Abstract
Hard, or stony, corals make rocks that can, on geological time scales, lead to the formation of massive reefs in shallow tropical and subtropical seas. In both historical and contemporary oceans, reef-building corals retain information about the marine environment in their skeletons, which is an organic–inorganic composite material. The elemental and isotopic composition of their skeletons is frequently used to reconstruct the environmental history of Earth's oceans over time, including temperature, pH, and salinity. Interpretation of this information requires knowledge of how the organisms formed their skeletons. The basic mechanism of formation of calcium carbonate skeleton in stony corals has been studied for decades. While some researchers consider coral skeletons as mainly passive recorders of ocean conditions, it has become increasingly clear that biological processes play key roles in the biomineralization mechanism. Understanding the role of the animal in living stony coral biomineralization and how it evolved has profound implications for interpreting environmental signatures in fossil corals to understand past ocean conditions. Here we review historical hypotheses and discuss the present understanding of how corals evolved and how their skeletons changed over geological time. We specifically explain how biological processes, particularly those occurring at the subcellular level, critically control the formation of calcium carbonate structures. We examine the different models that address the current debate including the tissue–skeleton interface, skeletal organic matrix, and biomineralization pathways. Finally, we consider how understanding the biological control of coral biomineralization is critical to informing future models of coral vulnerability to inevitable global change, particularly increasing ocean acidification.
Keywords: amorphous calcium carbonate, aragonite, biomineralization, calcite, calicoblastic cells, corals, crystal growth, skeletal organic matrix
1. Introduction
Although indigenous peoples have used coral reef resources for tens of thousands of years (Kirch, 2017) and coral fossils were known in Nicolaus Steno's time (the late 17th century; Rosenberg, 2009), coral reefs were first brought to wider European knowledge by accident, at 11 p.m. on 11 June 1770, when Lieutenant James Cook's ship, HM Bark Endeavour, “bumped” and ran aground on the Great Barrier Reef off the northeast coast of Australia (Cook, 1770). The ship was slightly damaged, but to free it, the crew had to throw about 50 tons of cargo, including six cannons, over the side; they subsequently repaired the damage and the ship continued on its voyage. From that time forward, however, most British captains of both naval and merchant ships became extremely cautious about approaching land in uncharted waters of tropical and subtropical seas. Some, who took short cuts, were unlucky, resulting in the multitude of shipwrecks that speak of the dangers of coral reefs, both to the vessels and to the reefs (Work, Aeby, & Maragos, 2008).
Seventy-two years after Cook's “bump,” in May 1842, Charles Darwin, at the age of 33, published his first monograph, a treatise entitled, “The Structure and Distribution of Coral Reefs” (Darwin, 1842). He realized that reefs, although considered (rightfully) geological structures, were the result of biological processes and subject to competition. He wrote,
In an old-standing reef, the corals, which are so different in kind on different parts of it, are probably all adapted to the stations they occupy, and hold their places, like other organic beings, by a struggle one with another, and with external nature; hence we may infer that their growth would generally be slow, except under peculiarly favourable circumstances. (p. 76)
This was an amazing observation. It was sheer intuitive logic that would later lead to his second treatise, “On the Origins of Species,” first published on 24 November 1859 (Darwin, 1859), the first printing of which sold out within 1 day.
It would take the rest of the 19th century to understand that reef-forming corals are geologically ancient organisms. Their evolutionary origins are still not well constrained. However, reef-building corals have profound influences on the evolution, geology, ecology, and geochemistry of the world's oceans. Here we examine the role of corals from the perspective of biological mineralization and their incredible history of survival, evolution, and adaptability throughout hundreds of millions of years of global climate change.
