Abstract
Background
Osteosarcoma (OS) is one of the most difficult cancers to treat due to its resistance to chemotherapy. The essential role played by Mcl-1 in promoting chemoresistance has been observed in a variety of cancers, including OS, while the underlying mechanism remains unclear.
Methods
We investigated the expression of Mcl-1 in 42 paired OS specimens obtained before and after adjuvant chemotherapy, and its correlation with clinicopathological characteristics. Loss and gain of function studies were performed to analyze the effects of Mcl-1 modulations on the chemosensitivity, and the mechanism involved in the deregulation of Mcl-1 in OS cells.
Results
In OS specimens, the expression of Mcl-1 was significantly upregulated after chemotherapy, and high Mcl-1 expression was associated with poorer overall survival and an increased recurrence rate. Furthermore, we demonstrated that chemotherapy-driven increased Mcl-1 decreased chemosensitivity by promoting tumour proliferation and inhibiting DNA damage. Moreover, Mcl-1 was found to be a direct target of miR-375 in OS cells. The knockdown of Mcl-1 phenocopied miR-375 downregulation, and the overexpression of miR-375 rescued the effects of cisplatin-induced DNA damage mediated by Mcl-1.
Conclusion
Our data indicated that chemotherapy-driven increase in the expression of Mcl-1 plays a critical role in chemoresistance, and the intervention of the miR-375/Mcl-1 axis may offer a novel strategy to enhance chemosensitivity in OS treatment.
Keywords: osteosarcoma, Mcl-1, chemotherapy resistance, miR-375, cisplatin
Introduction
Osteosarcoma (OS) is common in children and adolescents.1 The standard clinical treatment used for newly diagnosed OS patients consists of surgery in combination with multi-agent chemotherapy with an agent such as cisplatin (Cis), and this regimen has remained unchanged over the past 30 years.2 Despite great advancement in surgical techniques and neoadjuvant chemotherapy, the treatment failure rate of OS is still approximately 30%, which is mainly as a result of multidrug resistance.3 Hence, elucidating and targeting the mechanisms that promote cell survival during chemotherapy and lead to chemoresistance are necessary to improve the treatment efficacy of OS.
Our previous research has shown that chemotherapy by itself could stimulate the overexpression of multiple tumour-associated genes, which contributes to therapeutic resistance.4 Emerging evidence has found that the expression of MCL1 was significantly upregulated in OS patients with poor chemosensitivity and prognosis.5 MCL1 (Mcl-1 is a transcription product) is a member of the multi-BCL-2 homology (BH) domain (BH1−BH4)-containing family of antiapoptotic proteins that shows the potential to cause chemotherapy resistance in a variety of tumours.6–8 An increasing number of studies have shown that MCL1 ablation may be a promising strategy to attenuate chemoresistance in OS patients.9,10 However, the underlying mechanism by which Mcl-1 modulates chemoresistance in OS is still largely unknown.
In this study, we aimed to explore the molecular mechanisms that are involved in the effects of Mcl-1 on chemoresistance in OS. Our recent studies showed that the expression of Mcl-1 in OS tissue depends on whether the patient received chemotherapy and chemotherapy-driven increase in Mcl-1 could inhibit Cis responsiveness in OS cells. A comprehensive microRNA (miRNA) analysis found that Mcl-1 expression is regulated by miR-375 and that Mcl-1 knockdown or increased miR-375 expression significantly reduce the viability and migration of OS cells. Thus, the miR-375/Mcl-1 axis is tightly correlated with chemotherapy resistance and may serve as a novel chemotherapy target in human OS.
Materials and Methods
Human OS Tissues
We obtained 42 Paired OS tumor tissues before (by the needle acupuncture technique) and after (by surgery) adjuvant chemotherapy which were used for this research at The Third Xiangya Hospital of Central South University between 2012 and 2018. All patients provided written informed consent, a parent or legal guardian provided written informed consent for the patients under the age of 18 years. This study was approved by the ethics committee of the Third Xiangya Hospital of Central South University and was performed in accordance with the relevant guidelines. Overall survival was calculated from the date of surgery to the date of death or the last follow-up. Recurrence was defined as the emergence of a new lesion according to computed tomography. None of the patients died as a result of operative complications or other factors. The mean age of the patients was 18 years (range, 6–62 years). The last follow-up was May 2019, and the median follow-up was 22 months (range, 7–37 months).
Immunohistochemistry Staining
Tissue sections (5 μm) were immunostained with antibodies against Mcl-1 (1:200; Cell Signaling, USA). Two independent pathologists from the Second Xiangya Hospital who were blinded to the clinical data independently performed the immunohistochemical evaluations. A semiquantitative H-score ranging from 0 to 300 was calculated for each specimen by multiplying the area of the distribution (0–100%) of each staining intensity level by the staining intensity level, as described in previous reports.11
Cell Lines and Transfection
The human OS cell lines (HOS, U2OS and MG63) and foetal osteoblastic 1.19 cells (hFOB 1.19) were obtained from Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% foetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere with 5% CO2, as described in our previous work.12 Cis was purchased from Yuanye Biotech (Shanghai, China) and diluted in PBS.
