Abstract
Purpose of Review:
Inflammatory bowel disease (IBD) is a multifactorial disease caused by dysregulated immune responses to commensal or pathogenic intestinal microbes, resulting in chronic intestinal inflammation. However, a subset of patients with IBD diagnosed <6 years of age, known as very early-onset (VEO)-IBD, can be phenotypically and genetically distinct from older onset IBD. We aim to review the clinical presentation of children with VEO-IBD and recent discoveries that point to the underlying genomic and immunologic drivers of disease, and the significant impact on our therapeutic decisions.
Recent Findings:
VEO-IBD is increasing in incidence and is associated with more severe disease, aggressive progression, and poor response to most conventional therapies. This article will review some of the genetic findings in this population and the subsequent impact on therapy, with targeted approaches.
Summary:
Children with VEO-IBD may present with a different phenotype and more severe disease than older children and adults. An integrated approach combining genetics, immunology, and traditional IBD evaluations can lead to the identification of causal defects that directly impact management. These strategies can also be employed in older onset refractory IBD.
Keywords: very early-onset inflammatory bowel disease, whole-exome sequencing, immunodeficiency
Introduction
The genomic contribution to inflammatory bowel disease (IBD) is complex and polygenic, involving over 200 risk loci identified mainly through genome wide association studies.1,2 These studies have provided the genetic landscape of IBD and shed light on the delicate interaction between host defense and the gut microbiome in disease development. However, genetics can play a more dominant role in the very young children with IBD, diagnosed <6, known as very early-onset IBD (VEO-IBD), particularly in those who present in the first year of life, known as infantile onset IBD. Children with VEO-IBD can have a more severe and refractory disease course than older children and adults with IBD. Over the last decade, using advanced sequencing technology, monogenic defects have been identified in some of these children, often involving primary immunodeficiency genes.3–5
Epidemiology
Pediatric IBD has increased in incidence and prevalence, and this phenomenon has included very young children.6–8 Approximately 6% to 15% of the pediatric IBD population is less than 6-years-old,7,8 and recent studies have demonstrated a rapid rise in this cohort from 1.3 to 2.1 per 100 000 children from 1994 to 2009, with a mean annual increase of 7.2%.9 This rise may be, at least in part, secondary to changes in the environment and the gut microbiota, which develops between birth and 3 years of life, coincident with the age of onset of many VEO-IBD cases. Exposures such as route of delivery, gestational age, maternal diet, and infant feeding practices shape the colonization of an infant’s microbiota until age 3 and can impact disease risk at all ages.10 Simultaneously, the immune system develops during the first 3 years of life. The intestine is the largest immune organ, and it is at this interface that the microbiota and immune system shape and regulate one another in function and development. This interaction is well balanced in healthy children; however, disruptions in development may affect the formation of the microbial community structure and function (dysbiosis) and/or immune response, leading to devastating sequelae.10
Disease Classification
Because a subset of patients with VEO-IBD present with extensive colonic disease (pancolitis), it can be difficult to differentiate ulcerative colitis (UC) from Crohn’s disease (CD).8,11 In addition, the extent and location of disease can change and progress over time, and patients who present with exclusive colonic disease can later develop perianal and small bowel disease.12 Therefore, indeterminate colitis is diagnosed more often in patients with VEO-IBD (11%–22%) as compared to older onset IBD (4%–10%).9,13–15 Because of this colonic involvement, patients with VEO-IBD are also more commonly diagnosed with UC (35%–59%) as compared to older onset IBD (older children > 6 and adults) in which CD is more prevalent (55%–60%). In contrast, only 30% to 35% of VEO-IBD patients are diagnosed with CD.
Unique Genomics of VEO-IBD
Advances in sequencing technology, such as whole-exome sequencing (WES) and targeted sequencing panels, have radically changed our approach to VEO-IBD and provided an opportunity to investigate its unique genomic etiology.16–21 Since 2009, single gene defects have been identified in this cohort, and some of the discoveries have led to targeted therapy. Examples include the identification of IL-1022 and IL-10R23,24 gene mutations in infantile IBD, which result in the phenotype of severe perianal disease, colitis, and folliculitis.5,24 Although refractory to conventional therapies, these patients are successfully treated with hematopoietic stem cell transplantation (HSCT). Additional underlying immunodeficiencies or genetic disorders have been identified in children with VEO-IBD.4,11,18,25,26 These include genes integral to intestinal epithelial barrier function, phagocyte bacterial killing, hyper or autoimmune inflammatory pathways, and development and function of the adaptive immune system.11,18,25,27 These defects can impact the developing gut microbiome and thus progression of intestinal inflammation. As we learn more about this disease we will have the unique opportunity to develop therapeutic strategies that are directed toward the underlying impaired pathway in this cohort.
Genetic Variants in the IL-10-IL-10R Pathway and Related Cytokine Family Members
Homozygous loss-of-function mutations in IL10 ligand and receptors IL10RA and IL10RB were the first genes to be identified as causative for VEO-IBD.23 They are associated with severe intestinal inflammation, particularly in neonatal or infantile IBD, with a phenotype of severe enterocolitis and perianal disease.22,23 In addition, compound heterozygote loss-of-function mutations of IL10RA have been reported with neonatal IBD and enterocolitis.28 IL-10 is an anti-inflammatory cytokine secreted by a variety of cells, including dendritic cells, natural killer cells, eosinophils, mast cells, macrophages, B cells, and CD4+ T-cell subsets (including Th2 cells, Th1 cells, Th17 cells, and Treg).29,30 IL-10 maintains homeostasis through suppression of an excessive proinflammatory response and exerts its effect through binding to the IL-10 receptor, IL-10R, which is a tetrameric complex.31,32 IL-10 defects are associated with intestinal inflammation, arthritis, folliculitis, and increase risk of lymphoma,28,33 particularly large B-cell lymphoma. HSCT has proven to be a successful treatment for these patients and potentially life-saving.34,35
Genetic Variants Influencing Intestinal Epithelial Barrier Function
It is at the epithelial surface where the interaction between the immune cells and the intestinal environment must be perfectly tuned to prevent inappropriate responses. The intestinal barrier is necessary to maintain a physical separation between commensal bacteria and the host immune system, and any break in this defense can lead to chronic intestinal inflammation.36,37 Increased translocation of bacteria or translocation of inappropriate bacteria, as in the case in dysbiosis, drives an inflammatory loop.
