Abstract
From December 2005 to April 2007, we enrolled 60 adults starting antiretroviral therapy (ART) in Lima, Peru to receive community-based accompaniment with supervised antiretrovirals (CASA), consisting of 12 months of DOT-HAART, as well as microfinance assistance and/or psychosocial support group according to individuals’ need. We matched 60 controls from a neighboring district, and assessed final clinical and psychosocial outcomes at 24 months. CASA support was associated with higher rates of virologic suppression and lower mortality. A comprehensive, tailored adherence intervention in the form of community-based DOT-HAART and matched economic and psychosocial support is both feasible and effective for certain individuals in resource-poor settings.
Keywords: Adherence, HIV, Resource-poor setting, Poverty, DOT-HAART
Introduction
HAART nonadherence is among the strongest predictors of HIV clinical outcomes [1–6]. Meta-analyses support the efficacy of a wide range of adherence interventions on antiretroviral adherence and virologic suppression [7, 8]. Among the many adherence strategies that have been explored, direct observation of all or some doses of antiretroviral therapy (DOT-HAART) has been utilized for a broad range of populations, under programmatic and investigational settings [9, 10]. Randomized controlled trials assessing DOT-HAART efficacy have demonstrated variable results, but appear to suggest that DOT-HAART may be particularly effective for high-risk individuals, such as those with prior HAART nonadherence and substance use [11]. Recent innovations have paired DOT-HAART with other forms of adherence support, such as case management and adherence counseling [12, 13]. By combining interventions, the participant receives the structured support of supervised pill-taking while developing coping and problem-solving skills that would enable sustained adherence once DOT-HAART has stopped.
In Peru, we designed and implemented an adherence intervention consisting of Community-based Accompaniment with Supervised Antiretrovirals (CASA). We define the term accompaniment as medical, social and economic support delivered by paid community-based health workers [14]. In Peru, CASA support comprises 12 months of home-based DOT-HAART delivered by community health workers, along with emotional and instrumental support to help patients adhere to all aspects of HIV treatment (i.e. appointments, tests, medications). In addition, mental health and socioeconomic needs for each patient are assessed and matched social support is provided as needed. Because many HIV-positive individuals in this community have lost both economic and emotional sources of support in the course of their illness, we delivered microfinance and peer support groups as the matched forms of support to patients transitioning off DOT-HAART. The rationale for this combined, tailored intervention is to maximize the participant’s ability to maintain sustained treatment adherence, including self-administration of antiretroviral therapy.
We hypothesized that matched social support would result in improved clinical and psychosocial outcomes. We have previously reported preliminary outcomes at 12 months, which demonstrated greater rates of virologic suppression and improved psychosocial status among CASA participants, compared with controls [15]. Here, we describe final clinical and psychosocial outcomes at 24 months, compared with a matched control group selected from a neighboring health district.
Methods
Setting and Recruitment
In collaboration with the National HIV Program, we enrolled 95 adult patients between December 2005 and April 2007 to receive CASA support. All patients were referred from a tertiary public hospital, which provides HIV care and antiretroviral therapy to the health region of Lima Este (total population 1,856,514 inhabitants). Providers referred patients who were living in poverty and were about to start (or recently started) HAART based on WHO criteria [16]. Among the stakeholders of this participatory research, providers and health promoters requested that priority be given to particularly vulnerable groups—individuals who were co-infected with TB and women—although males without TB co-infection were not excluded. We matched controls among individuals who were eligible for HAART during the same period seen at a tertiary hospital serving a neighboring health region (Lima Ciudad), who did not receive community-based treatment support or DOT-HAART. We enrolled the first 60 controls that were successfully matched by age (±5 years), primary referral criteria (TB, woman, neither), and baseline CD4 cell count (≤ or >200 cells/ml3) to CASA cases. Patients were excluded if they lived outside their respective hospitals’ catchment area. All patients provided informed consent. The study protocol was reviewed by the Institutional Review Boards of the Brigham and Women’s Hospital, the Peruvian National Institute of Health, as well as the ethics committees of participating hospitals.
Intervention Description
A description of the community-based team, training and DOT-HAART procedures has been published elsewhere [15]. In brief, a team of nurses, field supervisors, and lay health workers provided DOT of all HAART doses, monitored for side effects and other potential threats to treatment adherence, provided emotional and logistic support to patients and their families, helped to coordinate appointments and diagnostic tests, and communicated patient issues to the medical team. The team participated in a four-day training course which reviewed Ministry of Health norms for HIV care; principles of HIV, antiretroviral medications, and side effects; teaching on adherence, DOT, and patient-centered adherence support; and issues in mental health, domestic violence, and substance use. Monthly meetings including DOT workers provided refresher training and emotional support to the team. DOT workers were compensated with monthly food baskets. DOT-HAART lasted 12 months, with tapered DOT visits during the last 3 months. Patients also received financial aide for diagnostic tests and medications to treat opportunistic infections and adverse reactions, transportation and nutritional support, as needed. At approximately 9 months, the psychosocial and economic status of each CASA participant was assessed using standardized forms, described in the following section. These forms were reviewed and discussed by the community-based team of nurses and health promoters to identify candidates for referral for microfinance assistance and/or peer support groups. Candidates were invited to participate in peer support groups and/or microfinance assistance, but participation was not required for CASA support.
