Abstract
Background
A Diagnostic Laboratory Hub (DLH) was set up in Guatemala to provide opportunistic infection (OI) diagnosis for people with HIV (PWH).
Methods
Patients newly presenting for HIV, PWH not receiving antiretrovirals (ARVs) for >90 days but returned to care (Return/Restart), and PWH on ARVs with symptoms of OIs (ARV treatment) were prospectively included. Screening for tuberculosis, nontuberculous mycobacteria (NTM), histoplasmosis, and cryptococcosis was done. Samples were couriered to the DLH, and results were transmitted electronically. Demographic, diagnostic results, disease burden, treatment, and follow-up to 180 days were analyzed.
Results
In 2017, 1953 patients were included, 923 new HIV infections (an estimated 44% of all new HIV infections in Guatemala), 701 on ARV treatment, and 315 Return/Restart. Three hundred seventeen (16.2%) had an OI: 35.9% tuberculosis, 31.2% histoplasmosis, 18.6% cryptococcosis, 4.4% NTM, and 9.8% coinfections. Histoplasmosis was the most frequent AIDS-defining illness; 51.2% of new patients had <200 CD4 cells/mm3 with a 29.4% OI incidence; 14.3% of OIs in new HIV infections occurred with CD4 counts of 200–350 cells/mm3. OIs were the main risk factor for premature death for new HIV infections. At 180 days, patients with OIs and advanced HIV had 73-fold greater risk of death than those without advanced disease who were OI-free.
Conclusions
The DLH OI screening approach provides adequate diagnostic services and obtains relevant data. We propose a CD4 screening threshold of <350 cells/mm3. Mortality remains high, and improved interventions are required, including expansion of the DLH and access to antifungal drugs, especially liposomal amphotericin B and flucytosine.
Keywords: cryptococcosis, histoplasmosis, HIV, opportunistic infections, tuberculosis
Globally, there are 37 million people with HIV (PWH), and nearly 1 million died from AIDS-related diseases in 2017 [1]. Guatemala had an estimated number of 46 000 PWH in 2017, with 2100 new HIV infections in adults and 200 children [1]. Antiretroviral (ARV) coverage encompasses 39% of PWH, and 28% have a suppressed viral load. It is estimated that 50% receive a diagnosis when they have advanced disease (CD4 cell count <200 cells/mm3) [1].
Public health care for HIV in Guatemala is organized in HIV units, most with limited diagnostic capability. We decided to set up a Diagnostic Laboratory Hub (DLH) to provide diagnostics for OIs in a timely manner and at acceptable cost. We expected accurate OI diagnosis to provide data on relative incidence. Here, we describe the DLH, the network, and the results from 2017, the first year of operation.
METHODS
Participants
In November of 2015, all HIV units were invited to a kick-off meeting. The objectives were to (i) organize a fast and reliable system to request diagnosis and deliver results; (ii) provide learning about diagnosis, treatment, and management of OIs, and (iii) prospectively screen a cohort of HIV patients for OIs. Thirteen out of the 16 HIV units agreed to participate in the network, which was called FUNGIRED. A secure website (http://fungired.gt/) was set up with the following design: (i) information module about the network and an epidemiology section with statistics; (ii) continuous learning module through which e-learning courses were delivered; (iii) electronic laboratory system to request diagnostic tests online from HIV units and provide results. Once entered, the system warns the DLH of sample delivery, which smooths laboratory workload and reduces the turnaround time.
Patients
We prospectively included patients (i) with newly diagnosed HIV infection, (ii) who abandoned ARVs for >90 days but returned to care (Return/Restart), and (iii) on ARVs with symptoms compatible with an OI (ARV treatment). In 2017, all patients had to be screened for tuberculosis (TB), NTM, histoplasmosis, and cryptococcosis, irrespective of their CD4 counts.
All ages were tested, but only patients >13 years old were analyzed.
Clinical Samples
The following samples were requested from all patients: whole blood in an isolator tube (Abbott Diagnostics, IL, USA; 10 mL), serum (5 mL), urine, and sputum. At the discretion of the physician, other clinical samples were taken. From Monday to Friday, samples were delivered to DLH by a courier. Personnel at each HIV unit were trained in sample packaging using a box with a portable cooler. The parcel was picked up the same day and delivered to the DLH in <24 hours.
Diagnostic Follow-up at the DLH and Treatment
The microbiological, immunological, and molecular tests as well as the treatment of the OIs are described in the Supplementary Data.
Statistical Analysis
Variables collected include demographic data, opportunistic infection diagnostic results, treatment, and follow-up to 180 days. Statistical Package for the Social Sciences, version 25.0 (SPSS Inc., Chicago, IL, USA), was used for analysis. The chi-square test or Fisher exact test and Mann-Whitney U test were used to compare the groups. Kaplan-Meier survival methodology was employed, and differences were assessed by log-rank test. Cox regression was used to establish hazard ratios (HRs). A P value <.05 was considered statistically significant.
RESULTS
Cohort of Patients
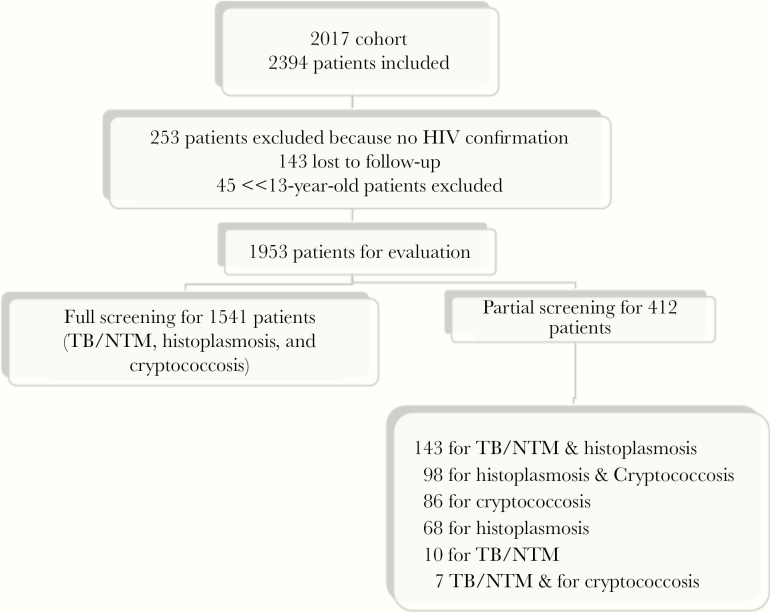
During 2017, 2394 patients were included, but 441 (18.42%) were excluded for different reasons (Figure 1). A total of 1953 PWH were available for analysis. Full screening was done in 1541 (79%) patients and partial in 412. Out of 1953 patients, 95% were tested for histoplasmosis, 87% for TB/NTM, and 89% for cryptococcosis (Figure 1).