Defects in the intestinal epithelial barrier function can be involved in VEO-IBD. These processes include loss-of-function mutations in ADAM17 resulting in ADAM17 deficiency,38,39 IKBKG (encoding NEMO) resulting in X-linked ectodermal dysplasia and immunodeficiency,40 COL7A1 resulting in dystrophic epidermolysis bullosa,41 FERMT1 resulting in Kindler syndrome,42–44 and TTC7A,19 or gain-of-function mutations in GUCY2 resulting in familial diarrhea.26,45 Many of these results in immune deficiency as well as additional extraintestinal manifestations including changes in skin, hair, and nails.
Homozygous mutations in tetratricopeptide repeat domain 7A (TTC7A) gene have been shown to lead to several phenotypes including different forms of severe combined immunodeficiency, multiple intestinal atresia, and infantile onset IBD (Figure 1).19,46,47 Other phenotypes linked to this gene also include immune deficiency-related enteropathy-lymphocytopenia-alopecia syndrome.48 There have been cases of severe intestinal disease in older patients with compound heterozygous mutations in TTC7A.49 Although the mechanism of disease seen in TTC7A defects has not been fully elucidated, the protein appears to repress RhoA signaling. Therefore, mutations lead to increased rho kinase activity, disrupting epithelial intestinal cell polarity and growth with subsequent multiple intestinal atresia and impairment of immune cell homeostasis resulting in combined immunodeficiency.19,46 Future therapeutic developments inhibiting rho kinase may be an effective treatment option for this condition.
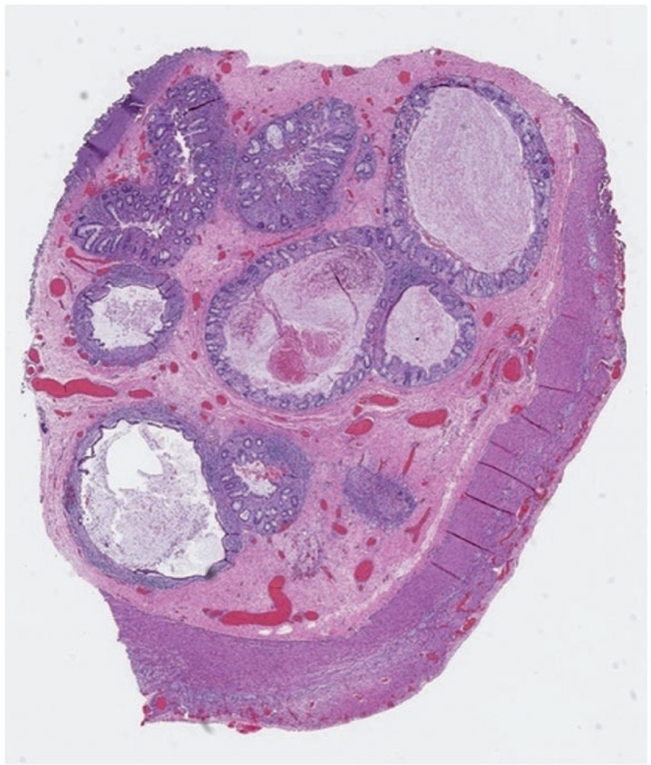
Figure 1.
Ascending colon resected from a male infant with biallelic mutation in TTC7A, multiple intestinal atresias, and severe combined immunodeficiency. Luminal obstruction was attributable to multiple minute lumina lined by mucosa and muscularis mucosae with expanded submucosa.
