Introduction
Multiple sclerosis (MS) is the most prevalent chronic inflammatory disease of the central nervous system (CNS), affecting >2 million people worldwide (~400,000 in the United States),1 and currently incurable. It is punctuated by fully or partially reversible episodes of neurological disability, usually lasting days to weeks. Typical presenting syndromes include, but are not limited to, monocular visual loss due to optic neuritis, limb weakness or sensory loss due to transverse myelitis, double vision due to brainstem dysfunction, or ataxia due to a cerebellar lesion.2 After typically 10–20 years, many of those affected develop a “progressive” clinical course, eventually manifesting impaired mobility and cognition; ~15% have a progressive course from onset. More than a dozen disease-modifying medications are available to reduce the frequency of transient episodes of neurological disability and limit the accumulation of focal white matter lesions on MRI. No medication fully prevents or reverses progressive neurological deterioration, characterized most commonly by impaired ambulation, loss of bladder control, and slowed cognitive processing, but whether disease-modifying medications can delay clinical progression is controversial.3–5 The annual economic cost in the United States is ~$10 billion.6
Pathology
The pathological conception of MS as a disseminated “plaque-like sclerosis” was established ~150 years ago; indeed, the demonstration of dissemination – in space (disease-related changes in multiple CNS regions, including white matter, gray matter, brainstem, spinal cord, and optic nerve; Figure 1) and time – forms the cornerstone of MS diagnosis. Our understanding of the details of that pathology, and especially how it evolves over time, has been revolutionized with modern techniques such as immunohistochemistry and magnetic resonance imaging (MRI).
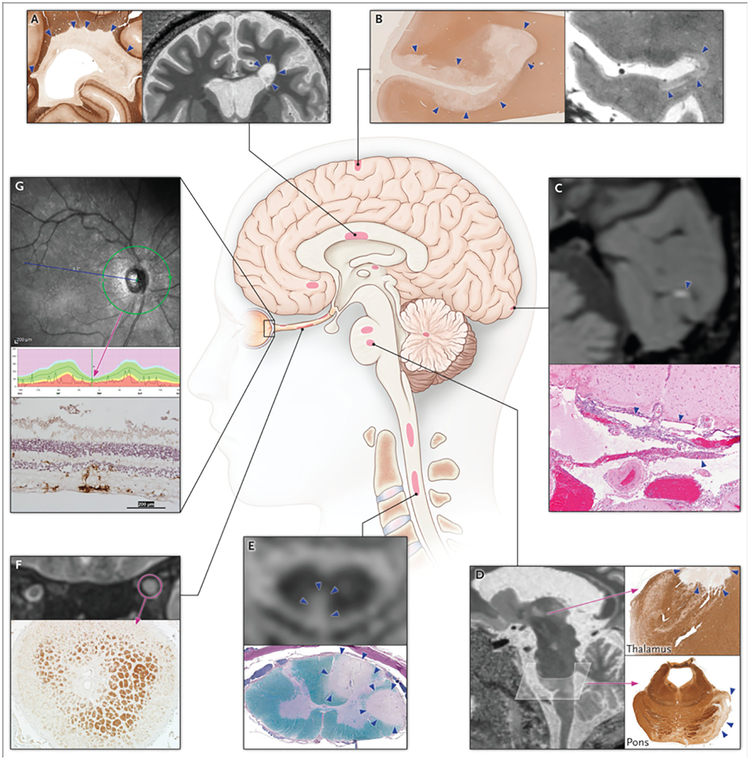
Figure 1. Topography of multiple sclerosis lesions.
Schematic of lesion location, calling out imaging and pathological examples, in the (A) periventricular white matter; (B) subpial cortex; (C) leptomeninges; (D) thalamus and pons; (E) spinal cord; (F) optic nerve; and (G) retina. (A, B, D) 7-tesla MRI of a 40-year-old woman with relapsing-remitting MS, with similar pathological findings (in different cases) highlighted by immunohistochemistry directed against myelin proteolipid protein. (C) 3-tesla post-gadolinium MRI of a 35-year-old woman with secondary progressive MS, with corresponding pathological findings in the meninges of a different case (hematoxylin and eosin stain). (E) 3-tesla MRI of a 60-year-old woman with relapsing-remitting MS and corresponding pathological findings in a different case (Luxol fast blue-periodic acid Schiff stain). (F) 3-tesla MRI of a 31-year-old woman with relapsing-remitting MS and corresponding pathological findings in a different case (anti-proteolipid protein immunohistochemistry). (G) Spectral-domain optical coherence tomography reconstruction showing thinning of the peripapillary retinal nerve fiber layer. The normal range of retinal thickness is shown in green, and for this particular individual (black line) the retina is thinner than 99% of control eyes. The bottom panel shows corresponding pathological findings in a different case (immunohistochemistry for Iba-1, a microglial marker, with hematoxylin counterstain). Lesions are denoted with arrows or circles.
