Introduction
Cough is a vital protective reflex preventing aspiration and enhancing airway clearance. Pathologically excessive and protracted cough is however a common and disabling complaint affecting perhaps 5 to 10 percent of the adult population[1]. When severe, it causes a major decrement in the quality of life with comorbidity such as incontinence, cough syncope and dysphonia leading to social isolation, depression, and difficulties in relationships[2].
Whilst a wide range of diseases may be associated with chronic cough it has become increasingly clear that the majority of adult patients presenting with chronic cough as the primary complaint have a common clinical presentation[3]. They often complain of exquisite sensitivity to inhalation of environmental irritants such as perfumes, bleaches, and cold air which result in sensations of tickling/irritation in the throat and an urge to cough; features suggestive of heightened sensitivity of the neuronal pathways mediating cough[4]. There is also a unique epidemiology with two thirds of patients being female and the peak prevalence in their fifties and sixties. These observations have led to the concept of cough hypersensitivity syndrome as a diagnosis[5]. In children chronic cough presents in a markedly different fashion with different aetiology. They are not miniature adults[6].
This guideline aims to improve diagnostic accuracy and promote evidence-based therapy for both paediatric and adult patients in both primary and secondary care. The guideline is intended for use by all health care professionals looking after patients with chronic cough. The guideline has been developed by a multidisciplinary international panel of clinicians and scientists with a published record of expertise in the field. Input on patient views and preferences was sought via the European Lung Foundation who provided an advisory group of patient representatives who expressed their preferences via teleconferences, attendance at the ERS Congress, and in writing. They contributed to formulating and prioritising the key questions.
Guideline scope and structure
This guideline follows the hybrid model of the ERS Guidelines Working Group and Science Council[7], which combines the scientific rigor of the GRADE framework for key questions of uncertainty with a narrative component to reflect the expert consensus of the guideline task force. The narrative covers clinically important aspects of chronic cough while the eight key questions systematically explore the evidence in areas of clinically important controversy.
Full details of the methodological process and the analysis of the individual questions can be found in the online supplement. Table 1 provides a summary of the eight questions (two diagnostic and six therapeutic questions), the level of evidence, and the recommendations arising from the systematic review. All other propositions should be regarded as narrative statements.
Table 1. Table of recommendations, strength and level of evidence, and supporting remarks.
| Recommendation | Strength of recommendation | Level of evidence | Values and preferences | Remarks |
|---|---|---|---|---|
| Question 1: Should chest CT scan be routinely performed on chronic cough patient with normal chest X-ray and physician examination? | ||||
| Recommendation 1: We suggest that clinicians do not routinely perform a chest CT scan in patients with chronic cough who have a normal chest X-ray and physical examination. | Conditional | Very low | This recommendation places relatively higher value on the impact on patient management and outcomes including adverse events from radiation exposure. Lower value was given to diagnostic sensitivity and specificity. |
|
| Question 2: Should FeNO/blood eosinophils be used to predict treatment response to corticosteroids/anti-leukotrienes in chronic cough? | ||||
| Recommendation 2: Research recommendation | - | Very low | This recommendation places relatively higher value on predictability for the treatment response and the impact on the treatment decision. Lower value was given to diagnostic sensitivity and specificity. |
|
| Question 3: Should anti-asthmatic drugs (anti-inflammatory or bronchodilator drugs) be used to treat patients with chronic cough? | ||||
| Recommendation 3a: We suggest a short-term ICS trial (2–4 weeks) in adult patients with chronic cough. | Conditional | Low | This recommendation is based on the higher value of the clinical benefits from ICS in some patients with asthmatic cough (or airway eosinophilic inflammation) and lower value on adverse events. |
|
| Recommendation 3b: We suggest a short-term ICS trial (2-4 weeks) in children with chronic dry cough. | Conditional | Low | This recommendation is based on a higher value of the clinical benefits from ICS in some patients with asthmatic cough (or eosinophilic inflammation) and a lower value on adverse events. |
|
| Recommendation 3c: We suggest a short-term antileukotriene trial (2–4 weeks) in adults with chronic cough, particularly in those with asthmatic cough. | Conditional | Low | This recommendation is based on higher value on the clinical benefits from anti-leukotriene in some patients with asthmatic cough (or airway eosinophilic inflammation) and lower value on adverse events. |
|
| Recommendation 3d: We suggest a short-term trial (2–4 weeks) of ICS and long-acting bronchodilator combination in adults with chronic cough and fixed airflow obstruction. | Conditional | Moderate | This recommendation is based on higher value on the clinical benefits from ICS and long-acting bronchodilator combination in some patients with chronic obstructive pulmonary disease and a lower value on adverse events. |
|
| Question 4: Should anti-acid drugs (PPIs and H2 antagonists) be used to treat patients with chronic cough? | ||||
| Recommendation 4: We suggest that clinicians do not routinely use anti-acid drugs in adult patients with chronic cough. | Conditional | Low | This recommendation is based on higher value of the clinical benefits from anti-acid drugs only in some patients with acid reflux and a lower value on adverse events. |
|
| Question 5: Should drugs with pro-motility activity (reflux inhibitors, prokinetics and macrolides with pro-motility activity) be used to treat patients with chronic cough? | ||||
| Recommendation 5: There is currently insufficient evidence to recommend the routine use of macrolide therapy in chronic cough. A one month trial of macrolides can be considered in the cough of chronic bronchitis refractory to other therapy, taking into account local guidelines on antimicrobial stewardship. | Conditional | Low | This recommendation is based on higher value on the clinical benefits from drugs with pro-motility activity only in some patients with chronic bronchitis and lower value on adverse events. |
|
| Question 6: Which cough neuromodulatory agents (pregabalin, gabapentin, tricyclics, and opiates) should be used to treat patients with chronic cough? | ||||
| Recommendation 6a: We recommend a trial of low dose morphine (5–10 mg bd) in adult patients with chronic refractory cough. | Strong | Moderate | This recommendation is based on a higher value of the clinical benefits and adverse events from opiates for chronic refractory cough. |
|
| Recommendation 6b: We suggest a trial of gabapentin or pregabalin in adult patients with chronic refractory cough. | Conditional | Low | This recommendation is based on a higher value of the clinical benefits and adverse events from gabapentin in chronic refractory cough. |
|
| Question 7: Should non-pharmacological therapy (cough control therapy) be used to treat patients with chronic cough? | ||||
| Recommendation 7: We suggest a trial of cough control therapy in adult patients with chronic cough. | Conditional | Moderate | This recommendation is based on a higher value of the clinical benefits from cough control therapy in chronic refractory cough, but lower value on adverse events. |
|
| Question 8: Should a trial of antibiotics be used in children with chronic wet cough with normal chest x ray, normal spirometry and no warning signs? | ||||
| Recommendation 8: We suggest a trial of antibiotics in children with chronic wet cough with normal chest X-rays, normal spirometry, and no warning signs. | Conditional | Low | This recommendation is based on higher value of the clinical benefit from antibiotics in chronic wet cough, but lower value on adverse events. |
|
Definition of chronic cough
To define a chronic cough on the basis of longevity is clearly an arbitrary paradigm. Early studies used three months based on the MRC definition of chronic bronchitis[8]. More recent guidelines have adopted eight weeks in adults[9] and four weeks in children[10]. Inclusion criteria for studies of novel antitussives require a cough refractory to treatment to be present for over a year. Whilst some patients cough on a daily basis over many years for others the disease has a relapsing and remitting course making a definition based purely on a temporal basis difficult to sustain. The diagnosis of chronic cough should be made on a global clinical assessment taking into account the other features of the phenotypes of cough detailed below. The failure to recognise that the patient is suffering from the syndrome of chronic cough may lead to misdiagnosis with the patient labelled as suffering from recurrent chest infections, treatment resistant asthma, or exacerbations of COPD.
The commonly used definition of chronic cough in children is 4 weeks, although cough in children lasting 3 to 8 weeks has been termed prolonged acute cough[10, 11]. Irrespective of the exact duration, chronic cough in children is different from that in adults due to differences in the airway morphology, a higher degree of vulnerability to noxious insults, reduced control of the cough reflex and differences in maturation of the neurological and immunological system in the different paediatric age groups[6]. Chronic cough in children is best seen as a symptom of an underlying disease.
Epidemiology
Cough is a common medical problem and the socioeconomic burden is substantial[12]. However, there is no precise data on the burden of chronic cough, probably because chronic cough was previously perceived not as a clinical entity but as the consequent symptom from other respiratory conditions. There is no agreed definition of chronic cough for use in epidemiological studies[8].
A meta-analysis estimated the global prevalence of chronic cough in the general adult population as about 10%[1]. It was more prevalent in Europe, America and Oceania than in Asia and Africa. Notably, the prevalence of chronic cough in adults is associated with a number of characteristics [13–16]. In a recent international survey of 10,032 adult patients attending specialist cough clinics, two-thirds were females and the most common age for presentation was in the sixth decade [3]. The distinct demographic pattern is thought to be related to sex differences in central processing of cough sensation. The most commonly associated conditions are irritable bowel syndrome, obesity and a variety of neuropathic syndromes. Iatrogenic chronic cough from drug treatments is frequently unrecognised.
About 35 % of preschool children report cough at any given time in a month[17]; however, so far, no studies have systematically compared the prevalence of chronic cough in children worldwide. Reports of chronic cough in populations vary between 1% in India[18], 9% in Eastern Europe[19] and 5-12 % in China with increases in areas with higher air pollution[20]. Subjective perception and parental reporting of symptoms further biases prevalence reports[21]. Studies comparing prevalence rates worldwide are warranted.
Impact on patients
Chronic cough is highly disruptive to the individual affected and those around them. The most common reasons why patients with cough seek medical attention include concern about a serious underlying illness, vomiting, exhaustion, sleep disruption, social embarrassment, difficulty speaking on the telephone, urinary incontinence and annoyance to family, friends and workmates[22].
The consequence of chronic cough is a wide range of complications of coughing[23]. Most impactful on Health-Related Quality of Life (HRQOL) are stress urinary incontinence, interference with speech and depression[24]. However, there are many others that can be equally bothersome, such as syncope. Individuals report, on average, eight adverse symptoms associated with cough[22].
