Abstract
The objective of the study was to characterize immunological responses to a Brazilian Jiu-Jitsu high-intensity interval training session. Neuromuscular function, blood, and salivary samples were obtained after a Brazilian Jiu-Jitsu high-intensity interval training session. Saliva and blood samples were collected at Pre- (before the warm-up) and immediately Post-training. Neuromuscular function was evaluated by lower body muscle testing. The horizontal countermovement jump was performed at Pre (after the warm-up) and immediately Post blood and saliva collection, and approximately 5 minutes Post-training. The horizontal countermovement jump performance did not present any significant changes Post-training, while blood leukocytes, urea, IgA and salivary alpha-amylase showed a significant increase. Salivary alpha-amylase activity increased more than six times immediately Post compared to Pre-training. Saliva volume, secretion rate, and uric acid were not significantly different between Pre and Post condition. A Brazilian Jiu-Jitsu high-intensity interval training session elicited an increase in the blood cells responsible for antibody production and muscle damage adaptation after exercise. On the other hand, neuromuscular performance was not significantly affected Post-training, suggesting that immunological and performance responses were not necessarily associated.
Key words: metabolism, neuromuscular function, martial arts
Introduction
Combining bouts of high-intensity cyclic efforts, interspersed with passive and/or active low-intensity periods, Brazilian Jiu-Jitsu (BJJ) is an intermittent combat sport. The sport is characterized by strenuous contact, demanding high levels of strength and power, combined with a well-developed endurance capacity (da Silva et al., 2014). Regarding the training methods reported in the literature to improve endurance capacity in athletes, high-intensity interval training (HIIT) is receiving significant attention. The method is characterized by alternating periods of high-intensity efforts and incomplete rest intervals. An implicit advantage of the method is the execution of higher volumes of training at higher intensities (Billat, 2001). Performed in a specific manner for the fights, one example of HIIT would be the execution of rounds lasting about 2 minutes, with rest intervals ranging from 1 to 4 minutes (Bounty et al., 2011). Fight simulation with different lengths and rest intervals, can be characterized as specific HIIT, so that the athlete’s endurance capacity is enhanced (Bounty et al., 2011).
Additionally, strenuous contact and high-intensity efforts may induce tissue damage, characterized by increases in plasma activity of
myofibrilar enzymes such as creatine kinase (Warren et al., 1999), inflammatory responses (Coutts et al., 2007a, 2007b) and decreases in muscle function (Allen et al., 2008; Warren et al., 2001). Muscles that are intensively stimulated, usually present a progressive decline in performance connoted as neuromuscular fatigue (Allen et al., 2008). The phenomenon is multifactorial, reversible and with magnitude dependent on the specificity of the tasks performed (Bishop et al., 2008; Chapman et al., 2006; Ide et al., 2011). Nowadays, considering that excessive training fatigue is a problem for athletes (Meeusen et al., 2013), monitoring of fatigue and recovery following both training and competitions has received significant attention (Bishop et al., 2008; Chapman et al., 2006; Chapman et al., 2008; Ispirlidis et al., 2008). Specifically the inflammatory response appears to involve the production of cytokines, immunoglobulins, enzymes, and acute phase proteins as well as leukocytes in the bloodstream (Walsh et al., 2011).
After a single bout of exercise, there is a rapid and pronounced neutrophilia due to demargination caused by shear stress and catecholamines, followed by a second delayed increase due to the cortisol-induced release of neutrophils from the bone marrow (Gleeson, 2007; McCarthy et al., 1992). The numbers of lymphocytes can increase during and immediately after an exercise bout. However, the lymphocyte count falls below Pre exercise levels during the early stages of recovery (Kakanis et al., 2010). This temporary immunosuppression state is related to increased incidence of upper respiratory tract infection (URTI) in athletes (Gleeson, 2007; Kakanis et al., 2010). Acute high-intensity exercise may also change the saliva constituents. Some proteins with immunological properties (e.g., Immunoglobulin A and alpha-amylase) and metabolites (urea and uric acid) can indicate changes in oral cavity immune protection and metabolic stress after exercise, respectively (Nunes et al., 2011; Walsh et al., 1999). Low concentrations of salivary IgA (IgA) have been implicated with increased susceptibility of URTI due to reduced defense against pathogens transmitted mainly via the oral cavity (Mackinnon et al., 1993).