To predict miRNAs with potential binding sites in the 3′ UTR of Mcl-1, TargetScan (http://www.targetscan.org/) algorithms were used. The miR-375-mimics, Mcl-1 knockdown plasmids (siMcl-1-1 and siMcl-1-2) and Mcl-1 overexpression plasmid (described as Mcl-1) were purchased from GenePharma (Shanghai, China). The detailed protocol used for cell transfection was described in our previous work.4
Luciferase Activity Assay
Luciferase activity was assayed with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) as described in previous reports.11 The activity of the co-expressed Renilla luciferase was used for the normalization of the transfection efficiency.
Western Blot Analysis
The cells were lysed using 1× buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 2 mM EDTA, 1.0% Triton X-100, 1.0% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)), and the protein concentration was detected using a bicinchoninic acid assay kit (BCA kit; CWBiotech). Samples containing 20 μg of total protein were separated on a 10% SDS-polyacrylamide gel by electrophoresis and then transferred onto nitrocellulose membranes (GE Healthcare, Chicago, USA). The membranes were then probed with the primary antibodies, and tubulin was used as an internal control. The following primary antibodies were used for Western blotting: anti-Mcl-1 (1:1000; Cell Signaling, USA) and anti-Tubulin (1:1000, Santa Cruz, USA).
Quantitative RT-PCR
Total RNA from OS cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) as described in our previous work.13 Quantitative real-time PCR was performed using specific primers targeting Mcl-1, miR-375 and U6. The primers used in this study are provided in Table 1.
Table 1.
Quantitative Real-Time PCR Primers
| Gene | Species | Forward Primer | Reverse Primer |
|---|---|---|---|
| MCL1 | Human | CAGCGACGGCGTAACAAAC | ACAAACCCATCCCAGCCTCTTT |
| U6 | Human | CTCGCTTCGGCAGCACATATACT | ACGCTTCACGAATTTGCGTGTC |
| miR-375 | Human | CACAAAATTTGTTCGTTCGGCT | GTGCAGGGTCCGAGGT |
Cell Proliferation Assay
The in vitro growth of cells treated with Cis (Yuanye Biotech, Shanghai, China) was detected with a CCK-8 assay kit (Sangon Biotech, Shanghai, China) as described in our previous reports.14 The cell proliferation curve was generated according to the corresponding normalized OD450 values.
Wound Healing Assay
Twenty-four hours after transfection and Cis treatment, the plates containing cells were scratched using a sterile pipette tip to generate a wound through the confluent monolayer. The wound healing rate was determined after 24 hrs and normalized according to the wound size at 0 hrs as described in our previous work.14
Transwell Migration Assays
The migration assays were conducted by using transwell chamber according to the manufacturer’s protocol (BD Science, Bedford, MA, USA). After treatment, MG63 cells were added to the upper chambers and incubated for 24 h. The migration rates were quantified by counting the migratory cells at least three random fields.
Comet Assay
A single cell gel electrophoresis (comet assay) kit was employed to evaluate DNA damage (Trevigen, Inc., Gaithersburg, MD, USA). After the treatment of cells with Cis, the genomic DNA was purified using a DNA isolation kit (Omega Bio-Tek, Inc., Norcross, GA, USA). Subsequently, the amount of DNA damage was quantified based on comet tail lengths (consisting of the nuclear region and tail) which were scored visually as described in previous work.15
Statistical Analysis
All data were included in statistical analysis performed with GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA). An unpaired Student’s t-test (two-tailed) was used for the comparison of two unpaired groups, and one-way ANOVA was applied for multi-group data comparison. The survival curves were estimated using the Kaplan–Meier method, and the log rank test was used to compute the differences between the curves. All quantified data are presented as the mean ± s.e.m., and the level of statistical significance was set to *p < 0.05.