Stress urinary incontinence is particularly impactful, as cough affects females disproportionately compared to males. Female patients with cough and urinary incontinence have worse HRQOL compared to those without incontinence[24]. In a quarter of patients, the incontinence is severe but rarely discussed. Thus incontinence should be enquired about during a consultation.
The impact of cough can be assessed and quantified formally with validated HRQOL tools, such as the Leicester Cough Questionnaire (LCQ) or the Cough-specific Quality of Life Questionnaire (CQLQ)[25, 26]. A strength of cough HRQOL tools is that they can be used to demonstrate the efficacy of anti-tussive therapy that is clinically meaningful. In the clinic simply asking “score your cough out of 10” is perhaps the easiest subjective measure of treatment success[27] and should be asked at each consultation.
In children, the caregiver’s worries about the underlying reason for the cough are a major driver to seek medical attention[28]. Paediatric cough is best considered as a symptom of an underlying disease. Therefore, the burden of disease is influenced by the quality of the health care system as well as health care independent factors such as age range[29–31], gender, and indoor and outdoor air pollution[32].
Aetiology and mechanisms
Cough is a vital protective reflex preventing aspiration into the lung. Patients with a poor cough reflex such as those who suffer from neurological conditions succumb to recurrent episodes of aspiration[33] frequently misdiagnosed as “chest infections”. Cough is a vagal reflex evoked by stimulation of afferents carried by the tenth cranial nerve, with their receptive fields primarily in the larynx and conducting airways, but also potentially in the alveolar septa and parenchyma of the lung (e.g. pulmonary embolism, heart failure, altitude sickness), the pharynx and oesophagus, and even the ear, with vagal afferents projecting to the auricular canal from the superior vagal (jugular) ganglia (Arnolds reflex)[34].
Noxious stimuli (e.g. gastric fluid, protons, cigarette smoke, particulates, hyper or hypotonicity) are detected through receptors and ion channels (e.g. TRPV1, TRPA1, TRPV4, ASIC, P2X3) localized to vagal afferent nerve terminations in the airways mucosa[35]. The vagal afferent nerves regulating cough are polymodal i.e. responding to a variety of different chemical and mechanical stimuli. Cellular stress releasing ATP appears to be an important stimulus[36]. Afferent neuronal traffic is relayed via vagal axons to the brainstem via at are least two different biochemical pathways[37]. Cortical influences modulate the reflex, with women having a greater area of the somatosensory cortex devoted to cough. The system is characterised by marked redundancy, plasticity and adaption. The neurobiology of cough has recently been comprehensively reviewed[38].
Cough may be caused by excessive stimulation of a normal cough reflex such as occurs following inhalation of a foreign body or noxious vapours. However, most patients presenting with a chronic cough have features of cough reflex hypersensitivity, responding to exposure to low levels of thermal, chemical, or mechanical stimulation[5]. The cough hypersensitivity syndrome has been adopted as an overarching diagnosis with the different phenotypes dependent on the type and location of the inflammation seen. Both central and peripheral mechanisms have been postulated for cough reflex hypersensitivity[39].
The aetiological mechanisms for cough hypersensitivity remain controversial and are dealt with in greater depth below. In the airways, T2 inflammation occurs in approximately a quarter of patients although this may be through stimulation of the innate immune system rather than atopy[40]. This gives rise to the phenotypes of cough variant asthma and eosinophilic bronchitis[41]. Reflux, particularly nonacid gaseous airway reflux, and oesophageal dysmotility are common features[42]. Central mechanisms for cough hypersensitivity have also been postulated, with circumstantial supportive evidence generated using fMRI[43]. It is suggested that there is an underlying neuropathic process responsible for cough hypersensitivity[44], a view that is supported by the development of cough in certain forms of hereditary somatosensory neuropathy[45].
Phenotypes of chronic cough
Asthmatic Cough I Eosinophilic Bronchitis
Asthma is a clinical diagnosis. There is no agreed single diagnostic test to diagnose or exclude asthma and because of its heterogeneous presentation opinions differ on how to describe the syndrome in patients with chronic cough. Eosinophilic inflammation may be a useful biomarker of asthmatic cough and may have utility in directing therapeutics. All adults and children with chronic cough may be assessed for eosinophilic inflammation. Sputum eosinophilia is perhaps the most accurate indicator, but is not routinely available, time-consuming, and requires expert interpretation. Exhaled nitric oxide can be used as a surrogate marker of eosinophilic airway inflammation and steroid responsiveness in classic asthma, but its role in asthma and chronic cough is questioned below. A meta-analysis of observational studies showed exhaled nitric oxide to have a relatively high specificity of 0.85 in predicting asthma among adult patients with chronic cough[46]; however, there is still no consensus on the cut-off level for the diagnosis. Blood eosinophilia is a simple and readily available measure, but is characterised by diurnal and seasonal variability[47] so multiple assessments should be made[48]. An eosinophil count of greater than 0.3 cells/µL may be taken to indicate eosinophilic airway inflammation[49, 50].
Three subgroups of asthmatic cough have been recognised. Classic asthma is characterised by airflow variability and bronchial hyperresponsiveness. Spirometry is thus an obligatory investigation. Cough variant asthma (CVA)) was originally described as those patients with asthma and cough as the sole symptom and where treatment with bronchodilators improved coughing [51]. Opinions vary as to whether this should be sought by performing bronchial provocation test. Some centres see this as an important part of the workup, whereas others find it adds little to the patient pathway. The third form of asthmatic cough is eosinophilic bronchitis (EB) without bronchoconstriction or hyperresponsiveness. The lack of these latter two features has been suggested to indicate that EB is a separate condition – Non-Asthmatic Eosinophilic Bronchitis[52]. However, in chronic cough communication with patients and other health care professionals may be enhanced if it is considered as part of an asthmatic spectrum, particularly as all three subgroups can respond to anti-inflammatory asthma therapy. The vital importance of establishing or refuting the diagnosis of asthmatic cough lies in the therapeutics (discussed in questions below) as it may be considered as a treatable trait.
Reflux cough
The role of reflux, oesophageal dysmotility, and aspiration in chronic cough is controversial. Its prevalence has been estimated from 0 to almost 100%. Early studies using the criteria of acid reflux found a low incidence and poor temporal relationship[53]. A systematic review[54] found no significant benefits over placebo of PPIs in patients without acid reflux and only modest benefits even in patients with acid reflux. It was suggested that non-acid reflux, both liquid and gaseous, may be an aetiological factor[55]. However no technology reliably detects such reflux and the diagnosis relies on the clinical history supported by validated questionnaires such as the Hull Airway Reflux Questionnaire (HARQ)[56] (see issc.info for multi lingual versions) or Reflux Symptom Index[57]. The picture is complicated by the observation that there is a high prevalence of oesophageal dysmotility in patients with chronic cough[42] and thus oesophago-pharyngeal reflux rather than GORD/GERD may be the problem.
Many of the signs and symptoms associated with chronic cough are explicable by reflux and aspiration. Voice change, nasal symptoms and dysgeusia are common[58]. Frequent “chest infections” bronchitis, and even frank bronchiectasis may be the consequence rather than the cause of cough through repeated aspiration. Unsurprisingly following aspiration of contents from the GI tract there is an inflammatory response. This might be neutrophilic or eosinophilic giving rise to asthmatic cough and mucus hypersecretion[59].
Postnasal drip syndrome/Upper airways cough syndrome
The 2006 American College of Chest Physicians (ACCP) cough management guidelines suggested the term upper airways cough syndrome (UACS) to describe the variety of signs and symptoms previously referred to by other synonyms including postnasal drip syndrome, rhinitis and rhinosinusitis[60]. The revised nomenclature however did not resolve ongoing controversy regarding the existence of this syndrome and the mechanism(s) by which it may induce chronic cough.
A first-generation antihistamine and decongestant were recommended as the treatment, in the absence of adequate randomised controlled trial (RCT) evidence. The first-generation antihistamines however are thought to be antitussive through their action as centrally penetrant anticholinergics[61].
However UACS could be accepted as an aetiology of chronic cough in some patients by acting as a trigger for cough hypersensitivity although the mechanism remains obscure. The absence of evidence for localised treatment might suggest that upper airway symptoms merely reflect generalised airway inflammation consequent to asthma or airway reflux.
Iatrogenic cough
Chronic cough occurs in approximately 15% of patients taking angiotensin-converting enzyme inhibitors (ACEI). ACEI increases the sensitivity of the cough reflex in most subjects[62] and it is probable that additional factors are required to produce clinical impact. Since the reflex is reset there may be no close temporal relation to drug administration or withdrawal and the cough[63]. No patient with a cough or who develops one should be given ACEI. Angiotensin II antagonists do not affect the cough reflex.
Drugs such as bisphosphonates or calcium channel antagonists may worsen pre-existing reflux disease causing increased cough. Prostanoid eye drops such as latanoprost may descend the lacrimal duct irritating the pharynx[64].