Since neuromuscular, blood, and salivary immunological responses to a BJJ HIIT session have not been investigated, we designed an experiment to study the phenomena. Our hypothesis was that the high-intensity and strenuous contact nature of the sport would induce acute muscle function impairment and immunological depression.
Methods
Participants
The inclusion criteria for the study were as follows: practicing BJJ for at least 3 years (technical level ranged from purple to black belt), training frequency of at least 4 times per week, no consumption of anabolic androgenic steroids, drugs, medications, or dietary supplements with potential effects on physical performance. Ten male BJJ athletes (3 black belts, 6 purple belts and 1 brown belt) participated in the study (age: 28.1 ± 6.6 years; body mass: 85.7 ± 16.1 kg; body height: 1.8 ± 0.1 m; BJJ training experience: 9.7 ± 5.2 years). Forty-eight hours prior to the testing session, participants were required to refrain from strenuous exercise and consumption of alcohol, tobacco, and caffeine. This study was approved by the local Ethics Committee (CAAE: 16562213.8.0000.5453) and was performed in accordance with international ethical standards. All participants received and signed an informed consent form.
Procedures
Neuromuscular function, blood, and salivary samples were obtained after a BJJ high-intensity interval (HIIT) training session. Saliva and blood samples were collected at Pre (before the warm-up) and immediately Post-training session. Neuromuscular function was evaluated by lower body muscle testing. The horizontal countermovement jump (HCMJ) was performed at Pre (Post warm-up) and immediately Post blood and saliva collection, as well as approximately 5 minutes Post-training.
Horizontal Countermovement Jump (HCMJ)
The Countermovement Jump was chosen as it is inexpensive, easy to administer, reliable, and a valid method of monitoring fatigue and supercompensation of in individual and team sports (Claudino et al., 2017; Maulder and Cronin, 2005). After completing a standardized warm up (three non-maximal and practice trials of the HCMJ, followed by lower limb stretching exercises), participants performed three maximal HCMJ. The protocol followed procedures recommended by(Maulder and Cronin, 2005). Athletes began by standing on the designated testing leg with their toe in front of the starting line. They were instructed to sink (approximately a 120° knee angle) as quickly as possible and then jump as far forward as possible and land on both feet. Participants were instructed and verbally encouraged to jump for maximal distance. Three attempts were performed, and the longest jump was recorded for analysis. The HCMJ was chosen because there has been early evidence of horizontal tests having greater stability within trials (coefficient of variability = 1.1-2.0%) and across testing occasions (test to test reliability = 0.80-0.97) compared to vertical tests (Maulder and Cronin, 2005).
Saliva and Blood Sample Collection
Unstimulated whole saliva samples were collected immediately before blood samples using the passive drool method. Distillated water was used for cleaning the volunteer’s oral cavity. Afterwards, the athletes were instructed to keep the saliva in the mouth for exactly two minutes, and then the entire contents of the oral cavity were spit into a plastic beaker. The saliva was then transferred into graduated plastic tubes and the volume was measured. Immediately after the saliva collection, capillary blood was collected from the middle finger pad after it was cleaned with ethyl alcohol (70%). A lancet device (Accu-chek Softclix Pro®) was used to guarantee proper depth of penetration for blood collection. The first blood drop was discarded. The puncture area was gently pressured without repetitive movements to allow free blood flow into the collecting tube. First, 200 μL of blood were collected in ethylenediamine tetraacetic acid (EDTAK3) by Microvette® Sarstedt 200. The saliva and blood samples were transported in a refrigerated box (4°C) and analyzed within 30 minutes.
