gray matter differences by means of two sample t-test with age, gender and total intracranial volume (TIV) as covariates. Subsequently, a multiple regression analysis was performed to determine the correlation between specific behavioural manifestation in bvFTD and gray matter volume. For that, the FrSBe scores of subscales of apathy (FrSBe A), and disinhibition (FrSBe D) were entered as regressors into the design matrix along with age, gender and TIV as confounding covariates. The analysis was conducted with an uncorrected significance level of p<0.001, with an additional cluster extend threshold of 30 contiguous voxels.
White matter Image Analysis
MRI images of all participants were read by a qualified and diagnostically blinded neuroradiologist (CK) before being subjected to any further processing and those with any white matter hyper intensities were excluded from the study. The FSL 5.0 (FMRIB Software Library tools, www.fmrib.ox.ac.uk/fsl) software package was used for processing raw diffusion-weighted images, including correcting motion artifacts and eddy current distortion and tensor fitting 16. The FA images were derived for each voxel and the resulting images were registered into the template space. The registered FA images were averaged to derive a mean FA which was further skeletonized for tract based spatial statistics (TBSS) (Smith et al., 2006). Then the FA skeleton was thresholded at 0.2 to include only the white matter and each subject’s FA data was projected on to this skeleton. Finally, the differences in FA between controls and bvFTD were analyzed in a voxel-wise fashion using FSL’s randomized with 5000 permutations and with age, gender and TIV as covariates. The group comparisons were observed with a family wise error (FWE) corrected Threshold-Free Cluster Enhancement (TFCE) at a significance level of p < 0.05. Furthermore, a multiple regression analysis was performed using the FrSBe subscale scores (FrSBeA, (FrSBeD and age and gender as nuisance variables in GLM to study the relationship between behavior score and FA values. In order to identify the differences in behavioural measures, oneway ANOVA analysis was used with a statistical significance of p<0.05.
RESULTS
Demographic, neuropsychological and Behavioural results
The patients with bvFTD did not differ in terms of age (p=0.70) and gender (p=0.3) in comparison controls (Table 1). Healthy controls showed higher scores on MMSE and ACE (p<0.001) than bvFTD. Table 2 summarizes the measures of apathy and disinhibition in patients and controls. Independent sample t-test showed significant differences between groups on FrSBeA (t=6.33, df=26, p<0.001) and disinhibition (t=3.72, df=38, p=0.001) score. We found a correlation between apathy and disinhibition subscale (r=0.33, p=0.03) but no significant correlation between any of the subscales and variables included in the SPM general linear model (age, gender, and TIV).
Table 2:
Mean (S.D.) subscores of Frontal System Behavioural Scale (FrSBe).
| FrSBe | Controls(n=20) | bvFTD(n=25) |
|---|---|---|
| Apathy | 16.40±2.28 | 32.32±12.31 |
| Disinhibition | 21.25±5.42 | 30.24±10.45 |
| Executive Dysfunction | 22.41±5.68 | 48.65±14.53 |
Voxel Based Morphometry results of gray matter atrophy
Compared with controls, bvFTD showed reduced gray matter volume predominantly involving orbitofrontal, middle and medial frontal regions, inferior, superior and anteromedial temporal areas, limbic regions, insula and basal ganglia (p<0.05, FWE corrected (Supplementary Fig. 1 and Supplementary Table 1).
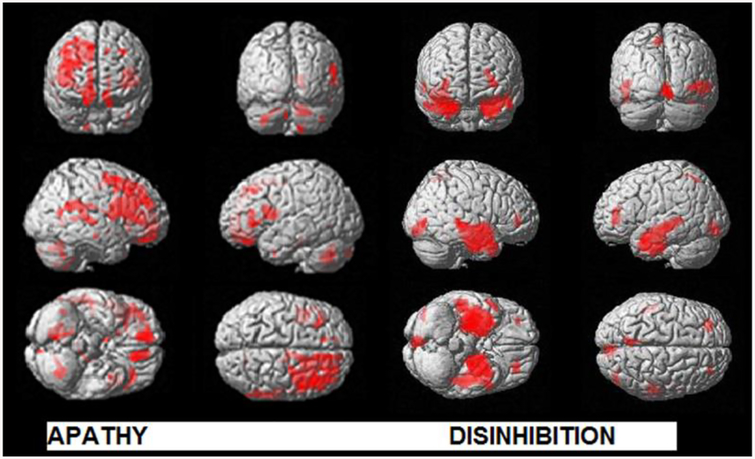
The results of regression analysis relating FrSBe subscores of apathy and disinhibition to reduced gray matter volume at an uncorrected threshold (P<0.001) with 30 extend threshold are illustrated in Fig. 1 and Supplementary Table 2. The increased apathy score correlated with reduced gray matter volume in several distinct brain areas of dorsolateral prefrontal cortex(DLPFC), medial prefrontal cortex (MPFC), orbitofrontal cortex (OFC), anterior cingulate, supplementary motor area, insula, superior and middle temporal gyrus and inferior parietal lobule with greater involvement of right hemisphere. The atrophied regions contained most significant clusters in the right middle frontal gyrus (MFG), right superior frontal gyrus (SFG), left gyrus rectus (GREC), bilateral anterior cingulate cortex (ACC), left inferior frontal gyrus (IFG), right supplementary motor area (SMA) and bilateral insula.
