Abstract
Use of immune index is a new potential approach for cancer classification and prediction. To investigate the status and clinical effect of immune index in gallbladder cancer (GBC), 238 GBC patients from Zhongshan Hospital affiliated to Fudan University were involved in the present study, including 113 patients in a training set and 125 patients in a validation set. Five immune cells (macrophages, neutrophils, regulatory T cells, cytotoxic T cells and mast cells) were selected based on a literature review and the immune index for each patient was calculated using the LASSO regression. A low immune index (<1) was defined as immunotype A and a high immune index (≥1) was defined as immunotype B. The 5‐year overall survival rate for immunotype A was higher than that for immunotype B in the training set and the validation set (70.0% vs 37.0%, P < 0.001; 68.9% vs 47.5%, P = 0.002; respectively). Moreover, the immune index showed higher prediction efficiency compared with all the single immune cells which we selected. When combined with the immune index, the areas under the curve (AUC) of the TNM staging system in both sets were elevated from 0.677 to 0.787 and from 0.631 to 0.694, respectively. Interestingly, gemcitabine‐based chemotherapy only benefits stage II patients of immunotype B and stage III patients of both immunotype A and immunotype B (P = 0.015, P = 0.030, P = 0.011, respectively) but does not work in stage II patients of immunotype A (P = .307). Taken together, the immune index could effectively predict prognosis and the benefits of gemcitabine‐based chemotherapy and might improve on the TNM staging system.
Keywords: chemotherapy, gallbladder cancer, immune cells, immune index, tumor microenvironment
we integrated multiple tumor‐infiltrating immune cells and constructed a new immune index model of gallbladder cancer by the largest single center cohort so far to predict the prognosis and the gemcitabine‐based chemotherapy response. In our study, patients with low immune index (immunotype A) could experience a more favorable survival, but a reduced response to gemcitabine‐based chemotherapy (especially for stage II patients), compared with patients with high immune index (immunotype B).
1. INTRODUCTION
Gallbladder cancer (GBC) is the sixth most common type of digestive tract carcinoma and the most common type of biliary cancer but is still a relatively infrequent malignancy, with a morbidity rate of 1/100,000‐23/100,000.1, 2 However, the incidence of GBC has steadily risen at the rate of 4% in the past 10 years.3 GBC is also a highly lethal disease, with a 5‐year survival rate of 5%‐10%. Complete surgical resection is the only curative therapy for GBC4, with only 10% of GBC patients (with early‐stage disease) having the opportunity to receive complete surgical resection.5 Therefore, chemotherapy is critical for GBC patients. Gemcitabine‐based chemotherapy is the first‐line treatment for advanced GBC patients in the National Comprehensive Cancer Network (NCCN) Guidelines.6, 7 Nevertheless, some studies report the negative impacts of gemcitabine‐based chemotherapy and potential shorter survival of GBC patients.8 The precise mechanism is unknown at present, but a practical risk stratification model for GBC to predict the prognosis after surgical resection and the benefit from gemcitabine‐based chemotherapy is urgently needed.
The most widely used model for GBC is the TNM staging system published by the American Joint Committee on Cancer (AJCC). The TNM staging system classifies GBC patients by the extent and size of the primary tumor (T), the involvement of regional lymph nodes (N), and the absence or presence of distant metastases (M).9 Although the TNM staging system has been validated and accepted worldwide, heterogeneous prognosis still exists for each stage. With the increasing knowledge of the tumor microenvironment (TME) and the rapid development of tumor immunology, the crucial role of tumor‐infiltrating immune cells in the TME is becoming clearer, improving the predictive efficiency of the TNM staging system.
As an essential hallmark of cancer, inflammation is the most common risk factor of GBC, which primarily presents as a gallbladder stone.1, 2, 10, 11 Inflammation inside tumors is induced by tumor‐infiltrating immune cells, which constitute the TME together with tumor‐associated fibroblasts (TAF) and vascular endothelial cells (VEC).12, 13, 14 The tumor‐infiltrating immune cells in the TME mainly include lymphoid lineage cells such as T cells and B‐cells, and myeloid lineage cells such as macrophages, neutrophils and mast cells (MC).12, 13, 14 These immune cells could communicate with each other and play a promotive or contrary function on the initiation, growth and invasion of tumor cells, directly or indirectly.15, 16, 17, 18, 19 Furthermore, some research suggests that the immune elements in TME could modulate chemotherapy response.20, 21, 22
In consideration of the important value of tumor‐infiltrating immune cells, we evaluated the different kinds of immune cells, constructed an immune index model using multiple linear regression and explored its prognostic value for clinical outcomes, especially for gemcitabine‐based chemotherapy. Our work illustrates the importance of tumor‐infiltrating immune cells and develops a promising system to predict prognosis and chemotherapy benefit in GBC.