Peer support groups were based on our prior experience conducting support groups for individuals in treatment for multidrug-resistant tuberculosis (MDR-TB) [17, 18]. Patients were ineligible if they had smear-positive tuberculosis, or were diagnosed with an antisocial personality disorder, active psychosis or mania. Sessions were held at the tertiary hospital where participants received their HIV care. The sessions lasted approximately 90 min and were moderately structured, covering pre-assigned themes: introductions and group contract; emotional responses to HIV; HIV and health; stigma and rejection; depression, anxiety and substance abuse; family and partner relationships; rights and responsibilities; session with participation of family and/or friends; work, economy and responsibilities; recreational event and closure. Participants were notified of each session by home visit, without informing them of the session theme. Prior to each session, we called participants to confirm their attendance. Transportation costs and sandwich lunches were provided. Ten closed monthly sessions were facilitated by a psychiatrist, nurse, and health promoter. Discussions developed in a spontaneous manner, with input from facilitators to guide discussion, seek opinions of all members of the group, and mediate any conflicts or difficult discussions. A closing recreational celebration was held upon completion of the sessions.
Our microfinance program was also based on our organization’s experience providing similar support to individuals with MDR-TB [19]. The microfinance project was supervised by an economist and social worker, in conjunction with the CASA team, and was conducted in five phases: selection, training, elaboration of business plan, implementation of business plan, and follow-up. Criteria for selection included physical and mental capacity to work, responsibility and motivation to participate, feasible business options, and economic need in the household. Training consisted of four 2-h sessions in didactic and workshop format covering business ideas and planning, marketing strategies, business organization, and investment planning and financing. Transportation and food were provided for training sessions. The microfinance team provided technical support, working with individual participants to elaborate a business plan. Upon plan approval, the no-interest loan was dispersed along with a payment calendar. To minimize loan diversion toward non-business expenditures, the microfinance team accompanied the participant on business purchases and the borrower received monthly food baskets worth approximately 35 USD for 4 months to reduce financial stressors in the household. Follow-up consisted of monthly visits to business sites to provide feedback, as well as group meetings to share experiences. The team worked with individuals to modify business and/or payment plans based on business and repayment success or failure.
Data Collection and Measures
A separate, non-blinded team collected data using standardized forms from charts and interview. We collected data on sociodemographic and clinical characteristics. We used the Hopkins Symptom Checklist to measure depression, the Berger Stigma Instrument, the Duke University of North Carolina Social Support Scale, and the Medical Outcomes Study HIV Quality of Life questionnaires [20–23]. For self-efficacy, we used a scale derived by our sister organization, Prevention and Access to Care and Treatment (PACT), which was adapted from the HIV Self-Efficacy Questionnaire [24], as well as the Confidence in Diabetes Self-Care Scale [25] and HIV self-management items specific to medication adherence developed and tested by Smith and colleagues [26]. With the exception of the Berger Stigma Instrument and HIV Self-efficacy Questionnaire, all instruments had been previously validated in Spanish. Our validation of the Berger instrument in Spanish and the internal reliability of these instruments in our cohort has been described elsewhere [27, 28]; for all instruments the Cronbach’s alpha was ≥0.80. We conducted interviews at baseline and 12 months and derived a change score (24 month score—baseline) for each scale. Adherence was defined as having taken at least 95% of prescribed days of HAART within the last month of observation, based on a modified AIDS Clinical Trials Group instrument that included 30-day recall [29, 30]. Individuals who were not taking HAART were considered to have 0% adherence.
Clinical outcomes were virologic suppression; clinical status (on HAART, died, stopped HAART); TB treatment outcomes; and mean change in CD4 cell count at 24 months compared with baseline. Our primary endpoint, virologic suppression, was defined as having a viral load of \400 copies per ml at 2 years. As per intent to treat analysis, individuals who died or were missing viral load data were counted as unsuppressed.
Data Analysis
To test significance, we reported the chi-square for categorical variables, unless there were <5 predicted participants per cell where Fisher’s exact test was used. For continuous variables, we reported t-tests or, for non-normally distributed, the Wilcoxon two-sample test. We assessed the effect of our intervention on proportion with virologic suppression using univariate and multivariable analysis. For multivariable analysis, logistic regression analyses were performed on datasets multiply imputed using Markov Chain Monte Carlo methods [31]. Rather than imputing a single value for each missing data point, this multiple imputation procedure replaces each missing value with a set of possible values that represent the uncertainty about the correct imputation value. Effect estimates are then pooled for each covariate and confidence intervals incorporate the uncertainty inherent in imputing missing values. We included in this model all significantly different baseline characteristics between the intervention and control group as well as baseline characteristics associated with favorable response on univariate analysis, excluding collinear variables, r > 0.60. We also assessed the association of the CASA intervention with survival using Kaplan–Meier estimates. Based on post-hoc calculation, we were powered to detect a difference of 20% with a power of 80% and α = 0.05.