Results
The Expression of Mcl-1 Was Upregulated in Human OS Tissue After Chemotherapy and Was Related to Poor Prognosis
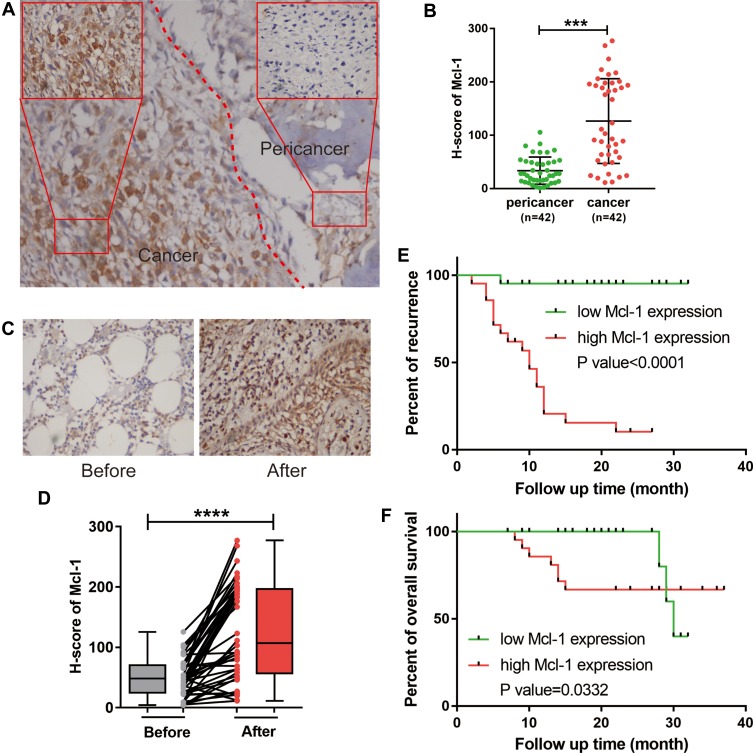
To investigate the expression of Mcl-1 in OS and its association with chemotherapy, we first evaluated Mcl-1 expression by immunohistochemical analysis in 42 paired OS specimens obtained before and after adjuvant chemotherapy. Mcl-1 was located diffusely throughout the cytoplasm and membranes of tumour cells (Figure 1A). However, the levels of cytoplasmic and membranous Mcl-1 expression were significantly higher in tumour than in peritumour tissues (p<0.001) (Figure 1B). Moreover, when compared to that in paired tissues before chemotherapy, the expression level of Mcl-1 was significantly upregulated in OS tissues after adjuvant chemotherapy (p<0.0001) (Figure 1C and D). Importantly, patients whose tissues showed high Mcl-1 staining intensity had significantly poorer overall survival and increased recurrence rates (Figure 1E and F). As shown in Table 2, the expression of Mcl-1 did not correlate with age, pathological type, tumour location or tumour size but significantly correlate with gender (p=0.0133), Enneking preoperative stage (p<0.001), metastasis (p<0.001), recurrence (p<0.001) and death (p=0.0405).
Figure 1.
Upregulation of Mcl-1 in OS after chemotherapy was correlated with poor patient survival. (A) Representative immunohistochemistry (IHC) staining of Mcl-1. (B) Scatter plots showing the evaluated IHC scores in tumour and corresponding peritumour OS specimens, n=42, Student’s t-test, ***p<0.001. (C) Representative images of the immunohistochemical staining of Mcl-1 in paired OS tissues before and after adjuvant chemotherapy. (D) Scatter plots showing the evaluated IHC scores in OS tissue before and after adjuvant chemotherapy, n=42, Student’s t-test, ****p<0.0001. (E, F) Kaplan–Meier plots showing the recurrence rate (E) and overall survival (F) in OS patients categorized by Mcl-1 expression (n=21 for the high Mcl-1 group versus n=21 for the low Mcl-1 group); the p value was determined by a log rank test.
Table 2.
Association Between Mcl-1 Expression and Clinicopathologic Factors of OS Patients
| Characteristics | Total | Mcl-1 Expression | p | ||
|---|---|---|---|---|---|
| 1+ (n, %) | 2+ (n, %) | 3+ (n, %) | |||
| Gender | 0.0133* | ||||
| Male | 23 | 7(30.43) | 12(52.18) | 4(17.39) | |
| Female | 19 | 13(68.42) | 2(10.53) | 4(21.05) | |
| Age (years) | 0.9923 | ||||
| <18 | 27 | 13(48.15) | 9(33.33) | 5(18.52) | |
| ≥18 | 15 | 7(46.67) | 5(33.33) | 3(20.00) | |
| Enneking preoperative staging | <0.001*** | ||||
| I/II stage | 25 | 20(80.00) | 5(20.00) | 0(0.00) | |
| III stage | 17 | 0(0.00) | 9(52.94) | 8(47.06) | |
| Pathological type | 0.1044 | ||||
| Osteoblastic | 24 | 11(45.83) | 10(41.67) | 3(12.50) | |
| Fibroblastic | 12 | 7(58.33) | 3(25.00) | 2(16.67) | |
| Chondroblastic | 4 | 1(25.00) | 0(0.00) | 3(75.00) | |
| Telangiectatic | 2 | 1(50.00) | 1(50.00) | 0(0.00) | |
| Tumor location | 0.2501 | ||||
| Femur | 20 | 12 (60.00) | 6 (30.00) | 2 (10.00) | |
| Tibia | 12 | 10 (83.33) | 2 (16.67) | 0 (0.00) | |
| Humerus | 6 | 4 (66.67) | 0 (0.00) | 2 (33.33) | |
| Other | 4 | 3(75.00) | 1(25.00) | 0(0.00) | |
| Tumor size | 0.5729 | ||||
| ≤50mm | 27 | 13(48.15) | 8(29.63) | 6(22.22) | |
| 50–100mm | 10 | 6(60.00) | 3(30.00) | 1(10.00) | |
| ≥100mm | 5 | 1(20.00) | 3(60.00) | 1(20.00) | |
| Metastasis | <0.001*** | ||||
| Yes | 17 | 0(0.00) | 9(52.94) | 8(47.06) | |
| No | 25 | 20(80.00) | 5(20.00) | 0(0.00) | |
| Recurrence | <0.001*** | ||||
| Yes | 19 | 0(0.00) | 11(57.89) | 8(42.11) | |
| No | 23 | 20(86.96) | 3(13.04) | 0(0.00) | |
| Death | 0.0405* | ||||
| Yes | 5 | 0(0.00) | 4(80.00) | 1(20.00) | |
| No | 37 | 20(54.05) | 10(27.03) | 7(18.92) | |
Note: *p<0.05, ***p< 0.001, one-way ANOVA.