Figure 1.
Description of the 2017 cohort. Abbreviations: NTM, nontuberculous mycobacteria; TB, tuberculosis.
Table 1 shows the sociodemographic data for the whole cohort of patients. There were 923 patients with new HIV infection, 701 on ARVs, and 315 Return/Restart. According to Guatemala UNAIDS estimates for 2017 [1], this study encompassed 44% (923 out of 2100 adults) of new adult HIV infections, 4% (701 out of 17 153) of those on ARVs, and 1.1% (315 out of 28 000) of Return/Restart.
Table 1.
Sociodemographic Data of Patients With and Without an OI in 2017 Cohort
| Total 1953 | With OI 317 (16.2%) | Without OI 1636 (83.8%) | |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Sex | |||
| Male | 1261 (64.6) | 211 (66.6) | 1050 (64.2) |
| Female | 679 (34.8) | 104 (32.8) | 575 (35.1) |
| Transsexual | 13 (0.7) | 2 (0.6) | 11 (0.7) |
| Age, y | |||
| No. | 1934 (99) | 310 (97.8) | 1624 (99.3) |
| Median | 34 | 36 | 34 |
| IQR | 17 | 15 | 17 |
| Unknown | 19 (1) | 7 (2.2) | 12 (0.7) |
| Sexual orientation | |||
| Heterosexual | 1502 (76.9) | 260 (82.0) | 1242 (75.9) |
| Homosexual | 292 (15.0) | 29 (9.1) | 263 (16.1) |
| Bisexual | 86 (4.4) | 11 (3.5) | 75 (4.6) |
| Unknown | 73 (3.7) | 17 (5.4) | 56 (3.4) |
| Ethnic group | |||
| Ladino | 1331 (68.2) | 224 (70.7) | 1107 (67.7) |
| Mayan | 280 (14.3) | 30 (9.5) | 250 (15.3) |
| Other | 9 (0.5) | 2 (0.6) | 7 (0.4) |
| Unknown | 333 (17.1) | 61 (19.2) | 272 (16.6) |
| Type of patient | |||
| Newly HIV infections | 923 (47.3) | 147 (46.4) | 776 (47.4) |
| On ART | 701 (35.9) | 100 (31.5) | 601 (36.7) |
| Restart/return | 315 (16.1) | 68 (21.5) | 247 (15.1) |
| Unknown | 14 (0.7) | 2 (0.6) | 12 (0.7) |
| CD4, cells/mm3 | |||
| No. | 1323 (67.7) | 218 (68.8) | 1105 (67.5) |
| Median | 210 | 63.5 | 246 |
| IQR | 307 | 153 | 297 |
| Unknown | 630 (32.3) | 99 (31.2) | 531 (32.5) |
| Viral load, copies/mL | |||
| No. | 1072 (54.9) | 191(60.3) | 881 (53.9) |
| Median | 50 477 | 138 585 | 42 322 |
| IQR | 154 376 | 375 132 | 123 730 |
| Unknown | 881 (45.1) | 126 (39.7) | 755 (46.1) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; OI, opportunistic infection.
For 630 patients (32.3%), CD4 cell counts were not available (Table 1). For 1323 with CD4 cell counts, 28.3% had <100 cells/mm3, 47.6% had <200 cells/mm3, 70.7% <350 cells/mm3, and 29.3% ≥350 cells/mm3. Women and men had similar numbers of CD4 cells (median women vs men, 220 vs 207; P = .478). This comparison between ethnic groups was significant (median Ladino vs Maya, 221 vs 176.5; P = .028).
Opportunistic Infections
Three hundred seventeen (16.2%) patients had an OI, and these PWH had lower counts of CD4 (median OI, 63.5; vs median non-OI, 246; P < .001) and higher viral loads (P < .001) (Table 1) than those without an OI.
Although CD4 counts were known for only 218 of 317 (68.8%) patients, there was a strong association between CD4 count and OI occurrence (Table 1). Overall, 198 (90.8%) OIs occurred in patients with CD4 counts <350 cells/mm3. Only 20 OIs (9.2%) were found in patients with ≥350 CD4 cells/mm3 (11 TB, 3 NTM, 4 cryptococcosis, and 2 histoplasmosis). Most coinfections occurred in those with very low CD4 cell counts; 17 (80.9%) were <100 cells/mm3, 3 (14.3%) 100–199 cells/mm3, and 1 (4.8%) 200–350 cells/mm3.
Table 2 shows the sociodemographic data of 317 patients classified by the OIs identified. There were 114 (35.9%) patients with TB, 14 (4.4%) with NTM, 99 (31.2%) with histoplasmosis, 59 (18.6%) with cryptococcosis, and 31 (9.8%) with coinfections. Coinfection incidence was 2%, and there were 13 histoplasmosis and TB, 8 cryptococcosis and TB, 7 cryptococcosis and histoplasmosis, 1 cryptococcosis and NTM, 1 TB and NTM, and 1 cryptococcosis and histoplasmosis and TB.
Table 2.