2. PATIENTS AND METHODS
2.1. Study design
The flow chart of the study is shown in Figure 1. This study was approved by the Institutional Review Board (IRB) of Fudan University Zhongshan Hospital and informed consent was obtained from each patient. A total of 238 consecutive patients with GBC undergoing radical resection or palliative surgery were enrolled in our study from Fudan University Zhongshan Hospital in China between 2004 and 2013. Among them, 113 patients with odd ID numbers were included in the training set and 125 patients with even ID numbers were included in the validation set. All the included patients met the following criteria: 8th AJCC TNM stage with confirmed postoperative histopathology diagnosis, available tumor specimens and complete follow‐up data. The exclusion criteria included: loss of follow up, or survival less than 1 month after surgery. The following clinicopathological factors were collected: age at surgery, gender, TNM stage, tumor differentiation, surgical margin, microvascular invasion and chemotherapy status. The follow up of postoperative patients included: medical history (symptoms and physical examination), laboratory studies and imaging examination every 3 months in the first 2 years and every 6 months in the subsequent years. Overall survival (OS) was confirmed from the day of operation to the date of death or the latest follow up. Baseline characteristics and 5‐year OS according to TNM stage and different sets are described in Table 1 and Figure S1.
Figure 1.
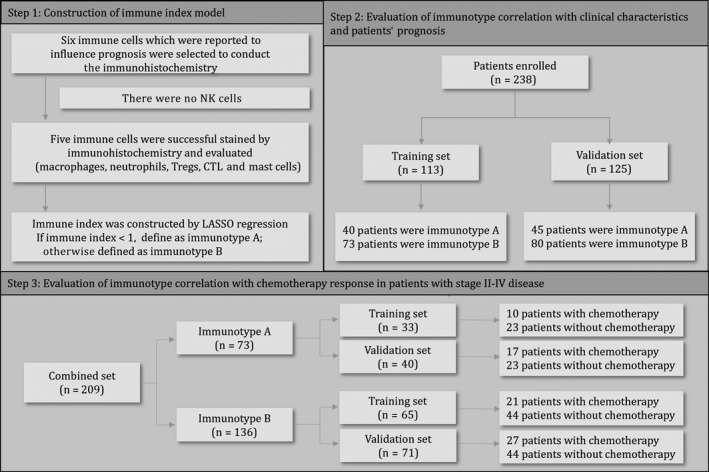
Flow chart of the study. Six immune cells were screened by literature review and immunohistochemistry was performed in the training set (N = 113) and the validation set (N = 125). Five immune features were ultimately selected to construct the immune index model. The associations of immunotype with overall survival (OS) and chemotherapy benefit were sequentially tested in both sets
Table 1.
Demographics and clinicopathologic characteristics of patients with gallbladder cancer
| Characteristic | Training set | Validation set | P | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| All patients | 113 | 100 | 125 | 100 | |
| Age at surgery, yearsa | |||||
| Mean ± SD | 62.8 ± 11.2 | 63.8 ± 11.5 | .485 | ||
| Gender | |||||
| Female | 81 | 71.7 | 84 | 67.2 | 0.543 |
| Male | 32 | 28.3 | 41 | 32.8 | |
| TNM stage | |||||
| I | 15 | 13.3 | 14 | 11.2 | 0.159 |
| II | 39 | 34.5 | 61 | 48.8 | |
| III | 38 | 33.6 | 34 | 27.2 | |
| IV | 21 | 18.6 | 16 | 12.8 | |
| pT‐stage | |||||
| T1 | 15 | 13.3 | 15 | 12.0 | 0.156 |
| T2 | 42 | 37.2 | 63 | 50.4 | |
| T3 | 32 | 28.3 | 31 | 24.8 | |
| T4 | 24 | 21.2 | 16 | 12.8 | |
| pN‐stage | |||||
| N0 | 78 | 69.0 | 103 | 82.4 | 0.069 |
| N1 + N2 | 35 | 31.0 | 22 | 17.6 | |
| Tumor differentiation | |||||
| Wessll | 12 | 10.6 | 6 | 4.8 | 0.118 |
| Moderate | 37 | 32.7 | 53 | 42.4 | |
| Poor | 64 | 56.6 | 66 | 52.8 | |
| Surgical margin | |||||
| Negative | 100 | 88.5 | 112 | 89.6 | 0.948 |
| Positive | 13 | 11.5 | 13 | 10.4 | |
| Microvascular invasion | |||||
| Absent | 85 | 75.2 | 90 | 72.0 | 0.680 |
| Present | 28 | 24.8 | 35 | 28.0 | |
| Chemotherapy | |||||
| Absent | 78 | 69.0 | 77 | 61.6 | 0.287 |
| Present | 35 | 31.0 | 48 | 38.4 | |
The results of continuous variables are presented as mean ± SD.