Results
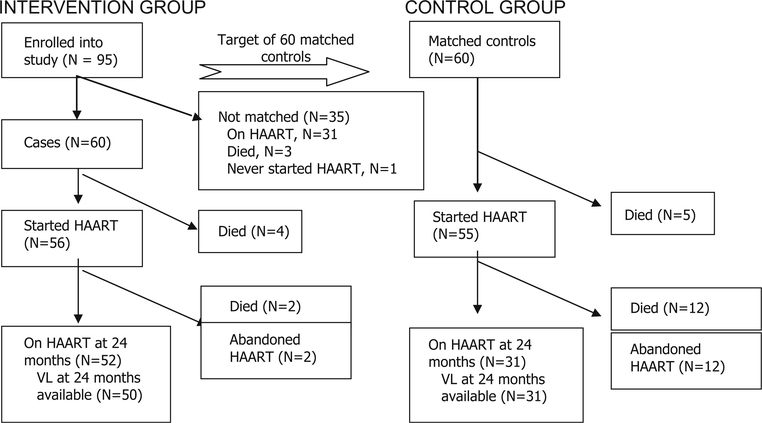
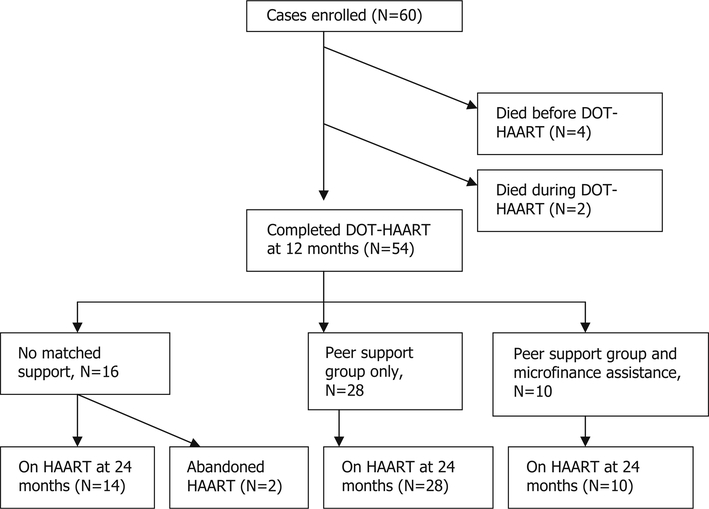
As shown in Fig. 1, a total of nine individuals among cases and controls died before starting HAART. Among CASA participants, 52 of the 56 individuals, 92.9%, who started HAART remained on HAART at 2 years, compared with 56.4%, 31 of 55, of individuals in the control group. As shown Fig. 2, 38 individuals, 63.3%, were enrolled in peer support groups, and 10 participants, 16.7%, received microfinance aid. Among the individuals selected for peer support groups, all but one attended at least one session. Participants attended a median of six support sessions, first and third quartiles four and eight. In terms of microfinance projects, 12 individuals were eligible for participation, but one did not complete training and one chose not to participate due to concerns of business failure. There were no adverse events reported associated with participation in the study.
Fig. 1.
Study flow
Fig. 2.
Matched support among intervention group, N = 60
Baseline characteristics, summarized in Tables 1 and 2, were similar between groups, except for a few respects: the socioeconomic status of the CASA group was lower than the control group, and substance use was more common within the control group. In addition, the control group was more likely to report a perceived benefit of HAART.
Table 1.
Baseline sociodemographic and clinical characteristics (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean SD | Control group, N = 60 N (%) or Mean SD | X-square, unless otherwise specified |
|---|---|---|---|
| Sex | |||
| Male | 28 (46.7) | 28 (46.7) | 0.00 |
| Female | 32 (53.3) | 32 (53.3) | |
| Age | 31.7 ± 7.8 | 31.9 ± 7.1 | −0.16 (t-test) |
| Civil status, 117 | 2.11 | ||
| Married or living together | 29 (48.3) | 20 (35.1) | |
| Single, separated, divorced, widowed | 31 (51.7) | 37 (64.9) | |
| Socioeconomic status | |||
| Limited educationa, 116 | 7 (11.9) | 5 (8.8) | 0.21 (Fisher’s exact) |
| Unemployed, 115 | 44 (75.9) | 32 (56.1) | 4.99* |
| Lacks basic servicesb, 119 | 20 (33.3) | 7 (11.9) | 7.82** |
| Food insecurityc, 106 | 34 (56.7) | 16 (34.8) | 5.00* |
| Difficulty accessing health services in past 3 months, 102 | 35 (62.5) | 29 (63.0) | 0.003 |
| HIV status | |||
| Months from diagnosis to HAART#, 111 | 3.2 [1.6, 13.0] | 3.3 [2.1, 11.1] | 0.64 (Kruskal–Wallis) |
| Months on HAART at enrollment# | 1.3 [0.2, 3.9] | 2.3 [0.0, 6.6] | 3.27 (Kruskal–Wallis) |
| Weight (kg), 116 | 53.9 ± 10.0 | 53.4 ± 11.9 | 0.17 (t-test) |
| CD4 (cells/ml3), 111 | 114.8 ± 87.2 | 109.7 ± 97.9 | 0.29 (t-test) |
| Viral load (copies/ml)#, 106 | 130,000 [29000, 230000] | 72,000 [26000, 284000] | 0.62 (Kruskal–Wallis) |
| Substance abuse (drug or alcohol) | 12 (20.0) | 24 (40.0) | 5.71* |
| Documented drug abuse | 3 (5.0) | 10 (16.7) | 0.03 (Fisher’s exact) |
| Documented alcohol abuse | 11 (18.3) | 22 (36.7) | 5.06* |
| TB co-infection | 33 (55.0) | 35 (58.3) | 0.14 |
| Suspected MDR TB | 8 (13.3) | 8 (13.3) | 0.00 |
Illiterate or no education beyond primary level
Home lacks at least one of the following: electricity, running water, or plumbing
Patient reported at least a day without food in the past 3 months due to poverty
If non-normal, median [1st and 3rd quartiles]
P < 0.05
P < 0.01
Table 2.