MS lesions can appear throughout the CNS and are most easily recognized in the white matter as focal areas of demyelination, inflammation, and glial reaction. Evidence from MRI and pathology (biopsies and autopsies) indicates that the earliest stages of white matter demyelination (known as “early active white matter lesions”) are heterogeneous7 and evolve over the course of months. Interestingly, regardless of the particular immunological pattern of early demyelination (Figure 2), analysis of active lesions, over both time and space, suggests that a single immune-effector mechanism dominates in each person.8 Consistent with this notion is the observation that plasma exchange, which removes pathogenic antibodies from the circulation, ameliorates relapses that are refractory to initial treatment with glucocorticoids only in patients whose active lesions contain immunoglobulin and complement,9 and that cerebrospinal fluid (CSF) profiles differ by lesion pattern.10 The identification of noninvasive biomarkers that correlate with active lesion patterns will facilitate the design of personalized therapeutic strategies, as current MS treatment algorithms may not adequately address the underlying pathogenic heterogeneity of this complex disease.
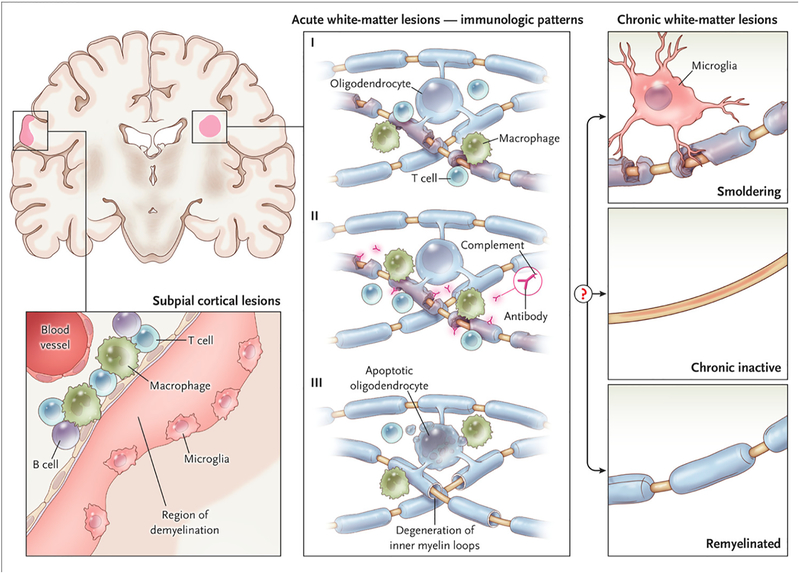
Figure 2. White and gray matter lesions.
Early active white matter demyelination falls into three major categories. The most common types (Patterns I and II) show a background of mononuclear phagocytes with perivascular and parenchymal T-cell infiltration; Pattern II is further distinguished by immunoglobulin and complement deposition. In ~25% of biopsied active lesions (Pattern III), oligodendrocyte apoptosis is accompanied by a “dying-back” oligodendrogliopathy, starting at the “inner tongue.” These lesions show resemble viral, toxic, and ischemic processes, and can be destructive. After the acute phase, factors that remain poorly understood determine whether surviving axons in a lesion are invested by a thin myelin sheath (“remyelinated”), whether inflammation resolves without remyelination (“chronic inactive”), or whether inflammation and slow myelin degeneration persist (“smoldering”). Smoldering lesions are most common in progressive MS. The subpial cortical lesion, which is also more common in progressive MS, is characterized by demyelination of the superficial cortex, possibly associated with inflammation in the overlying leptomeninges and sparse microglia at the border between demyelinated and myelinated neuropil.
What determines the long-term fate of a given lesion – whether inflammation resolves or “smolders,” or whether it remyelinates – is not well understood. Recent data from longitudinal imaging studies suggest that lesions that form in younger people may repair more effectively,11 consistent with preclinical work indicating that age strongly modulates immune-mediated regenerative processes.12,13 What remains unclear is whether lesions can remyelinate years after a smoldering lesion is established and whether remyelinated lesions have heightened susceptibility to recurrent demyelination. High-resolution, ultrahigh-field (7-tesla) MRI shows promise as a tool for noninvasive lesion staging,14 and future studies should investigate the relationship between lesion outcomes and clinical status.
Myelin is not exclusive to white matter, and demyelination in MS also involves gray matter.15–17 About half of cortical lesions are perivascular. Often, the inflamed vessel is near the leukocortical junction, and demyelination affects both gray and white matter; sometimes, a small penetrating cortical vein is involved, and only central cortical layers are affected. Cortical lesions are less inflammatory than their white matter counterparts and have substantially less blood-brain-barrier (BBB) permeability.18
The remaining cortical lesions do not arise from a single cortical vessel but rather appear to proceed inward from the pial surface of the brain. In autopsies after decades of disease, most such lesions are inactive, in contrast to subpial lesions from early MS, which are inflammatory and topographically associated with diffuse and focal leptomeningeal inflammatory aggregates (especially when captured in biopsies).17 Subpial lesions can be extensive and are often found on flanking cortical banks within a sulcus, strongly suggesting a leptomeningeal origin. Leptomeningeal inflammation can organize into self-sustaining structures akin to tertiary lymphoid follicles.19 Although MRI supports an association between leptomeningeal inflammation and subpial cortical demyelination,20 robust detection methods are lacking; the natural history of such lesions – and their responsiveness to therapy – remain unknown.
