Summary
Antigen-experienced IgM+ B cells with mutated V genes have emerged as important effectors of both adaptive and innate-like immune responses. While their precise role in recall responses appear to differ according to the nature of the immunogen or the infectious agent, they are able to achieve rapid plasma cell differentiation, germinal center re-initiation, as well as IgM and IgG memory pool replenishment, which establishes them as multi-lineage precursors of the various functional memory subsets. For innate-like responses, recent data have shown that activation by gut commensals is able to generate, both in mice and humans, a systemic IgM+ population with specificity against glycan epitopes, which displays broad cross-reactivity towards multiple micro-organisms, and ensures a first line of defense against systemic infections.
Introduction
B cell immune memory is the capacity to respond more rapidly to a re-encounter of the immunizing antigen with high affinity and exquisite specificity. Memory responses are classically assigned to switched IgG memory B cells whose unique cytoplasmic tail confers them enhanced reactivity [1]. IgM memory B cells have been described almost 40 years ago, but their precise functional role remains as today ill-defined. We will summarize in this review recent data on the behavior of IgM memory B cells in infectious and non-infectious settings, both in mice and humans, data that highlight their distinct functional features in innate-like and adaptive responses.
IgM memory B cells in adaptive responses
Mouse IgM vs IgG memory subsets in recall response against model antigens
A controversy still exists concerning the specific fate of IgM and IgG memory subsets in recall responses. Two studies initially proposed that IgM memory B cells are the privileged effectors of germinal center (GC) recall responses (using sheep red blood cells (SRBC) and phycoerythrin (PE) immunization, respectively), while IgG memory would be poised towards plasma cell differentiation [2,3]. A latter study, however, based on NP-KLH challenge with a TH1 polarizing adjuvant, favored a secondary GC reaction seeded by IgG memory B cells [4]. An alternate proposition was made by M. Shlomchik and colleagues, stating that the differentiation potential of memory subsets was in fact imparted by a gradient of memory markers, comprised of CD73, CD80 and PD-L2, with low-expressing subsets (“double-negative” for CD80 and PD-L2) undergoing GC differentiation upon transfer and challenge, while double- positive CD80/PD-L2 memory B cells would give rise to plasma cells [5,6]. As IgM memory B cells were mainly negative for these markers in their model (a transgenic NP-setting with transfer to a transgenic host), and IgG1 were mainly positive, this would seem a priori to go along the first proposition. However, these different models may not be comparable, as most memory B cells that go through a germinal center reaction (and are labelled through an AID-fate mapping system) are CD73+CD80+ (ref. 2 and discussion below), suggesting that IgM memory B cells negative for these markers are generated through a germinal center independent pathway, a pathway documented notably in Bcl6-deficient animals [7,8]. The function of this subset that displays an unmutated BCR is nevertheless still elusive, as its capacity to compete with affinity matured memory B cells upon antigen challenge remains to be established. It should also be noted that no clear equivalent of this germinal center-independent subset has been described so far in humans.
The debate concerning the differentiation potential of memory B cell subsets may not converge to a unique scheme in mouse model systems, as recall responses strongly depend on the immunization conditions, like the delay between prime and boost (and the level of circulating antibodies [3]), the nature of the antigen (and the persistence of germinal center reactions), the site of immunization (and the lymphoid tissue studied, lymph node vs. spleen), and finally, the type of adjuvant used (and the polarization of the response towards distinct IgG isotypes exerting different inhibitory functions).
Longevity of IgM vs. IgG memory
After immunization against PE, switched memory B cells, followed over 500 days, were shown to decay with faster kinetics than PE-specific IgM memory B cells [3]. Using the same immunization conditions, the group of M. Nussenzweig showed that both IgM and IgG memory B cells had the same survival capacity, as previously reported in an NP response [9], but that the higher mutation load of switched memory B cells increased the proportion of auto-reactive cells, which were progressively purged from the memory pool by post-germinal center tolerance mechanisms [10]. A different proposition emerged from the group of M. Jenkins, who linked the reduced longevity of anti-PE IgG+ B cells to the presence of high avidity IgM precursors in the naive B cell pool, the shortcut in affinity maturation induced by such a pre-adapted repertoire failing to generate a long-lived memory profile [11]. In humans, while IgG memory B cells have been shown to survive for decades in several infectious or vaccine conditions [12], no comparisons have been made with IgM memory B cells, whose systematic analysis, as mentioned in the next chapter, has rarely been performed.