Baseline psychosocial characteristics (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean SD | Control group, N = 60 N (%) or Mean SD | t-Test, unless otherwise specified |
|---|---|---|---|
| Perceives HAART benefit, 102 | 44 (78.6) | 44 (95.7) | 0.01* (Fisher’s Exact) |
| Quality of life, 102 | |||
| Quality of life | 40.7 ± 9.1 | 40.0 ± 8.9 | 0.58 |
| Physical health (PHS) | 40.1 ± 10.0 | 40.8 ± 11.3 | −0.11 |
| Mental health (MHS) | 41.3 ± 10.2 | 39.2 ± 9.8 | 1.17 |
| Depression | |||
| HSC depression, 102 | 2.00 ± 0.55 | 1.96 ± 0.42 | 0.41 |
| Suicidal ideation in past month, 102 | 14 (25.0) | 9 (19.6) | 0.43 |
| Stigma score, 102 | 51.6 ± 14.4 | 46.0 ± 16.4 | 1.83 |
| Social support, 102 | |||
| Social support | 62.3 ± 18.5 | 65.4 ± 20.7 | −0.58 |
| Emotional social support | 69.4 ± 19.4 | 67.6 ± 21.4 | 0.60 |
| Instrumental social support | 51.6 ± 27.8 | 62.1 ± 30.7 | −1.60 |
| Self-efficacy | |||
| Self-efficacy | 64.8 ± 19.3 | 66.0 ± 14.5 | −0.37 |
P < 0.05
P < 0.01
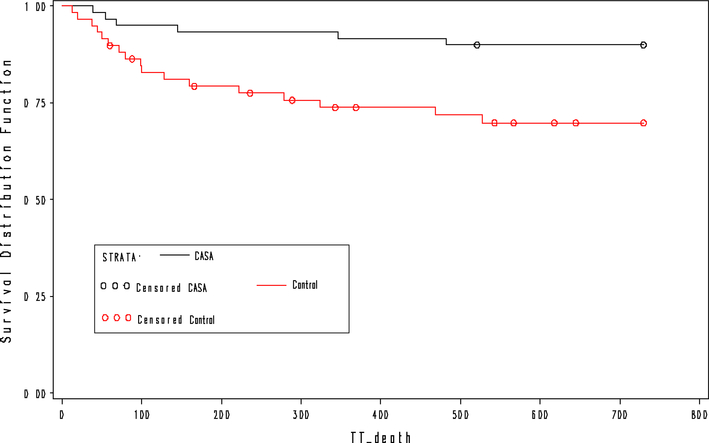
At 2 years, CASA participants were more likely to be on HAART, 86.7% versus 51.7%, X2 = 17.7, P < 0.01, and achieve virologic suppression, 66.7% versus 46.7%, X2 = 4.9, P = 0.03, and report adherence to HAART, 79.3% versus 44.1%, X2 = 15.3, P < 0.01, although CD4 cell counts did not differ significantly among groups (Table 3). Among TB patients, outcomes were better among CASA participants, with 81.8% of individuals completing treatment as a cure, compared with 48.6% among controls, X2 = 15.6, P < 0.01. Median time from enrollment to any death was 106.5 days, Q1 54, Q3 346 days, in the intervention group, compared with 99 days, Q1 50, Q3 222 days, in the control group; as shown in Fig. 3, time to death was significantly lower among individuals in the CASA group, compared with controls, hazard ratio 0.29, 95% CI 0.12, 0.75, X2 = 6.64. Changes in psychosocial indicators were also greater at 24 months for CASA participants compared with controls, in terms of reduced stigma, t(81) = 2.89, P < 0.01, and social support, t(81) = 3.97, P < 0.01, while changes in quality of life, depression and self-efficacy did not differ between groups (Table 4).
Table 3.