Sociodemographic Data of Patients per OI
| TB | NTM | H | C | Coinfections | |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| 114 (35.9) | 14 (4.4) | 99 (31.2) | 59 (18.6) | 31 (9.77) | |
| Sex | |||||
| Male | 77 (67.5) | 10 (71.4) | 65 (65.7) | 41 (69.5) | 18 (58) |
| Female | 37 (32.5) | 4 (28.6) | 33 (33.3) | 17 (28.8) | 13 (42) |
| Transsexual | 1 (1) | 1 (1.7) | |||
| Age, y | |||||
| No. | 111(97.4) | 14 (100) | 99 (100) | 55 (93.2) | 31 (100) |
| Median | 36 | 38 | 35 | 35 | 39 |
| IQR | 17 | 17 | 13 | 14 | 10 |
| Unknown | 3 (3.6) | 4 (6.8) | |||
| Sexual orientation | |||||
| Heterosexual | 86 (75.4) | 14 (100) | 88 (88.9) | 44 (74.6) | 28 (90.3) |
| Homosexual | 14 (12.3) | 6 (6.1) | 7 (11.9) | 2 (6.5) | |
| Bisexual | 8 (7) | 1 (1.0) | 2 (3.4) | ||
| Unknown | 6 (5.3) | 4 (4.0) | 6 (10.2) | 1 (3.2) | |
| Ethnic group | |||||
| Ladino | 79 (69.3) | 11 (78.6) | 69 (69.7) | 44 (74.6) | 21 (67.7) |
| Mayan | 13 (11.4) | 2 (14.3) | 11 (11.1) | 2 (3.4) | 2 (6.5) |
| Other | 2 (1.8) | ||||
| Unknown | 20 (17.5) | 1 (7.1) | 19 (19.2) | 13 (22.0) | 8 (25.8) |
| Type of patient | |||||
| Newly HIV infections | 48 (42.1) | 4 (28.6) | 56 (56.6) | 27 (45.8) | 12 (38.7) |
| On ART | 44 (38.6) | 6 (42.9) | 23 (23.2) | 19 (32.2) | 8 (25.8) |
| Restart/return | 21 (18.4) | 4 (28.6) | 20 (20.2) | 12 (20.3) | 11 (35.5) |
| Unknown | 1 (0.9) | 1 (1.7) | |||
| CD4, cells/mm3 | |||||
| No. | 76 (66.7) | 12 (85.7) | 62 (62.6) | 47 (79.7) | 21 (67.7) |
| Median | 94 | 213 | 36 | 56 | 27 |
| IQR | 174 | 297 | 102 | 144 | 279 |
| Unknown | 38 (33.3) | 2 (14.3) | 37 (37.4) | 12 (20.3) | 10 (32.3) |
| Viral load, copies/mL | |||||
| No. | 67 (58.8) | 8 (57.1) | 59 (59.6) | 39 (66.1) | 18 (58.1) |
| Median | 123 405 | 122 261 | 234 601 | 96 010 | 128 305 |
| IQR | 397 379 | 162 029 | 739 693 | 294 306 | 271 726 |
| Unknown | 47 (41.2) | 6 (42.9) | 40 (40.4) | 20 (33.9) | 13 (41.9) |
Abbreviations: C, cryptococcosis; H, histoplasmosis; NTM, nontuberculous mycobacteria; OI, opportunistic infection; TB, tuberculosis.
In total, there were 137 TB, 16 NTM, 120 histoplasmosis, and 76 cryptococcosis. These numbers were used to calculate the incidences of these infections in the cohort (Table 3).
Table 3.
Incidence of OIs in the Global Cohort and in the New HIV Infections Subset
| Global Cohort | |||||||
|---|---|---|---|---|---|---|---|
| All Cases | Unknown CD4a | <100 CD4a | <200 CD4a | <350 CD4a | ≥350 CD4a | ||
| TB | Cases/No. screened | 137/1701 | 46/518 | 51/343 | 70/572 | 80/843 | 11/340 |
| Incidence, % | 8.1 | 8.9 | 14.9 | 12.2 | 9.5 | 3.2 | |
| NTM | Cases/No. screened | 16/1701 | 2/518 | 6/343 | 8/572 | 11/843 | 3/340 |
| Incidence, % | 0.9 | 0.4 | 1.7 | 1.4 | 1.3 | 0.9 | |
| H | Cases/No. screened | 120/1850 | 45/569 | 55/368 | 65/615 | 73/910 | 2/371 |
| Incidence, % | 6.5 | 7.9 | 15.0 | 10.6 | 8.0 | 0.5 | |
| C | Cases/No. screened | 76/1732 | 16/530 | 38/336 | 52/568 | 56/853 | 4/349 |
| Incidence, % | 4.4 | 3.0 | 11.3 | 9.2 | 6.6 | 1.1 | |
| Total incidence, % | 19.9 | 20.2 | 42.9 | 33.4 | 25.4 | 5.7 | |
| Newly HIV infections | |||||||
| All Cases | Unknown CD4a | <100 CD4a | <200 CD4a | <350 CD4a | ≥350 CD4a | ||
| TB | Cases/No. screened | 55/825 | 15/180 | 25/207 | 31/332 | 36/485 | 4/160 |
| Incidence | 6.7 | 8.3 | 12.1 | 9.3 | 7.4 | 2.5 | |
| NTM | Cases/No. screened | 5/825 | 1/180 | 2/207 | 4/332 | 4/485 | 0/160 |
| Incidence | 0.6 | 0.6 | 1.0 | 1.2 | 0.8 | 0.0 | |
| H | Cases/No. screened | 65/872 | 21/186 | 36/220 | 41/352 | 44/516 | 0/170 |
| Incidence | 7.5 | 11.3 | 16.4 | 11.6 | 8.5 | 0.0 | |
| C | Cases/No. screened | 34/840 | 7/187 | 20/201 | 24/328 | 26/489 | 1/164 |
| Incidence | 4.0 | 3.7 | 9.9 | 7.3 | 5.3 | 0.6 | |
| Total incidence | 18.8 | 24.0 | 39.4 | 29.4 | 22 | 3.1 |
Abbreviations: C, cryptococcosis; H, histoplasmosis; NTM, nontuberculous mycobacteria; OI, opportunistic infection; TB, tuberculosis.
aNumber of CD4 cells/mm3.