2.2. Immunohistochemistry and evaluation of immune cells
Tissue microarray (TMA) was established using paraffin‐embedded tumor specimens and mounted on glass slides with 2.0‐mm tissue core. We selected six kinds of immune cells which might influence the prognosis of patients based on a literature review (ie, macrophages, neutrophils, regulatory T cells [Tregs], cytotoxic T cells [CTL], MC and natural killer cells [NK cells]) and marked them by immunohistochemistry stain of specific molecule markers. No NK cells were found after immunohistochemistry staining of two kinds of anti–CD56 antibody (Dako, clone 123C3; Abcam, ab9018) with different concentrations. The process of immunohistochemistry was described previously23 with single and specific antibodies (macrophage: anti–CD68 polyclonal antibody, Abcam, ab955, diluted 1:200; neutrophil: anti–CD66b polyclonal antibody, BD Biosciences, Clone G10F5, diluted 1:200; Treg: anti–Foxp3 polyclonal antibody, Abcam, ab22510, diluted 1:100; CTL: anti–CD8, DAKO, IR623, ready‐to‐use; mast cell: anti–tryptase polyclonal antibody, Abcam, ab2378, diluted 1:1000, respectively). Intensity of immune cells in TMA was defined as the mean number of positive markers per HPF (200X) from three random fields. Two independent pathologists (Dr Luo and Dr Chen) were blinded to the clinical information and assessed the intensity of different immune cells, respectively, and the counts were averaged.
2.3. Least Absolute Shrinkage and Selection Operator
Least absolute shrinkage and selection operator (LASSO) is a co–regression analysis method that can reduce the dimension of original data and produce a statistical model. It performs both variable selection and regularization and enhances the prediction accuracy and interpretability of the statistical model. Because LASSO regression has a low requirement for original data, it has been applied in many medical modeling processes.24, 25, 26, 27 We adopted LASSO regression to construct the prognostic model in our study.
2.4. Statistical analyses
Continuous variables were calculated using Student's t test, and categorical variables were analyzed using the χ2 test. The Kaplan‐Meier method, the log‐rank test, and univariate and multivariate Cox proportional hazards models were applied to evaluate the prognostic value of the immune index model. The prognostic efficiency of different predictive models was assessed by receiver operating characteristic (ROC) analysis. Statistical analysis was performed with SPSS 23.0 (IBM), MedCalc 15.6.1 (MedCalc Software bvba; https://www.medcalc.org; 2015) and R software packages 3.4.2 with the “glmnet” package (The R foundation for Statistical Computing, https://www.r-project.org/). A two‐sided P‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of immune cells
Tumor‐infiltrating immune cells were assessed by immunohistochemical staining of specific polyclonal antibodies (Figure 2A). As shown in Figure 2B, different kinds of immune cells infiltrated into gallbladder tumor tissues in totally different ways. The distribution of macrophages was spindle‐like, neutrophil distribution was TV‐tower‐like and Tregs distribution was lightning‐rod‐like. CTL distribution was in between macrophage and neutrophil distribution, and mast cell distribution was in between neutrophil and Treg distribution. Meanwhile, the cell counts per HPF of the same immune cell ranged widely among patients, and the median counts of macrophages, neutrophils, Tregs, CTL and mast cells were 50.3, 11.3, 2.5, 20.5 and 13.3, respectively.
Figure 2.
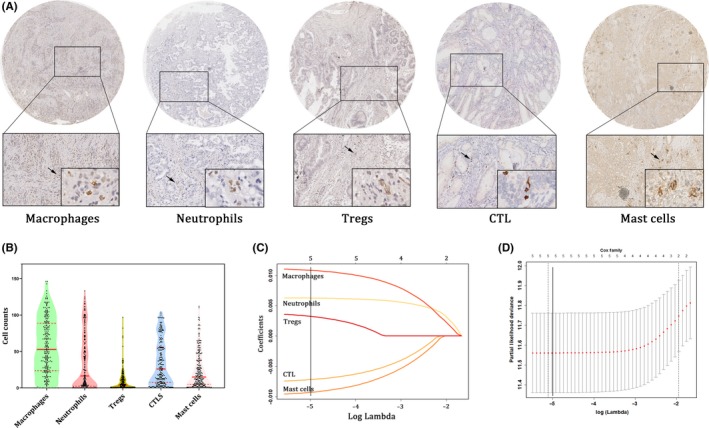
Construction of immune index model. A, Infiltration of macrophages, neutrophils, regulatory T cells (Tregs), cytotoxic T cells (CTL) and mast cells in GBC patients with 200× magnification. B, Violin plot showing the cell count distribution of the selected immune cells. C, Least absolute shrinkage and selection operator (LASSO) coefficient profiles of the selected immune cells. A solid vertical line is drawn at the value (log λ = −5) chosen by fivefold cross‐validation. D, Partial likelihood deviance for the LASSO coefficient profiles. A light dashed vertical line represents the minimum partial likelihood deviance. A solid vertical line represents for the partial likelihood deviance at the value (log λ = −5)
We also explored the relationship between TNM stage and tumor‐infiltrating immune cells. As shown in Figure S2, there was no difference between stages I‐II and stages III‐IV for the infiltration of macrophages, Tregs, CTL and mast cells (but there was a difference for neutrophils). Cell counts of infiltrating neutrophils in stages III‐IV were significantly higher than those in stages I‐II (P < 0.001).
Immune cells are not isolated from each other. As presented in Figure S3, significant Spearman's rank correlation coefficients occurred between immune cells. There were significant positive correlations between macrophages, neutrophils and Tregs, and between CTL and mast cells. A significant negative correlation also existed between neutrophils and mast cells.