Fig. 1:
Regions of reduced gray matter density (red) associated with apathy and disinhibition in behavioural variant FTD (P<0.001, uncorrected with an extent threshold of 30 voxels) in the whole brain analysis are presented. The results are displayed in neurological convention.
The disinhibition score correlated with gray matter volume loss in the limbic lobe including parahippocampus (PHP), hippocampus (HP) and amygdala (AMY), temporal lobe including bilateral middle temporal gyrus (MTG), right superior temporal gyrus (STG), bilateral middle and superior temporal pole (MTP), bilateral fusiform gyrus (FG) and right insular cortex as seen in Fig. 1 and Supplementary Table 2).
Tract Based Spatial Statistics Results of White Matter Integrity
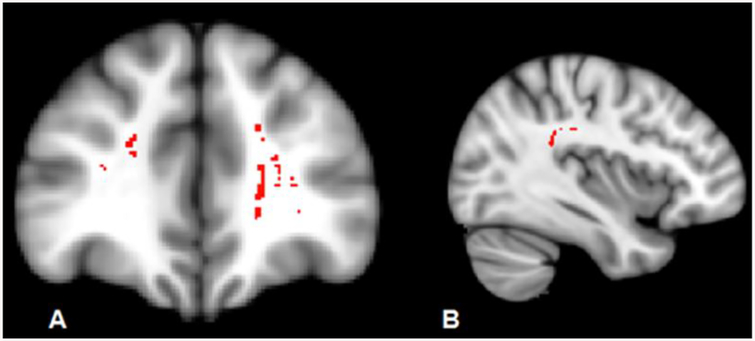
Compared with controls, bvFTD revealed reductions in FA in forceps minor, bilateral inferior fronto-occipital fasciculus, superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus and uncinate. Apathy was related to bilateral Inferior fronto-occipital fasciculus and forceps minor whereas disinhibition was related to temporal part of SLF (Fig. 2).
Fig. 2:
Regions of significant white matter loss (red) associated with A) apathy and B) disinhibition in behavioural variant FTD patients (P<0.001, uncorrected).
Discussion
The neural correlates of apathy and disinhibition in bvFTD are yet to be clearly established. This prospective study investigated the relationship between behaviour measured by FrSBe sub items and neural gray matter atrophy and white matter loss of integrity.
Neural substrate of apathy and disinhibition in behavioural variant FTD using Voxel Based Morphometry
In this study, we observed relatively distinct anatomic distributions of gray matter loss in each of the behaviors in patients with behavioural variant FTD. Our findings demonstrated that frontal cortex, limbic lobe, insula, and temporal structures are differentially involved in apathetic behaviour, whereas the temporo-limbic regions are more involved in disinhibited behaviour (Fig. 1, Table 1). In previous gray matter studies in bvFTD, VBM correlation analysis has shown an association between apathy and reduced gray matter volume in bilateral orbital, medial, inferior and dorsolateral frontal cortices along with that in bilateral anterior temporal and right caudate areas(Zamboni, Huey, Krueger, Nichelli, & Grafman, 2008); Massimo et al., 2015). Prior studies also reported frontal-limbic damage in association with disinhibition (Hornberger et al., 2011; Massimo et al., 2015; Rosen et al., 2005)). Rosen et al, in a heterogeneous group of dementia patients, demonstrated that apathy score on NPI covaried with ventromedial frontal atrophy and the disinhibition score covaried with subgenual cingulate gyrus atrophy (Rosen et al., 2005).