IgM memory B cells: broad effectors of immune responses in infectious contexts
New insights concerning recall responses have emerged from mouse infectious models, in which IgM memory B cells appear to play multiple roles. Mice injected with Plasmodium-infected RBCs generate Plasmodium-specific IgM+ and switched memory B cells which represent 20 and 30% respectively of the total specific B cell memory response, as well as a major IgMlowIgD+ subset (50%), all subsets being detectable up to one year after infection [13]. Both switched and IgM-only subsets were largely CD80+CD73+ with mutated Ig receptors, while the IgD+ subset, unmutated and CD80-CD73-, appears to represent a GC-independent subset. Interestingly, this subset did not proliferate upon reinfection. While memory and germinal center B cells engaged in the recall response were mainly isotype-switched, the authors did not specifically studied to what extent early switching contributed to this phenotype. Their focus was mainly on the description of an early plasma cell response, which was dominated by differentiation of IgM-only memory B cells in spite of being numerically lower initially, followed by IgG plasma cells a few days later, such plasma cells being generated from both switched memory B-cell differentiation and isotype switching of activated IgM+ B cells [13].
In the same Plasmodium chabaudi mouse model of infection, it was more recently shown that a major part of memory B cells, both switched and IgM+, expressed the FCRL5 marker several months after infection. These cells were positive for CD80 and CD73, as well as CD11b, CD11c and T-bet, although these markers were expressed at a much lower level than at 4 weeks after infection, and responded vigorously upon reinfection by expanding the pool of memory B cells and giving rise to ASC [14]. This observation clearly demonstrated that the so-called atypical memory B cells markers (FCRL5,T-bet, CD11b+, CD11c+) observed on CD21-CD27- memory B cells in many human chronic infections and autoimmune diseases (HIV, HCV, Malaria, Lupus) [15], do not systematically prevent memory B cells from being functional, and rather mark activated B cells.
The immune response against Ehrlichia muris, an obligate intra-cellular rickettsia infecting macrophages and dendritic cells, generates a strong extra-follicular plasmablast neutralizing IgM response, with a GC reaction that was delayed until 3 weeks post infection [16]. At later time points, this infection generated essentially IgM memory B cells, with some switched memory B cells in a 10:1 ratio. As described above for malaria infection, these IgM memory B cells were T-bet+, CD73+, CD80+, as well as, for most of them, CD11c+, CD11b+ and PD-L2+. The properties of IgM memory B cells labelled by the inducible AID fate mapping system in this infectious setting (AID-Cre-ERT2 crossed with a ROSA-loxP-reporter line) were followed over successive transfer and challenges. This IgM memory pool gave rise to all effector subsets after challenge, germinal center, memory B cells and plasma cells, both IgM+ or switched. The newly reconstituted IgM memory pool conserved its broad effector potential upon additional transfer, clearly demonstrated by shared clonal relationships between donor and differentiated subsets, thus confirming its multi-lineage differentiation capacity [17].
It was proposed that, despite the pentameric structure of the IgM antibody which increases its avidity, the lower affinity of IgM memory B cells could in fact afford a wider cross-specific protection in front of mutating pathogens. A striking example was reported showing that, when rapamycin was administrated during vaccination, it conferred cross-strain protection against lethal infection with influenza virus subtypes [18]. The proposition of the authors was that rapamycin, by inactivating the mTOR kinase and therefore reducing class switch recombination (CSR) and germinal centers (GC) expansion, favored the emergence of IgM antibodies (Ab) with lower affinity, which targeted more conserved elements of the HA stem and thus were more cross-reactive. Along the same line, it was shown that, in mice immunized with the Dengue virus envelope protein and boosted with variant protein subtypes, IgM memory B cells with fewer mutations were specifically recruited in germinal centers, giving rise to a cross-reactive antibody response in serum [19].