Genetic Variants Influencing Bacterial Recognition and Clearance
Chronic granulomatous disease (CGD) is a result of defective phagocytes, specifically the granulocytes responsible for bacterial killing and clearance.50 The NADPH oxidase complex within neutrophils is responsible for killing of ingested microbes through its production of the respiratory burst. Mutations in any part of the complex molecules, including X-linked and autosomal recessive inheritance (CYBB, CYBA, NCF1, NCF2, NCF4) can result in intestinal inflammation, autoimmune disease, and immunodeficiency.51,52 In addition to susceptibility to systemic bacterial and fungal infections, intestinal inflammation can be observed in as high as 40% of patients with CGD.53–56 Several variants have been associated with VEO-IBD, in particular defective NCF2 results in altered binding to RAC2.57 These patients can present in the neonatal or first year of life. With colitis, severe fistulizing perianal disease, and stricturing.57 Histology frequently demonstrates multiple granulomas that may not have associated inflammatory change.16,58 Other neutrophil defects that are associated with VEO-IBD include leukocyte adhesion defect, due to mutation in ITGB2.59,60 These patients can present with an IBD phenotype, history of bacterial infection, and increased peripheral blood granulocytes.61 Glycogen storage disease Type 1b, with the unique combination of neutropenia and neutrophil granulocyte dysfunction, can also present with intestinal inflammation.62
Therapies used to treat patients with these defects need to be carefully considered. For example, anti-TNFα therapy, an effective therapy for IBD, is contraindicated in CGD. These agents can increase the risk of severe infections in patients with CGD and can be fatal.63 Other therapies include sargramostim, antibiotics, and allogenic HSCT, which have demonstrated some success.64 IL-1R antagonists have been used in these patients with some positive results, by restoring autophagy and directly limiting inflammation.65
Genetic Variants Impairing Development of the Adaptive Immune System
Several genetic variants can alter the development or function of adaptive immune cells in a cell-intrinsic or -extrinsic manner. Multiple gene defects that impact the development or function of the adaptive immune system have been associated with severe combined immune deficiency (SCID).27,66,67 Defects that affect development or function of B cells and T cells by blocking either early lymphocyte survival or recombination of the B-cell receptor or T-cell receptor68–70 can occur with loss-of-function mutations in recombination activating genes (RAG1 or RAG2) or the IL-7 receptor (IL7R) causing Omenn syndrome and the PTEN gene causing PTEN syndrome.71 Omenn syndrome, a recessive form of SCID, can also be associated with intestinal disease as well as severe eczematous rash.67,72 Laboratory studies can show increased oligoclonal T cells and reduced B cells, and histology can show an intestinal graft versus host appearance, including epithelial cell apoptosis, eosinophilic and neutrophilic infiltrates, and villous atrophy in the small bowel.73,74 Defects in B-cell development lead to an absence of circulating mature B cells and antibody production, which have been linked to an IBD phenotype.66 This includes agammaglobulinemia, including X-linked agammaglobulinemia,75 common variable immune deficiency and IgA deficiency. These are complex and heterogeneous diseases, with the responsible mutations known for only a minority of cases.76 Even a mild immune deficiency such as IgA deficiency has a significantly higher rate of IBD than the general population.77 This may reflect changes to the microbiome due to the lack of selective pressure,78 increased microbial translocation, compromised signaling within the gastrointestinal tract, or stimulation of an aberrant response due to active infection.
Loss-of-function mutation in LRBA, resulting in multiple defects in immune cell populations, can result in a VEO-IBD phenotype with enteropathy in addition to autoimmune cytopenia, lymphoproliferation, immune deficiency, as well as endocrine manifestations.79 LRBA is responsible for regulating CTLA4, which is recycled by immune cells in order to control immune responses. Abatacept, a CTLA4 immunoglobulin fusion drug, has been effective in treating LRBA deficiency, but HSCT can be an option for severe cases.
In addition to hypogammaglobulinemia, defects leading to overproduction of immunoglobulins including hyper-IgM, hyper-IgD, and hyper-IgE syndromes have been associated increased infections, intestinal inflammation, dermatologic manifestations, and perianal disease.80
Wiskott-Aldrich syndrome (WAS) results from a loss-of-function mutation in Wiskott-Aldrich syndrome protein (WASP), affecting actin filament formation. These patients can exhibit thrombocytopenia, eczema, eosinophilia, and immune deficiencies along with intestinal inflammation.81 The clinical manifestation of patients with VEO-IBD with this genetic defect can be pancolitis in addition to other autoimmune processes. Genetic defects in ARPC1B can also result in a similar phenotype to WAS with intestinal inflammation.
Genetic Variants Impairing Regulatory T Cells
Defects in regulatory T cells (Tregs) can clinically present with colonic inflammation and well as an enteropathy. The prominence of villous atrophy is a clue to these disorders. Immunodysregulation, polyendocrinopathy, enteropathy X-linked syndrome (IPEX) is most often secondary to mutations of Forkhead box protein 3 (FOXP3) gene, a transcription factor that is essential for the development and immunosuppressive activity of CD4 Foxp3+ Tregs.72,82–84 There are over 20 mutations in FOXP3 that have been identified in patients with IPEX,83 and patients frequently present with neonatal severe secretory diarrhea, failure to thrive, infection (due to defects in immunoregulation), skin rash, insulin dependent diabetes, thyroiditis, cytopenias, and other autoimmune disorders.72 Tregs are absent or dysfunctional in these patients, and in the intestine, histologic analyses may reveal infiltration of inflammatory cells in the lamina propria and submucosa of the small bowel and colon as well as changes in the mucosa of the small bowel (ie, villous blunting).85 Other genetic defects have been found to cause IPEX-like disease, including loss-of-function mutations impacting IL-2-IL-2R interactions, STAT5b, and ITCH, or gain-of-function mutations in STAT1, all of which critically influence the development and function of Tregs.72 Furthermore, Zeissig et al. identified a novel loss-of-function mutation in CTLA4, a surface molecule of regulatory T cells that directly suppresses effector T-cell populations, in VEO-IBD.86
Autoimmune and Autoinflammatory Disorders
Immunologic considerations.
Autoimmune disease in general is strongly associated with variants related to immune deficiency, and VEO-IBD is similarly enriched with gene defects related to primary immune deficiencies. The study of primary immune deficiencies and their association with VEO-IBD has illuminated the critical and delicate interaction of the immune system with the luminal contents of the gastrointestinal tract.