Our results demonstrated that scores on apathy correlated with atrophy of dorsolateral prefrontal cortex, orbitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex, gyrus rectus, superior and middle temporal gyrus, and inferior parietal lobule. The atrophy of DLPFC is implicated in cognitive inertia (Levy & Dubois, 2006), which leads to a deficit in executing goal-directed behavior (GDB). Recent observations rely on the fact that apathy emanates from the difficulty in manipulating and integrating the various levels of a plan to execute an action (Eslinger, Moore, Antani, Anderson, & Grossman, 2012). Notably, our findings of an association between apathy and orbitofrontal cortex damage corroborate with that of Massimo et al., (Massimo et al., 2015), who reported a correlation between orbitofrontal cortex damage and poor motivation. The goal selection deficits in patients are strongly linked to poor motivation, thereby making them disreactive to positive reward and negative rewards or punishment signals.
The atrophy of anterior cingulate cortex is consistent with previous literature regarding apathy in bvFTD (Massimo et al., 2009), (Rosen et al., 2005). In addition to frontal atrophy, the insular damage was also noted in our subjects, which is consistent with apathy findings in Alzheimer’s disease and progressive supranuclear palsy (Massimo et al., 2015). Our analysis also identified a significant correlation of apathy with atrophic changes in the inferior parietal lobule. This extended evidence suggests a possible role for the inferior parietal lobule in mediating the integration of competing sources of situational data for the purpose of contextualizing and scene setting as proposed by Eslinger et al., (Eslinger et al., 2012).
In our study, the scores on disinhibition were associated with temporal limbic structures, consistent with the findings of Zamboni et al., (Zamboni et al., 2008). who reported a right hemisphere amygdale, hippocampus, middle temporal gyrus and nucleus accumbens atrophy on employing Frontal system behavioural scale and VBM. The hippocampus and amygdala are involved in the behavioural inhibition system (Levita et al., 2014; Walters & Kiehl, 2015) and have also been found to correspond with fear conditioning (Baeuchl, Meyer, Hoppstädter, Diener, & Flor, 2015) and their relationship with temperament dimensions are quite complex and interdependent. The lesions and hypoperfusion in amygdala lead to diminished fear response and weakened fear conditioning (moral-emotive deficit) (Sah, Faber, Lopez De Armentia, & Power, 2003), while on the other hand, hippocampal dysfunction leads to memory problems and behavioural control deficit (Walters & Kiehl, 2015). The investigation of the multimodal system of emotions such as anger, fear, disgust, happiness, sadness, and surprise in FTD showed the insula as a neural substrate for processing of disgust recognition (Baeuchl et al., 2015).
In contrast, Hornberger et al., identified orbitofrontal cortex atrophy as a candidate marker of disinhibition by using both subjective NPI and objective Hayling test (Hornberger et al., 2011). Furthermore, the same finding was replicated in a large sample of mixed dementia patients, including bvFTD using measures of the cortical volume of orbitofrontal cortex (Kumfor, Irish, Hodges, & Piguet, 2013). Prior reports in patients with temporal lesions or temporal lobe epilepsy clearly described the extensive behavioural symptoms, including mania, euphoria, and aggressive behaviour and it seems consistent with the gray matter loss in temporal structures (Krueger et al., 2011). Moreover, these behavioural deficits are thought to arise due to interruption of connecting pathways, between the temporal lobe and the orbitofrontal regions rather than exclusively as a consequence of dysfunction in the temporal lobe gray matter (Bakchine S,2000). According to Cummings, tempero-limbic structures are part of the orbitofrontal cortex, whose dysfunction leads to prominent disinhibitive syndromes (Cummings, 1995). There is convergent evidence from quantitative measures of whole brain gray matter loss, that disrupted anatomical connections are critical for the genesis of certain behavioural dysfunction. Our study did not find any frontal areas correlating with disinhibition, a finding of significance. This and the results of some of the earlier studies discussed above indicate that disinhibition arises from impaired reward/punishment attribution mechanisms that seems independent of prefrontal cortex dysfunction. These findings and our results suggest that integration of the prefrontal cortex and temporal limbic structures is necessary for the proper performance of the complex social behavior.