A high-throughput antibody screen in healthy donors was developed to study the humoral response to BK polyomaviruses (BKV), viruses that establish a dormant infection with almost 100% prevalence in humans. This study, the first of its kind that mined both the IgM and IgG memory repertoires, identified memory B cells expressing broadly cross-reactive and neutralizing antibodies for BK virus subtypes and related JC polyomaviruses [20]. Strikingly, the repertoires of IgM and IgG memory B cells were largely distinct, and, more surprisingly, the anti-viral specificity of IgM memory B cells was isotype-dependent, as binding was frequently lost by swapping mu and gamma heavy chain constant regions in a monomeric Ig. This study thus revealed an intrinsic function of the IgM memory subset, distinct from its switching potential.
Tissue-resident IgM memory B cells harbor a distinct antigen recognition profile
Local and systemic memory B cell responses against respiratory syncytial virus were studied in 2.5 to 4 years old children previously exposed to the virus [21]. There was no correlation between the frequency of virus-specific memory B cells in paired blood and adenoid tissues from the donor studied. Adenoid-specific antibodies showed higher binding affinities and neutralization capacities compared to those isolated from peripheral blood, and 1/4 of these high-affinity antibodies originated from an unswitched memory B cell population, harboring highly mutated VH genes but lacking CD27. A fraction of them expressed the CD45RBmem55 marker, which marks memory B cells and a marginal zone precursor subset [22,23]. Whether this specific phenotype is linked with the young age of the donors, or is the hallmark of such tissue-resident population remains to be established. This study anyway underlined the potency of tissue-resident memory B cells and the possible negative impact of adenoidectomy on anti-viral defense.
The overall picture emerging from IgM memory responses is complex, describing multiple functional facets of this subset: an early plasma cell differentiation potential in recall responses during which the pentameric secreted antibodies might be specially effective; a "stem-like" capacity to self-renew and generate all downstream effectors; a lower hypermutation load, often correlated with lower affinity and broader reactivity, endowing them with a specific capacity to cope with variant forms of pathogens, including through further maturation in germinal centers.
IgM memory B cells in mucosal and innate-like responses
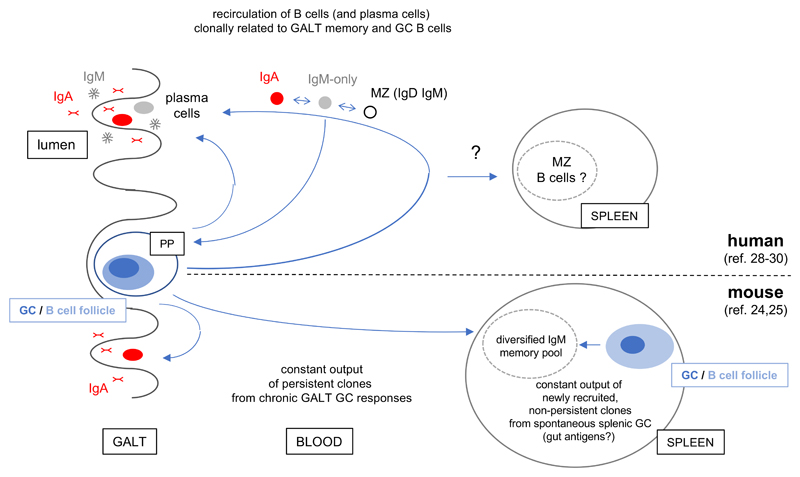
Recent studies have described the existence of innate-like IgM memory B cells, which are generated in the mouse in the absence of deliberate immunization, or which, in humans, are linked to mucosal activation (figure 1).