OI incidence was 19.9% (Table 3). TB incidence was 8.1%, followed by histoplasmosis with 6.5%. OI incidence in patients with a CD4 count ≥350 cells/mm3 was 5.7%, with TB being the most frequent (11 cases), followed by cryptococcosis. In these patients, CD4 ranged from 358 to 1142 cells/mm3, with only 4 having CD4 counts <400 cells/mm3.
New HIV Infections
We describe a group of 923 newly diagnosed HIV infections in detail because they represent 44% of the cases seen in Guatemala in 2017 [1]. Women and men arrived at HIV diagnosis with a similar median CD4 count (192 vs 197 cells/mm3; P = .711). The CD4 cell count was unknown for 23.2% of patients. Of the remainder, 31.7% had <100 CD4 cells/mm3, 19.5% 100–199 cells/mm3, 23.8% 200–350 cells/mm3, and 25% >350 cells/mm3. Thus, 51.2% of patients had advanced HIV disease (<200 CD4 cells/mm3).
The incidence of OIs was 18.8% but rose to 29.4% and 39.4% for patients with <200 CD4 cells/mm3 and <100 CD4 cells/mm3, respectively (Table 3). In this group, TB had an incidence of 6.7% and histoplasmosis of 7.5%—the most frequent AIDS-defining illness in all groups except in those with CD4 counts ≥350 cells/mm3 (Table 3). There were 12 coinfections (5 histoplasmosis and cryptococcosis, 4 histoplasmosis and TB, 2 cryptococcosis and TB, and 1 TB and NTM), 10 of which were in patients with <350 CD4 cells/mm3. For the remaining 2 patients, the CD4 count was unknown.
Performance of Diagnostic Techniques
Cases diagnosed by direct microscopy and culture were limited (Table 4). Polymerase chain reaction (PCR) in sputum was positive for 91.6% of the cases of TB but only 56.5% for histoplasmosis. However, 75% of histoplasmosis cases were diagnosed by urine antigen test. In those considered to have disseminated histoplasmosis diagnosed by a positive antigen and/or a positive Isolator blood culture, the urine antigen was positive in 94.4% of cases. Cryptococcal antigen in serum was positive in all cases, whereas culture was only positive for 39.6%. Except for cryptococcosis, >1 technique was required to diagnose 100% of cases (Table 4).
Table 4.
Diagnostic Performance for OIs
| Tuberculosisa,b | NMTc | Histoplasmosisd,e | Cryptococcosisf | ||
|---|---|---|---|---|---|
| No. cases | 137 | 16 | 120 | 76 | |
| Direct exam | No. tested | 133 | ND | ND | NDg |
| Positive, % | 18 | — | — | — | |
| Conventional culture | No. tested | 135 | 16 | 110 | 34h |
| Positive, % | 38.5 | 100 | 10.9 | 56 | |
| Isolator Blood culture | No. tested | 82 | ND | 69 | 59 |
| Positive, % | 19.5 | — | 30.4 | 17 | |
| Ag detection in serum | No. tested | ND | ND | ND | 76 |
| Positive, % | — | — | — | 100 | |
| Ag detection in CSF | No. tested | ND | ND | ND | 34 |
| Positive, % | — | — | — | 79.4 | |
| Ag detection in urine | No. tested | ND | ND | 112 | ND |
| Positive, % | — | — | 75 | — | |
| PCR | No. tested | 131 | ND | 92 | ND |
| Positive, % | 91.6 | — | 56.5 | — |
Abbreviations: CSF, cerebrospinal fluid; ND, not done; NTM, nontuberculous mycobacteria; OI, opportunistic infection; PCR, polymerase chain reaction.
aThere were 114 single tuberculosis and 23 coinfections.
bOne hundred percent tuberculosis diagnosis was reached with a combination of culture and PCR.
cThere were 14 single nontuberculous mycobacteria and 2 coinfections.
dThere were 99 single histoplasmosis and 21 coinfections.
eOne hundred percent histoplasmosis diagnosis was reached with a combination of Ag detection and polymerase chain reaction.
fThere were 59 single cryptococcosis and 17 coinfections.
gND: not done. For Cerebrospinal fluid, Indian ink was performed, but the results are not shown because of the 100% sensitivity of Ag detection.
hOnly cerebrospinal fluid culture.
Treatment of OIs and Survival
Twenty-one patients were lost to follow-up, 10 with OIs (3 TB, 1 NTM, 2 histoplasmosis, and 4 cryptococcosis) and 11 without OIs. Thirteen patients died immediately after admission and were not treated, 15 patients refused any treatment (death was confirmed by the National Registry), and 5 did not receive treament for other reasons. The actual therapies delivered are shown in Table 5.
Table 5.
Opportunistic Infection Treatment for 286 Patients Followed up to 180 Days
| 114 TB | 14 NTM | 99 Histoplasmosis | 59 Cryptococcosis | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| AmB | — | — | 21 (21.2) | 1 (1.7) |
| AmB followed by FZ | — | — | 4 (4.0) | 23 (39.0) |
| AmB followed by ITZ | — | — | 31 (31.3) | — |
| FZ | — | — | 3 (3.0) | 22 (37.3) |
| ITZ | — | — | 21 (21.2) | — |
| Antibiotics | — | 5 (35.7) | — | — |
| Anti-TB drugs | 98 (86) | 5 (35.7) | — | — |
| No treatment or unknowna | 16 (14) | 4 (28.6) | 19 (19.2) | 13 (22.0) |
Abbreviations: AmB, conventional amphotericin B; FZ, fluconazole; ITZ, itraconazole; NTM, nontuberculous mycobacteria; TB, tuberculosis.
aThirteen patients dead immediately after admission; 19 were lost to follow-up, and there is no evidence that they received treatment; 15 refused the treatment (death was confirmed using the National Registry), and 5 did not receive treatment for other reasons.