2. What are Stony Corals?
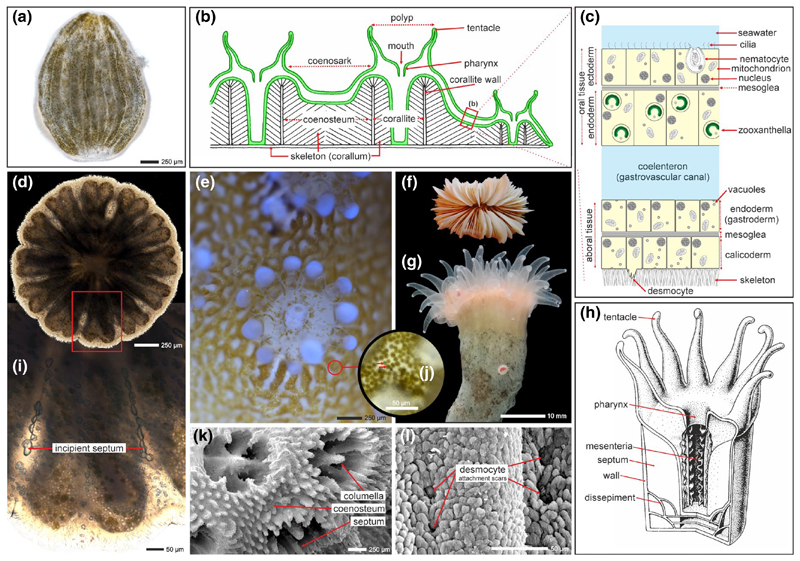
Together with several anatomically distinct groups, scleractinian corals (commonly called “hard” or “stony” corals; see Cairns, 2007) belong in the class Anthozoa which is in one of the oldest invertebrate phyla, the Cnidaria. Cnidarians are some of the earliest metazoans to possess an organized body structure (Erwin et al., 2011). A characteristic of these marine animals is a radially symmetrical body with only two cell layer types: an ectoderm and an endoderm, separated by the mesoglea, a noncellular gelatinous matrix (Pochon et al., 2010). Their life cycle begins from soft-bodied, planktonic, planula larvae that cannot eat, but rather rely on their yolk for nutrition (Figure 1a). The amount of yolk nutrients determines their ability to survive as plankton without feeding, and therefore, the distance they can travel as plankton from where they were spawned. Upon settlement on a hard substrate, however, the planulae undergo metamorphosis to form polyps with a mouth that allows them to capture zooplankton (Figure 1b,g). For some shallow-water stony corals, this serves to supplement the nutrition they receive from endosymbiotic dinoflagellates (Figure 1e,f), while other species, including deep-water corals, remain asymbiotic or aposymbiotic. Stony corals are sessile and remain at their site of settlement for the rest of their lives.
Figure 1.
The general organization of the scleractinian coral soft tissue and underlying skeleton. (a) Planktonic larva (planula, greenish color due to symbiotic algae) and newly settled polyp (d) with incipient skeletal deposits (i). (b) Main skeletal and soft tissue structures of colonial coral with (c) enlargement showing simplified section of two main tissue layers with symbiotic algae in oral endoderm. Such organization is exemplified by the symbiotic scleractinian Stylophora pistillata (e, with tissue cover, brown-green tiny dots (j, enlarged, arrows) are symbiotic algae; k, l, bare skeleton). (g) Solitary and asymbiotic coral Desmophyllum dianthus (f, bare skeleton, upper view). (h) 3D view of solitary corallum with main soft tissue and skeleton structures. Images (b) and (h) are courtesy of Ewa Roniewicz
Scleractinian corals (Figure 2a), which can form reefs in shallow tropical and subtropical seas, are the only extant anthozoans in which settlement and tissue reorganization during metamorphosis leads to deposition of an external mineral skeleton comprised of calcium carbonate. During metamorphosis, the aboral ectoderm of the planula transforms from a columnar epithelium into squamous cells called calicoblasts (the tissue layer composed of such cells is called the calicoblastic cell layer or calicoblastic epithelium [Von Heider, 1881]). The calicoblastic epithelium is in contact with the skeleton (Tambutté et al., 2011) and is mechanically anchored to it by specialized cells (desmocytes), which leave attachment scars on the skeleton (Muscatine, Tambutté, & Allemand, 1997; Figure 1c,l). The first mineral deposits of the initial polyp form a circular plate that shortly is supplemented by vertical blades known as septa and structures forming a cylindrical or cup-like wall (or theca; Figure 1d,h,i). In effect, the coral animals are a thin “glove” of a living organism on a biomineral skeleton of their making and that continuously grows as long as the animals live (Figure 2a). In colonial taxa, these polyps can ultimately form large reefs visible from space (Figure 2a,b).
Figure 2.
Coral reefs viewed underwater and by satellite. (a) Massive Porites building reef structures as observed by SCUBA divers (Photo credit: Hagai Nativ, University of Haifa, Israel). (b) Palau Atoll surrounding Babeldaob, Koror, and Peleliu islands in the Republic of Palau, as seen by NASA SeaWIFS satellite (courtesy of NASA: https://eoimages.gsfc.nasa.gov/images/imagerecords/87000/87423/palau_oli_2014080_wide.jpg)
3. Evolutionary History of Reef-Forming Corals
The transition from abiotic calcium carbonate deposition on microbial surfaces to biomolecule-mediated skeletal calcite and aragonite formation by eukaryotes is one of the most dramatic transitions in the evolution of life in the oceans. In the early Archean eon, 4,000 to 2,500 Ma (millions of years before present), prokaryotic photosynthetic microorganisms, such as cyanobacteria, formed vast deposits of calcium carbonate around their sheaths (Allwood, Walter, Kamber, Marshall, & Burch, 2006; Riding, 2006). This process, which occurs on some eukaryotic algae today, is primarily a result of elevated pH on the cell walls. In Archean time, photosynthetic microbes made layer upon layer of carbonates, forming vast stretches along the coastlines of primordial continental landmasses. These ancient reef-like structures, called stromatolites, record some of the earliest evolution of life on Earth (Awramik, 1984). The transition, 2,000 Ma later, to the Phanerozoic eon (“visible life,” 541 Ma to present) was marked by the rapid appearance and evolution of basal metazoa, including those that calcify, ending stromatolite dominance, and kick-starting multicellular animal biomineralization in the oceans (Valentine, 2002).