Several autoimmune/autoinflammatory diseases have been linked with intestinal inflammation in children with VEO-IBD. These include mevalonate-kinase deficiency,87 mutations in NLRC4,88 familial Mediterranean fever,89,90 Hermansky-Pudlak syndrome,91 and X-linked lymphoproliferative syndrome (type 1 and type 2).18,92–94 Although there are many additional clinical manifestations in these patients, 20% of patients with X-linked lymphoproliferative syndrome who have a loss-of-function defect in the gene X-linked inhibitor of apoptosis protein (XIAP) present with VEO-IBD.95 XIAP is involved in NOD2- mediated NF-?B signaling, and therefore these children may have an impaired ability to sense bacteria. In addition, as an inhibitor of apoptosis, it prevents apoptosis of activated T cells, thus allowing for expansion and survival of T cells in response to pathogens.96,97 Therefore, in XIAP deficiency, due to the inability to clear pathogens, there is a hyperinflammatory state, with increased production of cytokines resulting in an IBD phenotype.95,97 Children with these mutations can present with severe colonic and perianal fistualizing disease18,98 (Figure 2(A) to (C)), and of great concern, EBV infection can result in fatal hemophagocytic lymphohistiocytosis (HLH).98 HSCT is curative for the immune deficiency, prevents the risk of development of HLH, and in most cases, appears to be curative for the intestinal disease.
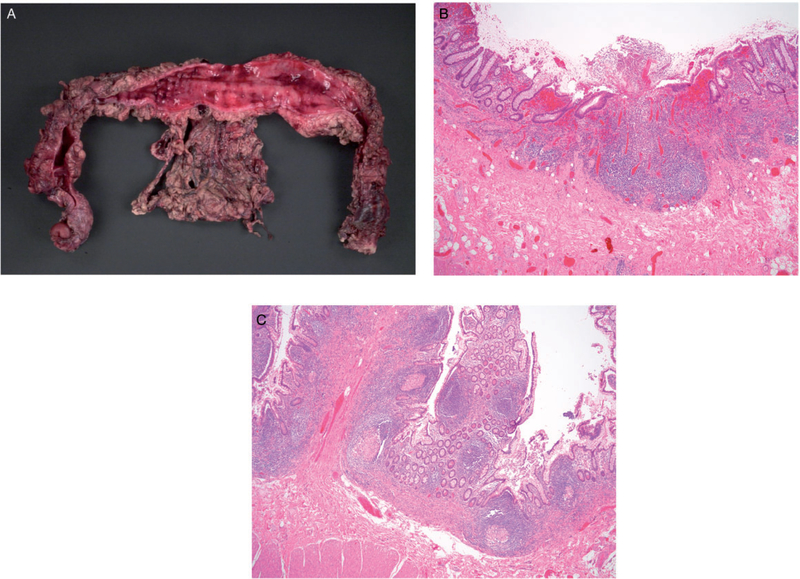
Figure 2.
Terminal ileum and colon from a male patient with infantile onset IBD, identified as XIAP deficiency at age 16 years. A, Colon resection from the patient at age 10 years: Extensive mucosal hemorrhage with nodule formation and structuring. B, Sections from the colon reveal a diffuse active chronic colitis with ulcerations. C, Sections from separately submitted segment of terminal ileum reveals multiple well-formed granulomas in the mucosa.
TRIM22 has recently been identified as a causal single gene defect in patients with a phenotype of severe perianal disease and granulomatous colitis.99 Tripartite motif (TRIM) proteins are important components of both the innate and adaptive immune system, including cell proliferation, apoptosis, and autoimmunity. Defects in these proteins are involved in malignancies, autoimmune disease, and familial Mediterranean fever and Opitz syndrome type 1. TRIM22 mutations result in impaired NOD2 binding and signaling. Monogenic defects can result in VEO-IBD and can play a role on older onset disease as well.
Histopathological findings associated with VEO-IBD and immunodeficiencies.
The diagnostic histopathology of children with VEO-IBD may provide some further understanding of the underlying etiology of the disease and may help guide the subsequent evaluation. In a recent clinical and histological review of subjects with VEO-IBD and older onset pediatric IBD, we identified findings characteristic of primary immunodeficiency in the VEO-IBD cohort on diagnostic endoscopic biopsies. Some of these features include increased frequency of apoptosis, moderate to severe chronic architectural changes, small intestinal villous blunting often in the absence of inflammation, and eosinophils in the crypts, lamina propria, and surface epithelium on the biopsies.100 These findings have been previously associated with immunodeficiencies and disorders of immune regulation, such as severe combined immunodeficiency or common variable immunodeficiency. Therefore, their presence in the histology of VEO-IBD patients may be indicators of different disease drivers than older onset IBD and should signal further investigation in these patients.16
A larger cohort study characterized the phenotypic and genotypic features of patients with infantile onset IBD (IO-IBD) diagnosed before 2 years of age. Of these subjects, 77% exhibited extensive disease with significant inflammation of both the upper and lower gastrointestinal tract. Similar to the aforementioned study, the epithelial abnormalities observed in these patients included abundant epithelial cell apoptosis as well as epithelial shedding and tufting. Furthermore, villous blunting was identified in at least 40% of the unclassifiable IO-IBD cases, similar to what we detected in our review.101
Conclusion
Although this is not an exhaustive description of the rare genomic drivers of VEO-IBD, this review highlights the different components of the immune system, including innate and adaptive response, involved in VEO-IBD. Treatments guided toward the specific defect, such as IL-1 antagonists, colchicine, sargramostim, or HSCT may lead to remission or even cure in some cases. In addition, identifying a defect will allow for essential monitoring for the associated potential complications, such as risk of HLH in XIAP, risk of lymphoma in IL-10 gene variants, and CGD. In addition to these monogenic diseases, VEO-IBD has been shown to have a high degree of genetic heterogeneity. It is therefore likely that there are more pathways involved in VEO-IBD, and the outcome of therapeutic intervention can be improved through further study and identification of the associated variants. Utilizing next-generation sequencing such as WES can improve detection of variants and diagnosis of disease. The combination of these genomic findings with translational studies looking at the functions of these genes will allow for better mechanistic insight into the role of immune dysregulation in intestinal inflammation and provide an opportunity to deliver true precision medicine to children with VEO-IBD.