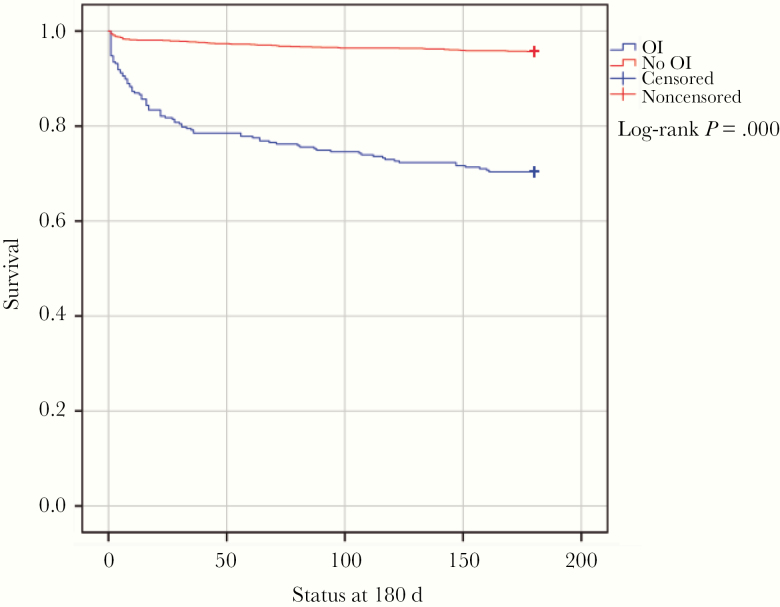
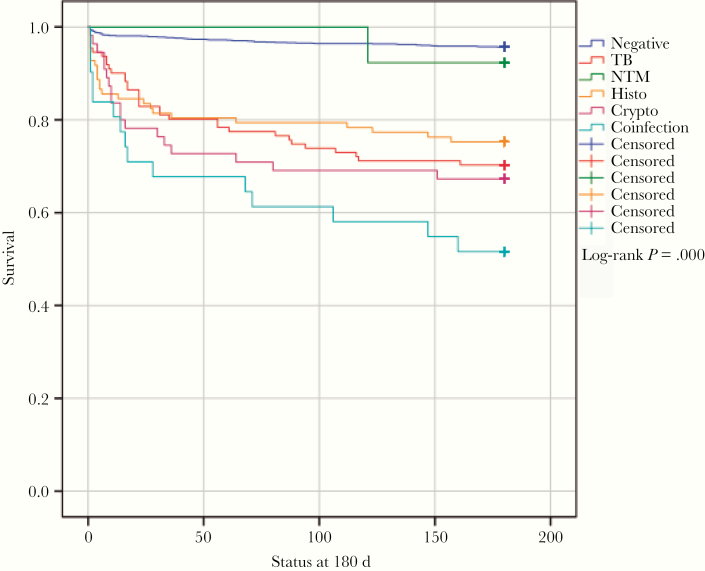
Figure 2 shows survival curves for patients with and without OIs. Mortality at 180 days of patients with OIs was 29.6%. The highest mortality at 180 days was caused by coinfections, at 48.4% (HR, 7.9), followed by cryptococcosis, 32.7% (HR, 4.9), TB, 29.7% (HR, 4.7), and histoplasmosis, 24.7% (HR, 3.7) (Figure 3).
Figure 2.
Kaplan-Meier survival curves of patients with and without opportunistic infections. Abbreviation: OI, opportunistic infection.
Figure 3.
Kaplan-Meier survival curves of opportunistic infection cases. Abbreviations: NTM, nontuberculous mycobacteria; TB, tuberculosis.
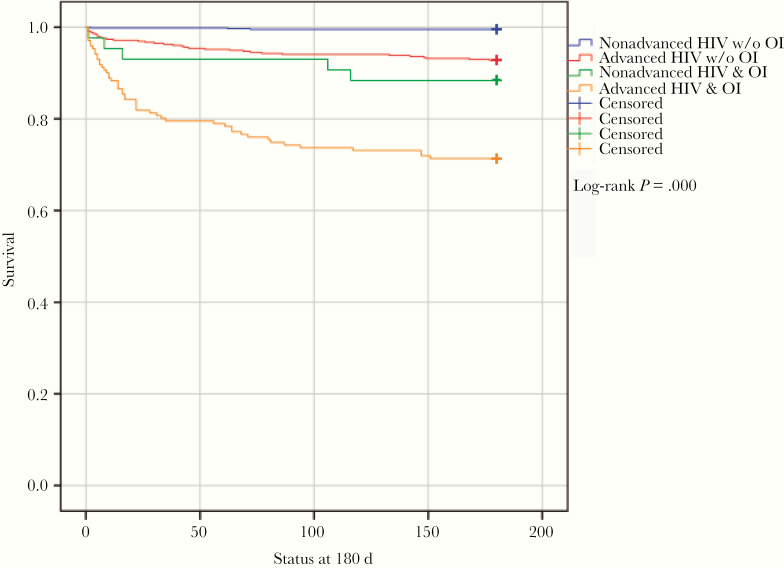
Mortality at 180 days in patients with nonadvanced disease without an OI was 0.5%; it was 11.6% in those with OIs (HR, 26.5). For patients with advanced disease without OIs, the mortality was 7.3% (HR, 16.2); it was 28.7% in those with OIs (HR, 72.9) (Figure 4).
Figure 4.
Survival curves for advanced vs nonadvanced HIV and opportunistic infections. Abbreviation: OI, opportunistic infection.
Log-rank testing was statistically significant for all survival comparisons (P < .0001) except for NTM. In the Cox regression model, age, gender, sexual orientation, ethnic group, and type of patient were included to ascertain interactions. None of these comparisons were statistically significant except age. When age and OIs were analyzed together, age was not statistically significant and thus was discarded as an interactive variable in survival.
DISCUSSION
Here we show that a national DLH can provide a high-quality diagnostic service for fungal disease and TB/NTM for an LMIC. This demonstration is the first of its kind in terms of population reach, timeliness, and clinical impact for a large population, as opposed to a local district. Not only did this new health care diagnostic system improve the diagnosis and management of OIs; it also provided crucial epidemiology data for these life-threatening infections in Guatemala.