Prior to the Cambrian “explosion” of animal life, the Ediacaran Period (635–541 Ma) marks a brief intermezzo (on geological timescales) with an abundance of enigmatic, soft-bodied organisms called the “Ediacaran Biota.” The Ediacarans represent an early stage in multicellular animal evolution; however, their relationships with marine animal phyla that subsequently emerged in the Cambrian (and represent the majority of extant phyla) remain enigmatic (Xiao & Laflamme, 2009). Most of the soft-bodied Ediacaran organisms went extinct at the Ediacaran–Cambrian boundary, but some probably continued to diversify (Cai, Xiao, Li, & Hua, 2019; Wood et al., 2019) and several are now considered to be basal Cnidaria (Gehling, 1988; Ivantsov & Fedonkin, 2002). Indeed, molecular clock analysis suggests that Cnidaria originated about 740 Ma, prior to the Ediacaran Period (Park et al., 2012). Cnidaria, therefore, rank among the most basal metazoan phyla dating back to the Neoproterozoic. Within Cnidaria is the class Anthozoa (Figure 3), a group that includes Hexacorallia (Scleractinia, Corallimopharia, Anthipatharia, Actiniaria, Zoanthiniaria), Ceriantharia, and Octocorallia and is thought to be a sister group to all other cnidarians, including Cubozoa (box jellyfish), Hydrozoa (jellyfish and hydroids), and Scyphozoa (true jellyfish; Collins, 2002; Kayal et al., 2018). All cnidarians share cnidae, or nematocytes; basically cellular “harpoons” that spear and poison zooplanktonic prey. Furthermore, the anthozoan life cycle is characterized by a planula larval stage that (Figure 1a), after settlement, develops into a sessile polyp (Figure 1d).
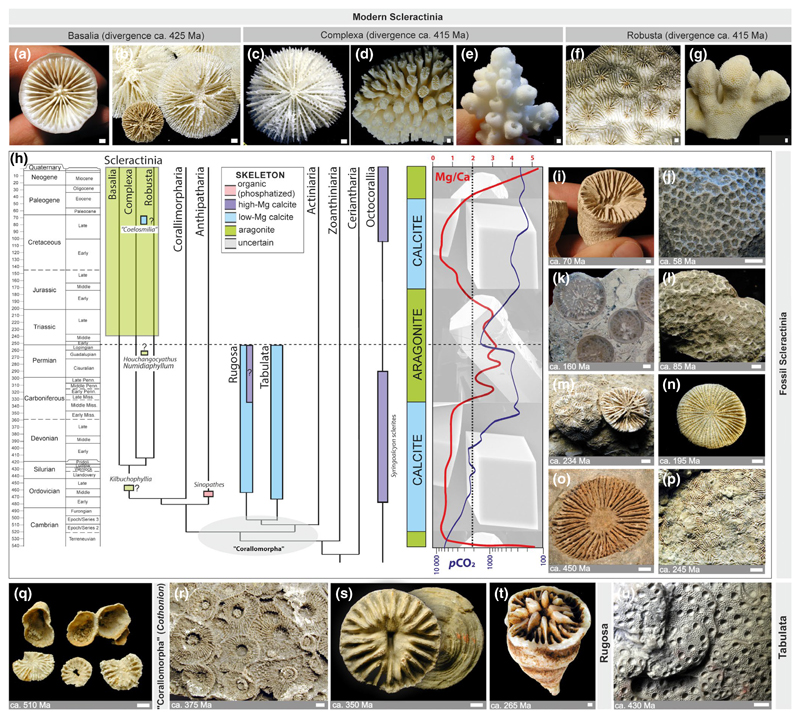
Figure 3.