Table 1.
Genetic Findings Associated With Very Early-Onset Inflammatory Bowel Disease.
| Gene | Disease | Pathogenic Inheritance | Phenotypic Features |
|---|---|---|---|
| Defect in epithelial barrier function | |||
| ADAM17 | Autosomal recessive | Psoriasiform erythroderma, pustules, broken hair, abnormal nails, small bowel, and colonic involvement | |
| IKBKG | NEMO | X-linked | Hypodontia, thin hair, frontal bossing, recurrent infections, and enteropathy |
| COL7A1 | Epidermolysis bullosa | Autosomal recessive | Recurrent blistering or erosions, esophageal stricture, anal fissures and stenosis, enteropathy, and hair and nail abnormalities |
| FERMT1 | Klinder syndrome | Autosomal recessive | Recurrent skin blisters, esophageal strictures, and colonic involvement |
| TTC7A | Hereditary multiple intestinal atresia | Autosomal recessive | Intestinal atresia, dermatitis, alopecia, and immunodeficiency |
| GUCY2 | Autosomal dominant Gain of function | Diarrhea, esophagitis, electrolyte abnormalities, ileal obstruction, dilated small bowel, and secretory diarrhea | |
| Defect in NADPH oxidase complex | |||
| CYBA | Chronic granulomatous disease | Autosomal recessive | Life threatening bacterial and fungal infections, excessive inflammation characterized by granulomas in any organ including small and large intestine, gastric outlet obstruction, and perianal disease |
| CYBB | X-linked | ||
| NCF1 | Autosomal recessive | ||
| NCF2 | Autosomal recessive | ||
| NCF4 | Autosomal recessive | ||
| NOX1 | Autosomal recessive Loss of function | Bacterial infections and colonic involvement | |
| Defect in adaptive immunity | |||
| IL-10 | IL-10 deficiency | Autosomal recessive | Neonatal onset, folliculitis, panenteric, perianal disease, and lymphoma |
| IL-10RA | |||
| IL-10RB | |||
| RAG1 | Severe combined immunodeficiency (typically T-B-) | Autosomal recessive | Recurrent severe infections, chronic diarrhea, failure to thrive, and variable intestinal involvement |
| RAG2 | Autosomal recessive | ||
| ZAP70 | Omenn syndrome | Autosomal recessive | Skin inflammation and variable intestinal involvement |
| IL7R | Autosomal recessive | ||
| PTEN | PTEN hamartoma tumor syndrome | Autosomal dominant | Thyroiditis, autoimmune hemolytic anemia, hamartomas, adenopathy, adenoid lymphoid hyperplasia, thymic hyperplasia, developmental delay, and enteropathy |
| LRBA | LRBA deficiency | Autosomal recessive | Enteropathy, lymphoproliferation, and immune deficiency |
| WASP | Wiskott Aldrich syndrome | X-linked, autosomal recessive | Thrombocytopenia, eczema, eosinophilia, immune deficiency, and colonic involvement |
| ARPC1B | Loss of function | ||
| BTK | X-linked agammaglobulinemia | X-linked | Recurrent infections after first few months of life and variable intestinal involvement |
| DKC1 | Dyskeratosis congenita | X-linked | Microcephaly, IUGR, nail dystrophy, abnormal skin pigmentation, leukoplakia of oral mucosa, enteropathy, and stricture formation |
| ITGB2 | Leukocyte adhesion deficiency-1 | Autosomal recessive | Delayed separation of umbilical cord, recurrent bacterial infections, leukocytosis, lack of pus and impaired wound healing, and enteropathy |
| ICOS | ICOS deficiency | Autosomal recessive | Common variable immunodeficiency, splenomegaly, autoimmune disease, recurrent bacterial infections, and enteropathy |
| DOCK8 | Hyper-IgE syndrome | Autosomal recessive | Skin abscesses, eczema, allergic diseases, sinopulmonary infections, broad nasal base and bridge, frontal bossing, deep set eyes, retained primary teeth, fractures, scoliosis, and enteropathy |
| PIK3CD | Activated PI3K-delta syndrome | Autosomal recessive | Simopulmonary infections, chronic active viral infections, lymphadenopathy and nodular lymphoid hyperplasia, risk of B-cell lymphoma, and enteropathy |
| Impaired regulatory T cells | |||
| FOXP3 | IPEX | X-linked | Neonatal-onset secretory diarrhea, failure, infection, skin rash, diabetes, thyroiditis, cytopenia, other autoimmune conditions, and enteropathy |
| IL-2RA | IPEX-like | X-linked | Enteropathy, eczema, and autoinflammatory disease |
| STAT1 | IPEX-like | Autosomal dominant gain of function | Enteropathy and arthritis |
| STAT3 | STAT3 GOF IPEX-like | Autosomal recessive Gain of function | Lymphadenopathy, autoimmune cytopenia, multiorgan autoimmunity, infection, eczema, short stature, and enteropathy |
| STAT5b | STAT5b deficiency | Autosomal recessive | Growth failure, IGF-I deficiency, chronic pulmonary disease, and enteropathy |
| ITCH | ITCH deficiency | Autosomal recessive | Autoimmune inflammatory cell infiltration of lungs, liver, gut, growth failure, diarrhea, hepatosplenomegaly, and enteropathy |
| IL-21 | Autosomal recessive | ||
| IL-21R | |||
| CTLA4 | Complex immune dysregulation syndrome | Autosomal dominant | Enteropathy and autoimmune disease |
| Autoinflammatory and hyperinflammatory defects | |||
| MVK | Hyperimmunoglobulin D syndrome | Autosomal recessive | Episodic nausea, fever, abdominal pain, oral ulcers, arthritis, splenomegaly, and enteropathy |
| NLRC4 | Autosomal dominant GOF | Inflammasome activation hemophagocytic lymphohistiocytosis, episodic inflammation, and panenteric disease | |
| MEFV | Familial Mediterranean fever | Autsomal dominant or Autosomal recessive | Serositis, periodic fevers, rash, arthritis, and enteropathy |
| HPS1 | Hermansky Pudlak syndrome | Autosomal recessive | Oculocutaneous albinism, pulmonary fibrosis, bleeding disorder, and colitis |
| HPS4 | |||