Blood and Saliva analyses
Hematological analyses were run in an automatic analyzer (Sysmex®, Kobe, Japan) and included white blood cell (WBC), lymphocyte, and neutrophil counts. Biochemical measurements were conducted with a semiautomated spectrophotometric analyzer (BioPlus 2000) with commercial kits (Biotecnica®; Varginha, Brazil). The IgA was quantified by the immunoturbidimetric assay, salivary alpha-amylase (SAA) activity by the 2-chloro-p-nitrophenyl-α-D-maltotrioside method at 405 nm wavelength, and uric acid and urea were analyzed by the enzymatic-colorimetric method. The saliva samples were centrifuged at 2,200 x g for 10 min under refrigeration (4°C) before saliva quantification. Urea, uric acid, and IgA concentration was assayed in undiluted saliva. The SAA samples were diluted with distillated water (basal samples x 100 times) and samples collected immediately after exercise x 1000 times. The saliva secretion rate (SR) was calculated by dividing collected volume by time collection. The salivary IgA results were corrected by the secretion rate (μg/min). All analyses were run in parallel with the commercial blood and control serum (Control Lab®, Rio de Janeiro, Brazil). The means and standard deviation derived from the control samples were used to calculate the coefficient of analytical variation (CVA).
Brazilian Jiu-Jitsu high-intensity interval training session
Before training each athlete performed a 10 min standardized BJJ warm-up consisting of 5 min of general stretching and 5 min of specific BJJ exercises with a work/rest ratio of 30 s each. The BJJ high-intensity interval training session consisted of 6 rounds of 2 min each with a 1 min rest interval. The exercise performed was the guard-pass as it represents a very specific and frequent BJJ fighting situation that is extensively trained during BJJ training sessions. During training sessions and competitions, athletes usually defend against the opponent ground attacks using both arms and legs. This situation is connoted in fights as the guard. At the same time, the opponent attacks the guard, attempting to undo the legs and arms work, in order to submit or immobilize the other athlete. In order to complete the initial simulated training volume, the match was continued into overtime when a submission occurred. During the exercise, athletes were verbally encouraged to perform their best attacks and defenses against the opponents, always trying to force submission. The opponents were approximately of the same weight class and technical level. A black belt BJJ teacher supervised the training session.
Statistical Analyses
The Shapiro-Wilk test was used to verify the normality of data. For normal data, the paired t-test was used to compare the Pre to Post condition. For non-normal data, the Wilcoxon matched-pairs signed rank test was employed. Data are presented as mean ± standard deviation. The level of significance was set at p ≤ 0.05. Statistical comparisons were performed using the software GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA).
Results
All athletes completed the training session without any restrictions or interruptions. None of them reported muscle pain, injuries, or any discomfort associated with the exercise. Results are presented as mean ± standard deviation.
Horizontal Countermovement Jump (HCMJ) performance
Figure 1 shows horizontal countermovement jump (HCMJ) performance at Pre and Post BJJ interval training session. There were no significant (p = 0.67) changes from Pre (2.20 ± 0.11 m) to Post training (2.20 ± .013 m) values.
Figure 1.
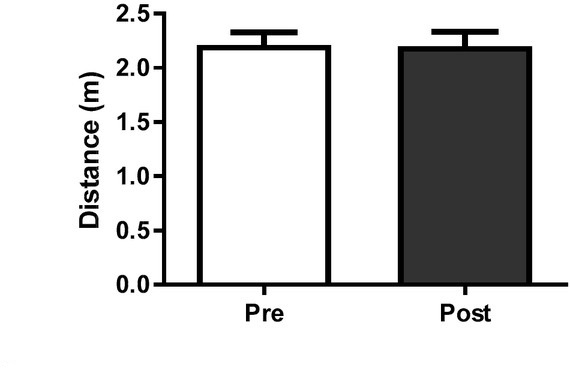
Horizontal countermovement jump (HCMJ) performance at Pre and Post the BJJ interval training session. No significant changes were observed. Data presented as mean ± standard deviation.