Overview of Phanerozoic anthozoan diversity. (a–g) Recent scleractinian corals are represented by ca. 1,500 species, of which about half are shallow-water, often colonial taxa living in symbiosis with dinoflagellate algae (Z, zooxanthellate), and half are shallow- and deep-water, often solitary taxa lacking symbiotic algae (AZ, azooxanthellate). Molecular phylogeny suggests the presence of three major clades among modern scleractinians that diverged more than 400 Mya: (1) Basalia (e.g., (a) Gardineria [AZ], (b) Letepsammia [AZ]), (2) Complexa (e.g., (c) Fungiacyathus [AZ], (d) Galaxea [Z], (e) Acropora [Z], and Robusta (e.g., (f) Acanthastrea [Z], (g) Stylophora [Z]). (h) Simplified anthozoan phylogeny: sudden appearance of highly diversified scleractinian corals about 14 Ma after the Permian–Triassic boundary (Middle Triassic) is preceded by the rare occurrence of scleractinian-like forms in Paleozoic (e.g., (o) Kilbuchophyllia). Despite of major shifts in seawater Mg/Ca ratio which dictates calcium carbonate polymorph selection in abiotic conditions (low Mg/Ca favors calcite, whereas high Mg/Ca favors aragonite [Berner, 2013]) scleractinians almost invariably form aragonite skeletons throughout their fossil record (possible exception is calcite “Coelosmilia” (i)): (j) Astrocoenia, (k) Donacosmilia, (l) Columnastrea, (m) Tropiastraea (left), Margarophyllia (right), (n) Haimeicyclus, (p) Pamiroseris. Paleozoic calcifying corals were represented by mostly calcite rugose (solitary and colonial: e.g., (r) Hexagonaria, (s) Hadrophyllum, (t) Tachylasma), and colonial tabulate corals (e.g., (u) Heliolites). There is a pre-Ordovician record of calcifying corals, but their exact taxonomic attribution is uncertain (“Corallomorpha,” e.g., (q) Cothonion). Approximate age of illustrated fossil corals is given in bottom-left corner of the image. Scale bars = 2 mm
The fossil record during the early Cambrian period is poor (Savarese, Mount, Sorauf, & Bucklin, 1993). Many early anthozoans appear to have been soft-bodied, or used chitin-like structures to build their skeletons (Baliński, Sun, & Dzik, 2012). However, some exceptionally well-preserved soft-bodied fossils provide glimpses of early anthozoan evolution. Tiny fossils of soft-bodied sea anemones have been described from the Lower Cambrian of China (Han et al., 2010). These fossils, from 538 Ma, share characteristics exhibited by all modern anthozoans, and are thus likely stem group representatives (Figure 3h).
Two groups of now extinct calcifying anthozoans emerged in the Ordovician (Figures 3 and 4). Tabulata, exclusively colonial corals, appeared in the early Ordovician (485 to 445 Ma). The first Tabulata were encrusting and small in size (Scrutton, 1999), and the group represented a first attempt of biomineralizing cnidarians to contribute to the construction of reef belts in the paleotropical seas (Copper, 2001; Webby, 1992). The Rugosa emerged during the middle Ordovician around 460 Ma (Baars, Ghobadi Pour, & Atwood, 2012). Although colonial forms of rugose corals occur in the fossil record, the overwhelming majority were solitary and composed of calcite, the more stable of the two major polymorphs of calcium carbonate. Unlike modern scleractinian corals, which are radially symmetric with septa inserted cyclically, rugose corals typically exhibit strong bilateral symmetry with septa inserted in a tetraradial fashion. Most likely, rugosans were monophyletic and evolved independently from soft-bodied anthozoans (Baars et al., 2012).
Figure 4.
Growth forms of the Triassic and Recent corals in direct comparison. Triassic scleractinian corals that emerged en masse after the Permian–Triassic boundary (extinction of Paleozoic rugosan corals) were highly diversified and showed solitary (a), phaceloid (d), cerioid (f), thamnasterioid (i), and meandroid (k) growth forms fully comparable to modern scleractinians (c, e, g, j, l, respectively). Some well-preserved Triassic corals (b) show regular banding of TD's (arrows) which characterize modern corals symbiotic with dinoflagellates (h); coral–dinoflagellate symbiosis was likely a key driver in the evolution and expansion of shallow-water Mesozoic scleractinians. Scale bars: a, c–g, i–l = 5 mm, b, h = 50 µm. Triassic (Carnian) specimens from (a, b, d, f, i, k) Carnian of Italian Dolomites and (b) Norian of Turkey
Overall, during Paleozoic time, tabulate and rugose corals built reefs and increased in size, diversity, and geographic distribution, peaking during the Devonian. The end-Devonian crisis, marked by the collapse of reef environments, led to a significant taxonomic decline of tabulates, which suffered a major extinction and did not fully recover during the Carboniferous. In contrast, Rugosa were less affected and diversity remained high until the end of the Permian (250 Ma), at which time the largest extinction event in the Phanerozoic occurred (Raup & John Sepkoski, 1982).
Molecular phylogenies (Kitahara, Cairns, Stolarski, Blair, & Miller, 2010; Stolarski et al., 2011) suggest the emergence of the predecessors of modern reef-forming scleractinian corals in the early Ordovician (ca. 445 Ma). The oldest scleractinian lineages were almost certainly solitary and asymbiotic. Such an ancient origin of scleractinian corals is supported by the occurrence of solitary, conical-to-discoidal corals with septal insertion patterns identical to modern scleractinian corals (Figure 3o). Surprisingly, these fossil corals are limited to the Ordovician period and, thus far have only been found in southern Scotland and Northern Ireland (Scrutton, 1998; Scrutton & Clarkson, 1991). Regardless, fossils of scleractinian corals reappear after the end-Permian extinction, in the mid-Triassic (ca. 245 Ma), and became highly diverse (Stanley & Fautin, 2001). These mid-Triassic ancestors exhibited all growth forms found in modern reef-building corals (Figure 4).