Chronic cough in children
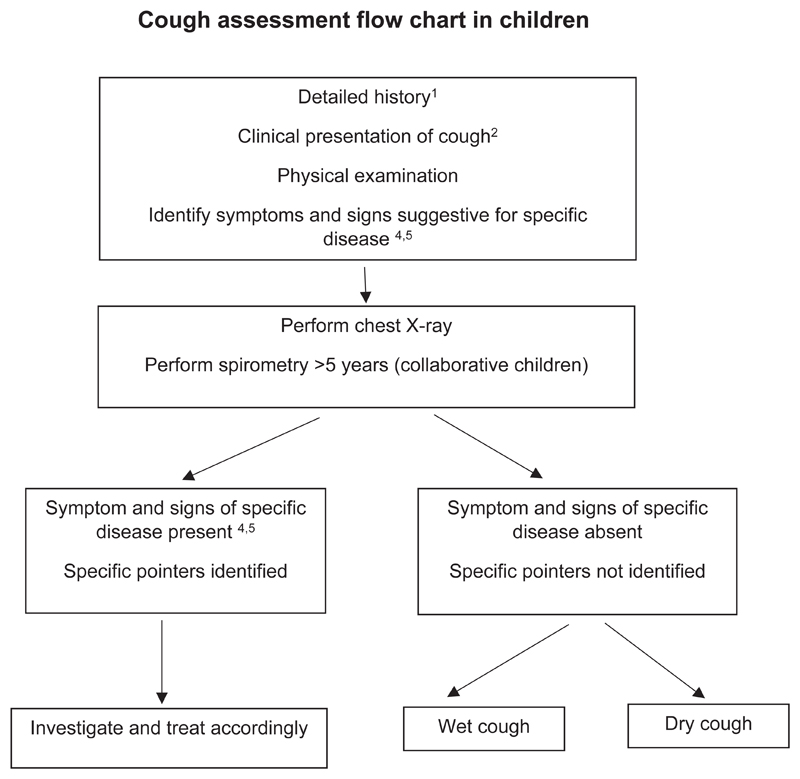
Chronic cough in children differs from that in adults in terms of common aetiologies and management and is increasingly defined as cough that lasts more than 4 weeks. Regardless of setting and age, children with chronic cough should be evaluated carefully using children-specific protocols[65].
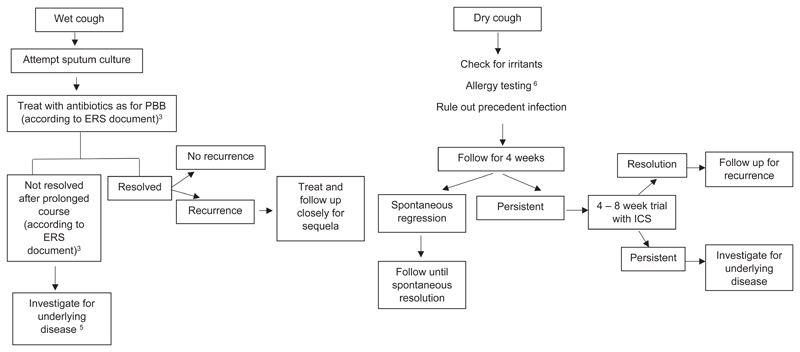
During childhood, the respiratory tract and nervous system undergo a series of anatomical and physiological maturation processes that influence the cough reflex. Additionally, immunological responses undergo developmental and memorial processes that make infection and congenital abnormalities the predominant causes of cough in children[66]. Thus, tracheomalacia, protracted bacterial bronchitis (PBB), and bronchiectasis occur, in addition to common aetiologies such as asthma and post-infectious cough[67]. PBB is not a new entity and PBB-like conditions were being reported in the 1980s[68]. An ERS task force has recently advanced a reliable definition of PBB for day-to-day clinical practice when all three of the following criteria are fulfilled: 1) Presence of continuous chronic (>4 weeks’ duration) wet or productive cough; 2) absence of symptoms or signs (i.e. specific cough pointers) suggestive of other causes of wet or productive cough; and 3) cough resolved following a 2–4-week course of an appropriate oral antibiotic[69]. PBB may be a precursor of bronchiectasis[70].
Initial assessment for chronic cough in children includes a detailed history and thorough physical examination to identify possible specific causes due to an underlying disease. A sudden onset of cough in an otherwise healthy preschool child may suggest foreign body aspiration and requires bronchoscopy. A chest x-ray as well as spirometry in collaborative children is essential. If specific cause for the chronic cough is suspected, further investigations are necessary. In case no specific pointers are detected and chest x-ray and spirometry are normal then the guideline panel considered that another period of observation of up to four weeks was indicated. In case of persistence of cough differentiate between dry and wet cough[71]. Exposure to airborne irritants (e.g. tobacco exposure, combustions, traffic related exposure etc.), allergen exposure or postinfectious cough may be a reason for dry chronic cough. In case of wet cough, sputum cultures should be attempted.
Habit/tic cough is another aetiology found particularly in children, manifesting the core clinical features of tics including suppressibility, distractibility, suggestibility, variability, and the presence of a premonitory sensation whether the cough is single or one of many tic. The formerly called psychosomatic cough should now be labelled somatic cough disorder and this diagnosis should only be made after an extensive evaluation that includes ruling out tic disorders and uncommon causes of chronic cough[72].
Psycho-morbidity is present in all patients with chronic cough with a variety of aetiologies, and tends to decrease following successful treatment[73]. There are limited criteria for the diagnosis of psychogenic (or somatic) cough and features of psychogenic cough reported in the literature are not unique to psychogenic cough[72]. Somatic cough disorder has been commonly used to describe cough without obvious aetiology. However, recent research has revealed neurobiological phenomena are responsible for psychogenic cough[43]. The presence of depression and/or anxiety cannot be used to diagnose psychogenic cough because, as in adults, patients with a persistently troublesome chronic cough can develop these psychologic symptoms when their coughs remain untreatable. Non-pharmacological trials of hypnosis or suggestion therapy or combinations of reassurance, counselling, or referral to a psychologist and/or psychiatrist have been suggested in management, but such strategies lack an evidence base.
Chronic refractory cough
A proportion of patients with chronic cough, particularly among adults, have persistent cough despite thorough investigation and treatment according to published practice guidelines. Terms such as idiopathic chronic cough, unexplained chronic cough and chronic refractory cough have been utilized to describe this clinical condition[74]. Successful trials of drugs with neuromodulatory effects such as opiates, gabapentin, and P2X3 antagonists suggest that aberrant neurophysiology is likely to underlying this condition. Here the term chronic refractory cough is used to indicate that the cough is refractory to conventional treatment of cough-associated conditions or traits.
Chronic cough in other diseases
Most chronic respiratory disease is associated with cough. Physical distortion of the airway such as occurs in lung cancer or the bronchorrhea of cystic fibrosis and chronic bronchitis produces cough by mechanical effects. However cough hypersensitivity through cell damage and inflammation underlies much of the increased cough seen in other pathologies. The different pathological processes in individual conditions contribute to the disease specific, heterogeneous, aetiology of cough in other lung disease.
As an example, cough in interstitial lung diseases (ILDs) is common with a prevalence of 30 to 90%. Patients with ILD often respond poorly to general anti-tussive therapy. In an open label study of idiopathic pulmonary fibrosis (IPF) pirfenidone reduced 24-hr objective cough counts and improved cough-related QoL[75]. Reformulated sodium cromoglicate improved 24-hr objective cough by 31% in patient with IPF whereas there was no effect in chronic idiopathic cough[76]. It seems likely that each individual respiratory condition will have its own profile dependent on the tussigenic factors expressed in that disease.
Chronic cough, tobacco and nicotine
Smoking is the major remediable cause of chronic cough and is inextricably linked to chronic obstructive pulmonary disease (COPD). Epidemiological studies have demonstrated a relationship between cumulative smoking exposure and chronic cough[77]. Furthermore, smoking history and current cigarette consumption are predictors of objectively-measured cough frequency[78]. A natural inference therefore would be to ascribe a protussive effect to tobacco smoke and its components. Research in otherwise healthy smokers and nonsmokers, however, has provided additional insights, some of which contradict general assumption.
Multiple studies of otherwise healthy smokers have demonstrated suppressed cough reflex sensitivity to inhaled capsaicin[79, 80]. The development of electronic cigarettes (e-cigs) provided a mechanism of non-combustible delivery of nicotine to the lungs. One tobacco cigarette equivalent induced significant suppression of cough reflex sensitivity[81]. These data are consistent with previous clinical observations of transient increase in cough within the first month after smoking cessation[82]. All patients should quit smoking and they should be warned there may be a transient increase in coughing. For those unable to quit because of excessive coughing e-cigs may be a supportive therapy[83].
Assessing cough in the clinic
Initial assessment
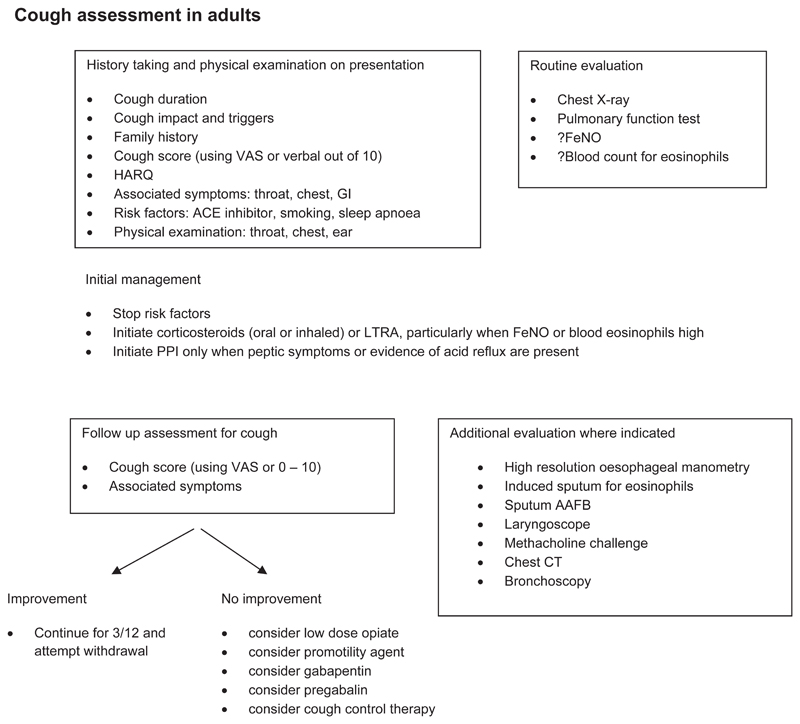
The history, examination, and investigations for patients with chronic cough are performed to exclude treatable traits of the disease for which directed therapy can be offered. The guideline panel placed higher value on control of any on-going pathology such as reflux or airway eosinophilia before currently available neuro-modulatory treatments are considered. A detailed history and examination should be directed to exclude malignancy, infection, foreign body inhalation or the use of an angiotensin converting enzyme (ACE) inhibitor. The impact of cough should be assessed either by recording simple measures such a cough score out of 10 or VAS or by more detailed, validated measures of cough quality of life (LCQ or CQLQ). Validated questionnaires may help to detect features of airway reflux (HARQ and RSI) and airway hypersensitivity[84].