3.2. Construction of immune index model
To explore the predictive ability of the different immune cells, we divided the patients into high or low infiltration on the basis of median counts of tumor‐infiltrating immune cells and conducted survival analysis. As shown in Figure S4, high infiltration of macrophages, neutrophils and Tregs predicted compromised prognosis (P < 0.001, P < 0.001, P = 0.011, respectively), while high infiltration of CTL and mast cells predicted favorable prognosis (P = 0.002 and P = 0.008, respectively).
We then constructed the immune index model for the training set. The coefficients for all five immune cells were calculated with LASSO regression when log λ = −5 (Figure 2C), and the partial likelihood deviance for the selected lambda was 11.632 (Figure 2D). The formula was generated where immune index = 2^(macrophage * 0.01105 + neutrophil * 0.006284 + Treg * 0.003582 − CTL * 0.007434 − mast cells * 0.0096). Each patient could obtain a unique immune index using the formula. Patients with a low immune index (<1) were defined as immunotype A, while other patients with a high immune index (≥1) were defined as immunotype B. The clinicopathological variables between immunotype A and immunotype B did not vary significantly, except for age (Table S1).
3.3. Correlation between immunotype and overall survival
To investigate the difference between immunotype A and immunotype B, we constructed a heat map according to the immune index, from low to high. As shown in Figure 3A, with the increase of the immune index and the conversion of immunotype, the main tumor‐infiltrating immune cells gradually changed from CTL and mast cells to macrophages, neutrophils and Tregs in the training set. The validation set had the same trend. Following this, we assessed the influence of the immune index on patients' prognosis using smooth hazard ratio (HR) curves of OS. As shown in Figure 3B, the Ln (HR) increased obviously with the gradual increase of the immune index in both sets. The HR and 95% confidence interval (CI) for both sets were 3.081(2.040‐4.654), P < 0.001 and 2.207(1.361‐3.581), P = 0.001, respectively.
Figure 3.
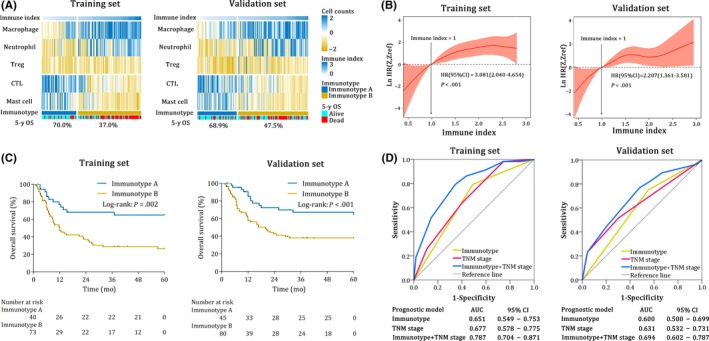
Prognostic efficiency of the immune index model in the training set and the validation set. A, Heat map classifying patients according to the levels of the immune index from left to right. The colors represent the relative counts of five immune cells in every patient. The 5‐year OS of different groups is indicated. B, Smooth hazard ratio (HR) curves show increasing HR of increasing immune index; immune index = 1 in the training set and the validation set. HR and 95% CI are calculated by univariate Cox regression. C, Kaplan‐Meier analysis of overall survival (OS) of gallbladder cancer (GBC) patients based on immunotype in the training set and the validation set. D, The receiver operating characteristic (ROC) and area under curve (AUC) of immunotype, TNM stage and TNM stage + immunotype. CI, confidence interval; HR, hazard ratio
As shown in Figure 3A, the 5‐year OS of immunotype A vs immunotype B in both sets were 70.0% vs 37.0% and 68.9% vs 47.5%, respectively. To further verify the predictive value of the immune index model, we compared the OS based on immunotypes using survival curves. Immunotype B patients demonstrated a more lower OS than immunotype A patients in the training set (Figure 3C, P = 0.002). The results were similar for the validation set (Figure 3C, P < 0.001). We also conducted a subgroup analysis of OS according to immunotype in the combined set, and found that immunotype was an effective prognostic predictor for OS in all the subgroups (Figure S5).
Univariate and multivariate analysis was performed to further verify the clinical value of the immune index model. Immunotype proved to be an effective predictive factor in both sets (P = 0.001 and P = 0.004, respectively; Table 2). Subsequently, all the statistically significant factors in univariate analysis were included in multivariate cox proportional hazards analysis, and immunotype (HR: 3.287; 95% CI: 1.720‐6.278; P < 0.001; and HR: 2.334; 95% CI: 1.275‐4.273; P = 0.006; respectively) and TNM stage (HR: 2.301; 95% CI: 1.312‐4.035; P = 0.004; and HR: 1.798; 95% CI: 1.065‐3.035; P = 0.029; respectively) were still independent prognostic indicators for OS in both sets (Table 2).
Table 2.