A common current diagnostic approach is to have laboratories close to the patients. However, in many LMICs such laboratories are not well equipped, the staff are undertrained, and the budget is severely constrained. On the other hand, in many countries there are reference laboratories, but they are focused on public health and development of surveillance programs, diagnosis of imported or infrequent diseases, analysis of local of national outbreaks, identification of rare species and new mechanisms of resistance, etc. However, the main objective of a DLH is to provide quick diagnostic services of high quality to a network of clinics or hospitals with a limited diagnostic capability. The implementation of DLH has (i) improved the training of staff utilizing several different diagnostic technologies; (ii) enhanced the quality of the results obtained; and (iii) reduced the cost per test for those that are formatted for multiple samples. Time to result is already fast but would be enhanced further with a 24-hour/7-day working pattern, but this is not currently feasible. A DLH that knows 24 hours in advance how many samples it will receive the next day can organize its workload appropriately, minimizing the turnaround time and cost per test while at the same time reaching a quality standard similar to high-income countries’ (HICs’) laboratories.
The main bottleneck was the time to deliver the samples to the DLH, which was solved with a frequent and rapid courier service. Currently, 70% of the HIV units are at 5 hours’ driving distance from the DHL, whereas the remote HIV units are at a maximum of 9 hours. In the near future, drones could be used to deliver parcels in a shorter time [10, 11].
The performance of the DLH has been analyzed with its 2017 activity providing diagnostics to a network of 13 HIV clinics. There were 2141 PWH originally included, with 45 children excluded and only 143 (6.8%) lost to follow-up. From the 3 groups of HIV patients evaluated, 923 were new presentations of HIV infection, a remarkable 44% of the estimated total of first PWH presentations in the country in 2017 [1]. For the first time, we reliably describe what the most frequent AIDS-defining illnesses are in Guatemala, with disseminated histoplasmosis taking top place. We have not formally documented other less common presentations such as Pneumocystis pneumonia, bacterial sepsis, toxoplasmosis, pneumonia, cytomegalovirus infections, and others, but our clinical experience places all these OIs at lower frequencies. Reliable data on the burden of different OIs, before and after ART initiation, are critical for planning of health services, including procurement of drugs and diagnostics, which will provide better care to PWH. In this group of patients, the epidemiological data are robust, giving a clear picture about their sociodemographic characteristics and the burden of OIs (Tables 1 and 2).
For the last 30 years of the HIV epidemic, CD4 count has been a key parameter to assess risk of opportunistic infections and define advanced HIV infection in adults. Although the CD4 cell count was unknown for 32.3%, for the 1323 PWH with known CD4 cell count, 47.6% had <200 cells/mm3 and 29.3% >350 cells/mm3. Specifically, for the 923 new HIV infections, 51.2% had a CD4 cell count <200 cells/mm3, which confirms prior data that PWH in Guatemala present very late, with a high mortality risk and cost of care, the worst in the region [12]. HIV awareness campaigns in Guatemala have clearly not achieved their intended objectives. A relevant finding is that women and men arrive with similar CD4 cell counts.
Recent World Health Organization (WHO) guidelines recommend specific care to patients with advanced disease (<200 CD4 cells/mm3 or WHO clinical stage 3 or 4 disease) [13]. Here, we have analyzed all patients, irrespective of CD4 count, but CD4 cell count provides a critical tool for stratifying the OI risk for PWH to reduce the cost of screening. It should be available in every single HIV clinic, or the DLH should offer it. Knowing the CD4 cell count in real time allows decisions on which patients should be screened.
Following the WHO’s advice to screen only patients with <200 CD4 cells/mm3, we would have missed 20.2% and 14.3% of OIs in the whole cohort and in the new HIV infection group, respectively. Therefore, our results support an OI screening threshold of <350 CD4 cells/mm3. We cannot make any recommendation for OIs other than TB, NTM, histoplasmosis, and cryptococcosis, because others were not addressed in this study.
In Guatemala, fungal infections are more prevalent than mycobacterial infections, 10.9% vs 9%, respectively. For newly presenting HIV infection, the situation is similar, 11.5% fungal infections vs 7.3% mycobacterial. These figures did not include Pneumocystis pneumonia, and molecular diagnosis for this infection will be included in the network in 2018. The resources devoted to diagnosis and management of mycobacterial diseases dwarf those dedicated to fungal infections—still neglected after >35 years of AIDS. For instance, in Guatemala flucytosine, an essential antifungal for the treatment of cryptococcal meningitis, is not available. The optimal timing of introducing ARVs in PWH with histoplasmosis has not been addressed at all, as another example.
Histoplasmosis and TB had similar incidence, although for new PWH, histoplasmosis was the most frequent OI associated with AIDS illness. Differentiating between histoplasmosis and TB by clinical means is difficult, and some patients have both infections. Until the implementation of the DLH in Guatemala, the epidemiology of both infections was unknown, and presumably many of the histoplasmosis cases were treated as TB, with a fatal outcome and a high cost of care. Using a screening strategy, independent of the physician’s requests, has proven to be useful for diagnosing OIs, especially the 31 coinfections that would not have been diagnosed without this approach. Concomitant TB and histoplasmosis are problematic to treat because of the interactions of rifampicin and itraconazole [9]. There were few NTM infections, but their diagnosis was important because the treatment is different from that of tuberculosis [9, 10].
Antigen detection was the best means of diagnosis of cryptococcosis and histoplasmosis. For TB, culture had a low sensitivity, whereas PCR was more useful. Xpert MTB/RIF and TB urinary lateral flow LAM assay were not available at the DLH. Despite limited sensitivity and slow response, culture should be performed because it is important for identification and antimicrobial susceptibility testing. The positive rate of histoplasmosis culture found in this study is low when compared with other studies [14, 15]. The reasons are unknown, but we can speculate about the length of time to deliver the samples from the HIV units to the DLH and the introduction of urine Ag detection, which could reduce the need for invasive samples like bone marrow, which is well known to increase the culture positivity rate. In addition, recovery of Histoplasma spp. in culture is a slow and risky procedure; resistant strains have not been detected so far, and antifungal susceptibility testing is not standardized for this species. Nowadays and after the introduction of Ag detection in urine, this diagnostic approach has limited clinical value.