Neural substrate of apathy and disinhibition in behavioural variant FTD using diffusion tensor imaging
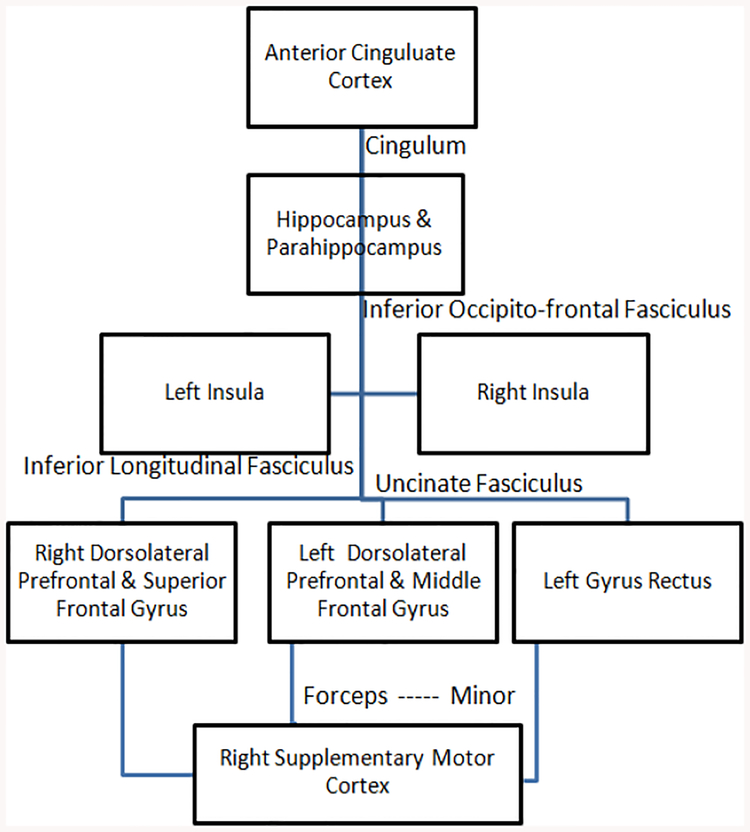
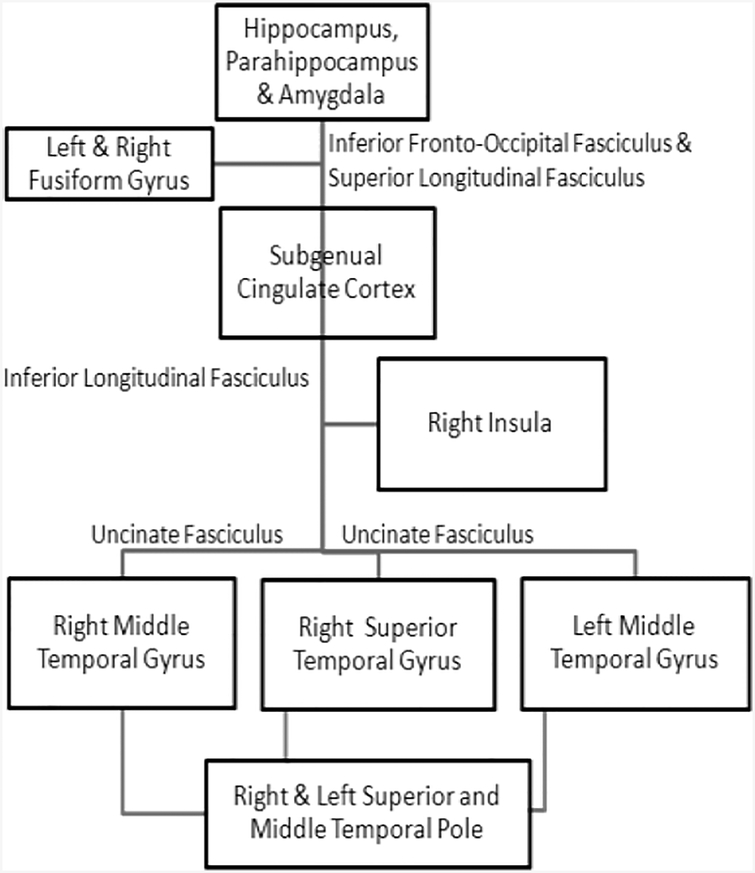
Our DTI findings suggest that similar to gray matter involvement, white matter FA alterations in specific brain regions are likely to be responsible for different clinical symptoms associated with bvFTD. It has been suggested that the gray matter and white matter structures in frontal and temporal parts of the brain comprise the critical components of a large scale network which is important in regulating the GDB (Brown & Pluck, 2000). The role of orbital and medial regions of inferior temporal lobe in value and reward-related information has been demonstrated previously (Grossman et al., 2010). Thus, it is possible that the degeneration of these frontal white matter projections to temporal areas may interfere with motivation and GDB. Previous studies in behavioural variant FTD described the white matter association with disinhibition using different methods and revealed inconsistent findings. Our study demonstrated that damage to inferior fronto-occipital fasciculus which passes through in depth of temporal lobe and insula (interconnecting occipital cortex, temporobasal areas, and superior parietal lobe to the frontal lobe), and forceps minor (connecting the medial and larteral prefrontal areas through the genu of corpous callosum) correlated with apathy and to temporal superior longitudinal fasciculus correlated with disinhibition. These findings corroborate with that of Borroni et al., who used an ROI approach and reported an association between superior longitudinal fasciculus damage and disinhibition measured by the frontal behavioural inventory (FBI) (Borroni et al., 2007). In contrast, Hornberger et al.,(Hornberger et al., 2011) reported a correlation between FA changes in the uncinate fasciculus, cingulum, and forceps minor with disinhibition measured by Hayling test. Moreover, we studied the correlation between mean diffusivity and behavioural changes in bvFTD, but we could not find any significant clusters. Thus, the disruption of distinct regions of white matter in the frontotemporal network leads to subtle behavioural abnormalities in bvFTD. The subtle differences in the results across studies may be attributable to either difference in measures used to assess the behavioural symptoms or may due to the differences in the clinical cohort. Based on the gray matter and white matter findings from some past studies and our study it is probable that apathy and disinhibition results from disruptions in the neural connections shown in Fig. 3 and Fig. 4 respectively that are responsible for controlling these behaviours.
Fig. 3:
Figure showing the neural connection, disruptions in which causes apathy.
Fig. 4:
Figure showing the neural connection, disruptions in which causes disinhibition.
The present study has some limitations. Although our sample size was larger than previous studies of apathy and disinhibition in bvFTD, we failed to reveal specific connections for corrected thresholds. Inorder to get the behavioural correlations with frontal, temporal and limbic regions we have used a liberal threshold which limits the findings. Also we are not able to perform the cortical thickness measurements using freeSurfer which is considered as another limitation of the study and the future studies will incorporate the freeSurfer findings in large cohorts. Finally, the lack of neuropathological confirmation of the diagnoses of these patients possibly is a limitation too.