Univariate and multivariate cox regression analysis of overall survival
| Characteristic | Training set | Validation set | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Univariate | ||||
| Age at surgery, years: >60 vs <60 | 0.647 (0.387‐1.080) | 0.097 | 1.806 (0.476‐1.364) | 0.424 |
| Gender: male vs female | 1.266 (0.726‐2.206) | 0.408 | 0.873 (0.496‐1.539) | 0.641 |
| TNM stage: III + IV vs I + II | 2.092 (1.225‐3.575) | 0.007 | 1.915 (1.136‐3.229) | 0.015 |
| Differentiation: poor vs well‐moderately | 1.617 (0.947‐2.761) | 0.080 | 1.505 (0.885‐2.558) | 0.133 |
| Surgical margin: positive vs negative | 2.343 (1.186‐4.630) | 0.015 | 1.861 (0.911‐3.800) | 0.090 |
| Microvascular invasion: present vs absent | 1.703 (0.977‐2.969) | 0.062 | 1.018 (0.578‐1.795) | 0.951 |
| Immunotype: Type B vs Type A | 2.892 (1.532‐5.462) | 0.001 | 2.449 (1.340‐4.477) | 0.004 |
| Macrophages: high vs low | 1.013 (1.006‐1.021) | <0.001 | 1.008 (1.001‐1.014) | 0.023 |
| Neutrophils: high vs low | 1.016 (1.009‐1.022) | <0.001 | 1.006 (1.001‐1.011) | 0.026 |
| Tregs: high vs low | 1.027 (1.012‐1.042) | <0.001 | 1.005 (0.998‐1.013) | 0.0858 |
| CTLs: high vs low | 0.990 (0.980‐0.999) | 0.029 | 0.991 (0.984‐0.998) | 0.029 |
| Mast cells: high vs low | 0.988 (0.979‐0.997) | 0.015 | 0.989 (0.979‐0.999) | 0.038 |
| Multivariate | ||||
| TNM stage: III + IV vs I + II | 2.301 (1.312‐4.035) | 0.004 | 1.798 (1.065‐3.035) | 0.029 |
| Surgical margin: positive vs negative | 1.748 (0.861‐3.548) | 0.124 | ||
| Immunotype: type B vs type A | 3.287 (1.720‐6.278) | <0.001 | 2.334 (1.275‐4.273) | 0.006 |
Bold indicates statistically significant values P < .05.
3.4. Efficiencies of prognostic models
Hence, we have proved the prognostic value of the immune index model, but the efficiency of our model was still obscure. Therefore, we calculated the C‐indexes of the immune index model and the single immune cell models. In the training set, the immune index model and all the single immune cell models were significant prognostic factors (all P < 0.05), while the C‐indexes of the single immune cell models (0.575‐0.623) were apparently lower than that of the immune index model (0.693) (Tables 2 and S2). Moreover, we extended the application of our immune index model using ROC analysis. The area under the curve (AUC) of the TNM stage was only 0.677 (95% CI: 0.578‐0.775) in the training set but was elevated to 0.787 (95% CI: 0.704‐0.871) when the immune index model was integrated (Figure 3D). All the results were similar in the validation set. Accordingly, we found that our immune index model was more effective than any single immune cell model and might improve on the TNM staging system.
3.5. Correlation between immunotype and chemotherapy
We evaluated the association between immunotype and gemcitabine‐based chemotherapy, which was widely used in gallbladder cancer patients with stage II‐IV disease. As shown in Figure 4A, the OS of the immunotype A patients in the training set was not improved (P = 0.345) after gemcitabine‐based chemotherapy, while the OS of the immunotype B patients was significantly elevated (P < 0.001). Similar findings were also found in the validation set (Figure 4B). Treatment with gemcitabine‐based chemotherapy was related to reduced risk of poor survival in immunotype B patients in both sets (HR, 0.421; 95%CI, 0.216‐0.820; P = 0.008; and HR, 0.459; 95%CI, 0.236‐0.894; P = 0.023; respectively), whereas similar risk reduction did not occur in immunotype A (HR, 0.663; 95%CI, 0.177‐2.485; P = .544; and HR, 0.680; 95%CI, 0.206‐2.246; P = .529; respectively) (Figure 4C).
Figure 4.
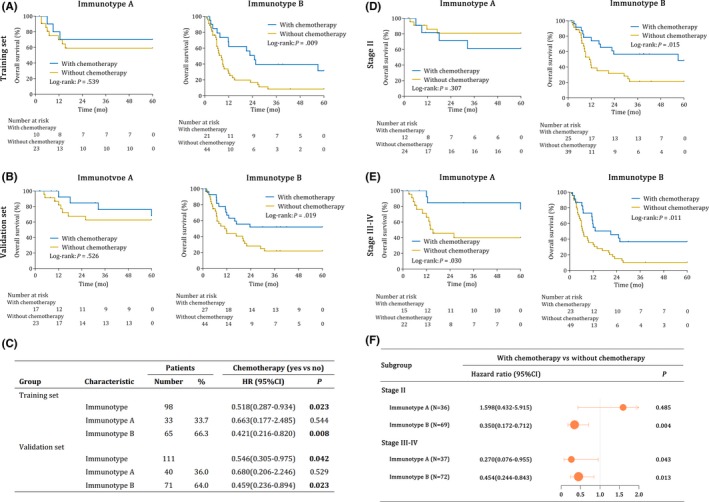
Chemotherapy benefits in stage II‐IV gallbladder cancer (GBC). A‐B, Kaplan‐Meier survival curves of GBC patients receiving chemotherapy or not for immunotype A and immunotype B in the training set (A) and the validation set (B). C, HR for overall survival in stage II‐IV gallbladder cancer patients receiving chemotherapy or not according to immunotype. Kaplan‐Meier survival curves of GBC patients receiving chemotherapy or not for immunotype A and immunotype B in stage II (D) and stage III‐IV (E) in the combined set. F, Subgroup analysis of chemotherapy benefits according to immunotype in stage II and stage III‐IV GBC patients. A combined set was generated by combing the training and validation set together. CI, confidence interval; HR, hazard ratio. P < 0.05 is considered statistically significant
We conduct a subgroup analysis to further explore the influence of immunotype on chemotherapy. As shown in Figure 4D‐F, for stage II patients with immunotype B and stage III‐IV patients with both immunotype A and immunotype B, gemcitabine‐based chemotherapy could significantly improve the 5‐year overall survival compared with those not receiving gemcitabine‐based chemotherapy (P = 0.015, P = 0.030, P = 0.011, respectively). However, for stage II patients with immunotype A, gemcitabine‐based chemotherapy could not improve the 5‐year overall survival compared with those not receiving gemcitabine‐based chemotherapy (P = .307).