Clinical outcomes at 2 years (N = 120)
| Variable N if not 120 | CASA cohort, N = 60 N (%) or Mean SD | Control group, N = 60 N (%) or Mean SD | X-square, unless otherwise specified |
|---|---|---|---|
| HAART status | |||
| On HAART | 52 (86.7) | 31 (51.7) | 17.7** |
| Abandoned HAART | 2 (3.3) | 12 (20.0) | |
| Died | 6 (10.0) | 17 (28.3) | |
| TB outcomes at 24 months among TB patients, 68 | |||
| Cure | 27 (81.8) | 17 (48.6) | 15.6** |
| Treatment completed | 0 (0) | 1 (2.9) | |
| Failure | 1 (3.0) | 1 (2.9) | |
| Death | 0 (0) | 9 (25.7) | |
| Default | 2 (6.1) | 1 (2.9) | |
| In treatment | 2 (6.1) | 1 (2.9) | |
| Not known | 1 (3.0) | 5 (14.3) | |
| Virologic suppression | 40 (66.7) | 28 (46.7) | 4.9* |
| CD4 cell count, 85 | 357 ± 163.1 | 414.9 ± 229.5 | −1.24 |
| Change CD4 cell count, 85 | 239.7 ± 133.6 | 300.7 ± 208.5 | −1.50 |
| HAART adherence (≥95% doses in past month), 116 | 46 (79.3) | 26 (44.1) | 15.3** |
P < 0.05
P < 0.01
Fig. 3.
Time to death by study status, N = 120
Table 4.
Psychosocial indicators at 24 months (N = 120)
| Variable N, if not 120 | CASA cohort, N = 60 N (%) or Mean SD | Control group, N = 60 N (%) or Mean SD | t-Test, unless otherwise specified |
|---|---|---|---|
| Perceives HAART benefit, 83 | 46 (90.2) | 29 (90.6) | 0.004 |
| Change in quality of life score, 82 | |||
| Quality of life | +17.0 ± 9.9 | +17.8 ± 10.4 | −0.35 |
| Physical health (PHS) | +19.3 ± 10.4 | +18.7 ± 11.6 | 0.25 |
| Mental health (MHS) | +14.7 ± 12.3 | +17.0 ± 11.8 | −0.81 |
| Change in depression score | |||
| HSC Depression, 83 | −0.59 ± 0.60 | −0.48 ± 0.49 | −0.89 |
| Suicidal ideation in past month, 83 | 2 (3.9) | 2 (6.3) | 0.23 |
| Change in Stigma score, 83 | −18.3 ± 16.0 | −8.1 ± 15.0 | −2.89** |
| Change in social support score, 83 | |||
| Social support | +13.3 ± 27.4 | −10.0 ± 23.5 | 3.97** |
| Emotional social support | +9.9 ± 26.4 | −5.0 ± 25.9 | 2.52* |
| Instrumental social support | +18.1 ± 37.0 | −17.8 ± 34.6 | 4.40** |
| Change in self-efficacy score, 83 | |||
| Self-efficacy | +25.1 ± 23.6 | +26.6 ± 19.1 | −0.29 |
P < 0.05
P < 0.01
Upon univariate analysis, male gender and baseline self-efficacy were marginally significant predictors of virologic suppression at 24 months (Table 5). On multivariable analysis including baseline differences of the cohorts, CASA support was associated with virologic suppression with an adjusted odds ratio of 2.46, 95% CI 1.03, 6.09, X2 = 4.01. A second model that additionally included gender and baseline self-efficacy yielded similar results (adjusted odds ratio for CASA intervention of 2.50, 95% CI 1.02, 6.17, X2 = 4.02.
Table 5.
Baseline characteristics associated with suppressed viral load at 24 months, univariate analysis (N = 120)
| Variable | Suppressed viral load, N = 68 N (%)/Mean SD | Death, unsuppressed viral load, N = 52 N (%)/Mean SD | X-square, unless otherwise specified |
|---|---|---|---|
| Male gender | 37 (54.4) | 19 (36.5) | 3.8 |
| Age | 32.9 ± 7.6 | 30.6 ± 7.1 | 1.66 |
| Unemployed | 46 (68.7) | 30 (62.5) | 0.47 |
| Lacks basic services | 16 (23.5) | 11 (21.6) | 0.06 |
| Food insecurity | 30 (44.1) | 20 (52.6) | 0.71 |
| Substance abuse | 21 (30.9) | 15 (29.9) | 0.06 |
| TB co-infection | 39 (57.4) | 29 (55.8) | 0.03 |
| Perceives HAART benefit at baseline | 57 (83.8) | 31 (91.2) | 1.03 |
| Difficulty accessing health services | 41 (60.3) | 23 (67.7) | 0.52 |
| Depression | 1.97 ± 0.51 | 1.99 ± 0.47 | −0.17 |
| Stigma | 49.9 ± 14.9 | 47.5 ± 16.7 | 0.72 |
| Social support | 64.0 ± 18.9 | 63.0 ± 20.8 | 0.25 |
| Self-efficacy | 67.7 ± 17.5 | 60.7 ± 15.9 | 1.95 |
| CD4 ≤ 200 | 56 (82.4) | 33 (76.7) | 0.52 |
Discussion
Consistent with published theory on matched social support, our results suggest that phase-appropriate matched support leads to improved physical and psychosocial outcomes. The “intensive” phase of our intervention was designed to stabilize the most critical physical and psychosocial issues during the first 8 months of HAART by providing one-to-one instrumental and emotional support via DOT-HAART. The “transition” phase began 9 months after HAART initiation and was designed to prepare the patient for self-administration by stabilizing economic and psychosocial environments for particularly vulnerable individuals. These phases are consistent with models of stressful life events [32]. In describing the process of bereavement, Weiss defines two phases [33]. In the crisis phase, the primary support needed is emotional. In the transitional phase, focus is on establishing a new identity and recovering from material losses associated with the lost partner, requiring both cognitive and tangible support. Qualitative research in our patient population and elsewhere [34, 35] confirm these phases of HIV illness, shifting from survival and physical recovery to strengthening social networks and coping with a chronic disease.