Initial evaluation should include spirometry and a recent chest x-ray (CXR) (Good Practice Statement).
Chest CT
Question 1: Should chest CT scan be routinely performed on chronic cough patients with normal chest X-ray and physical examination?
We suggest that clinicians do not routinely perform a chest CT scan in patients with chronic cough who have normal chest x-ray and physical examination (conditional recommendation, very low quality evidence).
Some prospective and retrospective cohorts identified CT findings in a range of 6.5% to 58% of patients with cough and normal CXR; however the causal relationship was either not specified or considered as unlikely related to cough [85–87]. There is a concern about potential cancer risk from CT radiation exposure [88, 89]. Thus the potential radiation risk needs to be weighed against possible diagnostic yields, particularly in susceptible populations such as children and females.
Further investigations to identify treatable traits in chronic cough
Further investigations for asthma, EB, reflux and oesophageal dysmotility, and rhinosinusitis should be considered depending on the clinical history.
Asthma and eosinophilic inflammation
Objective evidence of classic asthmatic cough conventionally requires some evidence of variable airflow obstruction such as peak flow variability, or reversibility to salbutamol of more than 12-15%. However, these investigations have a very low negative predictive value particularly in patients with normal lung function[86]. Further investigation of bronchial hyper-responsiveness (BHR) using either methacholine or histamine inhalational challenge is advocated by some although its utility in diagnosis is questioned. Evidence for ongoing airway eosinophilic inflammation can be sought by performing differential cell counts on samples from sputum induction or bronchoalveolar lavage. In such cases, elevated eosinophils (>3%) in the airways in the absence of BHR would suggests EB, which has been reported in up to 13% of patients attending cough clinics[41]. However, most centres do not have such facilities available, hence a non-invasive alternative is the use of fractional exhaled nitric oxide (FeNO) in breath or blood eosinophilia as a surrogate marker to assess airway eosinophilia. The clinical usefulness of FeNO or blood eosinophils in aiding diagnosis or predicting treatment response in patients with chronic cough has not yet been systematically evaluated[90].
Question 2: Should FeNO/blood eosinophils be used to predict treatment response to corticosteroids/anti-leukotrienes in chronic cough?
There is a need for convenient and practical tests for predicting anti-inflammatory treatment responses in patients with chronic cough. However, there is a still lack of quality evidence. Placebo-controlled trials are warranted to assess their utility and also consensus is required on threshold levels in patients with chronic cough.
One RCT [91] in adult non-smoking patients with chronic cough shows that baseline FeNO levels (greater than 30ppb or lower than 20 ppb) did not predict response to anti-leukotrienes. Cough frequency and quality of life were similar between high and low FeNO groups at the end of treatment. Observational studies suggested that non-responders to ICS may have significant lower levels of FeNO at baseline [92, 93], but the findings were not consistent[94]. Randomised placebo-controlled trials are required to validate the utility or otherwise of FeNO as a predictor of treatment response in chronic cough patients. Currently, there is no study examining the predictive utility of blood eosinophils in patients with chronic cough.
Given the uncertainty of diagnostic testing, a therapeutic trial may be indicated for asthmatic cough. In adults oral prednisolone for one week may cause a dramatic decrease in cough[95]. Inhaled corticosteroids (ICS) may be used when oral is contraindicated and is preferable in children. However it may be less effective since inflammation in CVA and EB is located in different parts of the airway from that seen in classic asthma[96], and may be driven by other pathways such as the innate immune system[97]. This also may explain the greater efficacy of systemic leukotriene antagonists such as montelukast in asthmatic cough[98].
Reflux and dysmotility
In the absence of peptic symptoms 24-hour pH monitoring for the investigation of reflux disease is not helpful. However abnormal oesophageal physiology is very common in patients with chronic cough and may be detected with poor sensitivity by a barium swallow. More accurately, high resolution oesophageal manometry provides diagnostic information as to the site and mechanism of dysmotility in the majority of patients[42].
The upper airways
In patients who report upper airway symptoms fibre optic laryngoscopy may be performed. The larynx is commonly found to be red and inflamed. However, the test has poor sensitivity and specificity. In select patients, laryngoscopy may be useful in identifying inducible laryngeal obstruction (ILO) associated with cough, and this may help plan the need for future cough control therapy[99]. Rhinoscopy may be helpful in identifying polyps and clearing mucus from blocked sinuses in patients with recurrent sinus and nasal inflammation, but routine laryngoscopy, rhinoscopy or CT sinuses is not advised as nasal findings are not directly associated with cough[100, 101].
Chronic cough in children
Chronic cough in children should be approached using paediatric-specific cough management protocols or algorithms and basing the management on the aetiology of the cough. The most common recognized aetiologies for chronic cough in children are post-infectious or natural resolution, asthma and PBB. Refer to the flow diagram on page x.
Treatment of chronic cough
Even after a thorough clinical assessment it may be impossible to identify which of the treatable traits is most likely to underlie the patient’s chronic cough. Individuals may vary in their response to the different modalities of treatment. The guideline panel considered that it was preferable to undertake sequential therapeutic trials of each agent in turn and if no responses were observed therapy should be stopped. The length of the trial depends on the pharmacology. Response to morphine occurs within one week. ICS may take a month. If successful, the guideline panel believes that treatment may be continued for several months to allow for resolution of neuronal hypersensitivity. Treatment may be then withdrawn to determine whether remission has occurred. The reader is referred to table 1 for commentary on the recommendations below.
Anti-asthmatic drugs
Question 3: Should anti-asthmatic drugs (anti-inflammatory or bronchodilator drugs) be used to treat patients with chronic cough?
We suggest a short-term ICS trial (2-4 weeks) in adult patients with chronic cough (conditional recommendation, low quality evidence).
Ten RCTs were identified for chronic cough, but with considerable heterogeneity in patient characteristics, intervention, measured outcomes and treatments responses. Two studies of chronic cough patients (unselected by airway hyper-responsiveness or sputum eosinophilia) found significant benefits from a 2-week high dose ICS treatment over placebo in reducing cough severity[102] and subjective cough frequency. However, in a study of patients with chronic cough and at least one additional respiratory symptom but with normal lung function, an 8-week medium dose ICS treatment did not produce a significant improvement in cough severity score over placebo. In two studies of patients with non-asthmatic chronic cough (defined by negative methacholine airway-hyper-responsiveness), ICS treatment was not superior to placebo in improving cough outcomes[103, 104]. In studies of patients with chronic bronchitis or chronic obstructive pulmonary disease (COPD), ICS treatment did not significantly improve subjective cough scores compared to placebo[105–108].
Although the original definition of CVA demonstrated improvement of coughing in a small number of asthmatic subjects with bronchodilator therapy, we do not recommend the use of a lone bronchodilator therapy as maintenance treatment for cough in asthmatic patients. The current GINA 2019 guideline recommend the use of low dose ICS-formoterol or low dose ICS. Effectiveness of these treatment regimes in CVA and asthmatic cough still requires further evaluation.
We suggest a short-term ICS trial in children with chronic dry cough (2-4 weeks) (conditional recommendation, low quality evidence).
Two RCTs were identified. A trial of 50 children aged 1-10 years with persistent nocturnal cough found that there is a modest but significant benefit in objective cough frequency from a 2-week course of high dose ICS over placebo[109]. Another study of 43 children aged 6-17 years with recurrent cough (two episodes of cough, each lasting two weeks in the past 12 months) found no significant effects of ICS in cough outcomes at 4-5 weeks; there was no association between ICS treatment response and airway hyper-responsiveness in hypertonic saline challenge[110].
We suggest a short-term anti-leukotriene trial (2-4 weeks) in adult patients with chronic cough, particularly in those with asthmatic cough (conditional recommendation, low quality evidence).
Three RCTs were identified. Two clinical trials[111, 112] of adults with cough variant asthma (defined by clinical history, absence of other common diseases, and presence of methacholine hyper-responsiveness) found significant benefits of oral anti-leukotriene (for 2-4 weeks) over placebo in subjective cough frequency or severity scores. However, a single trial of adults with atopic cough (defined as chronic cough with increased capsaicin cough sensitivity and atopic constitution but without bronchial hyper-responsiveness) did not find any significant benefits of 2-weeks montelukast over placebo in subjective cough score[113]. Adverse drug event was reported in one study, without any significant event related to the treatment[112]. There are no trials conducted in unselected chronic cough patients.
No RCTs are available for children. Mild, transient neuropsychiatric adverse events are common (>10%) in children[114].
We suggest a short-term trial (2-4 weeks) of ICS and long-acting bronchodilator combination in adults with chronic cough and fixed airflow obstruction (conditional recommendation, moderate quality evidence).
A single RCT[108] of COPD patients with chronic bronchitis, smoking history and at least one episode of COPD symptom exacerbation in the previous year found that the combination of 50 μg salmeterol and 500 μg fluticasone twice daily produced a significant improvement in cough severity score compared to placebo (scale: 0-4) (mean difference: −0.09; 95% CI: −0.17 to −0.01), whereas salmeterol or fluticasone monotherapy did not. The treatment was well-tolerated, except for an increased incidence of oropharyngeal candidiasis (8% in the combination treatment group vs. 2% in the placebo group).
Anti-acids
Question 4: Should anti-acid drugs (PPIs and H2 antagonists) be used to treat patients with chronic cough?
We suggest that clinicians do not routinely prescribe anti-acid drugs in adult patients with chronic cough (conditional recommendation, low quality evidence).