Mcl-1 Modulates the Sensitivity of OS Cells to Cis
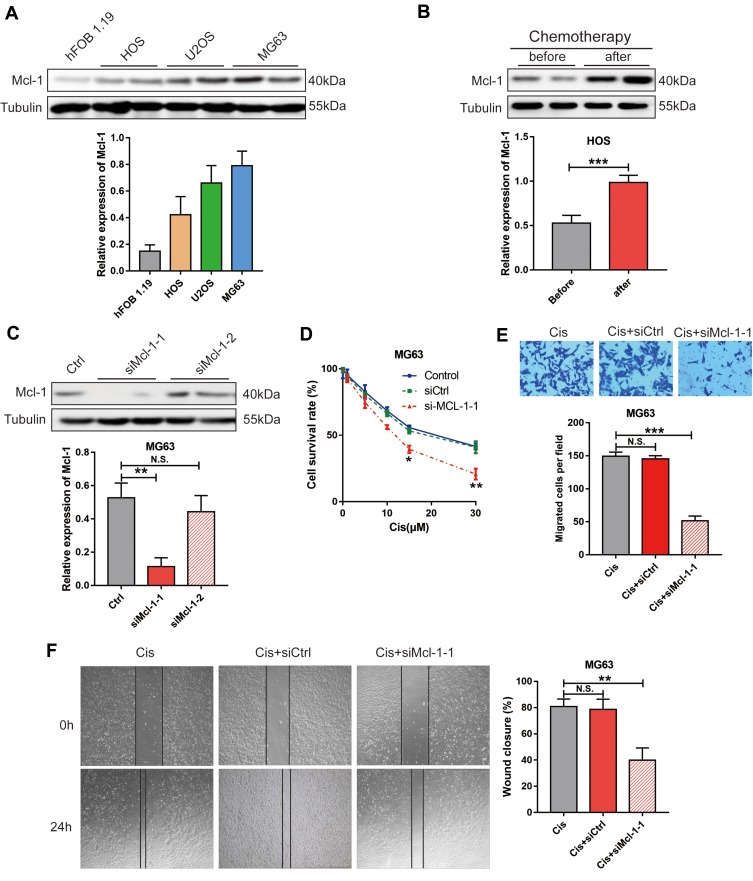
To explore the biological function of Mcl-1, we first determined the level of endogenous Mcl-1 expression in different OS cell lines via Western blot and found that Mcl-1 was expressed at higher levels in HOS, U2OS and MG63 cells than in foetal osteoblastic 1.19 cells (hFOB 1.19) (Figure 2A). We then determined that HOS and MG63 cells had the lowest and highest Mcl-1 expression, and these cells were used for subsequent experiments. We also found that the expression level of Mcl-1 was significantly increased in HOS cells after treatment with Cis (Figure 2B). To determine whether Mcl-1 was associated with Cis resistance, cell viability assays were performed by silencing Mcl-1 during Cis treatment. We confirmed the stable knockdown of Mcl-1 in MG63 cells with si-Mcl-1-1 (Figure 2C). The stable knockdown of Mcl-1 inhibited the proliferation and migration of MG63 cells (Figure 2D–F).
Figure 2.
Mcl-1 was involved in OS cell chemoresistance. Western blot analysis of Mcl-1 and Tubulin in hFOB 1.19, HOS, U2OS and MG63 cells (A). HOS cells treated with or without Cis (10 μM) (B). MG63 cells stably transfected with nonspecific shRNA (Ctrl) or Mcl-1-specific siRNA (si-Mcl-1-1 and si-Mcl-1-2). Student’s t-test, ***p< 0.001. (C). Representative blots are shown in the upper panel, and the summarized densitometry measurements are shown in the lower panel. Data are shown as the mean ± s.e.m., n = 5, **p< 0.01, N.S. means no significance, Student’s t-test. (D) Cell proliferation analysis of MG63 cells without or with stable Mcl-1 knockdown (n=3). Student’s t-test, *p<0.05, **p<0.01. Cell migration analysis of MG63 cells without or with stable Mcl-1 knockdown via transwell assay (E) or wound-healing assay (F), n=3 Student’s t-test, N.S. means no significance, **p<0.01, significantly different compared with the control group.