Spinal cord lesions are a major source of clinical disability. Perivascular and circumferential demyelination is often highly inflammatory and can involve gray matter.21 Spinal cord atrophy results from focal inflammatory demyelination and remote neuroaxonal degeneration.22 It is detectable by MRI, and the cross-sectional area of the spinal cord is therefore a promising outcome measure for clinical trials.23,24
Part of the CNS, the optic nerve is also a major target in MS, and loss of the contiguous retinal ganglion cells is well documented.25 Retinal damage can be assessed in vivo by optical coherence tomography,26 which shows, remarkably, substantial thinning of the retinal nerve-fiber and ganglion cell layers despite their lack of myelin. Thinning results from injury to axons in the optic nerve, which are derived from retinal ganglion cells, and which succumb to a dying-back process following retrobulbar inflammatory demyelination in acute optic neuritis. Recent studies clearly show concomitant retinal ganglion cell loss27 even in the absence of clinical optic neuritis, presumably reflecting either subclinical optic nerve inflammation or retrograde trans-synaptic degeneration.
Epidemiology
It is not known whether MS has a single or multiple causes, and rarely (if ever) has a specific etiological trigger been identified. Nonetheless, various genetic and environmental risk factors have been demonstrated (Figure 3).28 For unknown reasons, roughly three-quarters of people with MS are women, as is common in diseases that are considered autoimmune. Those with an affected first-degree relative have 2–4% risk for developing MS (compared to ~0.1% in the general population), and concordance in monozygotic twins is 30–50%. Genome-wide association studies, based on samples assembled from thousands of people with MS and matched controls, have identified >200 gene variants that raise the risk of MS, of which the most significant remains the human leukocyte antigen DRB1*1501 haplotype (odds ratio~3). Most risk alleles are associated with immune-pathway genes, consistent with the notion that autoimmune mechanisms are paramount in the development of clinical MS. To date, no validated genetic risk factor is known to influence clinical course, a limitation that reflects the difficulty in measuring disease severity in a disease that evolves over decades.
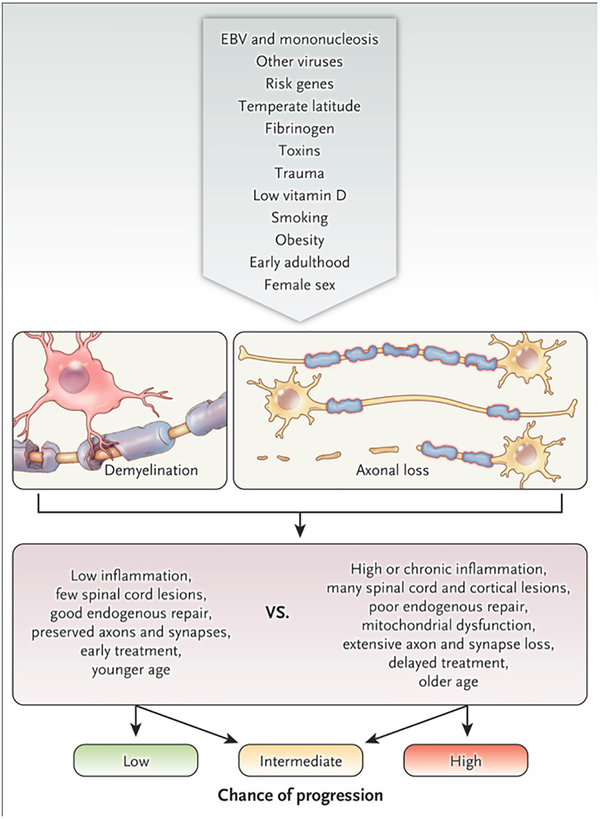
Figure 3. Risk factors, triggers, modifiers, and disease courses.
It is exceedingly unlikely that multiple sclerosis will ultimately be attributed to a single cause. Rather, the genetic and environmental factor or combination of factors that predispose to and initiate the disease, and that modify its course, are highly diverse from one person to the next. The top row of the figure depicts the funneling of proposed factors, for which varying levels of evidence exist, into the development of inflammatory, demyelinating lesions with heterogeneous axonal loss (second row). The third row lists features of the lesions and their consequences that are generally salutary or deleterious and that modify the chance of progression (bottom row).
On the environmental side, major risk factors include geographical latitude (higher incidence in more temperate climates), which may reflect seasonal changes in sunlight exposure influencing vitamin D levels or pathogens prevalent in these regions, although a genetic contribution is possible as well. Tobacco exposure, obesity, and mononucleosis are also associated with enhanced risk for developing MS. Mononucleosis results from infection by Epstein-Barr virus in the post-pubertal population, and only a minority of people with a history of mononucleosis (and a tiny minority of all those infected with the nearly ubiquitous Epstein-Barr virus) eventually develop MS. Viruses other than Epstein-Barr have been suggested as potential causes of MS or MS-related disease activity, but none has been definitively proven. Some of these may act as molecular mimics, whereas others may interfere with mechanisms that normally limit self-reactive cells. Differential susceptibility is reflected in the mouse model for MS, experimental autoimmune encephalomyelitis (EAE), such that specific myelin antigens are required to induce EAE in different strains of mice.29 Along these lines, an interesting set of experiments showed that components of the intestinal microbiome can also strongly influence the propensity to develop EAE, especially in genetically predisposed strains with transgenes for myelin recognition by B and T cells,30 and evidence for a similar phenomenon in MS patients is beginning to emerge.31,32 Overall, the mechanisms by which genetic polymorphisms and environmental exposures raise the risk of developing MS remain the subject of intense investigation.