| XIAP | X-linked lymphoproliferative syndrome | X-linked | Infectious mononucleosis or hemophagocytic lymphohistiocytosis secondary to EBV, splenomegaly, abnormal immunoglobulins, lymphoma, and enteropathy |
| TRIM22 | Autosomal recessive | Granulomatous colitis and severe perianal disease | |
| SKIV2L | Autosomal recessive | IUGR, failure to thrive, trichorrhexis nodosa, frontal bossing, and villous atrophy | |
| STXBP2 | Autosomal recessive | Hemophagocytic lymphohistiocytosis and enteropathy | |
| CASP8 | Caspase-8 deficiency | Autosomal recessive | Lymphadenopathy, splenomegaly, recurrent bacterial and viral infections, especially sinopulmonary infections, hypogam-maglobuilnemia, and enteropathy |
Abbreviations: EBV, Epstein-Barr virus; GOF, gain-of-function; IPEX, immunodysregulation, polyendocrinopathy, and enteropathy X-linked syndrome; IUGR, intrauterine growth restriction; NAPDH, nicotinamide adenine dinucleotide phosphate.
Key Points.
Children with VEO-IBD can present with a different phenotype and more severe disease than older children and adults, in some cases secondary to a monogenic defect.
Advances in sequencing technology have allowed for identification of causal gene defects in a subset of patients with VEO-IBD.
Identification of these causal variants has radically changed the approach in these children and provide an opportunity for true precision medicine by targeting the pathway responsible for disease.
It is critical to understand the underlying defect in children with VEO-IBD. Conventional therapy may not only be unsuccessful, but, as in cases of immunodeficiency, can be inappropriate as well.
Acknowledgment
The authors would like to thank Drs Devoto, Dawany, and Sullivan for their assistance with this study.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH K23DK100461.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Ridder L, Weersma RK, Dijkstra G, et al. Genetic susceptibility has a more important role in pediatric-onset Crohn’s disease than in adult-onset Crohn’s disease. Inflamm Bowel Dis. 2007;13:1083–1092. [DOI] [PubMed] [Google Scholar]
- 4.Biank V, Broeckel U, Kugathasan S. Pediatric inflammatory bowel disease: clinical and molecular genetics. Inflamm Bowel Dis. 2007;13:1430–1438. [DOI] [PubMed] [Google Scholar]
- 5.Begue B, Verdier J, Rieux-Laucat F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544–1555. [DOI] [PubMed] [Google Scholar]
- 6.Muise AM, Snapper SB, Kugathasan S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology. 2012;143:285–288. [DOI] [PubMed] [Google Scholar]
- 7.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. [DOI] [PubMed] [Google Scholar]
- 8.Uhlig HH, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benchimol EI, Mack DR, Nguyen GC, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. 2014;147: 803–813.e7; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 10.Ximenez C, Torres J. Development of the microbiota in infants and its role in maturation of the gut mucosa and the immune system. Arch Med Res. 2017;48:666–680. [DOI] [PubMed] [Google Scholar]
- 11.Glocker E, Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci. 2012;69:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell EC, Dawany N, Baldassano RN, et al. Diverting ileostomy for the treatment of severe, refractory, pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;65: 299–305. [DOI] [PubMed] [Google Scholar]
- 13.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. [DOI] [PubMed] [Google Scholar]
- 14.Mamula P, Telega GW, Markowitz JE, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–2010. [DOI] [PubMed] [Google Scholar]
- 15.Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:597–605. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol. 2013;11:1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao H, Yang W, Lee PP, et al. Exome sequencing identifies novel compound heterozygous mutations of IL-10 receptor 1 in neonatal-onset Crohn’s disease. Genes Immun. 2012;13: 437–442. [DOI] [PubMed] [Google Scholar]
- 18.Worthey EA, Mayer AN, Syverson GD, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13:255–262. [DOI] [PubMed] [Google Scholar]
- 19.Avitzur Y, Guo C, Mastropaolo LA, et al. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology. 2014;146:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kammermeier J, Drury S, James CT, et al. Targeted gene panel sequencing in children with very early onset inflammatory bowel disease-evaluation and prospective analysis. J Med Genet. 2014;51:748–755. [DOI] [PubMed] [Google Scholar]
- 21.Kelsen JR, Dawany N, Moran CJ, et al. Exome sequencing analysis reveals variants in primary immunodeficiency genes in patients with very early onset inflammatory bowel disease. Gastroenterology. 2015;149:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glocker EO, Frede N, Perro M, et al. Infant colitis—it’s in the genes. Lancet. 2010;376:1272. [DOI] [PubMed] [Google Scholar]
- 23.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran CJ, Walters TD, Guo CH, et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannioto Z, Berti I, Martelossi S, et al. IBD and IBD mimicking enterocolitis in children younger than 2 years of age. Eur J Pediatr. 2009;168:149–155. [DOI] [PubMed] [Google Scholar]
- 26.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795–1805. [DOI] [PubMed] [Google Scholar]
- 27.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519–533. [DOI] [PubMed] [Google Scholar]
- 28.Shim JO, Hwang S, Yang HR, et al. Interleukin-10 receptor mutations in children with neonatal-onset Crohn’s disease and intractable ulcerating enterocolitis. Eur J Gastroenterol Hepatol. 2013;25:1235–1240. [DOI] [PubMed] [Google Scholar]