miR-375 Directly Targets Mcl-1 and Downregulates Its Expression in MG63 Cells
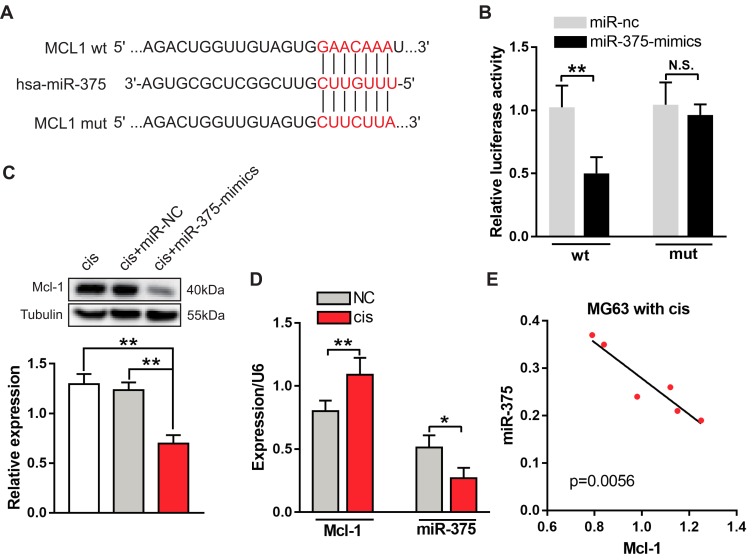
The presence of changes in the expression of miRNAs appears to be a common characteristic of cancers, including OS.3 The loss or suppression of miRNAs targeting Mcl-1 may cause aberrant overexpression of Mcl-1 in OS. To determine how Mcl-1 upregulation was involved in Cis resistance in OS cancer, we used a comprehensive bioinformatics analysis as a filter to generate a selective miRNA library for subsequent screening. The TargetScan algorithm showed that bases 901 to 907 in the MCL1 3′-UTR have perfect complementarity to the seed sequence of miR-375 (Figure 3A). To assess whether miR-375 directly regulates Mcl-1, we constructed a mutated MCL1 3′-UTR luciferase reporter, which completely restored luciferase activity induced by the miR-375 mimic (Figure 3B). Moreover, the transduction of the miR-375 mimic decreased the expression of Mcl-1 in MG63 cells treated with Cis (Figure 3C). In addition, we estimated the expression levels of miR-375 and Mcl-1 in different chemotherapeutic environments using quantitative PCR analysis. We found that there was a significant negative correlation between the expression levels of miR-375 and Mcl-1 after chemotherapy (Figure 3D and E). Based on these data, Mcl-1 is likely a novel direct target of miR-375 in OS cells.
Figure 3.
miR-375 directly targets Mcl-1 and is downregulated in MG63 cells treated with Cis. (A) Predicted binding site for hsa-miR-375 in the MCL1 3′-UTR. Wild type (wt) and mutant (mut) miR-375 binding sites are shown in red-colored word. (B) Luciferase activity 24 hrs after the co-transfection of MG63 cells with miR-375 mimics and the Mcl-1 WT or Mut 3′-UTR construct. Data are presented as the mean ± s.e.m. **p< 0.01. (C) Western blot analysis of Mcl-1 and tubulin in Cis-treated MG63 cells transiently transfected with miR-NC and miR-375-mimics (upper panel). The summarized densitometry measurements are shown in the lower panel. Data are shown as the mean ± s.e.m., n = 5, **p< 0.01, ANOVA. (D) RT-PCR analyses of the expression of miR-375 and Mcl-1 in MG63 cells with or without the treatment with Cis (10 μM). Data are shown as the mean ± s.e.m., n=6, *p< 0.05, **p< 0.01, Student’s t-test. (E) The correlation analyses of miR-375 and Mcl-1 expression in MG63 cells after treatment with Cis (10 μM) are shown, n=6, p< 0.001, Pearson’s r test.
miR-375 Modulates Cis-Induced DNA Damage in MG63 Cells and Affects Chemotherapy Sensitivity
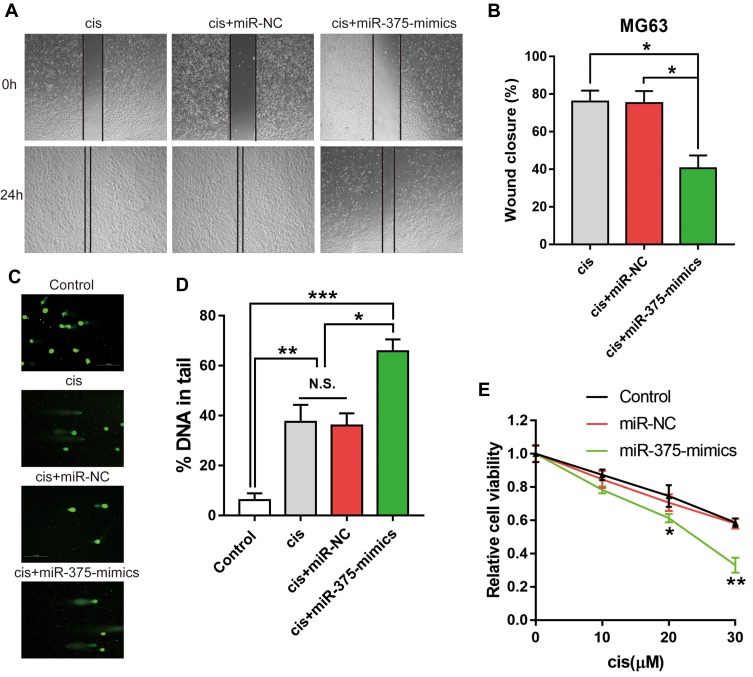
As we know, the role of chemotherapy, including Cis treatment, is to cause DNA damage in cancer cells. To determine whether miR-375 is associated with Cis resistance, cell viability, cell migration and DNA damage assays were performed by restoring miR-375 expression during Cis treatment. We performed a comet assay to assess DNA damage in MG63 cells after treatment with Cis (10 μM). In cells with DNA damage, negatively charged DNA fragments are released from the nucleus and migrate towards the anode.16 The restoration of miR-375 resulted in a significant increase in DNA damage compared to that inducted by the scrambled control or miR-NC (Figure 4C and Ds). Interestingly, we also observed that scratch closure and cell proliferation were significantly inhibited in MG63 cells transfected with miR-375-mimics (Figure 4A, B and E). Our results demonstrated the significantly increased chemosensitivity of OS cells transfected with miR-375-mimics.