In summary, using VBM and DTI, we observed widespread frontal and temporal gray matter and white matter disease in bvFTD. Gray matter atrophy in frontal, temporal and limbic structures and damage to inferior fronto-occipital fasciculus and forceps minor was associated with apathy, while disruption of the temporal limbic brain areas and white matter fiber integrity changes in superior longitudinal fasciculus were associated with disinhibition. Future studies targeting changes in structural-functional connectivity networks could provide valuable information about the relative contribution of grey and white matter to the behavioural manifestations in FTD.
Supplementary Material
Acknowledgements:
The authors wish to thank the participants in this study and their caregivers. Author Contribution: SR, BC and TV carried out the study and did data collection and data analysis. SR, KC, JV and PSM contributed to the preparation of the manuscript. PSM conceived the idea of and designed the study and got the grant for carrying out the study. JV contributed to getting the grant for the study.
Funding: This study was supported by grants provided by the National Institute on Aging (NIA), USA (grant no. R21AG029799 to JV and R01AG039330–01) to PSM.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors declare no conflict of interest.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Baeuchl C, Meyer P, Hoppstädter M, Diener C, & Flor H (2015). Contextual fear conditioning in humans using feature-identical contexts. Neurobiology of Learning and Memory, 121, 1–11. 10.1016/j.nlm.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Bakchine S Temporal lobe behavioral syndromes In: Bogousslavsky J Cummings J, eds. Behavior and Mood Disorders in Focal Brain Lesions. Cambridge: Cambridge University Press: 2000;369–398. [Google Scholar]
- Borroni B, Brambati SM, Agosti C, Gipponi S, Bellelli G, Gasparotti R, …Padovani A (2007). Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Archives of Neurology, 64(2), 246–251. 10.1001/archneur.64.2.246 [DOI] [PubMed] [Google Scholar]
- Brown RG, & Pluck G (2000). Negative symptoms: the “pathology” of motivation and goal-directed behaviour. Trends in Neurosciences, 23(9), 412–417. [DOI] [PubMed] [Google Scholar]
- Cummings JL (1995). Anatomic and behavioral aspects of frontal-subcortical circuits. Annals of the New York Academy of Sciences, 769, 1–13. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Antani S, Anderson C, & Grossman M (2012). Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behavioural Neurology, 25(2), 127–136. 10.3233/BEN-2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, …Perani D (2005). Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Annals of Neurology, 57(2), 216–225. 10.1002/ana.20365 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh P,R(1975). Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–98. [DOI] [PubMed] [Google Scholar]
- Grossman M, Eslinger PJ, Troiani V, Anderson C, Avants B, Gee JC, … Antani S (2010). The Role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia, 48(12), 3505–3512. 10.1016/j.neuropsychologia.2010.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C,P, Berg L, Danziger W,L, Coben L,A, Martin R,L (1982). A new clinical scale for the staging of dementia. Br J Psychiatry, 140:566–72. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Geng J, & Hodges JR (2011). Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain: A Journal of Neurology, 134(Pt 9), 2502–2512. 10.1093/brain/awr173 [DOI] [PubMed] [Google Scholar]