Anti-acid drugs are unlikely to be useful in improving cough outcomes, unless patients have peptic symptoms or evidence of acid reflux. Systematic reviews have found no significant benefits from PPI over placebo in adult patients without acid reflux and possible modest effect in those with acid reflux [54]. Faruqi et al. found no significant benefits of esomeprazole 20 mg twice daily therapy over placebo in subjective cough frequency, cough severity, or cough-specific quality of life scores at 8 weeks. There was a trend towards greater improvement in the PPI treatment arm in patients with dyspepsia[115]. In a study of chronic cough patients with rare or no heartburn, there were no benefits from a long-term high-dose PPI therapy (esomeprazole 40 mg twice daily for 12 weeks) in cough-specific quality of life or cough scores [116]. Whilst PPI is frequently considered safe observational studies reported potential risks of iron deficiency, vitamin B12 deficiency, hypomagnesemia, Clostridium difficile–associated diarrhoea, osteoporosis-related bone fracture, dementia, or pneumonia[117, 118]. However, direct evidence about the safety issues is lacking in chronic cough population. There is not enough evidence to draw a specific recommendation for PPI use in children.
Drugs with promotility activity
Question 5: Should drugs with promotility activity be used to treat patients with chronic cough?
There is currently insufficient evidence to recommend the routine use of macrolide therapy in chronic cough. A one month trial of macrolides can be considered in the cough of chronic bronchitis refractory to other therapy, taking into account local guidelines on antimicrobial stewardship. (conditional recommendation, low quality evidence).
No RCTs have been undertaken with pro-motility agents, such as baclofen, metoclopramide or domperidone, in patients with chronic cough. There are three RCTs with macrolides with promotility activity in adult patients with chronic cough. One study of patients with COPD GOLD stage ≥ 2 and chronic productive cough demonstrated a significant benefit of a 12-week low dose azithromycin (250 mg three times a week) over placebo for improving cough-specific quality of life (LCQ; MD 1.3; 95% CI 0.3 to 2.3; p=0.01)[119]. Adverse events were not significantly different. In two other trials of patients with unexplained cough or treatment-resistant cough, low-dose macrolide treatments (erythromycin 250 mg daily for 12 weeks or azithromycin 250 mg three times a week for 8 weeks) did not provide significant benefits over placebo for objective cough frequency, cough severity or cough-specific quality of life[120, 121].
Neuromodulators
Question 6: Which cough neuromodulatory agents (pregabalin, gabapentin, tricyclics and opiates) should be used to treat patients with chronic cough?
We recommend a trial of low dose slow release morphine (5-10 mg bd) in adult patients with chronic refractory cough (strong recommendation, moderate quality evidence).
A single RCT of low dose morphine (5 to 10 mg twice daily) in adults with chronic refractory cough found significant benefits over placebo in reducing cough severity (self-reported scale 0 to 9 points) (MD -1.96 points; 95%CI -1.09 to -2.11) and improving cough-specific quality of life (LCQ) (MD 2 points; 95%CI 0.93 to 3.07)[27]. Common adverse effects in this clinical trial were constipation and drowsiness in patients receiving morphine.
We suggest a trial of gabapentin or pregabalin in adults with chronic refractory cough (conditional recommendation, low quality evidence).
A single RCT of gabapentin therapy (maximum tolerable daily dose of 1800 mg) in adults with chronic refractory cough found significant benefits over placebo in improving LCQ (MD 1.8 points; 95%CI 0.56 to 3.04) and reducing cough frequency (although only of a single hour of observation) (MD -27.31%; 95%CI -2.87 to -51.75) and severity (VAS 0 to 100 points) (MD -12.33 points; 95%CI - 1.23 to -23.23)[122]. There is one RCT of pregabalin therapy (300 mg daily) in adult patients with chronic refractory cough alongside speech pathology therapy [123]. Pregabalin plus speech pathology therapy significantly improved cough-specific quality of life (LCQ) (MD 3.5 points; 95%CI 1.11 to 5.89: MID: 1.3 points) and cough severity (VAS 0 to 100 points) (MD -25.1 points; 95%CI -10.6 to -39.6) over placebo plus speech pathology therapy. There was no significant reduction in cough frequency. There is no comparison between pregabalin and placebo alone. An explanation for a lack of effect on cough frequency is that centrally acting therapies may be altering perception of cough rather than having truly anti-tussive effects. They could also be affecting the intensity of coughing without reducing the frequency. Dizziness, fatigue, cognitive changes, nausea, or blurred vision are common side effects of gabapentin and pregabalin. A systematic review revealed that the risk of withdrawal due to adverse events is 2.3 times higher than placebo[124].
Agents acting directly on cough hypersensitivity rather than the treatable traits causing hypersensitivity is a promising strategy for future developments. Current agents have been shown to be effective in adults, but the side effect profile is significant and may be mitigated by the use of lower doses than those used to treat pain.
Cough neuromodulators, such as opioids, gabapentin or pregabalin, are not used in children, due to reported adverse events, possible toxicity and lack of clinical trials[125].
Non-pharmacological cough control therapy
Question 7: Should non-pharmacological therapy (cough control therapy) be used to treat patients with chronic cough?
We suggest a trial of cough control therapy in adult patients with chronic cough (conditional recommendation, moderate quality evidence).
Two RCTs of physiotherapy/speech and language therapy (cough control therapy) in adult patients with chronic refractory cough have been reported. Vertigan et al. demonstrated that the 2-month intervention significantly reduced subjective cough score compared to placebo treatment (self-reported scale 2 to 10 points) (MD 2.8 points; 95% CI 1.3 to 4.0)[126]. In a multi-centre study by Chamberlain et al., the weekly intervention for 4 weeks showed benefits over placebo for cough-specific quality of life (LCQ; 1.53 points; 95% CI 0.21 to 2.85) and objective cough frequency (fold change) (0.59; 95% CI 0.36 to 0.95), but not for VAS severity or other quality of life outcomes. The improvements in the intervention group were sustained up to 3 months, but not beyond. No adverse effects were found[127]. There are no RCTs in children. This is a complex intervention that requires further study to determine which components are of value. Experienced practitioners should undertake cough-directed physiotherapy and speech and language therapy interventions.
Antibiotics for chronic wet cough in children
Question 8: In children with chronic wet cough with normal chest x-ray, normal spirometry and no warning signs, should a trial of antibiotics be used?
A trial of antibiotics is suggested in children with chronic wet cough with normal chest x-rays, normal spirometry and no warning signs (conditional recommendation, low quality evidence).
A single RCT of antibiotics in young children (mean age 1.9 years; IQR 0.9 to 5.1) with chronic wet cough (>3 weeks)[128]. A 2-week regimen of twice daily oral amoxycillin clavulanate treatment (22.5 mg/kg/dose) was compared. Cough resolution rates (defined as >75% reduction) were significantly higher in children who received amoxycillin clavulanate compared with those who received placebo (48% vs. 16%; p=0.015). Side effects were not significantly different between two groups; however, mild diarrhoea was found slightly more in the in the amoxycillin clavulanate group than in the placebo group (5/25 vs. 2/25)[128].
Future directions and new drugs
As the population ages the worldwide prevalence of chronic cough increases. This is partially due to an increasing awareness of the problem, changing diagnostic labels, air pollution, and increased efforts in educating health professionals. Changes in society such as the rise in obesity will also predispose to a greater incidence of causal factors related to chronic cough. Our understanding of the pathophysiological basis of chronic cough has dramatically advanced over the past decade with the realisation that neuronal hypersensitivity underlies the syndrome. Desensitisation through the use of agonists such as capsaicin has recently shown promise as a therapeutic strategy[129]. However, we are still grossly ignorant of the complex interplay in the peripheral and central nervous system. fMRI has given us insights into the central pathways of cough and will continue to do so in the future. However it is the pharmacology of the peripheral afferent vagus which has given us the most hopeful future therapeutic developments.
Much effort was devoted in the development of blockers of the nociceptors, mainly TRP receptors, which are responsible for the irritant sensation leading to the tickle that precedes cough. Whilst effective in animal models these agents have failed in the clinic[130, 131]. The substance P antagonist orvepitant has shown modest efficacy in phase 2 studies. One class of drugs has however produced a dramatic improvement in chronic cough in phase 2 studies[132]. ATP is released during cell damage and acts on afferent sensory nerves through P2X3 purinergic receptors. The first antagonist, gefapixant, has been studied in several hundred patients with chronic cough with resolution in the majority. Other compounds in this class are in development and we may have the first effective drugs for chronic cough in over 40 years.
Research gaps and recommendations for future studies
Because chronic cough has only recently been recognised as a separate entity a major challenge is the promulgation of the concept of cough hypersensitivity in adults and conditions such as protracted bacterial bronchitis in children. Whilst the aim of these guidelines is to further awareness we recognise the scale of the task and recommend the ERS should advocate chronic cough as a classification in the WHO ICD.
Very little is known of the natural history of chronic cough. We recommend observational cohort studies to identify: –
The true prevalence of chronic cough in the population.
The demographic characteristics of this patient population.
The natural history of chronic cough over time.
The clinical and psychosocial impact of chronic cough on patients.
The economic burden of chronic cough both to the individual and society.
The assessment of chronic cough in both the clinical and research settings needs further development. Current instruments to assess quality of life need refinement to be useful in routine clinical practice. Patient reported outcomes need to be developed and validated. There is an urgent need for fully automated cough recording technology that continuously monitors patients in real-time. Such devices may help confirm the diagnosis in the clinic, allow for objective assessment of clinical response, and ensure the entry of the correct population into clinical studies. In addition, the current clinical approach is largely based on sequential therapeutic trials; thus, practical biomarkers need to be developed to target treatable traits and guide treatment decisions in the clinic.
There are very few studies of cough in other diseases and currently patients with the syndrome of chronic cough are frequently mislabelled as suffering from other conditions. Studies of the overlap between respiratory disease and chronic cough are urgently need, particularly in view of the differences in pathophysiology and treatable traits.