Figure 4.
miR-375 promoted DNA damage, which inhibited MG63 cell proliferation and migration. MG63 cells, which were transfected with miR-NC or miR-375-mimics, were treated with Cis (10 μM). (A) Representative images of a wound healing assay 0 and 24 h after scratching. (B) Percentage of scratch closure is shown as the mean ± s.e.m., n = 5, *p< 0.05, ANOVA. (C) Representative images of the comet assay. (D) Percentage of DNA in the tail is shown as the mean ± s.e.m., n = 5, *p< 0.05, **p< 0.01, ***p< 0.001, ANOVA. (E) Cell viability was determined with a CCK-8 assay, data are shown as the mean ± s.e.m., n=5, *p< 0.05, **p< 0.01, Student’s t-test.
Mcl-1 Mediates the Effects of miR-375 on Cis Sensitivity in MG63 Cells
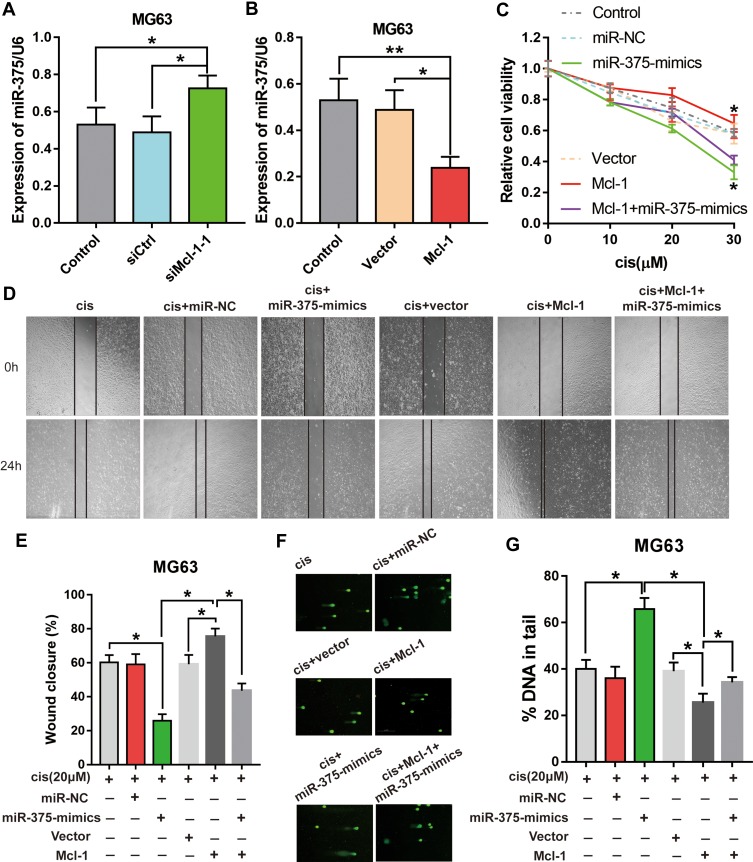
To prove that Mcl-1 mediates the effects of miR-375 on tumour promotion, miR-375 downregulation by Mcl-1 was prevented using siRNAs before assessment of cell growth, migration and DNA damage. To this end, miR-375 knockdown with siRNA was confirmed by RT-PCR (Figure 5A and B). We confirmed that MG63 cells transfected with miR-375-mimics showed attenuated growth and migration (Figure 4A and E); however, miR-375 knockdown prevented the increase in growth and migration induced by Mcl-1 overexpression (Figure 5C, D and E). Remarkably, the rescue of DNA damage similar to that described above was attained in MG63 cells transfected with the Mcl-1 overexpression plasmid (Figure 5F and G). The above data show that the inhibitory effects of miR-375 are partially mediated by targeting Mcl-1.
Figure 5.
Mcl-1 mediates the effects of miR-375 on MG63 cell growth, migration and DNA damage during Cis treatment. MG63 cells, which were transfected with siMcl-1-1 or Mcl-1 before transfection with miR-375-mimics, were treated with Cis (10 μM). (A, B) RT-PCR showing the rescue of the Mcl-1-induced downregulation of miR-375 by siMcl-1-1 in MG63 cells and the inhibition of miR-375 by Mcl-1 in MG63 cells. Data are shown as the mean ± s.e.m., n = 5, *p< 0.05, **p< 0.01, ANOVA. (C) Growth assay showing that Mcl-1 overexpression partially rescues the proliferative effects of miR-375 inhibition in MG63 cells, n=3, Student’s t-test, *p<0.05. (D) Representative images of a wound healing assay 0 and 24 h after scratching. (E) Percentage of scratch closure is shown as the mean ± s.e.m., n = 5, *p< 0.05, ANOVA. (F) Representative images of the comet assay. (G) Percentage of DNA in the tail is shown as the mean ± s.e.m., n = 5, *p< 0.05, ANOVA.