Blood and saliva analysis
The training session influenced all blood variables and some of the saliva variables. Table 2 presents the saliva biochemical analysis at Pre and Post BJJ HIIT condition. Compared to Pre, the mean salivary alpha-amylase activity increased 576% immediately Post (p ≤ 0.001). In addition, there was a higher range in SAA values Post (minimum value = 83 U/mL and maximum value = 2523 U/mL) compared to Pre (minimum value = 39 U/mL and maximum value = 176 U/mL) condition. Urea and salivary IgA increased more than 100% Post training (Table 2). Saliva volume, secretion rate and uric acid were not significantly different considering Pre and Post BJJ HIIT .
Figures 2, 3 and 4 show the absolute leucocyte (WBC), lymphocyte and neutrophil count at Pre and Post-BJJ HIIT, respectively. The total WBC, lymphocyte and neutrophil count showed significant (p ≤ 0.05) changes Post compared to Precondition.
Figure 2.
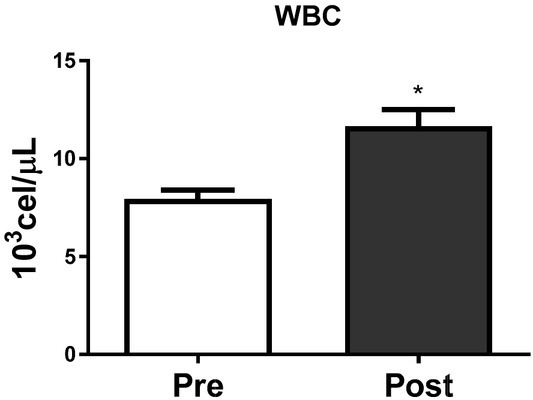
Leucocytes count at Pre and Post the BJJ interval training session. WBC = Leucocytes count (CVA = 1.5%); *CVA = analytical coefficient of variation. *Significant change (p ≤ 0.05) to Pre. Data presented as mean ± standard deviation.
Figure 3.
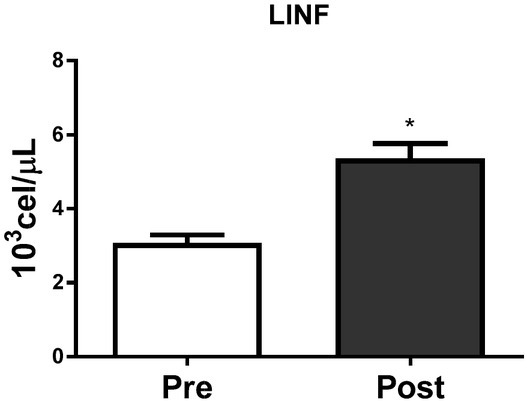
Lymphocytes count at Pre and Post the BJJ interval training session. LINF = Lymphocytes count (CVA = 3.0%); *CVA = analytical coefficient of variation. *Significant change (p ≤ 0.05) to Pre. Data presented as mean ± standard deviation.
Figure 4.
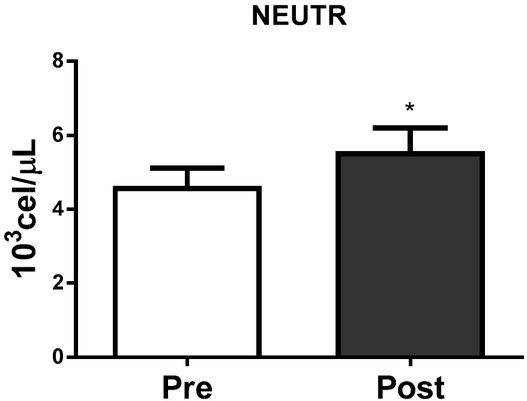
Neutrophils count at Pre and Post BJJ interval training session. NEUTR= Neutrophils count (CVA = 2.2%). *CVA = analytical coefficient of variation. *Significant change (p ≤ 0.05) to Pre. Data presented as mean ± standard deviation.
Table 1.