For diagnosis of histoplasmosis by antigen, urine is required, and the ease of acquiring urine explains why 95% of the patients were antigen tested. But the timing of urine sampling for histoplasmosis diagnosis could be important. An early morning urine collection improves the sensitivity of TB lateral flow LAM assay [16]. Another report shows an increased sensitivity after urine concentration [17]. The impact of these parameters on the sensitivity and specificity of Histoplasma antigen testing require research. Further improving the performance of the test would be valuable because culture is so slow and has a low sensitivity and there is no commercial PCR. The in-house PCR used in this work had a limited sensitivity (Table 4), although it was essential in the diagnosis of 26 cases (21.7%).
In addition to the diagnostic techniques employed in this work, the first WHO Model List of Essential In Vitro Diagnostics List (EDL) include Gen Xpert, LPA resistance, and LAM antigen [18], which we believe should be included in the portfolio of the DLH. In 2019, Histoplasma antigen detection was added to the EDL [19].
Cryptococcosis mortality of 32.7% (HR, 4.9) is similar to that of sub-Saharan Africa [20], where flucytosine is also not available and amphotericin B not routinely used. For histoplasmosis, we found a mortality of 24.7% at 180 days; 2 previous studies from that Guatemala and French Guyana showed mortality rates of 24.8% and 16.8%, respectively, but at 30 days of follow-up [14, 21]. An international cohort study evaluating TB-related mortality in PWH from Mexico, Chile, and Argentina found an 11% mortality at 1 year of follow-up, better than the 29.7% mortality at 6 months described here. Mortality in Eastern Europe (27%) is similar [22]. Notably, 9.8% of PWH here had lethal co-infections because only 51.6% were alive at 180 days (HR, 7.9).
As 52% of patients in Guatemala with HIV present with advanced disease, it is critical to establish a nationwide screening strategy with 2 main objectives, to diagnose and treat patients with OIs and to start safe and rapid HIV ART within 7 days of the day of HIV diagnosis, with the documented exception of cryptococcal meningitis (5 weeks’ delay) and tuberculosis (2 weeks’ delay) [11, 23]. As we show, OIs are the main causes of premature death of PWH, regardless of the CD4 count. Besides, with this screening strategy, 1636 patients who tested negative for the OIs screened were candidates for immediate ARVs. Several recent randomized trials have indicated that rapid ART initiation, including same-day start, can improve patients’ outcomes, especially by reducing loss to care in the pre-ART period [13].
Now validated in terms of clinical and survival impact, we propose a new health care diagnostic system, the diagnostic laboratory hub. This kind of organization is simpler, cheaper, and of higher quality than setting-up laboratories in every medical center. Courier companies and current Internet technology minimize the delays for delivery of samples and results, although there is room for improvement. In addition, the continuously high workload of a DLH ensures high-quality results, easier quality control, low cost per sample and diagnostic assay, and simpler continuing education for the staff. Even with full 24-hour/7-day operations, the DLH laboratory would be cheaper than any other kind of diagnostic system. In addition, an economic evaluation of the DLH is planned for 2020; conversations with the Ministry of Health have also started to analyze the sustainability of the project in the near future. For the time being, tuberculosis/NTM diagnosis is fully covered by the government, as well as the patient’s treatment for all OIs.
Strong technical and clinical leadership is a key feature of the DLH and other successful diagnostic laboratories, and this is easier to deliver with a focused program compared with a distributed one. With a good configuration and organization, a DLH, in limited-resource setting, can deliver similar performance as a laboratory in an HIC but at a lower cost. Depending on the size of the country, careful planning is required to ensure DLH coverage of the whole territory, taking into consideration the main bottleneck of this structure, the elapsed time clinical samples spend in transit to the DLH. Network communication is now possible through different software systems, allowing online meetings, conference rooms, and video webinars at affordable prices. This facilitates continuing education, discussions of clinical cases, and a permanent update of the DLH and its network of clinics. Other countries may choose to adopt this model of care to reduce deaths in PWH on a national or regional basis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgements
Juan Pablo Figueroa (ASI) for the support on data collection, Berta Taracena (Intrahealth) Estuardo Diaz (Intrahealth), Saúl Paau (AHF) and the multidisciplinary team that collaborate with Fungired.
Fungired members. (i) Oscar Eduardo López Pérez. Hospital La Amistad Japón-Guatemala, Izabal; (ii) Brenan Ortiz Barrientos. Hospital General San Juan de Dios, Guatemala city; (iii) Vilma Alejandrina Reyes Muñoz. Hospital Nacional “Dr Jorge Vides Molina,” Huehuetenango; (iv) Gladys Sajché Aguilar. Hospital Nacional “Juan José Ortega” Coatepeque, Quetzaltenango; (v) Aura Marina Méndez Andrade. Hospital Nacional de Escuintla, Escuintla; (vi) Luis Roberto Santa Marina de León. Hospital Nacional de Malacatán, San Marcos; (vii) Ana Lucía Gómez Alcázar. Hospital Nacional de Occidente, Quetzaltenango; (viii) Eduardo Celada González. Hospital Nacional de Retalhuleu, Retalhuleu; (ix) Gustavo A. Quiñónez M. Hospital Nacional Infantil “Elisa Martínez,” Izabal; (x) Germán Orlando Cuyuch Sontay. Hospital Regional “Hellen Lossi de Laugerud,” Alta Verapaz; (xi) Alba Virtud Contreras Marín. Hospital Regional de Cuilapa, Santa Rosa; (xii) María de Lourdes Fong Araujo. Hospital Regional de San Benito, Petén, (xiii) Claudia Mazariegos L. Hospital Regional de Zacapa, Zacapa and (xiv) Brenda Guzmán. Diagnostic Laboratory Hub, Asociación de Salud Integral, Guatemala City.
Financial support. This work was supported by Global Action Fund for Fungal Infections and JYLAG, a charity Foundation based in Switzerland (E.A. received this funding under the proposal: “Minimising HIV deaths through rapid fungal diagnosis and better care in Guatemala”). Other contributions came from AIDS Health Foundation (AHF) Guatemala, Intrahealth International and Ministry of health in Guatemala (MSPAS).