Discussion
Overcoming chemoresistance is the greatest challenge in treating OS. Chemoresistance is classified into two types: inherent resistance to the drug or acquired resistance, which is the result of prolonged exposure to the drug. Cisplatin remains the first-line chemotherapeutic used for OS treatment; however, OS patients with acquired resistance have a 5-year survival rate of <30%.17 Although several molecular mechanisms underlying cisplatin resistance have been reported, these mechanisms are complex and involve multiple steps and multiple genes, and they have not yet been fully clarified.
In this study, we revealed that the expression of Mcl-1 was significantly higher in human OS tissue after receiving chemotherapy compared with that in cancer tissue from the same patient prior to chemotherapy. Furthermore, the same results were found in OS cell lines; Mcl-1 expression was increased in OS cells after treatment with Cis. Although there is no direct evidence that clarifies the mechanism involved in the chemotherapy-driven increases in Mcl-1 expression, chemotherapy may promote the activity of a deubiquitinating enzyme, which may protect Mcl-1 from degradation in OS cells.18 Our results strongly suggest the importance of Mcl-1 in the chemoresistance of OS. In agreement with these findings, MCL1 was shown to limit the efficacy of anticancer agents and to function as a recombinant human tumour necrosis factor-related apoptosis-inducing ligand (rh-TRAIL). Treatments targeting MCL1 could be an effective strategy to overcome rh-TRAIL resistance in Triple Negative Breast Cancer (TNBC).19 Other studies also identified the essential role of Mcl-1 in promoting chemoresistance and showed that Mcl-1 could be a promising target for the treatment of ovarian cancer.18,20
MicroRNAs (miRNAs) are small noncoding RNAs (21–25 nucleotides) that act post-transcriptionally to suppress gene expression. The accumulating evidence of the involvement of dysregulated miRNAs in malignancy has provided new directions for research on the mechanisms underlying responses to chemotherapy.21 Recently, an increasing number of studies have identified aberrantly expressed miRNAs involved in many aspects of OS progression, such as tumour initiation, drug resistance, and metastasis.22 miR-375 has been identified as a tumour suppressor and was found to be significantly downregulated in multiple types of cancer, including hepatocellular carcinoma and glioma.23,24 Liu et al found that downregulated miR-375 could be used as a potential serum biomarker for the diagnosis and the prediction of the prognosis and chemosensitivity of OS.25 Similarly, Shi et al and Liu et al found that the expression level of miR-375 was significantly decreased in OS tissues compared with chondroma tissues or adjacent noncancerous tissues, besides, the reduction of miR-375 correlated with poor prognosis.26,27 In this study, we revealed the novel function of miR-375 in regulating chemotherapy sensitivity in cancer cells. Consistent with previous reports, we found that the expression of miR-375 was significantly inhibited in OS cells after treatment with Cis. We also confirmed that when they were transfected with miR-375-mimics, OS cells showed attenuated growth and migration. This suggests that the dysregulation of miRNAs could provide new targets for the prevention of chemotherapy resistance in tumour cells.
We found that OS patients with high intensity Mcl-1 staining showed significantly poorer overall survival and an increased recurrence rate. Based on our mechanistic studies, miR-375 exerts its tumour suppressor function by directly targeting and repressing Mcl-1, thereby reducing Cis-induced DNA damage. However, we only observed the tumour-promoting effects of the miR-375/Mcl-1 axis in vitro in the present study, and only 42 pairs of OS tissues were analysed in this study because of the limited number of available OS samples. To determine whether other factors exist in OS cells that affect the expression of Mcl-1 during treatment with Cis, we need more evidence. Therefore, more evidence related to the therapeutic potential of the miR-375/Mcl-1 axis should be obtained in vitro and in vivo in the future.
Our study was the first to reveal that miR-375 and its target gene Mcl-1 are dysregulated in human OS after chemotherapy and are correlated with clinical outcomes. Furthermore, miR-375 depletion suppressed chemosensitivity and Cis-induced DNA damage via Mcl-1. The miR-375/Mcl-1 axis provides a new avenue that could aid in the understanding of the mechanism of chemoresistance and may be a potential target for OS intervention.