The success of our program suggests that adherence strategies should vary over time to reflect the needs of the individual. Furthermore, social needs vary widely among individuals, depending on existing networks, degree of poverty, and severity of physical and mental illness. Individual patterns and determinants of nonadherence also vary widely [36]. In developed countries, social support adherence interventions have demonstrated conflicting results [25, 37–41]. The limited or variable impact of these interventions may be due to the heterogeneous needs of each individual and the change in needs over time [42]. Although common in clinical practice in developed countries, a tailored approach matching adherence strategies to individual patient needs has not previously been described [43]. A dynamic model of adherence [44, 45] is consistent with other models of life crises in which stressors, coping demands and support needs evolve over time [32, 46]. The matching hypothesis of social support interventions postulates that social support needs are dynamic and multidimensional, and the benefit of an intervention depends on whether it addresses the individual’s needs at a given time [47, 48].
Matched interventions introduce additional complexity, which can be challenging to implement. However, given that overwhelming poverty, social isolation, and depression pose significant barriers to adherence for many individuals living in resource-poor settings [49–51], the impact of DOT-HAART alone could wane once the patient transitions to self-administration. Despite the intervention’s complexity, our pilot experience suggests that a comprehensive matched approach may be acceptable to participants and feasible in a resource-limited setting. Despite initial concerns raised by some patients about accepting community-based DOT-HAART, all patients eventually accepted DOT-HAART and the majority of DOT was delivered in patient homes. Once accepted into patients’ homes, community health workers were able to witness daily household dynamics, enabling them to assess patient needs and match them to additional support as they transitioned off DOT-HAART. Based on this experience, our community-based team was able to designate and deliver matched adherence support to HIV-positive individuals in a resource-poor setting. However, because we did not collect data on the rate of completion of DOT encounters, we cannot make firm conclusions regarding the feasibility of this intervention. In addition, the feasibility of programmatic implementation beyond pilot scale remains to be determined.
The data reported here suggest that comprehensive, individualized CASA support can improve clinical and psychosocial outcomes for certain populations. These findings reinforce our preliminary results [15], which reported similar benefits at 12 months. Our intervention had an impact not only on adherence but also on mortality within the first 2 years. Although psychosocial outcomes were similar among survivors at 24 months in terms of quality of life, depression, and self-efficacy, CASA participants reported greater social support and reduced stigma compared with controls. It is possible that community health workers and peer support groups provided new forms of social support and reduced perceived stigma among participants. In addition, through home visits, community health workers could also work to strengthen existing sources of support and reduce enacted stigma among household members.
Our study is limited in its small size and non-randomized control group, which did not allow us to control for unmeasured and measured differences between the two groups. However, the impact of CASA support on virologic suppression is significant even when controlling for baseline differences, including substance use. Our assessment of adherence was limited in that it relied on self-report and used a single value without taking into account pill taking patterns or type of antiretroviral regimen. The intervention combined several modalities of social support (DOT-HA-ART, psychotherapy, and microfinance support), and the study assessed the impact of the intervention as a “package.” This observational study was not designed to understand whether each intervention was effective by itself, nor was it able to tease out effect interactions, such as additive or synergistic benefits from combined supports. Larger studies would be needed to determine which components of this complex intervention contribute the most to intervention effect. Finally, whether social support was actually the mediating mechanism of this intervention’s success requires additional exploration.
Our study may have limited generalizability because we prioritized TB co-infected and female patients, in response to the community’s perception of greatest need. Based on our understanding of the HIV-affected population in Peru and other resource-poor settings, we feel that these individuals likely represent the most vulnerable groups who may most benefit from comprehensive accompaniment. Both women and co-infected individuals often “fall through the cracks” because of socioeconomic marginalization and poor coordination of care. In developed countries, there is growing consensus that DOT-HAART may be more appropriate for individuals at greatest risk of nonadherence, rather than providing such an intensive intervention to “all-comers” [11, 52]. In resource-poor settings, randomized controlled trials of DOT-HAART have enrolled all individuals starting HAART [53, 54], although the experience in Kenya suggested that individuals with baseline depression benefited most from modified DOT-HAART [54]. In settings in which HIV care is not integrated with other services (e.g. TB services, obstetrics, family planning, pediatric care), individuals who need to navigate multiple programs may particularly benefit from community-based support to increase the coordination and communication between different services.
In summary, we present a model of care that combines community-based DOT-HAART with additional forms of matched economic and psychosocial support. Our experience has demonstrated the feasibility of delivering this intervention in a resource-poor setting using a team primarily consisting of lay community health workers. Final outcomes at 24 months support the effectiveness of CASA support on virologic outcomes, mortality and psychosocial well-being among vulnerable populations in Peru.