Pathogenesis
Tissue damage in MS results from a complex and dynamic interplay between the immune system, glia (myelin-making oligodendrocytes and their precursors, microglia, and astrocytes), and neurons (Figure 4). Although there is debate about whether the root cause of MS is intrinsic to the CNS or extrinsic, studies in animal models, particularly EAE in mice and marmosets, together with analysis of immune cells and their products in CSF and blood of humans, have disclosed a critical role for adaptive immunity.29 However, despite the fact that some disease-modifying therapies first shown to ameliorate EAE eventually reached clinical practice, differences between EAE and MS are myriad and have a variety of causes, including the genetic and environmental heterogeneity of human beings relative to laboratory mouse strains, as well as a complex immune process in MS that clearly involves T cells (the major driver of EAE) as well as B cells, antibodies, and cells of the innate immune system (as described below). Moreover, although some animal models show clinical progression, none recapitulates the spectrum of critical pathological features of MS.33 Genetic data suggests that the pathogenesis of MS shares important features with a variety of non-CNS autoimmune diseases.34
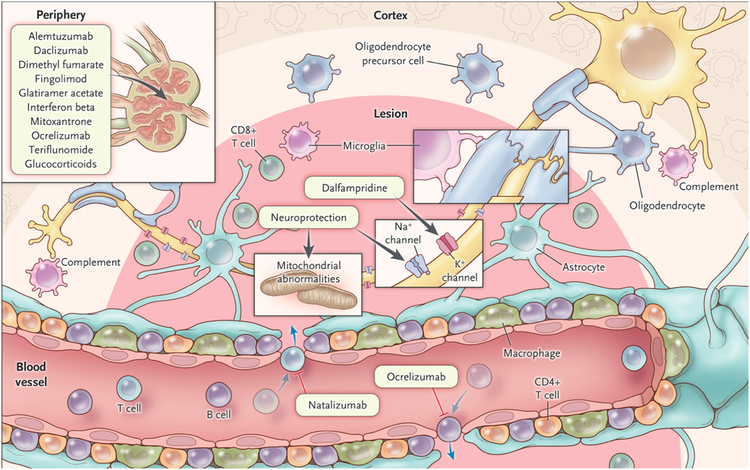
Figure 4. Cells, molecules, and therapies.
Simplified schematic depiction of major cell types within white matter MS lesions, along with several current and promising therapeutic targets in the central nervous system and in the periphery. More detailed descriptions can be found in the text.
On the T-cell side, both helper (CD4+) and cytotoxic (CD8+) T cells have been described in MS lesions: CD4s are more concentrated in the perivascular “cuff,” whereas CD8s are widely distributed within the parenchyma.35 Drugs that limit T-cell access to the CNS can reduce or eliminate new MS lesions. However, T cells reactive to myelin antigens have been observed in similar proportions in individuals with and without MS, suggesting either that these cells are dysfunctional in MS or that other immune factors also play critical roles.
Due to the early and dramatic success of B-cell-depleting antibodies in limiting MS lesion formation and clinical disease activity, there is renewed attention on the role of B cells.36 It has long been known that the CSF of most people with MS harbors unique antibodies (“oligoclonal bands”) produced within the CNS. There is evidence that the antibody-producing function of B-lineage cells is important in some MS lesions.7 However, due to the rapidity of the clinical response to B-cell depletion (as early as 8–12 weeks), even before the reduction of circulating immunoglobulin, it seems more likely that other functions of B cells, including antigen presentation to helper T cells and cytokine production, are more relevant.
Cells of the innate immune system are especially important in MS pathogenesis.37 Blood-borne macrophages infiltrate active MS lesions and remove myelin debris and inflammatory byproducts; classically and alternatively activated macrophages, as well as mixed populations, have been described in these lesions. Microglia, the primary endogenous phagocytes of the CNS, are abundant in MS lesions, but whether their role is pathogenic or protective – or both – remains uncertain.38 Microglial activation has been observed in the white matter of MS autopsy specimens, often remote from established lesions,39 and may represent the earliest stage of lesion development (as is the case in animal models40). Once activated, microglia and macrophages are pathologically indistinguishable, but recent progress using gene-expression technology has opened the door to unraveling their separate contributions, potentially enabling targeted therapy development.41 Studies in animals have suggested that monocyte/macrophage populations strongly influence myelin regeneration.13,42
Disturbance in the blood brain barrier is an important step in the development of white matter lesions, which show evidence of gadolinium extravasation early in their development. Abnormal vascular permeability precedes inflammatory demyelination in animal models40 and potentially in MS.43 Provocative studies in mice have shown that leakage of a key plasma protein (fibrinogen),44 or even secretion of a bacterial toxin,45 can trigger inflammatory demyelination by a cascade that involves microglial activation and subsequent adaptive immunity. In early MS lesions, vessels near the lesion center become permeable to gadolinium, which then diffuses passively into enlarged interstitial spaces; days later, the central breach in the blood brain barrier begins to repair, while small capillaries at the lesion edge become permeable – perhaps as part of the early wound-healing process.46 Leptomeningeal inflammation can also contribute to vascular permeability, but this appears to be a chronic process.20
Glial cell biology
Acute MS plaques demonstrate activation of astrocytes and microglia and sometimes caspase-independent oligodendrocyte apoptosis.7 Microglia are prominent in white matter lesions but are less activated in gray matter.18 Importantly, microglia play dual roles, sometimes mediating inflammation but in other circumstances promoting repair by clearance of myelin debris.47 In gray matter, microglia may limit damage through pruning of dysfunctional synapses that express classical complement cascade proteins (C1Q and C3). This pruning process may become pathological if activated astrocytes promote aberrant expression of complement at synapses, thereby accelerating degeneration.48 Since astrocytes are a major component of the MS plaque, they are well positioned to enhance inflammation by releasing effector molecules, but they may also limit damage by taking up glutamate, providing metabolic support to axons, and maintaining the blood brain barrier.49
An under-emphasized but surprisingly common cell (~10% of all CNS cells) is the oligodendrocyte precursor cell, which expresses the proteoglycan NG2.50 Oligodendrocyte precursor cells can differentiate into oligodendrocytes and are present even late in life,51 but in MS they are often arrested at the plaque edge, or they may differentiate into premyelinating oligodendrocytes but fail to wrap myelin.52 Thus, promoting oligodendrocyte precursor cell differentiation is an attractive strategy to enhance endogenous remyelination, but this must be balanced against the potential of oligodendrocyte precursor cells to respond to cytokines and thereby participate in inflammation themselves.53,54 Furthermore, oligodendrocytes may become dysfunctional even without dying, causing tissue damage through loss of trophic support to axons; whether such dysfunctional oligodendrocytes can participate in repair is unclear.