These guidelines were constructed with editorial independence from the ERS. Conflicts of interest were recorded and disclosures can be found alongside this article at erj.ersjournals.com
Supplementary Material
References
- 1.Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, Cho SH, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain SA, Garrod R, Douiri A, Masefield S, Powell P, Bucher C, Pandyan A, Morice AH, Birring SS. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193(3):401–408. doi: 10.1007/s00408-015-9701-2. [DOI] [PubMed] [Google Scholar]
- 3.Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, Smith JA, Parker SM, Chung KF, Lai K, Pavord ID, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. The European respiratory journal. 2014;44(5):1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 4.Millqvist E. The airway sensory hyperreactivity syndrome. PulmPharmacolTher. 2011;24(3):263–266. doi: 10.1016/j.pupt.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, Dal Negro RW, Dicpinigaitis P, Kantar A, McGarvey LP, Pacheco A, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. The European respiratory journal. 2014;44(5):1132–1148. doi: 10.1183/09031936.00218613. [DOI] [PubMed] [Google Scholar]
- 6.Chang AB. Pediatric cough: children are not miniature adults. Lung. 2010;188(Suppl 1):S33–40. doi: 10.1007/s00408-009-9166-2. [DOI] [PubMed] [Google Scholar]
- 7.Miravitlles M, Tonia T, Rigau D, Roche N, Genton C, Vaccaro V, Welte T, Gaga M, Brusselle G. New era for European Respiratory Society clinical practice guidelines: joining efficiency and high methodological standards. Eur Respir J. 2018;51(3) doi: 10.1183/13993003.00221-2018. [DOI] [PubMed] [Google Scholar]
- 8.Song WJ, Chang YS, Faruqi S, Kang MK, Kim JY, Kang MG, Kim S, Jo EJ, Lee SE, Kim MH, Plevkova J, et al. Defining Chronic Cough: A Systematic Review of the Epidemiological Literature. Allergy Asthma Immunol Res. 2016;8(2):146–155. doi: 10.4168/aair.2016.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, Kastelik JA, McGarvey LP, Smith JA, Tatar M, Widdicombe J. ERS guidelines on the assessment of cough. EurRespirJ. 2007;29(6):1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 10.Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):260s–283s. doi: 10.1378/chest.129.1_suppl.260S. [DOI] [PubMed] [Google Scholar]
- 11.Shields MD, Bush A, Everard ML, McKenzie S, Primhak R, British Thoracic Society Cough Guideline G BTS guidelines: Recommendations for the assessment and management of cough in children. Thorax. 2008;63(Suppl 3):iii1–iii15. doi: 10.1136/thx.2007.077370. [Clinical presentation of cough: How and when the cough started, time-course of cough, nature and quality of cough, symptoms associated with cough, triggers of cough, diurnal and nocturnal variations, cough associated with indoor and outdoor irritants.] [DOI] [PubMed] [Google Scholar]
- 12.Morice AH. Epidemiology of cough. Pulm Pharmacol Ther. 2002;15(3):253–259. doi: 10.1006/pupt.2002.0352. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61:975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colak Y, Nordestgaard BG, Laursen LC, Afzal S, Lange P, Dahl M. Risk Factors for Chronic Cough Among 14,669 Individuals From the General Population. Chest. 2017;152(3):563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Kang MG, Song WJ, Kim HJ, Won HK, Sohn KH, Kang SY, Jo EJ, Kim MH, Kim SH, Kim SH, Park HW, et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: The Korean National Health and Nutrition Examination Survey 2010-2012. Medicine (Baltimore) 2017;96(13):e6486. doi: 10.1097/MD.0000000000006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latti AM, Pekkanen J, Koskela HO. Defining the risk factors for acute, subacute and chronic cough: a cross-sectional study in a Finnish adult employee population. BMJ Open. 2018;8(7):e022950. doi: 10.1136/bmjopen-2018-022950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US preschool-age children. JAMA. 1994;272(13):1025–1030. [PubMed] [Google Scholar]
- 18.Singh D, Arora V, Sobti PC. Chronic/recurrent cough in rural children in Ludhiana, Punjab. Indian Pediatr. 2002;39(1):23–29. [PubMed] [Google Scholar]
- 19.Leonardi GS, Houthuijs D, Nikiforov B, Volf J, Rudnai P, Zejda J, Gurzau E, Fabianova E, Fletcher T, Brunekreef B. Respiratory symptoms, bronchitis and asthma in children of Central and Eastern Europe. Eur Respir J. 2002;20(4):890–898. doi: 10.1183/09031936.02.00260802. [DOI] [PubMed] [Google Scholar]
- 20.Pan G, Zhang S, Feng Y, Takahashi K, Kagawa J, Yu L, Wang P, Liu M, Liu Q, Hou S, Pan B, et al. Air pollution and children's respiratory symptoms in six cities of Northern China. RespirMed. 2010;104(12):1903–1911. doi: 10.1016/j.rmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Dales RE, White J, Bhumgara C, McMullen E. Parental reporting of childrens' coughing is biased. Eur J Epidemiol. 1997;13(5):541–545. doi: 10.1023/a:1007311912777. [DOI] [PubMed] [Google Scholar]
- 22.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657–1661. doi: 10.1001/archinte.158.15.1657. [see comments] [DOI] [PubMed] [Google Scholar]
- 23.Raj AA, Birring SS. Clinical assessment of chronic cough severity. Pulm Pharmacol Ther. 2007;20(4):334–337. doi: 10.1016/j.pupt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.French CL, Crawford SL, Bova C, Irwin RS. Change in Psychological, Physiological, and Situational Factors in Adults After Treatment of Chronic Cough. Chest. 2017;152(3):547–562. doi: 10.1016/j.chest.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 25.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of cough-specific quality of life questionnaire. Chest. 2002;121:1123–1131. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 26.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morice AH, Menon MS, Mulrennan SA, Everett CF, Wright C, Jackson J, Thompson R. Opiate therapy in chronic cough. AmJRespirCrit Care Med. 2007;175(4):312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 28.Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families? Chest. 2008;134(2):303–309. doi: 10.1378/chest.07-2236. [DOI] [PubMed] [Google Scholar]
- 29.Kantar A, Bernardini R, Paravati F, Minasi D, Sacco O. Chronic cough in preschool children. Early HumDev. 2013 doi: 10.1016/j.earlhumdev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Luyt DK, Burton PR, Simpson H. Epidemiological study of wheeze, doctor diagnosed asthma, and cough in preschool children in Leicestershire. BMJ. 1993;306(6889):1386–1390. doi: 10.1136/bmj.306.6889.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang AB, Landau LI, Van Asperen PP, Glasgow NJ, Robertson CF, Marchant JM, Mellis CM. Cough in children: definitions and clinical evaluation. MedJAust. 2006;184(8):398–403. doi: 10.5694/j.1326-5377.2006.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 32.Demoulin-Alexikova S, Plevkova J, Mazurova L, Zatko T, Alexik M, Hanacek J, Tatar M. Impact of Air Pollution on Age and Gender Related Increase in Cough Reflex Sensitivity of Healthy Children in Slovakia. Front Physiol. 2016;7:54–54. doi: 10.3389/fphys.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29–33. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 34.Canning BJ. Functional implications of the multiple afferent pathways regulating cough. PulmPharmacolTher. 2011;24(3):295–299. doi: 10.1016/j.pupt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Belvisi MG, Birrell MA, Khalid S, Wortley MA, Dockry R, Coote J, Holt K, Dubuis E, Kelsall A, Maher SA, Bonvini S, et al. Neurophenotypes in Airway Diseases. Insights from Translational Cough Studies. Am J Respir Crit Care Med. 2016;193(12):1364–1372. doi: 10.1164/rccm.201508-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonvini SJ, Birrell MA, Grace MS, Maher SA, Adcock JJ, Wortley MA, Dubuis E, Ching YM, Ford AP, Shala F, Miralpeix M, et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: Role of adenosine triphosphate. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morice AH, Kitt MM, Ford AP, Tershakovec AM, Wu WC, Brindle K, Thompson R, Thackray-Nocera S, Wright C. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1) doi: 10.1183/13993003.00439-2019. [DOI] [PubMed] [Google Scholar]
- 38.Mazzone SB, Farrell MJ. Heterogeneity of cough neurobiology: Clinical implications. Pulm Pharmacol Ther. 2019 doi: 10.1016/j.pupt.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Mazzone SB, Chung KF, McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir Med. 2018;6(8):636–646. doi: 10.1016/S2213-2600(18)30150-4. [DOI] [PubMed] [Google Scholar]
- 40.Lund S, Walford HH, Doherty TA. Type 2 Innate Lymphoid Cells in Allergic Disease. Curr Immunol Rev. 2013;9(4):214–221. doi: 10.2174/1573395510666140304235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic bronchitis is an important cause of chronic cough. American Journal of Respiratory Critical Care Medicine. 1999;160(2):406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 42.Burke JM, Jackson W, Morice AH. The role of high resolution oesophageal manometry in occult respiratory symptoms. Respir Med. 2018;138:47–49. doi: 10.1016/j.rmed.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Ando A, Smallwood D, McMahon M, Irving L, Mazzone SB, Farrell MJ. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-207425. [DOI] [PubMed] [Google Scholar]
- 44.Chung KF, McGarvey LP, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respiratory Medicine. 2013;1(5):412–422. doi: 10.1016/S2213-2600(13)70043-2. [DOI] [PubMed] [Google Scholar]
- 45.Spring PJ, Kok C, Nicholson GA, Ing AJ, Spies JM, Bassett ML, Cameron J, Kerlin P, Bowler S, Tuck R, Pollard JD. Autosomal dominant hereditary sensory neuropathy with chronic cough and gastro-oesophageal reflux: clinical features in two families linked to chromosome 3p22-p24. Brain. 2005;128(Pt 12):2797–2810. doi: 10.1093/brain/awh653. [DOI] [PubMed] [Google Scholar]