4. DISCUSSION
In this study, we integrated five tumor‐infiltrating immune cells in GBC tissue, which we selected based on literature review, and constructed the immune index model using LASSO regression. Our model could effectively predict the prognosis and the gemcitabine‐based chemotherapy benefit for GBC patients. More specifically, immunotype A patients with a low immune index (Macrophagelow Neulow Treglow CTLhigh MChigh) had a favorable survival rate, but there was a reduced benefit on 5‐year OS from gemcitabine‐based chemotherapy, especially for stage II patients. In contrast, immunotype B patients with a high immune index (Macrophagehigh Neuhigh Treghigh CTLlow MClow) would have a lower survival rate but greater chemotherapy benefit (Figure S6). Compared with single immune cell models, our integrated model had a more precise prediction efficiency, and could elevate the discriminatory power of the TNM staging system.
Applying an “immunoscore,” that is, using immunostaining of CD3+ cells and CD8+ cells, was first proposed by Galon.28 Based on the adaptive immune response, the immunoscore model proved to be a strong prognostic factor for survival of colon cancer in a multi‐center clinical trial.29 Galon also suggested that additional markers could be added subsequently to refine the method even further.30, 31 Thanks to the great effort of scientists, the important roles of innate immune and myeloid‐derived cells were discovered recently.32, 33, 34, 35, 36, 37, 38, 39, 40 However, an effective model for GBC based on both adaptive immune cells and innate immune cells, both lymphoid‐derived cells and myeloid‐derived cells, and both pro–tumor cells and anti–tumor cells, is urgently needed. Our immune index model for GBC was constructed with this background. Compared with Galon's immunoscore model, our immune index model includes additional types of immune cells and is constructed with a more complicated formula, so that it is more comprehensive and can reflect the TME more precisely, although the complicated formula might be a hinderance for widespread clinical application.
In our immune index model, macrophages, neutrophils and Tregs were negative predictors while CTL and mast cells were positive predictors. This result is in accord with previous literature. CTL are the main effector cells in TME, which can induce tumor cell apoptosis by expressing FasL or secreting perforin, granzyme or IFN‐γ.15 Mast cells have been reported to activate CTL by releasing TNF‐α and OX40L,41, 42 while Tregs can directly inhibit the killing function of CTL in a cell‐cell contact‐dependent manner.43, 44, 45 Macrophages can inhibit the effector function of CTL directly through secretion of PD‐L1, IL‐10, TGF‐β or ROS, or indirectly by recruiting Tregs or inhibiting the recruitment and recognition of CTL.46, 47, 48, 49 Neutrophils can secrete ROS, RNS and neutrophil elastase (NE), IL‐1RA, ARG‐1 to promote tumor initiation and tumor growth.50, 51, 52 The role of these immune cells in TME and their different distribution in patients might be the reason why GBC patients with same stage had totally different prognoses.
Interestingly, we found positive correlations between the poor predictors (macrophages, neutrophils and Tregs) and between the favorable predictors (CTL and mast cells). There was also a negative correlation between mast cells and neutrophils. The driving factor of this result was hard to determine in our study, but it proved that the immune cells in the TME did not exist independently. Correspondingly, these immune cells might keep communicating with each other through direct contact or various cytokines, as mentioned above.41, 43, 44, 45, 46, 47, 48, 49, 50, 51
The gemcitabine‐based chemotherapy has been proved to prolong the survival time of advanced GBC patients and has remained the first‐line regimen since 2004.53, 54, 55 However, there is conflicting evidence regarding the role of gemcitabine for GBC. In a randomized controlled trial, Edeline (2019) reported that the gemcitabine‐based treatment not only did not bring a survival benefit for GBC but might even promote tumor recurrence and shorten patients’ survival.8 In our opinion, this situation might be related to the patient's immune index. In our study, gemcitabine‐based chemotherapy could significantly improve the 5‐year OS for stage II patients with immunotype B and stage III‐IV patients with both immunotype A and immunotype B. Nevertheless, for stage II patients with immunotype A, gemcitabine‐based chemotherapy did not improve the 5‐year overall survival rate and even led to (insignificant) worse survival. It is reported that favorable anticancer immunity occurred after chemoradiotherapy, and elevated CD8 density after chemoradiotherapy was associated with a favorable clinical outcome.55 While the effect of chemotherapy on the elevation of CD8 density might be rather weak for the immunotype A (CTLhigh) patients in stage II, insufficient benefit but more side effects might be the possible reasons for the worse prognosis of immunotype A patients who received chemotherapy in stage II compared to patients without chemotherapy. This result is helpful for the clinical decision of who should receive gemcitabine‐based chemotherapy, and for the prevention of excessive toxicity and unnecessary resource consumption. However, we also noticed that the 5‐year OS of immunotype B patients receiving effective chemotherapy was still lower compared with immunotype A patients who benefit from gemcitabine‐based chemotherapy. Therefore, new treatment options for them, such as effective targeted therapy and immunotherapy, are urgently needed.