Figure 1. IgM memory B cells in mucosal and innate-like responses: recent advances.
This figure describes recent data that demonstrated linkage of systemic or circulating IgM+ B cells harboring mutated receptors with B cells diversifying in germinal centers from gut-associated lymphoid tissues. In the mouse (lower part), a splenic IgM memory population is shown to accumulate with time, generated by the constant output of chronic GC responses in Peyer's patches as well as from spontaneous splenic GC, triggered by antigens of endogenous or commensal origin (24,25). These splenic IgM memory B cells show cross-reactivity against glycan antigens of both commensal and infectious bacterial species. Such broad cross-reactivity is also observed in humans for clonally related anti-Klebsiella gut IgA plasmablasts and circulating IgM+ B cells with mutated Ig receptors (26). Also in humans (upper part), an IgM plasma cell subset, together with IgA, is generated from gut memory responses, while only IgA plasma cells are detected in the mouse gut (29). CD27+ IgA, IgM-only and MZ (IgM+IgD+) showed clonal relationships in blood, while, clonal relationships among gut memory B cells were restricted to the IgM-only/MZ and IgM-only/IgA subsets, as IgA and MZ B cells seem to lack such common clonal origin. All gut subsets showed some clonal relatedness with germinal center B cells (30). Whether MZ B cells in the spleen show similar relatedness with B cell populations diversifying against gut antigens remains to be determined.
A spontaneous IgM memory subset in the mouse
Spontaneous germinal centers are observed in spleen and Peyer's patches from non-immunized mice [24]. The output of these responses was identified by the inducible AID fate mapping mouse line, allowing the long-term follow-up of cells labelled by AID in the absence of exogenous activation. This analysis identified a persistent subset, composed mainly of IgM memory B cells in the spleen (together with IgA+ B cells), as well as plasma cells which, in bone marrow, are mainly IgA-secreting cells, present several months after their initial labeling [25]. Multiple approaches performed to determine their persistence and renewal, led us to conclude that maintenance of peripheral EYFP+ B cells and plasma cells was due to a continuous replenishment from ongoing immune responses taking place at mucosal sites. Splenic IgM memory B cells were CD73+ and CD80+ and accumulated with time, reaching 4-6% of splenic B cells at 5-8 months of age. One should stress that accumulation of the CD73+CD80+IgM+ subset in the spleen has nevertheless two different origins: one from chronic activation in Peyer's patches, where activated B cells persist for extended periods of time and continuously seed the periphery, accounting for most of the EYFP+ pool analyzed several months after their initial AID-dependent labeling; a second one from spontaneous germinal centers in the spleen, where B cells initially labelled more rarely persist, and thus feed continuously the splenic IgM "memory" pool from constantly renewed germinal center reactions. The antibodies produced by the IgM splenic memory B cells displayed extensive cross-reactivity against multiple bacterial species, including those that animals did not encountered in the animal facility [25]. This suggested that conserved glycans could be privileged antigens recognized by this innate-like IgM population activated in mucosal tissues, endowing them with broad cross-reactivity towards bacteria, of either commensal or infectious origin [26].
Recently, an exhaustive repertoire analysis of B1 cells described a major shift in the B1 repertoire in old animals, harboring N additions and somatic mutations, together with dominant clones retaining the original B1 profile [27]. These data somewhat contradicted the widely held notion of B1 cells originating from a fetal lineage and persisting throughout life by self-replenishment. One should mention however that the innate-like IgM memory B cells we observed, and which accumulate with time, harbor activation markers like CD43 which are used to identify splenic B-1 cells, and may therefore account for the late emergence of a repertoire with mutations and N-additions.