Axon biology
Although relative axonal sparing in the face of profound demyelination is a hallmark of MS pathology, axonal transections are frequent, especially acutely.55 Studies with 2-photon microscopy in animal models have begun to elucidate relevant cellular and molecular processes, some potentially reversible.56 In chronically demyelinated lesions, denuded axons remain vulnerable and can degenerate slowly; possible mechanisms include impaired axonal transport, mitochondrial dysfunction, and increased energy demands related to upregulation of ion channels.57 Importantly, adaptive immunity – critical for new white matter lesion formation – is much less prominent in the slow neurodegeneration of progressive MS, highlighting the importance of glial activation and secondary mechanisms of injury.
Biomarkers
The most important diagnostic and prognostic MS biomarker – particularly early in the disease course – is MRI, which is currently the only technique that can interrogate the entire CNS in vivo. Unfortunately, the slow rate of progression in time frames relevant for clinical monitoring or clinical trials, together with heterogeneous pathogenic mechanisms and the impracticality of directly sampling CNS tissue (as opposed to blood or cerebrospinal fluid), have limited biomarker development for progressive MS.
Magnetic resonance imaging
By MRI, Inflammatory demyelination is easily visible, as are blood brain barrier changes that accompany its early development. Figure 1 shows the in vivo MRI appearance of lesions in the periventricular white matter (A), thalamus and brainstem (D), spinal cord (E), and optic nerve (F). Since 2000, MRI has been the key diagnostic test when individuals present with a clinical syndrome suggestive of MS, and the most recent criteria58 – when applied carefully59 – allow accurate diagnosis with a single scan. MRI diagnostic criteria are revised as new data accumulate, and standardized protocols for routine use have been proposed.60,61 MRI is also critical in the development of new disease-modifying therapies, as new lesions are an order of magnitude more frequent than clinical relapses.62 Indeed, the effect on new lesion formation by MRI in small proof-of-concept studies strongly predicts the effect on relapses in definitive trials.63 Furthermore, MRI findings consistent with MS have been observed in healthy individuals scanned for other purposes (such as research), and up to 50% of individuals with this so-called “radiologically isolated syndrome” ultimately develop clinical MS, sometimes with a primary progressive course.64,65
Neurodegeneration in MS is best captured on MRI by measuring the size of the brain or spinal cord. An abnormally low “brain parenchymal fraction” – a measure of brain size relative to intracranial capacity – can be taken as surrogate evidence of prior disease-related brain atrophy. In cohort studies, CNS atrophy has been documented even before clinical presentation.66,67 Atrophy complements lesion-based biomarkers,68 and proof-of-concept clinical trials using atrophy as primary outcome have begun to appear.69,70 Recent studies of CNS atrophy have focused on specific gray matter structures (neocortex, thalamus).71–73
As conventional MRI biomarkers have not achieved strong correlation with clinical status on a population level, likely due to the heterogeneous presentation and course of MS and to the inherent variability of clinical measures, there has been a trend toward the use of imaging to investigate MS pathology and pathogenesis, including perivascular inflammation, cortical and spinal cord lesion development, myelin loss and regeneration, innate immune activation, leptomeningeal inflammation, and network function.14 Such research has been facilitated by the advent of 7-tesla MRI, and to a lesser extent molecular tracers detectable by positron emission tomography. A particularly exciting innovation has been the use of optical coherence tomography to assess the retina rapidly at micron-level resolution. Retinal ganglion cell axon loss results in easily detectable retinal thinning, which importantly tracks with MRI changes in the brain74 and can predict disability evolution on a cohort level.75
Blood and cerebrospinal fluid
Clonal expansion of immunoglobulin-secreting B cells and plasma cells in the CNS results in the characteristic finding of CSF-specific oligoclonal bands.76 While the targets of these immunoglobulins are probably multifaceted, their presence implies a CNS-restricted immune response. However, the specificity of oligoclonal bands for MS is poor, and infections can cause the same pattern. Currently, no externally validated blood immune marker has adequate sensitivity and specificity to be used for MS diagnosis, probably reflecting the genetic and environmental heterogeneity of MS. CSF and serum neurofilament light chains are promising in their ability to reflect axonal pathologic processes in the CNS at the cohort level,77 and there is ongoing interest in various types of noncoding RNA molecules that can affect gene expression.78 Whether these approaches are useful in individuals remains unclear.