3.1. Extinction and biomineralization of reefforming corals
Although reef-forming corals were present throughout most of the Phanerozoic period, they underwent several mass extinction events, sometimes followed by so-called “reef gaps” of millions of years, during which time they were absent in the fossil record (Stanley, 1988, 2003). For example, the end of the Cambrian is marked by an extinction and led to a period of nearly 30 Ma without significant reef formation by metazoans (Copper, 2001). The end-Devonian extinction affected mainly colonial tabulate corals wiping out 80%–90% of taxa (Zapalski & Berkowski, 2012), but solitary Rugosa, living in deeper waters, were less affected. Following the complete extinction of all Rugosa and Tabulata during the end-Permian extinction, there was a second “reef gap” during the first few million years of the Triassic. During this period, Scleractinia rose to prominence and became prolific reef builders along the margins of the Tethys Sea (Flugel & Senowbari-Daryan, 2001), an environment similar to many modern, tropical marine regions (Wang et al., 2016). The fossil remnants of these reefs can be seen in the Alpine mountain range in Austria and Italy (Bosellini, Gianolla, & Stefani, 2003; Figure 5). The end-Permian extinction also marked a prominent shift in the mineralogy of coral biomineralization: while Tabulata and Rugosa generated calcite, scleractinian corals began to produce aragonite which they continue to do today. Fine-scale skeletal features and stable isotope skeletal signatures, such as δ15N, suggest that symbiosis with photosynthetic dinoflagellates was a key driver of their evolutionary success (Frankowiak et al., 2016; Muscatine et al., 2005; Figure 1e,f). This intracellular symbiotic association with photosynthetic algae marked the beginning of modern-type scleractinian coral reefs.
Figure 5.
View on the Sassolungo (Langkofel) mountain in the Dolomites (northern Italy). These steep cliffs are 1,000 m high, rising up to nearly 3,200 m above sea level and represent remnants of middle Triassic (Ladinian) reef atolls that formed over millions of years after the end-Permian extinction with the help of the first scleractinian corals. Despite extensive alpine deformation, these Triassic atolls are still in their original position with respect to the surrounding deep-water clays. The extensive build-ups were the result of sea level rise, basin subsidence, and rapid growth of reef organisms
Perhaps the most famous, but not the most extreme, of all Phanerozoic extinctions at the end of the Cretaceous (66 Ma), led to the demise of sauropod dinosaurs as well as extensive ocean acidification (Henehan et al., 2019). However, this event only resulted in a moderate loss of scleractinians; approximately 45% of all taxa went extinct (Kiessling & Baron-Szabo, 2004). The extinction was more severe for highly integrated zooxanthellate corals, but these also recovered quite rapidly on geologic time scales (Kiessling & Baron-Szabo, 2004).
The causes that led to coral extinction throughout the Phanerozoic are likely varied. Ocean acidification, due to strong increases in atmospheric CO2, has been suggested (Greene et al., 2012), but the patterns shown in the fossil record of corals are more complex. Development of extensive ice caps, decreases in global temperature, and sea-level fluctuations were likely responsible for end-Ordovician coral extinctions that could also have led to their disappearance from the fossil record and potentially led to their migration into deep waters. The extinction of the Tabulata at the end of the Devonian and reef decline several million years prior to the end-Permian extinction suggest other factors. Suessialean dinoflagellates, which includes the family Symbiodiniaceae (LaJeunesse et al., 2018), the main symbiont of extant corals, almost completely disappeared at the end of the Triassic (Stanley & van de Schootbrugge, 2009). A breakdown in symbiotic relationships could have led to preferential extinction of colonial corals during several mass extinction events. In turn, the rise of scleractinians during the Triassic was likely linked to the evolution of photosymbiosis (Frankowiak et al., 2016; Kiessling, 2010).
Changes in atmospheric pCO2 may have indirectly shaped the trajectories of corals and other calcifying organisms through changes in temperature. This is perhaps most clearly illustrated by the large carbon cycle perturbation that occurred 55 Ma, at the boundary between the Paleocene and Eocene periods, and which serves as a deep time analog for current anthropogenic carbon emissions. The Paleocene–Eocene Thermal Maximum (PETM) was marked by the rapid release (<20,000 years) of 1,000s Pg of carbon, likely through the destabilization of seafloor gas hydrates or volcanism, driving an increase in global temperature of 5–8°C (Gutjahr et al., 2017; Sluijs et al., 2006; Zachos et al., 2005, 2003; Zeebe, Zachos, & Dickens, 2009). Surprisingly, most shallow-water marine calcifiers, including scleractinian corals, were not severely affected (Gibbs, Stoll, Bown, & Bralower, 2010).