Potential conflicts of interest. Blanca Samayoa declares no conflict of interest. Luis Aguirre declares no conflict of interest. Oscar Bonilla declares no conflict of interest. Narda Medina declares no conflict of interest. Dalia Lau-Bonilla declares no conflict of interest. Danicela Mercado declares no conflict of interest. Anneliese Moller declares no conflict of interest. Juan Carlos Pérez declares no conflict of interest. Ana Alastruey-Izquierdo has received in the past 5 years grants from GILEAD, F2G and Scynexis and personal fees for educational conferences from Pfizer, Astellas, MSD and GILEAD outside the submitted work. Eduardo Arathoon has received honoraria from GILEAD for educational conferences and participation in Advisory board meeting. David Denning and family hold Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company. He acts or has recently acted as a consultant to Scynexis, Pulmatrix, Zambon, iCo Therapeutics, Roivant, Biosergen and Fujifilm. In the last 3 years, he has been paid for talks on behalf of Dynamiker, Hikma, Gilead, Merck, Mylan, and Pfizer. He is a longstanding member of the Infectious Disease Society of America Aspergillosis Guidelines group, the European Society for Clinical Microbiology and Infectious Diseases Aspergillosis Guidelines group and the British Society for Medical Mycology Standards of Care committee. Juan Luis Rodriguez-Tudela declares no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
“Fungired”:
Oscar Eduardo López Pérez, Brenan Ortiz Barrientos, Vilma Alejandrina Reyes Muñoz, Gladys Sajché Aguilar, Aura Marina Méndez Andrade, Luis Roberto Santa Marina de León, Ana Lucía Gómez Alcázar, Eduardo Celada González, Gustavo A Quiñónez M, Germán Orlando Cuyuch Sontay, Alba Virtud Contreras Marín, María de Lourdes Fong Araujo, L Claudia Mazariegos, and Brenda Guzmán
References
- 1. UNAIDS. Fact sheet - latest global and regional statistics on the status of the AIDS epidemic 2018. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed May 19, 2019.
- 2. Global Laboratory Initiative Advancing TB Diagnosis. A publication of the Global Laboratory Initiative a Working Group of the Stop TB Partnership mycobacteriology laboratory manual 2014. Available at: https://www.who.int/tb/laboratory/mycobacteriology-laboratory-manual.pdf. Accessed November 30, 2019.
- 3. Montenegro SH, Gilman RH, Sheen P, et al. Improved detection of Mycobacterium tuberculosis in Peruvian children by use of a heminested IS6110 polymerase chain reaction assay. Clin Infect Dis 2003; 36:16–23. [DOI] [PubMed] [Google Scholar]
- 4. Muñoz C, Gómez BL, Tobón A, et al. Validation and clinical application of a molecular method for identification of Histoplasma capsulatum in human specimens in Colombia, South America. Clin Vaccine Immunol 2010; 17(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bialek R, Feucht A, Aepinus C, et al. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNA in human tissue. J Clin Microbiol 2002; 40:1644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Treatment of tuberculosis: guidelines for treatment of drug-susceptible tuberculosis and patient care.2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf;jsessionid=8B82E1F8D678BD6DA19310B13848EC62?sequence=1. Accessed May 19, 2019.
- 7. Ministerio de Salud Pública y Asistencia Social de Guatemala. Manual de Atención Para el Manejo del Paciente con Tuberculosis. Guatemala: Ministerio de Salud Pública y Asistencia Social Guatemala; 2018. . [Google Scholar]
- 8. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Available at: https://aidsinfo.nih.gov/e-news. Accessed June 27, 2019. [Google Scholar]
- 10. Lippi G, Mattiuzzi C. Biological samples transportation by drones: ready for prime time? Ann Transl Med 2016; 4:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amukele TK, Sokoll LJ, Pepper D, et al. Can unmanned aerial systems (drones) be used for the routine transport of chemistry, hematology, and coagulation laboratory specimens? PLoS One 2015; 10:e0134020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UNAIDS. AIDSinfo | UNAIDS 2017. Available at: https://aidsinfo.unaids.org/. Accessed May 19, 2019.
- 13. World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy.2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255884/9789241550062-eng.pdf?sequence=1. Accessed May 19, 2019.
- 14. Adenis A, Nacher M, Hanf M, et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. Hamill RJ, editor. PLoS Negl Trop Dis 2014; 8(8):e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arango-Bustamante K, Restrepo A, Cano LE, De Bedout C, Tobón AM, González A. Diagnostic value of culture and serological tests in the diagnosis of histoplasmosis in HIV and non-HIV Colombian patients. Am J Trop Med Hyg 2013; 89(5):937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gina P, Randall PJ, Muchinga TE, et al. Early morning urine collection to improve urinary lateral flow LAM assay sensitivity in hospitalised patients with HIV-TB co-infection. BMC Infect Dis 2017; 17:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savolainen L, Kantele A, Sandboge B, et al. Modification of clear view tuberculosis (TB) enzyme-linked immunosorbent assay for TB patients not infected with HIV. Clin Vaccine Immunol 2013; 20(9):1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nacher M, Blanchet D, Bongomin F, et al. Histoplasma capsulatum antigen detection tests as an essential diagnostic tool for patients with advanced HIV disease in low- and middle-income countries: a systematic review of diagnostic accuracy studies. PLoS Negl Trop Dis 2018; 12(10):e0006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Second WHO Model List of Essential In Vitro Diagnostics. Geneva: World Health Organization; 2019. [Google Scholar]
- 20. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis 2017; 17(11):e334–e343. [DOI] [PubMed] [Google Scholar]
- 21. Samayoa B, Roy M, Cleveland AA, et al. High mortality and coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. Am J Trop Med Hyg 2017; 97(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Podlekareva DN, Efsen AMW, Schultze A, et al. Tuberculosis-related mortality in people living with HIV in Europe and Latin America: an international cohort study. Lancet HIV 2016; 3(3):e120–31. [DOI] [PubMed] [Google Scholar]
- 23. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370(26):2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.