- 29.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 30.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelhardt KR, Grimbacher B. IL-10 in humans: lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Curr Top Microbiol Immunol. 2014;380:1–18. [DOI] [PubMed] [Google Scholar]
- 32.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102:8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neven B, Mamessier E, Bruneau J, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013;122:3713–3722. [DOI] [PubMed] [Google Scholar]
- 34.Engelhardt KR, Shah N, Faizura-Yeop I, et al. Clinical outcome in IL-10-and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131:825–830. [DOI] [PubMed] [Google Scholar]
- 35.Murugan D, Albert MH, Langemeier J, et al. Very early onset inflammatory bowel disease associated with aberrant trafficking of IL-10R1 and cure by T cell replete haploidentical bone marrow transplantation. J Clin Immunol. 2014;34:331–339. [DOI] [PubMed] [Google Scholar]
- 36.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. [DOI] [PubMed] [Google Scholar]
- 37.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. [DOI] [PubMed] [Google Scholar]
- 38.Chalaris A, Gewiese J, Paliga K, et al. ADAM17-mediated shedding of the IL6R induces cleavage of the membrane stub by gamma-secretase. Biochim Biophys Acta. 2010;1803: 234–245. [DOI] [PubMed] [Google Scholar]
- 39.Blaydon DC, Biancheri P, Di WL, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365:1502–1508. [DOI] [PubMed] [Google Scholar]
- 40.Karamchandani-Patel G, Hanson EP, Saltzman R, Kimball CE, Sorensen RU, Orange JS. Congenital alterations of NEMO glutamic acid 223 result in hypohidrotic ectodermal dysplasia and immunodeficiency with normal serum IgG levels. Ann Allergy Asthma Immunol. 2011;107:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmer KP, Schumann H, Mecklenbeck S, Bruckner-Tuderman L. Esophageal stenosis in childhood: dystrophic epidermolysis bullosa without skin blistering due to collagen VII mutations. Gastroenterology. 2002;122:220–225. [DOI] [PubMed] [Google Scholar]
- 42.Sadler E, Klausegger A, Muss W, et al. Novel KIND1 gene mutation in Kindler syndrome with severe gastrointestinal tract involvement. Arch Dermatol. 2006;142:1619–1624. [DOI] [PubMed] [Google Scholar]
- 43.Ussar S, Moser M, Widmaier M, et al. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern JS, Herz C, Haan E, et al. Chronic colitis due to an epithelial barrier defect: the role of kindlin-1 isoforms. J Pathol. 2007;213:462–470. [DOI] [PubMed] [Google Scholar]
- 45.Fiskerstrand T, Arshad N, Haukanes BI, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. [DOI] [PubMed] [Google Scholar]
- 46.Chen R, Giliani S, Lanzi G, et al. Whole-exome sequencing identifies tetratricopeptide repeat domain 7A (TTC7A) mutations for combined immunodeficiency with intestinal atresias. J Allergy Clin Immunol. 2013;132:656–664. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuels ME, Majewski J, Alirezaie N, et al. Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia. J Med Genet. 2013;50:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemoine R, Pachlopnik-Schmid J, Farin HF, et al. Immune deficiency-related enteropathy-lymphocytopenia-alopecia syndrome results from tetratricopeptide repeat domain 7A deficiency. J Allergy Clin Immunol. 2014;134:1354–1364.e6. [DOI] [PubMed] [Google Scholar]
- 49.Lawless D, Mistry A, Wood PM, et al. Bialellic mutations in tetratricopeptide repeat domain 7A (TTC7A) cause common variable immunodeficiency-like phenotype with enteropathy. J Clin Immunol. 2017;37:617–622. [DOI] [PubMed] [Google Scholar]
- 50.Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127:1319–1326; quiz 27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. [DOI] [PubMed] [Google Scholar]
- 52.Matute JD, Arias AA, Wright NA, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114: 3309–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol. 2009;104:117–124. [DOI] [PubMed] [Google Scholar]
- 54.Jones LB, McGrogan P, Flood TJ, et al. Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol. 2008;152:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenzweig SD. Inflammatory manifestations in chronic granulomatous disease (CGD). J Clin Immunol. 2008; 28(Suppl 1):S67–S72. [DOI] [PubMed] [Google Scholar]
- 56.Foster CB, Lehrnbecher T, Mol F, et al. Host defense molecule polymorphisms influence the risk for immune-mediated complications in chronic granulomatous disease. J Clin Invest. 1998;102:2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muise AM, Xu W, Guo CH, et al. NADPH oxidase complex and IBD candidate gene studies: identification of a rare variant in NCF2 that results in reduced binding to RAC2. Gut. 2012; 61:1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhillon SS, Fattouh R, Elkadri A, et al. Variants in nicotinamide adenine dinucleotide phosphate oxidase complex components determine susceptibility to very early onset inflammatory bowel disease. Gastroenterology. 2014;147:680–689.e2. [DOI] [PubMed] [Google Scholar]
- 59.Roos D, Law SK. Hematologically important mutations: leukocyte adhesion deficiency. Blood Cells Mol Dis. 2001;27: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 60.van de Vijver E, Maddalena A, Sanal O, et al. Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol Dis. 2012;48:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. [DOI] [PubMed] [Google Scholar]