4. Skeletal Structure and Formation
The principal crystalline “building block” of the approximately 1,500 listed species of modern Scleractinia (Cairns, 2007; Veron, 2000) consists almost exclusively of calcium carbonate crystals in the form of aragonite (e.g., Clode, Lema, Saunders, & Weiner, 2011; Foster & Clode, 2016; Von Euw et al., 2017). However, the presence of extracrystalline phases coexisting with aragonite has sometimes been reported. These include not only calcite (Frankowiak, Mazur, Gothmann, & Stolarski, 2013; Gladfelter, 1983; Houck, Buddemeier, & Chave, 1975; Lazareth et al., 2016; Wainwright, 1963) but also other noncarbonate minerals such as brucite (Mg(OH)2; Nothdurft et al., 2005). Nonetheless, these extracrystalline phases are not considered as the direct result of the coral biomineralization process since their presence could have different origins such as a mineral deposition due to the activity of boring organisms present within the skeleton (Macintyre & Towe, 1976), diagenetic alteration of the primary skeletal deposits (Frankowiak et al., 2013), secondary deposition of calcareous matter (Cusack et al., 2008; Dalbeck et al., 2011; Enmar et al., 2000), or even the result of an inorganic precipitation upon drying.
4.1. Contrasting the geological and biological model of biomineralization in corals
Although seawater is supersaturated with respect to calcium and bicarbonate ions, carbonates do not form spontaneously in seawater. However, they do form in a reliable, species-specific manner between coral tissue and the substrate. To examine this paradox, geochemists have investigated the chemistry of coral calcifying fluid, biologists have widely examined the skeletal organic matrix (SOM), while materials scientists have used various physical techniques to characterize the skeletal inorganic material. However, there is ongoing debate on the degree to which the biomineralization process in corals is directly related to seawater carbonate chemistry versus biological control.
One of the motivations to understand biomineralization in stony corals is that their skeletons incorporate atoms of elements other than calcium, carbon, and oxygen, often in relation to various environmental parameters such as pH (e.g., Hönisch et al., 2004; McCulloch et al., 2018), temperature (e.g., Dunbar, Wellington, Colgan, & Glynn, 1994; Mitsuguchi, Matsumoto, Abe, Uchida, & Isdale, 1996; Weber, 1973), and salinity (e.g., Giri, Swart, & Devlin, 2018; review by Corrège, 2006). Thus, through so-called geochemical “proxies,” the chemistry of coral skeletons can potentially allow reconstruction of past ocean conditions. However, with very few exceptions, trace element and stable isotope incorporation in coral skeletons is not in thermodynamic equilibrium with abiotically precipitated aragonite. The offsets are termed “vital effects” (e.g.; Adkins, Boyle, Curry, & Lutringer, 2003; Erez, 1978; Hönisch et al., 2004), a phenomenon that is often viewed as a geochemical process that has been slightly modified by the living coral. Such modifications include increased concentrations of Ca2+ (Al-Horani, Al-Moghrabi, & De Beer, 2003; Ohno et al., 2017; Sevilgen et al., 2019; Taubner, Hu, Eisenhauer, & Bleich, 2019) and (Chen, Gagnon, & Adkins, 2018; Comeau et al., 2017; McCulloch et al., 2018; Sevilgen et al., 2019; Zoccola et al., 2015, among others). These are then reflected in altered ratios of element/Ca and offsets in the isotopic ratio of elements such as C, O, and B as compared to seawater. These vital effects arise from the biomineralization process.
Coral biomineralization is geochemically framed in the context of aragonite saturation state, Ωarag, which relates the concentrations of Ca2+ and dissolved in seawater to their equilibrium concentrations as (Zeebe & Wolf-Gladrow, 2001):
Therefore, geochemists usually consider the chemical composition of the calcifying fluid with respect to Ωarag, and hence the ability of calcium carbonate to precipitate abiotically (e.g., Cohen & Holcomb, 2009; Figure 6a). In this context, decreased pH due to the release of anthropogenic CO2 to the atmosphere leads to lower Ωarag and therefore should, logically, result in decreased calcification rates (e.g., Doney, Fabry, Feely, & Kleypas, 2009; Erez, Reynaud, Silverman, Schneider, & Allemand, 2011; Kleypas et al., 1999; Silverman, Lazar, Cao, Caldeira, & Erez, 2009). Although, there is evidence that this simplified model can explain calcification in some coral species in response to decreased pH (e.g., Anthony, Kline, Diaz-Pulido, Dove, & Hoegh-Guldberg, 2008; Drenkard et al., 2013; Strahl et al., 2015), in many others there appear to be mechanisms to resist this stressor to some extent (e.g., Comeau, Edmunds, Spindel, & Carpenter, 2013; Crook, Potts, Rebolledo-Vieyra, Hernandez, & Paytan, 2012; Fabricius et al., 2011; Ries, Cohen, & McCorkle, 2009; Shamberger et al., 2014; Strahl et al., 2015).