Therapies
As of October 2017, the US Food and Drug Administration has approved 15 medications for modifying the course of MS: 5 preparations of interferon beta; 2 preparations of glatiramer acetate; the monoclonal antibodies natalizumab, alemtuzumab, daclizumab, and ocrelizumab (the first B-cell targeted therapy); the chemotherapy mitoxantrone; and the small-molecule oral agents fingolimod, dimethyl fumarate, and teriflunomide. Dalfampridine has been approved as a symptomatic therapy to improve walking speed. It is beyond the scope of this article to discuss the relative benefits, risks, modes of action, and routes of administration of these various medications (though some targets are depicted in Figure 4), except to say that all are approved for relapsing-remitting MS and reduce, to various extents, the likelihood of developing new white matter lesions, clinical relapses, and stepwise accumulation of disability. Based on the ability of several of these medications to delay a formal diagnosis of MS following an initial attack, there has been a general move toward early treatment, though as discussed above, the long-term value of this approach with respect to preventing progressive MS remains uncertain. The recent approval of ocrelizumab for primary progressive MS is a promising step, but the reasons for ocrelizumab’s ability to slow progression79 remain uncertain. Another important trend has been to escalate treatment with a target of “no evidence of disease activity,” as evidenced by absence of new lesions, relapses, disability progression and, more recently, tissue atrophy;80,81 however, it is doubtful that MS can be fully arrested with current therapies. Several incipient multicenter studies will compare early intensive treatment with more conventional treatment escalation approaches.
Small-scale studies have shown that immunoablation followed by autologous hematopoietic stem cell transplantation may be a highly durable and effective – and increasingly safe – therapy.82 The tolerability and high efficacy of B-cell modulating therapies is a welcome development, though opportunistic infections can occur rarely, and post-marketing studies will need to monitor long-term side-effects. There are early-stage efforts to interfere with specific T-cell populations thought to drive MS, stemming from data that certain key subsets of helper T cells, including those that express both interferon-gamma and interleukin-17, are important.83,84 Such approaches may involve specific inhibition, clonal deletion, or tolerization. Prior attempts at targeting cytokines have been unsuccessful85 or even deleterious,86 probably due to incomplete understanding of the roles of different forms of cytokines and their receptors, as well as compensatory pathways. The innate immune system has not been specifically targeted in large-scale MS trials, and given the high likelihood that this system can be both protective and deleterious, such efforts must be approached cautiously. Nonetheless, the ubiquity of innate immune cells in and around MS lesions underscores the need for further research.
Beyond the immune system, a great deal of work has revolved around tissue repair and protection. On the repair side, small studies have preliminarily reported mixed results for therapies that promote endogenous remyelination through various pathways.87 Interestingly, based on preclinical data including in vitro screens and testing in models such as EAE, several approved drugs (targeting, for example, nuclear hormone receptor, histaminic, cholinergic (muscarinic), and adrenergic pathways) are being tested for remyelination or myelin protection. Transplantation of neural or oligodendrocyte precursor cells into the brain is effective in animal models, but well-designed clinical trials have not been undertaken in MS, and it is likely that promotion of endogenous remyelination will prove more fruitful and feasible, especially if the inhibitory factors inherent in the MS plaque can be overcome.52 A challenge for remyelination trials is the lack of a robust, easily deployable biomarker of success. Visual evoked potentials have been used in small studies, but standardization is difficult and technical variability high. The specificity of high-resolution imaging-based markers for myelin regeneration remains questionable. Nevertheless, MRI is highly sensitive to changes in myelin, and such sensitivity can be exploited in early proof-of-concept trials.88
Axonal protection is actively being examined. Results from initial clinical trials of a wide variety of drugs have been published or reported, with several medium-to-large studies currently underway.89 There is an emerging consensus that slowing the rate of cerebral or spinal cord atrophy is a feasible goal, which at the proof-of-concept stage can be undertaken in several hundred people over a 1–2-year period.90 However, definitive proof of neuroprotection – an elusive goal in many neurological conditions – awaits larger studies with clinical endpoints.
Conclusions and future directions
Meaningful advances in basic immunology, myelin biology, and neuroscience, together with a global focus on halting progressive accumulation of disability,91 have opened the promise of a multipronged understanding of, and therapeutic attack on, MS. At the same time, a renewed focus on lesion development and repair – more broadly conceived to include lesions in white matter, gray matter, and leptomeninges – should ultimately unify lines of research, particularly on the side of fluid and imaging biomarkers and clinical outcomes, which have sometimes strayed too far from the causative biology. The richest conception of MS will allow appreciation of common pathology, which, in the setting of variable triggers and clinical courses, makes MS among the most heterogeneous, and remarkable, of all neurological disorders.