- 46.Song WJ, Kim HJ, Shim JS, Won HK, Kang SY, Sohn KH, Kim BK, Jo EJ, Kim MH, Kim SH, Park HW, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: A systematic review and meta-analysis. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 47.Mathur SK, Fichtinger PS, Evans MD, Schwantes EA, Jarjour NN. Variability of blood eosinophil count as an asthma biomarker. Ann Allergy Asthma Immunol. 2016;117(5):551–553. doi: 10.1016/j.anai.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamad GA, Cheung W, Crooks MG, Morice AH. Eosinophils in COPD: how many swallows make a summer? Eur Respir J. 2018;51(1) doi: 10.1183/13993003.02177-2017. [DOI] [PubMed] [Google Scholar]
- 49.Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, Sterk PJ. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2014 doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 50.Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, Towse L, Finnigan H, Dahl R, Decramer M, Chanez P, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 51.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300(12):633–637. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 52.Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989;1(8651):1346–1348. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- 53.Irwin RS, French CL, Curley FJ, Zawacki JK, Bennett FM. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104(5):1511–1517. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 54.Kahrilas PJ, Howden CW, Hughes N, Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143(3):605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patterson N, Mainie I, Rafferty G, McGarvey L, Heaney L, Tutuian R, Castell D, Johnston BT. Nonacid reflux episodes reaching the pharynx are important factors associated with cough. JClinGastroenterol. 2009;43(5):414–419. doi: 10.1097/MCG.0b013e31818859a3. [DOI] [PubMed] [Google Scholar]
- 56.Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189(1):73–79. doi: 10.1007/s00408-010-9272-1. [DOI] [PubMed] [Google Scholar]
- 57.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) JVoice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 58.Everett CF, Morice AH. Clinical history in gastroesophageal cough. RespirMed. 2007;101(2):345–348. doi: 10.1016/j.rmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Pacheco A, Faro V, Cobeta I, Royuela A, Molyneux I, Morice AH. Gastro-oesophageal reflux, eosinophilic airway inflammation and chronic cough. Respirology. 2011;16(6):994–999. doi: 10.1111/j.1440-1843.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 60.Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dicpinigaitis PV, Morice AH, Birring SS, McGarvey L, Smith JA, Canning BJ, Page CP. Antitussive drugs--past, present, and future. PharmacolRev. 2014;66(2):468–512. doi: 10.1124/pr.111.005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morice AH, Lowry R, Brown MJ, Higenbottam T. Angiotensin converting enzyme and the cough reflex. Lancet. 1987;2(8568):1116–1118. doi: 10.1016/s0140-6736(87)91547-9. [DOI] [PubMed] [Google Scholar]
- 63.Yeo WW, Chadwick IG, Kraskiewicz M, Jackson PR, Ramsay LE. Resolution of ACE inhibitor cough: Changes in subjective cough and responses to inhaled capsaicin, intradermal bradykinin and substance-P. Br J Clin Pharmacol. 1995;40:423–429. [PMC free article] [PubMed] [Google Scholar]
- 64.Fahim A, Morice AH. Heightened cough sensitivity secondary to latanoprost. Chest. 2009;136(5):1406–1407. doi: 10.1378/chest.09-0070. [DOI] [PubMed] [Google Scholar]
- 65.Chang AB, Oppenheimer JJ, Weinberger MM, Rubin BK, Weir K, Grant CC, Irwin RS, Panel CEC Use of Management Pathways or Algorithms in Children With Chronic Cough: CHEST Guideline and Expert Panel Report. Chest. 2017;151(4):875–883. doi: 10.1016/j.chest.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 66.Kantar A. Update on Pediatric Cough. Lung. 2016;194(1):9–14. doi: 10.1007/s00408-015-9815-6. [DOI] [PubMed] [Google Scholar]
- 67.Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Masters IB, Teoh L, Mellis CM, Landau LI, Marchant JM, Morris PS. A cough algorithm for chronic cough in children: a multicenter, randomized controlled study. Pediatrics. 2013;131(5):e1576–1583. doi: 10.1542/peds.2012-3318. [DOI] [PubMed] [Google Scholar]
- 68.Taussig LM, Smith SM, Blumenfeld R. Chronic bronchitis in childhood: what is it? Pediatrics. 1981;67(1):1–5. [PubMed] [Google Scholar]
- 69.Kantar A, Chang AB, Shields MD, Marchant JM, Grimwood K, Grigg J, Priftis KN, Cutrera R, Midulla F, Brand PLP, Everard ML. ERS statement on protracted bacterial bronchitis in children. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.02139-2016. [Symptoms and signs of specific diseases: chest pain, history suggestive of inhaled foreign body, dyspnoea, exertional dyspnoea, haemoptysis, failure to thrive, feeding difficulties (including choking/vomiting), cardiac or neurodevelopmental abnormalities, recurrent sinopulmonary infections, immunodeficiency, epidemiological risk factors for exposure to tuberculosis, signs of respiratory distress, digital clubbing, chest wall deformity, auscultatory crackles, chest radiographic changes (other than perihilar changes).Specific conditions and diseases: cystic fibrosis, primary ciliary dyskinesia, immune deficiency, tuberculosis, aspiration syndromes, tracheobronchomalacia, somatic and tic cough, bronchiectasis, children's interstitial lung disease, upper airway syndrome, asthma, ACE-inhibitor induced cough.Testing for allergy not to be routinely performed, should be undertaken in presence of features and signs of allergy] [DOI] [PubMed] [Google Scholar]
- 70.Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Petsky HL, Smith-Vaughan H, Masters B, Buntain H, Chang AB. Protracted Bacterial Bronchitis in Children: Natural History and Risk Factors for Bronchiectasis. Chest. 2016;150(5):1101–1108. doi: 10.1016/j.chest.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 71.Chang AB, Oppenheimer JJ, Weinberger M, Rubin BK, Irwin RS. Children With Chronic Wet or Productive Cough--Treatment and Investigations: A Systematic Review. Chest. 2016;149(1):120–142. doi: 10.1378/chest.15-2065. [DOI] [PubMed] [Google Scholar]
- 72.Haydour Q, Alahdab F, Farah M, Barrionuevo P, Vertigan AE, Newcombe PA, Pringsheim T, Chang AB, Rubin BK, McGarvey L, Weir KA, et al. Management and diagnosis of psychogenic cough, habit cough, and tic cough: a systematic review. Chest. 2014;146(2):355–372. doi: 10.1378/chest.14-0795. [DOI] [PubMed] [Google Scholar]
- 73.Vertigan AE. Somatic cough syndrome or psychogenic cough-what is the difference? J Thorac Dis. 2017;9(3):831–838. doi: 10.21037/jtd.2017.03.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGarvey L, Gibson PG. What Is Chronic Cough? Terminology. J Allergy Clin Immunol Pract. 2019;7(6):1711–1714. doi: 10.1016/j.jaip.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 75.van Manen MJG, Birring SS, Vancheri C, Vindigni V, Renzoni E, Russell A-M, Wapenaar M, Cottin V, Wijsenbeek MS. Effect of pirfenidone on cough in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.01157-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birring SS, Wijsenbeek MS, Agrawal S, van den Berg JWK, Stone H, Maher TM, Tutuncu A, Morice AH. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med. 2017;5(10):806–815. doi: 10.1016/S2213-2600(17)30310-7. [DOI] [PubMed] [Google Scholar]
- 77.Freund KM, Belanger AJ, D'Agostino RB, Kannel WB. The health risks of smoking. The Framingham Study: 34 years of follow-up. Ann Epidemiol. 1993;3(4):417–424. doi: 10.1016/1047-2797(93)90070-k. [DOI] [PubMed] [Google Scholar]
- 78.Sumner H, Woodcock A, Kolsum U, Dockry R, Lazaar AL, Singh D, Vestbo J, Smith JA. Predictors of Objective Cough Frequency in Chronic Obstructive Pulmonary Disease. AmJRespirCrit Care Med. 2013 doi: 10.1164/rccm.201211-2000OC. [DOI] [PubMed] [Google Scholar]
- 79.Millqvist E, Bende M. Capsaicin cough sensitivity is decreased in smokers. Respir Med. 2001;95(1):19–21. doi: 10.1053/rmed.2000.0965. [DOI] [PubMed] [Google Scholar]
- 80.Dicpinigaitis PV. Cough reflex sensitivity in cigarette smokers. Chest. 2003;123(3):685–688. doi: 10.1378/chest.123.3.685. [DOI] [PubMed] [Google Scholar]
- 81.Dicpinigaitis PV, Lee Chang A, Dicpinigaitis AJ, Negassa A. Effect of e-Cigarette Use on Cough Reflex Sensitivity. Chest. 2016;149(1):161–165. doi: 10.1378/chest.15-0817. [DOI] [PubMed] [Google Scholar]
- 82.Cummings KM, Giovino G, Jaen CR, Emrich LJ. Reports of smoking withdrawal symptoms over a 21 day period of abstinence. Addict Behav. 1985;10(4):373–381. doi: 10.1016/0306-4603(85)90034-6. [DOI] [PubMed] [Google Scholar]
- 83.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med. 2019;380(7):629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 84.Nordin S, Palmquist E, Bende M, Millqvist E. Normative data for the chemical sensitivity scale for sensory hyperreactivity: the Vasterbotten environmental health study. IntArchOccupEnvironHealth. 2012 doi: 10.1007/s00420-012-0812-2. [DOI] [PubMed] [Google Scholar]