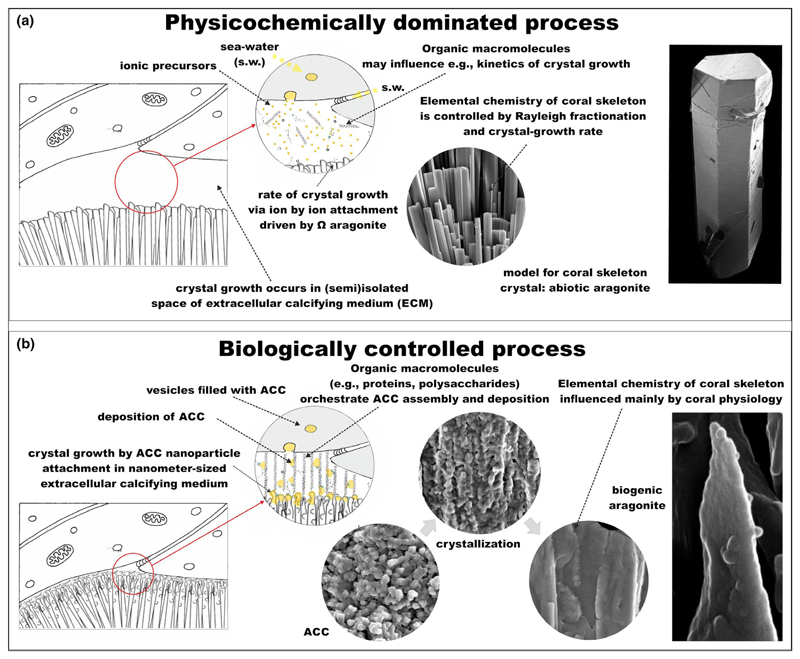
Figure 6.
Coral aragonite crystal growth as a physicochemically dominated process (a) versus biologically controlled process (b). In the often-held view of a physicochemically dominated process, the calicoblastic ectoderm and skeleton provide a wide space (Gagnon, Adkins, & Erez, 2012; see criticism in Brahmi et al., 2016) in which biological processes determine the extracellular calcifying medium (ECM) composition, but from there on, the process is dominated by crystal growth by a classical pathway of ion-by-ion attachment yielding a smooth, faceted surface. This is in contrast to the biologically controlled process, in which Ca2+ and bicarbonate, as well as ACC and biomolecules, are transported, in some cases by vesicles (yellow spheres), to the narrow ECM (width based on microsensor dimensions in Ammann, Bührer, Schefer, Müller, & Simon, 1987; de Beer, Kühl, Stambler, & Vaki, 2000). Structural molecules such as the proteins coadhesin, peroxidasin, and collagen, which form functional triple helices, adhere calicoblastic cells to the skeleton and may have a role in mineral formation. ACC nanoparticles are deposited on both ECM biomolecules and the growing mineral surface, and crystallization proceeds through a nonclassical pathway to crystallization by ACC particle attachment to yield a biogenic aragonite crystal with characteristic nanocomposite appearance
Preparation of cross sections of coral branches or nubbins in which the tissue can be preserved using a chemical fixative have allowed for increased understanding of how the calcifying milieu is established (e.g., Tambutté et al., 2007; Vandermeulen, 1975). This and other approaches focusing on the skeleton allow for investigation of the skeletal surface and/or internal regions (Benzerara et al., 2011; Cuif & Dauphin, 2005b; Cuif, Dauphin, Doucet, Salome, & Susini, 2003; DeVol et al., 2015; Gutner-Hoch et al., 2016; Isa, 1986; Nothdurft & Webb, 2007; Stolarski, 2003; van de Locht et al., 2013; Von Euw et al., 2017), along with the putative “calcification site” on the skeleton's surface or possibly within the animal tissue. Sites of biomineralization are any locations in which nucleation of the solid mineral phase occurs, including vesicles present within the calicoblastic cells (Hayes & Goreau, 1977; Mass, Drake, Heddleston, & Falkowski, 2017; Neder et al., 2019) or in the subectodermal space potentially present at the interface between the calicoblastic cells and the established skeleton (Clode & Marshall, 2002, 2003a, 2003b).
4.2. Composition of the coral SOM
There is significant organic matter in coral skeletons. Indeed, the organic portion of the coral skeleton has been studied for over 150 years. Silliman described the skeletal composition of 30 species in the mid-1840s (Silliman, 1846) and calicoblastic cells were recognized as being responsible for skeleton formation as early as 1881 (Von Heider, 1881; reviewed in Bourne, 1899). Furthermore, Duerden describes the remaining “colloidal material” following skeleton decalcification as bearing a resemblance to the mesoglea (Duerden, 1905), but that the appearance of this matrix becomes finer in older portions of the skeleton (Duerden, 1904). Mucopolysaccharides including chitin (Goreau, 1956, 1959; Wainwright, 1963), proteins (Johnston, 1980; Mitterer, 1978; Young, 1971; Young, O'Connor, & Muscatine, 1971), and lipids (Johnston, 1980; Young et al., 1971) were subsequently extracted from coral skeleton and described, with symbiont-derived photosynthate as one ultimate source of this fixed carbon (Muscatine & Cernichiari, 1969; Young, 1973; Young et al., 1971).