The present study has some limitations. First, this study is a single‐center and retrospective study with a relatively small number of patients. Therefore, more prospective studies or multi‐center studies are needed to verify our results. Second, this study only included five immune cells, and some other immune cells that could affect patients’ prognosis might be missed because of the lack of single and specific markers. We will update our model with further development of cell identification methods in the future.
In conclusion, we constructed a new immune index model of GBC by integrating multiple tumor‐infiltrating immune cells. Our immune index model is practical for predicting the prognosis of GBC patients and might improve on the current TNM staging system. Furthermore, our data suggest that the immune index model might be useful for determining which patients are most likely to benefit from gemcitabine‐based chemotherapy.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
This study was funded by grants from the National Natural Science Foundation of China (81872352 and 81600630), the Foundation of Shanghai Science and Technology Committee (16411952000), the JianFeng Project of XuHui Provincial Commission of Health and Family Planning (SHXH201703) and the Shanghai Medical Discipline of Key Programs for General Surgery (2017ZZ02007).
Wang J, Bo X, Wang C, et al. Low immune index correlates with favorable prognosis but with reduced benefit from chemotherapy in gallbladder cancer. Cancer Sci. 2020;111:219–228. 10.1111/cas.14239
Jie Wang, Xiaobo Bo and Changcheng Wang contributed equally to this work.
Contributor Information
Yueqi Wang, Email: yueqiwang@fudan.edu.cn.
Houbao Liu, Email: houbaoliu@aliyun.com.
REFERENCES
- 1. Lazcano‐Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer[J]. CA Cancer J Clin. 2001;51(6):349‐364. [DOI] [PubMed] [Google Scholar]
- 2. Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour[J]. Nat Rev Cancer. 2004;4(9):695‐706. [DOI] [PubMed] [Google Scholar]
- 3. Are C, Ahmad H, Ravipati A, et al. Global epidemiological trends and variations in the burden of gallbladder cancer[J]. J Surg Oncol. 2017;115(5):580‐590. [DOI] [PubMed] [Google Scholar]
- 4. Rakic M, Patrlj L, Kopljar M, et al. Gallbladder cancer[J]. Hepatobiliary Surg Nutr. 2014;3(5):221‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy[J]. Am J Gastroenterol. 2000;95(6):1402‐1410. [DOI] [PubMed] [Google Scholar]
- 6. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med. 2010;362(14):1273‐1281. [DOI] [PubMed] [Google Scholar]
- 7. National Comprehensive Cancer Network (NCCN) . Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers, Version 2. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx#hepatobiliary. Accessed March 6, 2019.
- 8. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12‐ACCORD 18‐UNICANCER GI): a randomized phase III study[J]. J Clin Oncol. 2019;37(8):658‐667. [DOI] [PubMed] [Google Scholar]
- 9. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual, 8th edn New York: Springer; 2017. [Google Scholar]
- 10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation[J]. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 11. Miquel JF, Covarrubias C, Villaroel L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris[J]. Gastroenterology. 1998;115(4):937‐946. [DOI] [PubMed] [Google Scholar]
- 12. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment[J]. Cancer Cell. 2012;21(3):309‐322. [DOI] [PubMed] [Google Scholar]
- 13. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis[J]. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges[J]. Nat Rev Clin Oncol. 2018;15(5):325‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Betts BC, Veerapathran A, Pidala J, et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function[J]. Sci Transl Med. 2017;9(372). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer[J]. Nat Rev Immunol. 2016;16(10):599‐611. [DOI] [PubMed] [Google Scholar]
- 17. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more[J]. Nat Rev Cancer. 2016;16(7):431‐446. [DOI] [PubMed] [Google Scholar]
- 18. Rigoni A, Colombo MP, Pucillo C. Mast cells, basophils and eosinophils: from allergy to cancer[J]. Semin Immunol. 2018;35:29‐34. [DOI] [PubMed] [Google Scholar]
- 19. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy[J]. Nat Rev Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+ regulatory T‐cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer[J]. Br J Cancer. 2015;113(12):1677‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H, Liu H, Shen Z, et al. Tumor‐infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer[J]. Ann Surg. 2018;267(2):311‐318. [DOI] [PubMed] [Google Scholar]