Funding
This work was supported by grants from the Fundamental Research Funds for the Central Universities of Central South University (2019zzts320), the Key Research and Development Programme of Hunan Province (No. 2018SK2090), the National Key Research and Development Programme of China (No. 2016YFC1201800), the National Natural Science Foundation of China (81671225 and 81871089), the National Natural Science Foundation of China (81600461), and the Xiangya Hospital Foundation for Young Scholars (2018Q01).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Anderson ME. Update on survival in Osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Sun W, Wang H, Zuo D, Hua Y, Cai Z. Research progress on the multidrug resistance mechanisms of osteosarcoma chemotherapy and reversal. Tumour Biol. 2015;36(3):1329–1338. doi: 10.1007/s13277-015-3181-0 [DOI] [PubMed] [Google Scholar]
- 3.Maximov VV, Akkawi R, Khawaled S, et al. MiR-16-1-3p and miR-16-2-3p possess strong tumor suppressive and antimetastatic properties in osteosarcoma. Int J Cancer. 2019;145:3052–3063. doi: 10.1002/ijc.v145.11 [DOI] [PubMed] [Google Scholar]
- 4.Huang P, Ouyang DJ, Chang S, et al. Chemotherapy-driven increases in the CDKN1A/PTN/PTPRZ1 axis promote chemoresistance by activating the NF-kappaB pathway in breast cancer cells. Cell Commun Signal. 2018;16(1):92. doi: 10.1186/s12964-018-0304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Li Z, Zhu X, Xu R, Xu Y. miR-29 family inhibits resistance to methotrexate and promotes cell apoptosis by targeting COL3A1 and MCL1 in Osteosarcoma. Med Sci Monit. 2018;24:8812–8821. doi: 10.12659/MSM.911972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253 [DOI] [PubMed] [Google Scholar]
- 8.Tron AE, Belmonte MA, Adam A, et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9(1):5341. doi: 10.1038/s41467-018-07551-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osaki S, Tazawa H, Hasei J, et al. Ablation of MCL1 expression by virally induced microRNA-29 reverses chemoresistance in human osteosarcomas. Sci Rep. 2016;6:28953. doi: 10.1038/srep28953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B, Li L, Li Y, Sun H, Zeng C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother. 2018;106:850–857. doi: 10.1016/j.biopha.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Mao LF, Zhang ZP, et al. Down-regulated miR-125a-5p promotes the reprogramming of glucose metabolism and cell malignancy by increasing levels of CD147 in thyroid cancer. Thyroid. 2018;28(5):613–623. doi: 10.1089/thy.2017.0401 [DOI] [PubMed] [Google Scholar]
- 12.Huang P, Huang FZ, Liu HZ, Zhang TY, Yang MS, Sun CZ. LncRNA MEG3 functions as a ceRNA in regulating hepatic lipogenesis by competitively binding to miR-21 with LRP6. Metabolism. 2019;94:1–8. doi: 10.1016/j.metabol.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Kaluba B, Jiang XL, et al. Liver X receptor inverse agonist SR9243 suppresses nonalcoholic steatohepatitis intrahepatic inflammation and fibrosis. Biomed Res Int. 2018;2018:8071093. doi: 10.1155/2018/8071093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Long M, Huang P, et al. Emerging integrated nanoclay-facilitated drug delivery system for papillary thyroid cancer therapy. Sci Rep. 2016;6:33335. doi: 10.1038/srep33335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia O, Romero I, Gonzalez JE, et al. Visual estimation of the percentage of DNA in the tail in the comet assay: evaluation of different approaches in an intercomparison exercise. Mutat Res. 2011;720(1–2):14–21. doi: 10.1016/j.mrgentox.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 16.Wu JL, Zhou SX, Zhao R, et al. MTHFR c.677C>T inhibits cell proliferation and decreases prostate cancer susceptibility in the Han Chinese population in Shanghai. Sci Rep. 2016;6:36290. doi: 10.1038/srep36290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14(11):722–735. doi: 10.1038/nrc3838 [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Luo Q, Zhao P, et al. MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer. Proc Natl Acad Sci U S A. 2019;116(8):2961–2966. doi: 10.1073/pnas.1814742116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Blasio A, Pratelli G, Drago-Ferrante R, et al. Loss of MCL1 function sensitizes the MDA-MB-231 breast cancer cells to rh-TRAIL by increasing DR4 levels. J Cell Physiol. 2019. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Chen W, Jin Y, et al. miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Biochem Pharmacol. 2019;161:98–112. doi: 10.1016/j.bcp.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Tormo E, Ballester S, Adam-Artigues A, et al. The miRNA-449 family mediates doxorubicin resistance in triple-negative breast cancer by regulating cell cycle factors. Sci Rep. 2019;9(1):5316. doi: 10.1038/s41598-019-41472-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Hu L, Li S, et al. Long non-coding RNA Taurine upregulated gene 1 promotes osteosarcoma cell metastasis by mediating HIF-1alpha via miR-143-5p. Cell Death Dis. 2019;10(4):280. doi: 10.1038/s41419-019-1509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XX, Chang Y, Meng FY, et al. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31(28):3357–3369. doi: 10.1038/onc.2011.500 [DOI] [PubMed] [Google Scholar]
- 24.Chang C, Shi H, Wang C, et al. Correlation of microRNA-375 downregulation with unfavorable clinical outcome of patients with glioma. Neurosci Lett. 2012;531(2):204–208. doi: 10.1016/j.neulet.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Zhao X, Zhang YJ, Fang GW, Xue Y. MicroRNA-375 as a potential serum biomarker for the diagnosis, prognosis, and chemosensitivity prediction of osteosarcoma. J Int Med Res. 2018;46(3):975–983. doi: 10.1177/0300060517734114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi ZC, Chu XR, Wu YG, et al. MicroRNA-375 functions as a tumor suppressor in osteosarcoma by targeting PIK3CA. Tumour Biol. 2015;36(11):8579–8584. doi: 10.1007/s13277-015-3614-9 [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Huang K, Jie Z, et al. CircFAT1 sponges miR-375 to promote the expression of Yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17(1):170. doi: 10.1186/s12943-018-0917-7 [DOI] [PMC free article] [PubMed] [Google Scholar]