- 62.Davis MK, Rufo PA, Polyak SF, Weinstein DA. Adalimumab for the treatment of Crohn-like colitis and enteritis in glycogen storage disease type Ib. J Inherit Metab Dis. 2008;31(Suppl 3):505–509. [DOI] [PubMed] [Google Scholar]
- 63.Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51:1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato K, Kojima Y, Kobayashi C, et al. Successful allogeneic hematopoietic stem cell transplantation for chronic granulomatous disease with inflammatory complications and severe infection. Int J Hematol. 2011;94:479–482. [DOI] [PubMed] [Google Scholar]
- 65.de Luca A, Smeekens SP, Casagrande A, et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A. 2014;111:3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131:959–971. [DOI] [PubMed] [Google Scholar]
- 67.Pai SY, Cowan MJ. Stem cell transplantation for primary immunodeficiency diseases: the North American experience. Curr Opin Allergy Clin Immunol. 2014;14:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. [DOI] [PubMed] [Google Scholar]
- 69.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. [DOI] [PubMed] [Google Scholar]
- 70.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Driessen GJ, IJspeert H, Wentink M, et al. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J Allergy Clin Immunol. 2016;138: 1744–1747.e5. [DOI] [PubMed] [Google Scholar]
- 72.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(–)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. [DOI] [PubMed] [Google Scholar]
- 74.Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N Engl J Med. 2003;349:1821–1828. [DOI] [PubMed] [Google Scholar]
- 75.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. [DOI] [PubMed] [Google Scholar]
- 76.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93:190–197. [DOI] [PubMed] [Google Scholar]
- 77.Ludvigsson JF, Neovius M, Hammarstrom L. Association between IgA deficiency & other autoimmune conditions: a population-based matched cohort study. J Clin Immunol. 2014;34:444–451. [DOI] [PubMed] [Google Scholar]
- 78.Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alangari A, Alsultan A, Adly N, et al. LPS-responsive beigelike anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130:481–488.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nielsen C, Jakobsen MA, Larsen MJ, et al. Immunodeficiency associated with a nonsense mutation of IKBKB. J Clin Immunol. 2014;34:916–921. [DOI] [PubMed] [Google Scholar]
- 81.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. [DOI] [PubMed] [Google Scholar]
- 82.Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2012. J Allergy Clin Immunol. 2013; 131:675–682. [DOI] [PubMed] [Google Scholar]
- 83.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeissig S, Petersen BS, Tomczak M, et al. Early-onset Crohn’s disease and autoimmunity associated with a variant in CTLA-4. Gut. 2015;64:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bianco AM, Girardelli M, Vozzi D, Crovella S, Kleiner G, Marcuzzi A. Mevalonate kinase deficiency and IBD: shared genetic background. Gut. 2014;63:1367–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Canna SW, Girard C, Malle L, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuloglu Z, Kansu A, Ustundag G, Birsin Ozcakar Z, Ensari A, Ekim M. An infant with severe refractory Crohn’s disease and homozygous MEFV mutation who dramatically responded to colchicine. Rheumatol Int. 2012;32:783–785. [DOI] [PubMed] [Google Scholar]
- 90.Beser OF, Kasapcopur O, Cokugras FC, Kutlu T, Arsoy N, Erkan T. Association of inflammatory bowel disease with familial Mediterranean fever in Turkish children. J Pediatr Gastroenterol Nutr. 2013;56:498–502. [DOI] [PubMed] [Google Scholar]
- 91.Mora AJ, Wolfsohn DM. The management of gastrointestinal disease in Hermansky-Pudlak syndrome. J Clin Gastroenterol. 2011;45:700–702. [DOI] [PubMed] [Google Scholar]
- 92.Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147:155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Speckmann C, Lehmberg K, Albert MH, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol. 2013;149:133–141. [DOI] [PubMed] [Google Scholar]
- 94.Kelsen JR, Dawany N, Martinez A, et al. A de novo whole gene deletion of XIAP detected by exome sequencing analysis in very early onset inflammatory bowel disease: a case report. BMC Gastroenterol. 2015;15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin Cell Dev Biol. 2015;39:115–123. [DOI] [PubMed] [Google Scholar]
- 96.Pedersen J, LaCasse EC, Seidelin JB, Coskun M, Nielsen OH. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20:652–665. [DOI] [PubMed] [Google Scholar]
- 97.Aguilar C, Latour S. X-linked inhibitor of apoptosis protein deficiency: more than an X-linked lymphoproliferative syndrome. J Clin Immunol. 2015;35:331–338. [DOI] [PubMed] [Google Scholar]
- 98.Filipovich AH. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol. 2011; 11:512–516. [DOI] [PubMed] [Google Scholar]
- 99.Li Q, Lee CH, Peters LA, et al. Variants in TRIM22 that affect NOD2 signaling are associated with very-early-onset inflammatory bowel disease. Gastroenterology. 2016;150: 1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conrad MA, Carreon CK, Dawany N, Russo P, Kelsen JR. Distinct histopathological features at diagnosis of very early onset inflammatory bowel disease [published online ahead of print December 14, 2018]. J Crohns Colitis 2018; doi: 10.1093/ecco-jcc/jjy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kammermeier J, Dziubak R, Pescarin M, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis. 2017; 11:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]