Saliva variables at Pre and Post BJJ HIIT session
| Analyses | CVA (%) | Pre | Post | Δ% | p |
|---|---|---|---|---|---|
| SAA (U/mL) | 0.4 | 110 ± 49 | 744 ± 785 | 576% | < 0.001 |
| Salivary IgA (μg/min) | 0.7 | 62.50 ± 29.60 | 134.20 ± 53.70 | 115% | < 0.001 |
| Salivary IgA (μg/mL) | 0.7 | 85.5 ± 38.7 | 172.6 ± 53.1 | 102% | < 0.001 |
| Uric Acid (mg/dL) | 2.8 | 0.21 ± 0.06 | 0.30 ± 0.21 | 43% | 0.130 |
| Urea (mg/dL) | 1.7 | 9.74 ± 3.60 | 21.0 ± 9.70 | 116% | < 0.001 |
| Secretion Rate (mL/min) | - | 0.80 ± 0.20 | 0.80 ± 0.30 | 0% | 0.716 |
| Saliva Volume (mL) | - | 1.50 ± 0.50 | 1.60 ± 0.70 | 7% | 0.716 |
Data presented as mean ± standard deviation; Values of p ≤ 0.05 were considered significant. CVA = analytical coefficient of variation. SAA = Salivary alpha-amylase activity. Δ% = Post/Pre
Discussion
The objective of the study was to observe the neuromuscular, blood and salivary immunological responses to a BJJ high-intensity interval training session. Our main findings were: 1) the horizontal countermovement jump (HCMJ) performance did not change significantly Post-training; 2) blood leukocytes, urea, IgA and salivary alpha-amylase (SAA) increased significantly at Post; SAA activity increased more than six times immediately Post compared to Pre-training. Saliva volume, secretion rate and uric acid were not significantly different between Pre and Post-training evaluations.
The literature reports that high-intensity exercises may induce acute decreases in neuromuscular performance (Allen et al., 2008; Byrne et al., 2004; Warren et al., 1999). Since horizontal countermovement jump performance did not result in any significant changes at Post-training, our initial hypothesis that the high-intensity nature of the sport would induce acute muscle function decrements was not confirmed. Meanwhile, the results corroborate with other previously reported research (Bonitch-Dominguez et al., 2010; da Silva et al., 2014).
Bonitch-Dominguez et al. (2010) investigated changes in peak leg power as a result of four judo bouts (5-min judo bouts each separated by 15 min of passive rest). The results showed no effect of successive bouts on peak leg power and no differences when comparing the power measured at Pre and Post each bout. The authors concluded that successive judo bouts had no effect on the peak power developed. Additionally, Barbas et al. (2011) investigated physiological and performance responses of well-trained adult wrestlers to a simulated one-day tournament following a typical weight loss regimen. Vertical jump performance presented a significant decrease from baseline values only before match 4, but increased prior to the final match of the tournament. Post-match vertical jump performance did not differ from their respective Pre-values. Recently, we have reported neuromuscular performance and blood lactate responses after simulated BJJ combats (da Silva et al., 2014). In the study, twenty-three BJJ athletes undertook three simulated BJJ fights (10 min duration each separated by 15 min of rest). Neuromuscular performance was assessed by the bench press throw and vertical countermovement jump tests, before the first (Pre) and after the last fight (Post). Blood lactate was measured at Pre, 1 min Post, and 15 min Post fights. The successive simulated BJJ fights demanded considerable anaerobic contribution of ATP supply, reinforcing the high-intensity intermittent nature of the sport. Nevertheless, no negative impact on acute neuromuscular performance was observed. One of the main differences between the present and our previous study (da Silva et al., 2014) was the training intervention. The present study was characterized by six rounds of two minutes, with one minute of rest in-between. The total training volume was of 17 minutes, and the exercise required was the guard-pass only. Additionally, the athletes were verbally encouraged to perform their best attacks and defenses, always trying to force submission on the opponents. This was a situation that may have contributed to the high-intensity efforts performed by the athletes. The training intervention of our previous study consisted of three fights of 10 min, with 15 min rest intervals between them. The total training volume was of 60 minutes and the exercise was performed without verbal encouragement to perform attacks and defenses. This represented a situation where athletes would feel more comfortable in following a “self-paced” fighting rhythm, involving lowering exercise intensity to support a higher exercise volume.