Acknowledgements
We would like to acknowledge the Office for AIDS Research at the National Institutes for Health; the Eleanor and Miles Shore Fellowship at Harvard Medical School; David Rocke-feller Center for Latin American Studies at Harvard University, and Partners In Health for support of this project. We also thank Christian Rojas, Miriam Callacna, Julio Acha, Humberto Castillo, Eduardo Rodriguez, and Mayler Albujar for their efforts with the intervention. No conflicts of interest exist.
Contributor Information
Maribel Muñoz, Socios En Salud Sucursal Perú, Lima, Peru.
Jaime Bayona, Socios En Salud Sucursal Perú, Lima, Peru.
Eduardo Sanchez, Hospital Nacional Hipólito Unanue, Lima, Peru.
Jorge Arevalo, Hospital Dos de Mayo, Lima, Peru.
Jose Luis Sebastian, Peruvian HIV Program, Ministerio de Salud, Lima, Peru.
Fernando Arteaga, Socios En Salud Sucursal Perú, Lima, Peru.
Dalia Guerra, Socios En Salud Sucursal Perú, Lima, Peru.
Jhon Zeladita, Socios En Salud Sucursal Perú, Lima, Peru.
Betty Espiritu, Socios En Salud Sucursal Perú, Lima, Peru.
Milagros Wong, Socios En Salud Sucursal Perú, Lima, Peru.
Adolfo Caldas, Division of Global Health Equity, Brigham and Women’s, Hospital, FXB Building, 7th Floor, 651 Huntington Avenue, Boston, MA 02115, USA.
Sonya Shin, Harvard Medical School, Boston, MA, USA; Division of Global Health Equity, Brigham and Women’s, Hospital, FXB Building, 7th Floor, 651 Huntington Avenue, Boston, MA 02115, USA.
References
- 1.Chesney M Adherence to HAART regimens. AIDS Patient Care STDS. 2003;17(4):169–77. [DOI] [PubMed] [Google Scholar]
- 2.de Olalla PG, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30(1):105–10. [DOI] [PubMed] [Google Scholar]
- 3.Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: a critical link between behavioral and biomedical sciences. J Acquir Immune Defic Syndr. 2002;31 (Suppl3): S98–102. [DOI] [PubMed] [Google Scholar]
- 4.Jain MK, Skiest DJ, Cloud JW, Jain CL, Burns D, Berggren RE. Changes in mortality related to human immunodeficiency virus infection: comparative analysis of inpatient deaths in 1995 and in 1999–2000. Clin Infect Dis. 2003;36(8):1030–8. [DOI] [PubMed] [Google Scholar]
- 5.Munakata J, Benner JS, Becker S, Dezii CM, Hazard EH, Tierce JC. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care. 2006;44(10):893–9. [DOI] [PubMed] [Google Scholar]
- 6.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43(1):78–84. [DOI] [PubMed] [Google Scholar]
- 7.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3): 285–97. [DOI] [PubMed] [Google Scholar]
- 9.Goggin K, Liston RJ, Mitty JA. Modified directly observed therapy for antiretroviral therapy: a primer from the field. Public Health Rep. 2007;122(4):472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10(6):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374(9707):2064–71. [DOI] [PubMed] [Google Scholar]
- 12.Bradley-Ewing A, Thomson D, Pinkston M, Goggin KJ. A qualitative examination of the indirect effects of modified directly observed therapy on health behaviors other than adherence. AIDS Patient Care STDS. 2008;22(8):663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangsberg D, Hammer G, Reynolds M, Ragland K, Barnberg J, Riley ED. Adherence case management (ACM) does not sustain the effect of modified directly observed therapy (MDOT) in HIV-positive homeless and marginally housed (H/M) individuals. 4th International Conference on HIV Treatment Adherence Miami, FL;2009. [abstract #159]. [Google Scholar]
- 14.Farmer P, Leandre F, Mukherjee JS, et al. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358(9279):404–9. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M, Finnegan K, Zeladita J, et al. Community-based DOT-HAART Accompaniment in an Urban Resource-Poor Setting. AIDS Behav. 2009. doi: 10.1007/s10461-009-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. S caling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. 2003. Available from http://www.who.int/3by5/publications/documents/arv_guidelines/en/. Accessed December 1, 2005.