Acknowledgments
The authors thank Ms. Erina He for drafting the figures and Drs. Martina Absinta, Carlos Pardo, and Yong Guo for providing radiological and histopathological images.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Daniel S. Reich, Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA
Claudia F. Lucchinetti, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA
Peter A. Calabresi, Departments of Neurology and Neuroscience, Johns Hopkins School of Medicine, Baltimore, Maryland, USA
References
- 1.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet 2017;389(10076):1336–46. [DOI] [PubMed] [Google Scholar]
- 3.Zhang T, Shirani A, Zhao Y, et al. Beta-interferon exposure and onset of secondary progressive multiple sclerosis. Eur J Neurol 2015;22(6):990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Signori A, Gallo F, Bovis F, Di Tullio N, Maietta I, Sormani MP. Long-term impact of interferon or Glatiramer acetate in multiple sclerosis: A systematic review and meta-analysis. Multiple Sclerosis and Related Disorders 2016;6:57–63. [DOI] [PubMed] [Google Scholar]
- 5.University of California, San Francisco MS-EPIC Team:, Cree BAC, Gourraud P-A, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Annals of Neurology 2016;80(4):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. Journal of Medical Economics 2013;16(5):639–47. [DOI] [PubMed] [Google Scholar]
- 7.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Annals of Neurology 2000;47(6):707–17. [DOI] [PubMed] [Google Scholar]
- 8.Metz I, Weigand SD, Popescu BFG, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Annals of Neurology 2014;75(5):728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan M, König F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 2005;366(9485):579–82. [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, König FB, Metz I, et al. Pattern II and pattern III MS are entities distinct from pattern I MS: evidence from cerebrospinal fluid analysis. Journal of Neuroinflammation 2017;14(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016;126(7):2597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawji KS, Mishra MK, Yong VW. Regenerative Capacity of Macrophages for Remyelination. Front Cell Dev Biol 2016;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruckh JM, Zhao J-W, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012;10(1):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol 2016;12(6):358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bö L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 2003;62(7):723–32. [DOI] [PubMed] [Google Scholar]
- 16.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128(Pt 11):2705–12. [DOI] [PubMed] [Google Scholar]
- 17.Lucchinetti CF, Popescu BFG, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011;365(23):2188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Annals of Neurology 2001;50(3):389–400. [DOI] [PubMed] [Google Scholar]
- 19.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004;14(2):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015;85(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore CP, Geurts JJG, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler 2009;15(2):180–8. [DOI] [PubMed] [Google Scholar]
- 22.DeLuca GC, Williams K, Evangelou N, Ebers GC, Esiri MM. The contribution of demyelination to axonal loss in multiple sclerosis. Brain 2006;129(Pt 6):1507–16. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Nair G, Vuolo L, et al. In vivo imaging of spinal cord atrophy in neuroinflammatory diseases. Annals of Neurology 2014;76(3):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearney H, Miller DH, Ciccarelli O. Spinal cord MRI in multiple sclerosis--diagnostic, prognostic and clinical value. Nat Rev Neurol 2015;11(6):327–38. [DOI] [PubMed] [Google Scholar]
- 25.Green AJ, Mcquaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 2010;133(Pt 6):1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2017;16(10):797–812. [DOI] [PubMed] [Google Scholar]
- 27.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 2012;135(Pt 2):521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ascherio A, Munger KL. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin Neurol 2016;36(2):103–14. [DOI] [PubMed] [Google Scholar]
- 29.Kipp M, van der Star B, Vogel DYS, et al. Experimental in vivo and in vitro models of multiple sclerosis: EAE and beyond. Multiple Sclerosis and Related Disorders 2012;1(1):15–28. [DOI] [PubMed] [Google Scholar]
- 30.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011;479(7374):538–41. [DOI] [PubMed] [Google Scholar]
- 31.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA 2017;363:201711235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proceedings of the National Academy of Sciences 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 2012;8(11):647–56. [DOI] [PubMed] [Google Scholar]
- 34.Farh KK-H, Marson A, Zhu J, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015;518(7539):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassmann H Mechanisms of white matter damage in multiple sclerosis. Glia 2014;62(11):1816–30. [DOI] [PubMed] [Google Scholar]
- 36.Michel L, Touil H, Pikor NB, Gommerman JL, Prat A, Bar-Or A. B Cells in the Multiple Sclerosis Central Nervous System: Trafficking and Contribution to CNS-Compartmentalized Inflammation. Front Immunol 2015;6(6):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra MK, Yong VW. Myeloid cells - targets of medication in multiple sclerosis. Nat Rev Neurol 2016;12(9):539–51. [DOI] [PubMed] [Google Scholar]
- 38.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 2011;14(10):1227–35. [DOI] [PubMed] [Google Scholar]
- 39.van der Valk P, Amor S. Preactive lesions in multiple sclerosis. Curr Opin Neurol 2009;22(3):207–13. [DOI] [PubMed] [Google Scholar]
- 40.Maggi P, Macri SMC, Gaitán MI, et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Annals of Neurology 2014;76(4):594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci 2014;17(1):131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miron VE, Boyd A, Zhao J-W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 2013;16(9):1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Absinta M, Nair G, Sati P, Cortese ICM, Filippi M, Reich DS. Direct MRI detection of impending plaque development in multiple sclerosis. Neurology: Neuroimmunology & Neuroinflammation 2015;2(5):e145–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu JK, Petersen MA, Murray SG, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun 2015;6:8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linden JR, Ma Y, Zhao B, et al. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. MBio 2015;6(3):e02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaitán MI, Shea CD, Evangelou IE, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Annals of Neurology 2011;70(1):22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science 2013;339(6116):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541(7638):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludwin SK, Rao VT, Moore CS, Antel JP. Astrocytes in multiple sclerosis. Mult Scler 2016;22(9):1114–24. [DOI] [PubMed] [Google Scholar]