On the other hand, Kraemer et al. (2001) investigated performance responses to a simulated freestyle wrestling tournament. Throughout the study, a battery of tests was performed at Pre and immediately Post each individual match. Lower body power and upper body isometric strength were significantly reduced as the tournament progressed. The authors concluded that a wrestling tournament potentialized physiological and performance decrements. However, a careful interpretation of these results must be done since wrestlers investigated adopted typical weight loss techniques ( 6% of total body weight). Nowadays, muscle function measurements are considered the most recommended methods for quantifying damage because the event results in an immediate and prolonged reduction in these variables, persisting over the entire span of the progression of the degenerative and regenerative processes (Byrne et al., 2004; Warren et al., 1999). The literature is well consolidated to associate muscle damage and neuromuscular function impairment to eccentric muscle actions (Lieber and Friden, 1999; Lieber et al., 2002). Due to the grappling nature of BJJ, we can speculate that during matches the athlete’s performance was not impaired because the eccentric component may be attenuated compared to other sports where muscle function is acutely decreased.
6% of total body weight). Nowadays, muscle function measurements are considered the most recommended methods for quantifying damage because the event results in an immediate and prolonged reduction in these variables, persisting over the entire span of the progression of the degenerative and regenerative processes (Byrne et al., 2004; Warren et al., 1999). The literature is well consolidated to associate muscle damage and neuromuscular function impairment to eccentric muscle actions (Lieber and Friden, 1999; Lieber et al., 2002). Due to the grappling nature of BJJ, we can speculate that during matches the athlete’s performance was not impaired because the eccentric component may be attenuated compared to other sports where muscle function is acutely decreased.
Regarding blood and salivary immunological responses, previous studies have showed that an acute BJJ session promotes physiological and psychological disturbances that may affect immune system biomarkers (Moreira et al., 2012). After the BJJ interval training session, we observed a significant increase in SAA activity, IgA secretion rate and concentration. Salivary IgA is responsible for inhibiting bacterial attachment and causing bacterial aggregation, initiating phagocytosis protecting the oral mucosa against viral and bacterial infections (Mackinnon et al., 1993). Our results indicate that salivary IgA and SAA may enhance the immune mucosal protection, since the increase of antibodies and SAA in the oral mucosal is important in bacterial and viral infection prevention, mainly in URTI (Gleeson, 2007; Laing et al., 2005). Salivary glands are innervated by both parasympathetic and sympathetic nerves. The sympathetic nervous system increases progressively activity with the intensity of exercise and is primarily responsible for the increase in SAA after training (de Oliveira et al., 2010). SAA has been successfully correlated with blood lactate during exercise (Bocanegra et al., 2012), intensity of training and load (Diaz et al., 2013). As such SAA could be an effective noninvasive biomarker to monitor BJJ athletes during training. The frequency, intensity and duration of exercise as well as the Pre exercise fitness level are all important determinants of the response to exercise (Nieman, 1997). Among well trained and elite athletes, high-intensity training periods and strenuous physical exercise are associated with increased susceptibility to URTIs (Ortega, 2003a; Tidball, 2005). Neutrophils and lymphocytes increase in the bloodstream after an acute exercise bout (Kakanis et al., 2010). Neutrophils play a fundamental role in the adaptive inflammatory response to training (Tidball, 2005). Neutrophils can directly damage the muscle through reactive oxygen species production. Our results showed elevated numbers of blood neutrophils immediately after the BJJ HIIT session (Figure 2). Catecholamines, cortisol, and growth hormone are mainly responsible for increasing the neutrophil number and activity in the bloodstream (Ortega, 2003b). Neutrophilia depends on the duration and intensity of exercise, and it decreases progressively returning to baseline levels 24 hours after exercise (Kakanis et al., 2010).