- 17.Acha J, Sweetland A, Guerra D, Chalco K, Castillo H, Palacios E. Psychosocial support groups for patients with multidrug-resistant tuberculosis: five years of experience. Glob Public Health. 2007;2(4):404–17. [DOI] [PubMed] [Google Scholar]
- 18.Sweetland A, Acha J, Guerra D. Enhancing adherence: the role of group psychotherapy in the treatment of MDR-TB in urban Peru In: Cohen A, Kleinman A, Saraceno B, editors. World mental health casebook: social and mental health programs in low-income countries. New York: Kluwer Academic/Plenum Press; 2002. p. 51–79. [Google Scholar]
- 19.Shin SS, Furin JJ, Alcantara F, Bayona J, Sanchez E, Mitnick CD. Long-term follow-up for multidrug-resistant tuberculosis. Emerg Infect Dis. 2006;12(4):687–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC functional social support questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26(7):709–23. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15. [DOI] [PubMed] [Google Scholar]
- 22.Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care. 1991;29(8):786–98. [DOI] [PubMed] [Google Scholar]
- 23.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–29. [DOI] [PubMed] [Google Scholar]
- 24.Shively M, Smith T, Bormann J, Gifford A. Evaluating self-efficacy for HIV disease management skills. AIDS Behav. 2002;6:371–9. [Google Scholar]
- 25.Jones DL, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15(4): 463–74. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50(2):187–99. [DOI] [PubMed] [Google Scholar]
- 27.Franke MF, Munoz M, Finnegan K, et al. Validation and abbreviation of an HIV stigma scale in an adult spanish-speaking population in urban Peru. AIDS Behav. 2010;14(1):189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin S, Munoz M, Espiritu B, et al. Psychosocial impact of poverty on antiretroviral nonadherence among HIV-TB coinfected patients in Lima, Peru. J Int Assoc Physicians AIDS Care. 2008;7(2):74–81. [DOI] [PubMed] [Google Scholar]
- 29.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–66. [DOI] [PubMed] [Google Scholar]
- 30.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–66. [DOI] [PubMed] [Google Scholar]
- 31.Schafer J Analysis of incomplete multivariate data. New York: Chapman and Hall; 1997. [Google Scholar]
- 32.Jacobson DE. Types and timing of social support. J Health Soc Behav. 1986;27(3):250–64. [PubMed] [Google Scholar]
- 33.Weiss R Transition states and other stressful situations: their nature and programs for their management In: Caplan G, Killilea M, editors. Support systems and mutual help: multidisciplinary explorations. New York: Grune and Stratton; 1976. [Google Scholar]
- 34.Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S127–33. [DOI] [PubMed] [Google Scholar]
- 35.Leaver CA, Bargh G, Dunn JR, Hwang SW. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007;11(6 Suppl):85–100. [DOI] [PubMed] [Google Scholar]
- 36.Levine AJ, Hinkin CH, Castellon SA, et al. Variations in patterns of highly active antiretroviral therapy (HAART) adherence. AIDS Behav 2005;9(3):355–62. [DOI] [PubMed] [Google Scholar]
- 37.Antoni MH, Carrico AW, Duran RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68(1):143–51. [DOI] [PubMed] [Google Scholar]
- 38.Carrico AW, Antoni MH, Duran RE, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-Positive gay men treated with HAART. Ann Behav Med. 2006;31(2):155–64. [DOI] [PubMed] [Google Scholar]
- 39.Jones DL, McPherson-Baker S, Lydston D, et al. Efficacy of a group medication adherence intervention among HIV positive women: the SMART/EST Women’s Project. AIDS Behav. 2007;11(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–62. [DOI] [PubMed] [Google Scholar]
- 41.van Servellen G, Carpio F, Lopez M, et al. Program to enhance health literacy and treatment adherence in low-income HIV-infected Latino men and women. AIDS Patient Care STDS. 2003;17(11):581–94. [DOI] [PubMed] [Google Scholar]
- 42.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon CM. Commentary on meta-analysis of randomized controlled trials for HIV treatment adherence interventions. Research directions and implications for practice. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S36–40. [DOI] [PubMed] [Google Scholar]
- 44.Castro A Adherence to antiretroviral therapy: merging the clinical and social course of AIDS. PLoS Med. 2005;2(12):e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spire B, Duran S, Souville M, Leport C, Raffi F, Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: from a predictive to a dynamic approach. Soc Sci Med. 2002;54(10):1481–96. [DOI] [PubMed] [Google Scholar]
- 46.Lazarus R, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- 47.Cohen S, McKay G. Social support, stress, and the buffering hypothesis: a theoretical analysis In: Baum A, Singer JE, Taylor SE, editors. Handbook of psychology and health. 4th ed. Hillsdale, NJ: Erlbaum; 1984. p. 253–67. [Google Scholar]
- 48.Cutrona C Stress and social support: in search of optimal matching. J Soc Clin Psychol. 1990;9:3–14. [Google Scholar]
- 49.Boyer S, Marcellin F, Ongolo-Zogo P, et al. Financial barriers to HIV treatment in Yaounde, Cameroon: first results of a national cross-sectional survey. Bull World Health Organ. 2009;87(4): 279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byakika-Tusiime J, Crane J, Oyugi JH, et al. Longitudinal antiretroviral adherence in HIV? Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav 2009;13(Suppl 1):82–91. [DOI] [PubMed] [Google Scholar]
- 51.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in Southwestern Uganda: a qualitative study. AIDS Behav. 2009. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangsberg DR. Modified directly observed therapy to improve HIV treatment outcomes: little impact with potent, once-daily therapy in unselected antiretroviral-naive patients. Curr HIV/ AIDS Rep. 2009;6(4):173–4. [DOI] [PubMed] [Google Scholar]
- 53.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007; 46(2):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48(5):611–9. [DOI] [PubMed] [Google Scholar]