Does contribution of such glycan-specific IgM memory B cells of mucosal origin to the systemic B cell pool also takes place in humans? Klebsiella pneumoniae colonizes mucosal surfaces of healthy people, but can generate severe infections in immune-compromised patients. Wardemann and colleagues reported the presence of Klebsiella LPS-O3 antigen specific memory B cells in the blood of healthy individuals, which display clonal relationship with intestinal plasmablasts [28]. These circulating memory B cells were mainly IgM, but also IgA and IgG, while they were essentially IgA in the lamina propria. They harbored on average 10 mutations on their VH gene for IgM and up to 20-25 mutations for IgA and IgG. These antibodies were glycan-specific as they did not show any polyreactivity against E. coli LPS, dsDNA and insulin, and reversion to their germline counterpart abolished their binding to the O3 antigen. Some of them showed exclusive specificity against a single O-mannose structure while others could recognize variant O antigens. Some of these antibodies were able to bind mannose residues present at the surface of other microorganisms, such as Saccharomyces cerevisiae, HIV, as well as several mouse and human commensal Gram+ and Gram- bacteria, and to provide protection against variant K. pneumoniae serotypes in a mouse model of septicemia. Whether such cells also reside at systemic lymphoid locations, notably in the human spleen, remains however to be established.
Both mouse and human studies thus report the presence in the periphery of a diversified IgM population educated in gut-associated lymphoid tissues (GALT), displaying cross-reactivity against multiple microbial epitopes and being able to act as a first line of defense against systemic infections.
A large IgM+ plasma cell compartment in the human gut, generated from IgM memory precursors
IgM plasma cells were recently shown to account for 10 to 20 % of plasma cells (PC) in the human gut, in contrast to the mouse lamina propria where they are negligible [29]. These PC are clonally related to a large IgM memory B cell population present in the intestine. Like IgA PC and IgA memory B cells, IgM PC recirculate, along with a subset of IgM-only memory B cells expressing gut homing receptors, suggesting that they migrate back to these sites through the general circulation. Gut IgM memory B cells were essentially found in the ileum where they expressed a tissue specific signature differing from their splenic counterpart, as well as from marginal zone B cells, with a trend toward B cell activation and PC differentiation. Clonal relationships were observed between gut IgM memory B cells and IgM PC but also between IgM memory B cells and IgA memory B cells and PC, suggesting an ongoing differentiation and diversification process. Secreted IgMs co-target with IgAs a rich and complex microbiota, and show reactivity against a broad array of epitopes including capsular polysaccharides, while IgA-only-coated bacteria appear less diverse [29].
A gut/splenic marginal zone axis inferred from high dimensional analysis in humans
While the previous study was mainly focused on IgM-only memory B cells, a high dimensional analysis of spleen and gut B cell subsets confirmed the specific differentiation pathway of marginal zone (MZ) B cells (IgM+IgD+CD27+) from a CD45RBmem+ precursor [30]. In GALT tissues, they moreover appear spatially segregated from switched B cells. Among gut B cell subsets, IgM-only CD27+ B cells harbor clonal relationships with either IgA+ or MZ populations, while MZ B cells are not related to IgA+ B cells, highlighting the heterogeneity of the IgM-only compartment. IgD thus appears as a partial, but imperfect discriminant of marginal zone vs. IgM memory subsets, with overlap patterns depending possibly upon the tissue of origin, or the isolation procedure [30,31]. Interestingly, MZ B cells were also related to gut GC B cells, suggesting that mucosal sites could be the place where MZ B cells diversify. This study reinforce the notion of the independent lineage formation of MZ B cells, and the analogy with species that generate their pre-immune repertoire by diversification in gut-associated lymphoid tissues [22,32].
Altogether, these different studies highlight the formation, in both mice and humans, of IgM B cell subsets that are pre-diversified against gut commensals and ensure protective functions either at the gut barrier site, or at the systemic level, notably through their participation in immune responses against pathogens harboring cross-reactive, conserved glycan epitopes (figure 1).
Conclusions
Antigen-experienced IgM+ B cells harboring mutated Ig genes appear to be generated along multiple activation pathways, and to display a wide array of effector functions. No clear marker has emerged so far to delineate functional subsets, and activation markers often differ between mice and humans. Repertoire analysis, single-cell gene profiling, and more thorough analysis of tissue-specific responses in humans may help to decipher the complexity of the unswitched B cell population and its contribution to immune defenses.