Conclusions
In conclusion, the BJJ HIIT session elicited increases in the blood cells responsible for antibody production and muscle damage adaptation after exercise. In conjunction with salivary immunological variables after exercise, our findings suggest that this kind of effort may modify the mucosal immunity, increasing the immune protection against viral and bacterial infections. On the other hand, neuromuscular performance did not present any significant changes Post-training, suggesting that immunological and performance responses are not necessarily associated. To the best of our knowledge, this is the first study to monitor an acute session of BJJ high-intensity interval training and the concurrent changes in neuromuscular, blood, and salivary immunological responses. The present findings may help athletes and coaches in programing HIIT sessions for BJJ. Since neuromuscular performance was unaffected Post-training, we can speculate that rest intervals between the present HIIT sessions proposed could be shorter. After the BJJ high-intensity interval training session, the main changes were observed in the SAA activity. In conjunction with salivary IgA, acute training effects can temporarily increase the mucosal immune protection preventing URTI. Saliva alpha amylase, IgA and urea assessments are less invasive methods to monitor athlete's immune function during training and competition.
Acknowledgements
We would like to thank all the Brazilian Jiu-jitsu athletes for the engagement in the research and the Alliance Campinas for providing the dojo for data collection.
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Barbas I, Fatouros IG, Douroudos II, Chatzinikolaou A, Michailidis Y, Draganidis D, Jamurtas AZ, Nikolaidis MG, Parotsidis C, Theodorou AA. Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur J Appl Physiol. 2011;111:1421–1436. doi: 10.1007/s00421-010-1761-7. [DOI] [PubMed] [Google Scholar]
- Billat LV. Interval Training for Performance: A Scientific and Empirical Practice: Special Recommendations for Middle-and Long-Distance Running. Part I: Aerobic Interval Training. Sports Medicine. 2001;31:13–31. doi: 10.2165/00007256-200131010-00002. [DOI] [PubMed] [Google Scholar]
- Bishop PA, Jones E, Woods AK. Recovery from training: a brief review: brief review. J Strength Cond Res. 2008;22:1015–1024. doi: 10.1519/JSC.0b013e31816eb518. [DOI] [PubMed] [Google Scholar]
- Bonitch-Dominguez J, Bonitch-Gongora J, Padial P, Feriche B. Changes in peak leg power induced by successive judo bouts and their relationship to lactate production. J Sports Sci. 2010;28:1527–1534. doi: 10.1080/02640414.2010.512641. [DOI] [PubMed] [Google Scholar]
- Bounty PL, Campbell BI, Galvan E, Cooke M, Antonio J. Strength and Conditioning Considerations for Mixed Martial Arts. Strength and Cond J. 2011;33:56–67. [Google Scholar]
- Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34:49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- Chapman D, Newton M, Sacco P, Nosaka K. Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med. 2006;27:591–598. doi: 10.1055/s-2005-865920. [DOI] [PubMed] [Google Scholar]
- Chapman DW, Newton M, McGuigan M, Nosaka K. Effect of lengthening contraction velocity on muscle damage of the elbow flexors. Med Sci Sports Exerc. 2008;40:926–933. doi: 10.1249/MSS.0b013e318168c82d. [DOI] [PubMed] [Google Scholar]
- Claudino JG, Cronin J, Mezencio B, McMaster DT, McGuigan M, Tricoli V, Amadio AC, Serrao JC. The countermovement jump to monitor neuromuscular status: A meta-analysis. J Sci Med Sport. 2017;20:397–402. doi: 10.1016/j.jsams.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Coutts A, Reaburn P, Piva TJ, Murphy A. Changes in selected biochemical, muscular strength, power, and endurance measures during deliberate overreaching and tapering in rugby league players. Int J Sports Med. 2007a;28:116–124. doi: 10.1055/s-2006-924145. [DOI] [PubMed] [Google Scholar]
- Coutts AJ, Wallace LK, Slattery KM. Monitoring changes in performance, physiology, biochemistry, and psychology during overreaching and recovery in triathletes. Int J Sports Med. 2007b;28:125–134. doi: 10.1055/s-2006-924146. [DOI] [PubMed] [Google Scholar]