- 85.Kastelik JA, Aziz I, Ojoo JC, Thompson RH, Redington AE, Morice AH. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25(2):235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 86.McGarvey LP, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, Shepherd DR, MacMahon J. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic. Thorax. 1998;53(9):738–743. doi: 10.1136/thx.53.9.738. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.French CT, Diekemper RL, Irwin RS, Adams TM, Altman KW, Barker AF, Birring SS, Blackhall F, Bolser DC, Boulet LP, Braman SS, et al. Assessment of Intervention Fidelity and Recommendations for Researchers Conducting Studies on the Diagnosis and Treatment of Chronic Cough in the Adult: CHEST Guideline and Expert Panel Report. Chest. 2015;148(1):32–54. doi: 10.1378/chest.15-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berrington de González A, Mahesh M, Kim K-P, Bhargavan M, Lewis R, Mettler F, Land C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 90.Sadeghi MH, Wright CE, Hart S, Crooks M, Morice A. Phenotyping patients with chronic cough: Evaluating the ability to predict the response to anti-inflammatory therapy. Ann Allergy Asthma Immunol. 2018;120(3):285–291. doi: 10.1016/j.anai.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Sadeghi MH, Wright CE, Hart S, Crooks M, Morice AH. Does FeNO Predict Clinical Characteristics in Chronic Cough? Lung. 2018;196(1):59–64. doi: 10.1007/s00408-017-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe K, Shinkai M, Shinoda M, Hara Y, Yamaguchi N, Rubin BK, Ishigatsubo Y, Kaneko T. Measurement of eNO with portable analyser might improve the management of persistent cough at primary care practice in Japan. Clin Respir J. 2016;10(3):380–388. doi: 10.1111/crj.12228. [DOI] [PubMed] [Google Scholar]
- 93.Hahn PY, Morgenthaler TY, Lim KG. Use of exhaled nitric oxide in predicting response to inhaled corticosteroids for chronic cough. Mayo Clin Proc. 2007;82(11):1350–1355. doi: 10.4065/82.11.1350. [DOI] [PubMed] [Google Scholar]
- 94.Prieto L, Ferrer A, Ponce S, Palop J, Marin J. Exhaled nitric oxide measurement is not useful for predicting the response to inhaled corticosteroids in subjects with chronic cough. Chest. 2009;136(3):816–822. doi: 10.1378/chest.08-2942. [DOI] [PubMed] [Google Scholar]
- 95.Doan T, Patterson R, Greenberger PA. Cough variant asthma: usefulness of a diagnostic-therapeutic trial with prednisone. Ann Allergy. 1992;69(6):505–509. [see comments] [PubMed] [Google Scholar]
- 96.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 97.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, Wang X, Hu M, Tang R, Chen Z. IL-13+ Type 2 Innate Lymphoid Cells Correlate with Asthma Control Status and Treatment Response. Am J Respir Cell Mol Biol. 2016 doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 98.Takemura M, Niimi A, Matsumoto H, Ueda T, Matsuoka H, Yamaguchi M, Jinnai M, Chin K, Mishima M. Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration. 2012;83(4):308–315. doi: 10.1159/000332835. [DOI] [PubMed] [Google Scholar]
- 99.Vertigan AE, Kapela SM, Kearney EK, Gibson PG. Laryngeal Dysfunction in Cough Hypersensitivity Syndrome: A Cross-Sectional Observational Study. J Allergy Clin Immunol Pract. 2018;6(6):2087–2095. doi: 10.1016/j.jaip.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 100.O'Hara J, Jones NS. "Post-nasal drip syndrome": most patients with purulent nasal secretions do not complain of chronic cough. Rhinology. 2006;44(4):270–273. [PubMed] [Google Scholar]
- 101.Pratter MR, Bartter T, Lotano R. The role of sinus imaging in the treatment of chronic cough in adults. Chest. 1999;116(5):1287–1291. doi: 10.1378/chest.116.5.1287. [DOI] [PubMed] [Google Scholar]
- 102.Chaudhuri R, McMahon AD, Thomson LJ, Macleod KJ, McSharry CP, Livingston E, McKay A, Thomson NC. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. JAllergy ClinImmunol. 2004;113(6):1063–1070. doi: 10.1016/j.jaci.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 103.Boulet LP, Milot J, Boutet M, St Georges F, Laviolette M. Airway inflammation in nonasthmatic subjects with chronic cough. Am J Respir Crit Care Med. 1994;149(2 Pt 1):482–489. doi: 10.1164/ajrccm.149.2.8306050. [DOI] [PubMed] [Google Scholar]
- 104.Pizzichini MM, Pizzichini E, Parameswaran K, Clelland L, Efthimiadis A, Dolovich J, Hargreave FE. Nonasthmatic chronic cough: No effect of treatment with an inhaled corticosteroid in patients without sputum eosinophilia. Can Respir J. 1999;6(4):323–330. doi: 10.1155/1999/434901. [DOI] [PubMed] [Google Scholar]
- 105.Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. EurRespirJ. 1989;2:935–939. [PubMed] [Google Scholar]
- 106.Wesseling GJ, Quaedvlieg M, Wouters EF. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. EurRespirJ. 1991;4:1101–1105. [PubMed] [Google Scholar]
- 107.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet. 1998;351:773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 108.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 109.Davies MJ, Fuller P, Picciotto A, McKenzie SA. Persistent nocturnal cough: randomised controlled trial of high dose inhaled corticosteroid. Arch Dis Child. 1999;81(1):38–44. doi: 10.1136/adc.81.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child. 1998;79(1):6–11. doi: 10.1136/adc.79.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough-variant asthma. J Asthma. 2002;39:291–297. doi: 10.1081/jas-120002285. [DOI] [PubMed] [Google Scholar]
- 112.Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol. 2004;93(3):232–236. doi: 10.1016/S1081-1206(10)61493-7. [DOI] [PubMed] [Google Scholar]
- 113.Kita T, Fujimura M, Ogawa H, Nakatsumi Y, Nomura S, Ishiura Y, Myou S, Nakao S. Antitussive effects of the leukotriene receptor antagonist montelukast in patients with cough variant asthma and atopic cough. Allergol Int. 2010;59(2):185–192. doi: 10.2332/allergolint.09-OA-0112. [DOI] [PubMed] [Google Scholar]
- 114.Benard B, Bastien V, Vinet B, Yang R, Krajinovic M, Ducharme FM. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.00148-2017. [DOI] [PubMed] [Google Scholar]
- 115.Faruqi S, Molyneux ID, Fathi H, Wright C, Thompson R, Morice AH. Chronic cough and esomeprazole: a double-blind placebo-controlled parallel study. Respirology. 2011 doi: 10.1111/j.1440-1843.2011.02014.x. [DOI] [PubMed] [Google Scholar]
- 116.Shaheen NJ, Crockett SD, Bright SD, Madanick RD, Buckmire R, Couch M, Dellon ES, Galanko JA, Sharpless G, Morgan DR, Spacek MB, et al. Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. AlimentPharmacolTher. 2011;33(2):225–234. doi: 10.1111/j.1365-2036.2010.04511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Rev Clin Pharmacol. 2012;5(3):337–344. doi: 10.1586/ecp.12.20. [DOI] [PubMed] [Google Scholar]
- 118.Moayyedi P, Leontiadis GI. The risks of PPI therapy. Nature Reviews Gastroenterology &Amp; Hepatology. 2012;9:132. doi: 10.1038/nrgastro.2011.272. [DOI] [PubMed] [Google Scholar]
- 119.Berkhof FF, Doornewaard-ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. Azithromycin and cough-specific health status in patients with chronic obstructive pulmonary disease and chronic cough: a randomised controlled trial. Respir Res. 2013;14:125. doi: 10.1186/1465-9921-14-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yousaf N, Monteiro W, Parker D, Matos S, Birring S, Pavord ID. Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax. 2010;65(12):1107–1110. doi: 10.1136/thx.2010.142711. [DOI] [PubMed] [Google Scholar]
- 121.Hodgson D, Anderson J, Reynolds C, Oborne J, Meakin G, Bailey H, Shaw D, Mortimer K, Harrison T. The Effects of Azithromycin in Treatment-Resistant Cough: A Randomized, Double-Blind, Placebo-Controlled Trial. Chest. 2016;149(4):1052–1060. doi: 10.1016/j.chest.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 123.Vertigan AE, Kapela SL, Ryan NM, Birring SS, McElduff P, Gibson PG. Pregabalin and Speech Pathology Combination Therapy for Refractory Chronic Cough: A Randomized Controlled Trial. Chest. 2016;149(3):639–648. doi: 10.1378/chest.15-1271. [DOI] [PubMed] [Google Scholar]
- 124.Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol. 2017;73(7):811–817. doi: 10.1007/s00228-017-2245-z. [DOI] [PubMed] [Google Scholar]
- 125.Gardiner SJ, Chang AB, Marchant JM, Petsky HL. Codeine versus placebo for chronic cough in children. Cochrane Database Syst Rev. 2016;7 doi: 10.1002/14651858.CD011914.pub2. CD011914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006;61(12):1065–1069. doi: 10.1136/thx.2006.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chamberlain S, Garrod R, Birring SS. Cough suppression therapy: does it work? Pulm Pharmacol Ther. 2013;26(5):524–527. doi: 10.1016/j.pupt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 128.Marchant J, Masters IB, Champion A, Petsky H, Chang AB. Randomised controlled trial of amoxycillin clavulanate in children with chronic wet cough. Thorax. 2012;67(8):689–693. doi: 10.1136/thoraxjnl-2011-201506. [DOI] [PubMed] [Google Scholar]
- 129.Ternesten-Hasseus E, Johansson EL, Millqvist E. Cough reduction using capsaicin. Respir Med. 2015;109(1):27–37. doi: 10.1016/j.rmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 130.Belvisi MG, Birrell MA, Wortley MA, Maher SA, Satia I, Badri H, Holt K, Round P, McGarvey L, Ford J, Smith JA. XEN-D0501, a Novel Transient Receptor Potential Vanilloid 1 Antagonist, Does Not Reduce Cough in Patients with Refractory Cough. Am J Respir Crit Care Med. 2017;196(10):1255–1263. doi: 10.1164/rccm.201704-0769OC. [DOI] [PubMed] [Google Scholar]
- 131.Morice AH. TRPA1 receptors in chronic cough. Pulm Pharmacol Ther. 2017;47(Supplement C):42–44. doi: 10.1016/j.pupt.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 133.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. NEnglJMed. 2005;352(21):2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 134.Price DB, Buhl R, Chan A, Freeman D, Gardener E, Godley C, Gruffydd-Jones K, McGarvey L, Ohta K, Ryan D, Syk J, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med. 2018;6(1):29–39. doi: 10.1016/S2213-2600(17)30424-1. [DOI] [PubMed] [Google Scholar]
- 135.Cheng S-L. Blood eosinophils and inhaled corticosteroids in patients with COPD: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:2775–2784. doi: 10.2147/COPD.S175017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mertens V, Blondeau K, Pauwels A, Farre R, Vanaudenaerde B, Vos R, Verleden G, Van Raemdonck DE, Dupont LJ, Sifrim D. Azithromycin reduces gastroesophageal reflux and aspiration in lung transplant recipients. DigDisSci. 2009;54(5):972–979. doi: 10.1007/s10620-009-0725-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.