- Krueger CE, Laluz V, Rosen HJ, Neuhaus JM, Miller BL, & Kramer JH (2011). Double dissociation in the anatomy of socioemotional disinhibition and executive functioning in dementia. Neuropsychology, 25(2), 249–259. 10.1037/a0021681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, & Piguet O (2013). Discrete Neural Correlates for the Recognition of Negative Emotions: Insights from Frontotemporal Dementia. PLOS ONE, 8(6), e67457 10.1371/journal.pone.0067457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, … Dubois B (2006). Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain: A Journal of Neurology, 129(Pt 11), 3051–3065. 10.1093/brain/awl288 [DOI] [PubMed] [Google Scholar]
- Levita L, Bois C, Healey A, Smyllie E, Papakonstantinou E, Hartley T, & Lever C (2014). The Behavioural Inhibition System, anxiety and hippocampal volume in a non-clinical population. Biology of Mood & Anxiety Disorders, 4(1), 4 10.1186/2045-5380-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, & Dubois B (2006). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex (New York, N.Y.: 1991), 16(7), 916–928. 10.1093/cercor/bhj043 [DOI] [PubMed] [Google Scholar]
- Malloy P, Tremont G, Grace J, & Frakey L (2007). The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 3(3), 200–203. 10.1016/j.jalz.2007.04.374 [DOI] [PubMed] [Google Scholar]
- Mathuranath P,S, Hodges JR, Mathew R, Cherian PJ, George A, Bak TH, (2004). Adaptation of the ACE for a Malayalam Speaking Population in Southern India. International Journal of Geriatric Psychiatry, 19,1–7. [DOI] [PubMed] [Google Scholar]
- Massimo L, Powers JP, Evans LK, McMillan CT, Rascovsky K, Eslinger P, … Grossman M (2015). Apathy in Frontotemporal Degeneration: Neuroanatomical Evidence of Impaired Goal-directed Behavior. Frontiers in Human Neuroscience, 9, 611 10.3389/fnhum.2015.00611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden J, & Mann D (2005). Frontotemporal dementia. The Lancet. Neurology, 4(11), 771–780. 10.1016/S1474-4422(05)70223-4 [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, … Salmon E (2006). Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders, 21(5–6), 373–379. 10.1159/000091898 [DOI] [PubMed] [Google Scholar]
- Powers JP, Massimo L, McMillan CT, Yushkevich PA, Zhang H, Gee JC, & Grossman M (2014). White matter disease contributes to apathy and disinhibition in behavioral variant frontotemporal dementia. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 27(4), 206–214. 10.1097/WNN.0000000000000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: A Journal of Neurology, 134(Pt 9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, Tible O, … Bedoucha P (2002). The apathy inventory: assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. International Journal of Geriatric Psychiatry, 17(12), 1099–1105. 10.1002/gps.755 [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, & Miller BL (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain: A Journal of Neurology, 128(Pt 11), 2612–2625. 10.1093/brain/awh628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, & Power J (2003). The amygdaloid complex: anatomy and physiology. Physiological Reviews, 83(3), 803–834. 10.1152/physrev.00002.2003 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TEJ (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Walters GD, & Kiehl KA (2015). Limbic Correlates of Fearlessness and Disinhibition in Incarcerated Youth: Exploring the Brain-Behavior Relationship with the Psychopathy Checklist: Youth Version. Psychiatry Research, 230(2), 205–210. 10.1016/j.psychres.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, & Warren JD (2007). VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. NeuroImage, 35(1), 207–213. 10.1016/j.neuroimage.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, & Grafman J (2008). Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology, 71(10), 736–742. 10.1212/01.wnl.0000324920.96835.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.