- da Silva BV, Ide BN, de Moura Simim MA, Marocolo M, da Mota GR. Neuromuscular responses to simulated brazilian jiu-jitsu fights. J Hum Kinet. 2014;44:249–257. doi: 10.2478/hukin-2014-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira VN, Bessa A, Lamounier RP, de Santana MG, de Mello MT, Espindola FS. Changes in the salivary biomarkers induced by an effort test. Int J Sports Med. 2010;31:377–381. doi: 10.1055/s-0030-1248332. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- Ide BN, Leme TC, Lopes CR, Moreira A, Dechechi CJ, Sarraipa MF, Da Mota GR, Brenzikofer R, Macedo DV. Time course of strength and power recovery after resistance training with different movement velocities. J Strength Cond Res. 2011;25:2025–2033. doi: 10.1519/JSC.0b013e3181e7393f. [DOI] [PubMed] [Google Scholar]
- Ispirlidis I, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Michailidis I, Douroudos I, Margonis K, Chatzinikolaou A, Kalistratos E, Katrabasas I, Alexiou V, Taxildaris K. Time-course of changes in inflammatory and performance responses following a soccer game. Clin J Sport Med. 2008;18:423–431. doi: 10.1097/JSM.0b013e3181818e0b. [DOI] [PubMed] [Google Scholar]
- Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, Marshall-Gradisnik SM. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. 2010;16:119–137. [PubMed] [Google Scholar]
- Kraemer WJ, Fry AC, Rubin MR, Triplett-McBride T, Gordon SE, Koziris LP, Lynch JM, Volek JS, Meuffels DE, Newton RU, Fleck SJ. Physiological and performance responses to tournament wrestling. Med Sci Sports Exerc. 2001;33:1367–1378. doi: 10.1097/00005768-200108000-00019. [DOI] [PubMed] [Google Scholar]
- Laing SJ, Gwynne D, Blackwell J, Williams M, Walters R, Walsh NP. Salivary IgA response to prolonged exercise in a hot environment in trained cyclists. Eur J Appl Physiol. 2005;93:665–671. doi: 10.1007/s00421-004-1270-7. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Mechanisms of muscle injury after eccentric contraction. J Sci Med Sport. 1999;2:253–265. doi: 10.1016/s1440-2440(99)80177-7. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Shah S, Friden J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin Orthop Relat Res. 2002;403:90–99. doi: 10.1097/00003086-200210001-00011. [DOI] [PubMed] [Google Scholar]
- Mackinnon LT, Ginn E, Seymour GJ. Decreased salivary immunoglobulin A secretion rate after intense interval exercise in elite kayakers. Eur J Appl Physiol Occup Physiol. 1993;67:180–184. doi: 10.1007/BF00376664. [DOI] [PubMed] [Google Scholar]
- Maulder P, Cronin J. Horizontal and vertical jump assessment: reliability, symmetry, discriminative and predictive ability. Physical therapy in Sport. 2005;6:74–82. [Google Scholar]
- McCarthy DA, Macdonald I, Grant M, Marbut M, Watling M, Nicholson S, Deeks JJ, Wade AJ, Perry JD. Studies on the immediate and delayed leucocytosis elicited by brief (30-min) strenuous exercise. Eur J Appl Physiol Occup Physiol. 1992;64:513–517. doi: 10.1007/BF00843760. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen A. European College of Sport S, American College of Sports M. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013;45:186–205. doi: 10.1249/MSS.0b013e318279a10a. [DOI] [PubMed] [Google Scholar]
- Moreira A, Franchini E, de Freitas CG, Schultz de Arruda AF, de Moura NR, Costa EC, Aoki MS. Salivary cortisol and immunoglobulin A responses to simulated and official Jiu-Jitsu matches. J Strength Cond Res. 2012;26:2185–2191. doi: 10.1519/JSC.0b013e31823b8702. [DOI] [PubMed] [Google Scholar]
- Nieman DC. Immune response to heavy exertion. J Appl Physiol (1985) 1997;82:1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- Nunes LA, Brenzikofer R, Macedo DV. Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin Biochem. 2011;44:1440–1444. doi: 10.1016/j.clinbiochem.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Ortega E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev. 2003a;9:70–93. [PubMed] [Google Scholar]
- Ortega E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev. 2003b;9:70–93. [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Blannin AK, Clark AM, Cook L, Robson PJ, Gleeson M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J Sports Sci. 1999;17:129–134. doi: 10.1080/026404199366226. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev. 2001;29:82–87. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]