- 22. Wang JT, Li H, Zhang H, et al. Intratumoral IL17‐producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer[J]. Ann Oncol. 2019;30(2):266‐273. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Bo X, Suo T, et al. Tumor‐infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer[J]. Cancer Sci. 2018;109(7):2266‐2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bloniarz A, Liu H, Zhang CH, Sekhon JS, Yu B. Lasso adjustments of treatment effect estimates in randomized experiments[J]. Proc Natl Acad Sci USA. 2016;113(27):7383‐7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies H, Glodzik D, Morganella S, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures[J]. Nat Med. 2017;23(4):517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Backes Y, Elias SG, Groen JN, et al. Histologic factors associated with need for surgery in patients with pedunculated T1 colorectal carcinomas[J]. Gastroenterology. 2018;154(6):1647‐1659. [DOI] [PubMed] [Google Scholar]
- 27. Fu H, Zhu Y, Wang Y, et al. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle‐invasive bladder cancer[J]. Clin Cancer Res. 2018;24(13):3069‐3078. [DOI] [PubMed] [Google Scholar]
- 28. Galon J, Costes A, Sanchez‐Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome[J]. Science. 2006;313(5795):1960‐1964. [DOI] [PubMed] [Google Scholar]
- 29. Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study[J]. Lancet. 2018;391(10135):2128‐2139. [DOI] [PubMed] [Google Scholar]
- 30. Galon J, Pages F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer[J]. J Transl Med. 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours[J]. J Pathol. 2014;232(2):199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy[J]. Nature. 2019;574(7776):45‐56. [DOI] [PubMed] [Google Scholar]
- 33. Benci JL, Johnson LR, Choa R, et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade[J]. Cell. 2019;178(4): 933‐948.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy[J]. Nature. 2019;572(7769):392‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liew PX, Kubes P. The Neutrophil's role during health and disease[J]. Physiol Rev. 2019;99(2):1223‐1248. [DOI] [PubMed] [Google Scholar]
- 36. Barrow AD, Edeling MA, Trifonov V, et al. Natural killer cells control tumor growth by sensing a growth factor[J]. Cell. 2018;172(3):pp. 534–48 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Canadas I, Thummalapalli R, Kim JW, et al. Tumor innate immunity primed by specific interferon‐stimulated endogenous retroviruses[J]. Nat Med. 2018;24(8):1143‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lavin Y, Kobayashi S, Leader A, et al. Innate immune landscape in early lung adenocarcinoma by paired single‐cell analyses[J]. Cell. 2017;169(4):pp. 750–65 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y, Souza‐Fonseca‐Guimaraes F, Bald T, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells[J]. Nat Immunol. 2017;18(9):1004‐1015. [DOI] [PubMed] [Google Scholar]
- 40. Podlech J, Ebert S, Becker M, Reddehase MJ, Stassen M, Lemmermann NA. Mast cells: innate attractors recruiting protective CD8 T cells to sites of cytomegalovirus infection[J]. Med Microbiol Immunol. 2015;204(3):327‐334. [DOI] [PubMed] [Google Scholar]
- 41. Bo X, Wang J, Suo T, et al. Tumor‐infiltrating mast cells predict prognosis and gemcitabine‐based adjuvant chemotherapeutic benefit in biliary tract cancer patients[J]. BMC Cancer. 2018;18(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thommen DS, Schumacher TN. T cell dysfunction in cancer[J]. Cancer Cell. 2018;33(4):547‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer[J]. Immunity. 2019;50(2):302‐316. [DOI] [PubMed] [Google Scholar]
- 44. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3[J]. Nat Rev Immunol. 2017;17(11):703‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment[J]. Trends Immunol. 2012;33(3):119‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM. TH2‐polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy[J]. Cancer Immunol Res. 2015;3(5):518‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruffell B, Chang‐Strachan D, Chan V, et al. Macrophage IL‐10 blocks CD8+ T cell‐dependent responses to chemotherapy by suppressing IL‐12 expression in intratumoral dendritic cells[J]. Cancer Cell. 2014;26(5):623‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm[J]. Nat Immunol. 2010;11(10):889‐896. [DOI] [PubMed] [Google Scholar]
- 49. Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis[J]. Trends Immunol. 2019;40(3):228‐242. [DOI] [PubMed] [Google Scholar]
- 50. Blaisdell A, Crequer A, Columbus D, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells[J]. Cancer Cell. 2015;28(6):785‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antonio N, Bonnelykke‐Behrndtz ML, Ward LC, et al. The wound inflammatory response exacerbates growth of pre‐neoplastic cells and progression to cancer[J]. EMBO J. 2015;34(17):2219‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park JO, Oh DY, Hsu C, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review[J]. Cancer Res Treat. 2015;47(3):343‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hezel AF, Noel MS, Allen JN, et al. Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild‐type unresectable or metastatic biliary tract and gallbladder cancer[J]. Br J Cancer. 2014;111(3):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta‐analysis of two randomised trials[J]. Ann Oncol. 2014;25(2):391‐398. [DOI] [PubMed] [Google Scholar]
- 55. Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor‐infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414‐S421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