- 50.Bergles DE, Richardson WD. Oligodendrocyte Development and Plasticity. Cold Spring Harb Perspect Biol 2015;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 2000;20(17):6404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin RJM, Goldman SA. Glia Disease and Repair-Remyelination. Cold Spring Harb Perspect Biol 2015;7(7):a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia 2015;63(8):1429–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitic M, Karram K, Israel N, et al. NG2 plays a role in neuroinflammation but is not expressed by immune cells. Acta Neuropathol 2017;97:595. [DOI] [PubMed] [Google Scholar]
- 55.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338(5):278–85. [DOI] [PubMed] [Google Scholar]
- 56.Nikić I, Merkler D, Sorbara C, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 2011;17(4):495–9. [DOI] [PubMed] [Google Scholar]
- 57.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. The Lancet Neurology 2015;14(2):183–93. [DOI] [PubMed] [Google Scholar]
- 58.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology 2016;87(13):1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. American Journal of Neuroradiology 2016;37(3):394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rovira A, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471–82. [DOI] [PubMed] [Google Scholar]
- 62.Harris JO, Frank JA, Patronas N, McFarlin DE, McFarland HF. Serial gadolinium-enhanced magnetic resonance imaging scans in patients with early, relapsing-remitting multiple sclerosis: implications for clinical trials and natural history. Annals of Neurology 1991;29(5):548–55. [DOI] [PubMed] [Google Scholar]
- 63.Sormani MP, Sormani MP, Bruzzi P, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 2013;12(7):669–76. [DOI] [PubMed] [Google Scholar]
- 64.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS ONE 2014;9(3):e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kantarci OH, Lebrun C, Siva A, et al. Primary Progressive Multiple Sclerosis Evolving From Radiologically Isolated Syndrome. Annals of Neurology 2016;79(2):288–94. [DOI] [PubMed] [Google Scholar]
- 66.De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010;74(23):1868–76. [DOI] [PubMed] [Google Scholar]
- 67.Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm 2015;2(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sormani MP, Sormani MP, Arnold DL, Arnold DL, De Stefano N, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Annals of Neurology 2014;75(1):43–9. [DOI] [PubMed] [Google Scholar]
- 69.Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 2014;383(9936):2213–21. [DOI] [PubMed] [Google Scholar]
- 70.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016;50:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher E, Nakamura K, Lee JC, You X, Sperling B, Rudick RA. Effect of intramuscular interferon beta-1a on gray matter atrophy in relapsing-remitting multiple sclerosis: A retrospective analysis. Mult Scler 2015;22(5):668–76. [DOI] [PubMed] [Google Scholar]
- 72.Zivadinov R, Havrdova E, Bergsland N, et al. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology 2013;268(3):831–41. [DOI] [PubMed] [Google Scholar]
- 73.Schlaeger R, Papinutto N, Panara V, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Annals of Neurology 2014;76(4):568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saidha S, Louzi Al O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Annals of Neurology 2015;78(5):801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martínez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 2016;15(6):574–84. [DOI] [PubMed] [Google Scholar]
- 76.Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol 2015;161(1):51–8. [DOI] [PubMed] [Google Scholar]
- 77.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016;22(12):1550–9. [DOI] [PubMed] [Google Scholar]
- 78.Guerau-de-Arellano M, Alder H, Ozer HG, Lovett-Racke A, Racke MK. miRNA profiling for biomarker discovery in multiple sclerosis: From microarray to deep sequencing. Journal of Neuroimmunology 2012;248(1–2):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2017;376(3):209–20. [DOI] [PubMed] [Google Scholar]
- 80.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Multiple Sclerosis and Related Disorders 2015;4(4):329–33. [DOI] [PubMed] [Google Scholar]
- 81.Kaunzner UW, Al-Kawaz M, Gauthier SA. Defining Disease Activity and Response to Therapy in MS. Curr Treat Options Neurol 2017;19(5):20. [DOI] [PubMed] [Google Scholar]
- 82.Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol 2017;13(7):391–405. [DOI] [PubMed] [Google Scholar]
- 83.Carbajal KS, Mironova Y, Ulrich-Lewis JT, et al. Th Cell Diversity in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J Immunol 2015;195(6):2552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwong B, Rua R, Gao Y, et al. T-bet-dependent NKp46(+) innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. The Lancet Neurology 2008;7(9):796–804. [DOI] [PubMed] [Google Scholar]
- 86.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1999;53(3):457–65. [PubMed] [Google Scholar]
- 87.Tran JQ, Rana J, Barkhof F, et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurology: Neuroimmunology & Neuroinflammation 2014;1(2):e18–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reich DS, White R, Cortese IC, et al. Sample-size calculations for short-term proof-of-concept studies of tissue protection and repair in multiple sclerosis lesions via conventional clinical imaging. Mult Scler 2015;21(13):1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nandoskar A, Raffel J, Scalfari AS, Friede T, Nicholas RS. Pharmacological Approaches to the Management of Secondary Progressive Multiple Sclerosis. Drugs 2017;77(8):885–910. [DOI] [PubMed] [Google Scholar]
- 90.Altmann DR, Jasperse B, Barkhof F, et al. Sample sizes for brain atrophy outcomes in trials for secondary progressive multiple sclerosis. Neurology 2009;72(7):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fox RJ, Thompson A, Baker D, et al. Setting a research agenda for progressive multiple sclerosis: The International Collaborative on Progressive MS. Mult Scler 2012;18(11):1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]