Highlights.
-
-
IgM memory B cells can achieve multiple fates upon recall adaptive responses, constituting multipotent progenitors of various memory effector cells.
-
-
Innate-like IgM cells educated in gut-associated lymphoid tissues can provide broad systemic protection against cross-reactive glycan epitopes, in both mice and humans.
Acknowledgments
The team "Development of the immune system" is supported by an ERC Advanced Grant "B-response" and by the Fondation Princesse Grace de Monaco.
Footnotes
The authors declare no conflict of interest
References
- 1.Engels N, Wienands J. Memory control by the B cell antigen receptor. Immunol Rev. 2018;283:150–160. doi: 10.1111/imr.12651. [DOI] [PubMed] [Google Scholar]
- 2.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 3.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, Shlomchik MJ. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209:2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DD, Wilmore JR, Allman D. Cellular Dynamics of Memory B Cell Populations: IgM+ and IgG+ Memory B Cells Persist Indefinitely as Quiescent Cells. J Immunol. 2015;195:4753–4759. doi: 10.4049/jimmunol.1501365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pape KA, Maul RW, Dileepan T, Paustian AS, Gearhart PJ, Jenkins MK. Naive B Cells with High-Avidity Germline-Encoded Antigen Receptors Produce Persistent IgM(+) and Transient IgG(+) Memory B Cells. Immunity. 2018;48:1135–1143 e1134. doi: 10.1016/j.immuni.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, Pepper M. Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity. 2016;45:402–414. doi: 10.1016/j.immuni.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CC, Baccarella AM, Bayat A, Pepper M, Fontana MF. FCRL5(+) Memory B Cells Exhibit Robust Recall Responses. Cell Rep. 2019;27:1446–1460 e1444. doi: 10.1016/j.celrep.2019.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017;321:18–25. doi: 10.1016/j.cellimm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates JL, Racine R, McBride KM, Winslow GM. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol. 2013;191:1240–1249. doi: 10.4049/jimmunol.1300062. 191:1240-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenderes KJ, Levack RC, Papillion AM, Cabrera-Martinez B, Dishaw LM, Winslow GM. T-Bet(+) IgM Memory Cells Generate Multi-lineage Effector B Cells. Cell Rep. 2018;24:824–837 e823. doi: 10.1016/j.celrep.2018.06.074. [• Serial cell transfers and lineage tracing demonstrated that the same IgM clone can generate all effector subsets of a recall response against Ehrlichia muris ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14:1266–1276. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton BR, Tennant RK, Love J, Titball RW, Wraith DC, White HN. Variant proteins stimulate more IgM+ GC B-cells revealing a mechanism of cross-reactive recognition by antibody memory. Elife. 2018;7:e26832. doi: 10.7554/eLife.26832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner JM, Cornacchione V, Sathe A, Be C, Srinivas H, Riquet E, Leber XC, Hein A, Wrobel MB, Scharenberg M, et al. Human Memory B Cells Harbor Diverse Cross-Neutralizing Antibodies against BK and JC Polyomaviruses. Immunity. 2019;50:668–676 e665. doi: 10.1016/j.immuni.2019.02.003. [•• Unbiased screening of the memory repertoire reveals both IgM and IgG polyomavirus-specific memory B cells in human blood, with IgM+ clones harboring a distinct repertoire and antigenic specificities linked with their isotyp ] [DOI] [PubMed] [Google Scholar]
- 21.Shehata L, Wieland-Alter WF, Maurer DP, Chen E, Connor RI, Wright PF, Walker LM. Systematic comparison of respiratory syncytial virus-induced memory B cell responses in two anatomical compartments. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09085-1. 1126. [• Comparison of RSV-specific B cells from blood and adenoids reveal that resident memory B cells from mucosal tissues, which include an IgM component, have a more potent and broader recognition potential than those from blood.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descatoire M, Weller S, Irtan S, Sarnacki S, Feuillard J, Storck S, Guiochon-Mantel A, Bouligand J, Morali A, Cohen J, et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J Exp Med. 2014;211:987–1000. doi: 10.1084/jem.20132203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koethe S, Zander L, Koster S, Annan A, Ebenfelt A, Spencer J, Bemark M. Pivotal advance: CD45RB glycosylation is specifically regulated during human peripheral B cell differentiation. J Leukoc Biol. 2011;90:5–19. doi: 10.1189/jlb.0710404. [DOI] [PubMed] [Google Scholar]
- 24.Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, Cooper TK, Kitamura D, Rahman ZS. IFN-gamma receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gallou S, Zhou Z, Thai LH, Fritzen R, de Los Aires AV, Megret J, Yu P, Kitamura D, Bille E, Tros F, et al. A splenic IgM memory subset with antibacterial specificities is sustained from persistent mucosal responses. J Exp Med. 2018;215:2035–2053. doi: 10.1084/jem.20180977. [•• A large IgM+ memory subset, with broad anti-glycan specificities, is shown to accumulate with time in the mouse spleen in the absence of immunization, originating from chronic, persistent germinal center reactions in Peyer's patches and from transient, spontaneous reactions in the spleen ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel P, Kearney JF. Immunological Outcomes of Antibody Binding to Glycans Shared between Microorganisms and Mammals. J Immunol. 2016;197:4201–4209. doi: 10.4049/jimmunol.1600872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, Herzenberg LA. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 2015;4:e09083. doi: 10.7554/eLife.09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollenske T, Szijarto V, Lukasiewicz J, Guachalla LM, Stojkovic K, Hartl K, Stulik L, Kocher S, Lasitschka F, Al-Saeedi M, et al. Cross-specificity of protective human antibodies against Klebsiella pneumoniae LPS O-antigen. Nat Immunol. 2018;19:617–624. doi: 10.1038/s41590-018-0106-2. [•• IgM memory B cells against Klebsiella mannose residues can be isolated from the blood of healthy donors, harboring mutated V genes, clonal relationships with mucosal IgA+ plasmablasts, and crossreactivity against glycan epitopes of various microorganisms.] [DOI] [PubMed] [Google Scholar]
- 29.Magri G, Comerma L, Pybus M, Sintes J, Llige D, Segura-Garzon D, Bascones S, Yeste A, Grasset EK, Gutzeit C, et al. Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity. 2017;47:118–134 e118. doi: 10.1016/j.immuni.2017.06.013. [•• This study shows that the human gut, in contrast to the mouse, contains a large IgM+ plasma cell population, related to gut IgM+ memory B cells that can switch to IgA, and which secrete antibodies that dually coat with IgA a gut bacterial population with increased diversity compared to IgA-only coated bacteria.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Uduman M, Siu JHY, Tull TJ, Sanderson JD, Wu YB, Zhou JQ, Petrov N, Ellis R, Todd K, et al. Spatiotemporal segregation of human marginal zone and memory B cell populations in lymphoid tissue. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06089-1. 3857. [•• High-dimensional histological analysis and repertoire sequencing reveals lineage relationships between IgM+IgD+CD27+ from blood and gut, including Peyer's patch germinal center B cells, while correlation with IgA+ B cells is restricted to IgM-only CD27+ B cells ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagnara D, Squillario M, Kipling D, Mora T, Walczak AM, Da Silva L, Weller S, Dunn-Walters DK, Weill JC, Reynaud CA. A Reassessment of IgM Memory Subsets in Humans. J Immunol. 2015;195:3716–3724. doi: 10.4049/jimmunol.1500753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weill JC, Weller S, Reynaud CA. A bird's eye view on human B cells. Semin Immunol. 2004 doi: 10.1016/j.smim.2004.08.007. [DOI] [PubMed] [Google Scholar]