The most extensively studied component of the coral SOM is the protein complex retained in the inorganic aragonite skeleton even after the overlying animal dies. These proteins are secreted by the calicoblastic ectoderm (Puverel, Tambutté, Zoccola, et al., 2005; Yamashiro & Samata, 1996), serve to connect that cell layer to the pre-existing skeleton (Clode & Marshall, 2002, 2003a), and pattern both the physical and chemical environment of the calcifying medium (Chen et al., 2018; Clode & Marshall, 2003a, 2003b; Drake et al., 2018). The SOM allows coral aragonite precipitation even under calcite-promoting conditions (Higuchi et al., 2014; Higuchi, Shirai, Mezaki, & Yuyama, 2017; Yuyama & Higuchi, 2019). The amino acid composition of the SOM proteins across Scleractinia is heavily biased toward aspartic and glutamic acids (Mass et al., 2012; Mitterer, 1978; Young, 1971), a phenomenon not observed for tissue protein complexes (Yamashiro & Samata, 1996). Due to the proliferation of sequenced coral genomes and proteomes, the identities of approximately 30 skeleton proteins have been determined and functions of several of these proteins have been established.
The most interesting of the coral SOM proteins are the coral acid-rich proteins (CARPs), or alternatively called skeletal aspartic acid-rich proteins (SAARPs; Drake et al., 2013; Mass et al., 2013; Ramos-Silva et al., 2013; Takeuchi, Yamada, Shinzato, Sawada, & Satoh, 2016). To dispel confusion introduced by using two different names for some members of this class of proteins, CAPR4 and SAARP1 are homologs, CARP5 and SAARP2 are homologs, and a previously described partial protein sequence, P27 (Drake et al., 2013) and SAARP3 are homologs. Together, these proteins group as a coral-specific clade exhibiting an expansion of two polyaspartic acid domains (Drake et al., 2013), part of which was originally sequenced by Edman degradation (Puverel, Tambutté, Periera-Mouries, et al., 2005). CARPs have pH values between ca. 3 and 4.5, lead to calcium carbonate precipitation when added to unamended seawater at physiologically relevant concentrations (Mass et al., 2013), and are upregulated upon settling of larvae as spat on hard substrate (Akiva et al., 2018; Mass et al., 2016). The discovery of these proteins strongly suggests that the control of carbonate precipitation within the calicoblastic space is not a simple function of Ωarag. There is a distinct spatial patterning of the CARPs in the skeleton (Mass, Drake, Peters, Jiang, & Falkowski, 2014) and on growing aragonite crystals in coral cell cultures (Mass, Drake, et al., 2017), suggesting specific roles for each of the CARPs in coral biomineralization. An additional group of acidic coral skeletal proteins about which little is known are the skeletal acidic proteins (SAPs), several of which appear to be restricted to the family Acroporidae (Ramos-Silva et al., 2013; Shinzato et al., 2011; Takeuchi et al., 2016). CARPs and other highly acidic proteins such as the SAPs may also function to transport concentrated Ca2+ from intracellular pools to the extracellular calcifying medium (Mass, Drake, et al., 2017).
One of the other best-studied nonacidic proteins in coral skeleton is α-carbonic anhydrase (CA), an enzyme responsible for the hydration of CO2 to bicarbonate and a proton (H+), an important process for biomineralization, and the dehydration of to CO2, which is necessary for aquatic carbon fixation. The function and evolution of CAs across life and their specialization in corals has been well-detailed elsewhere (Bertucci et al., 2013). Metazoan αCAs appear to have undergone multiple duplication events and those involved in biomineralization typically have a low complexity domain in the C-terminal half of the protein (Le Roy, Jackson, Marie, Ramos-Silva, & Marin, 2014). Their total activity in coral tissue has been determined to be sufficient, particularly when highly concentrated in certain areas, to supply CO2 in Symbiodinium-containing cells for photosynthesis and in the calicoblastic space for biomineralization (Hopkinson, Tansik, & Fitt, 2015). Although it was originally proposed to be limited to the cytosol of calicoblastic ectodermal cells (Bertucci, Tambutté, Supuran, Allemand, & Zoccola, 2011), a carbonic anhydrase, STPCA2, has been detected in coral skeleton (Drake et al., 2013; Ramos-Silva et al., 2013). STPCA2 and its scleractinian homologs belong to a clade of secreted αCAs with a cnidarian-specific secondary structure (Le Goff et al., 2016), exhibit significantly increased expression after larval settlement (Akiva et al., 2018; Mass et al., 2016), and are highly active when compared to other known CAs (Bertucci et al., 2011). While STPCA2’s catalytic activity exhibits a persistent decrease under decreased pH conditions, its expression shows a hyperbolic response with increased expression under moderate to low pH (~7.9 and 7.6, respectively; Drake et al., 2018), but greatly reduced expression at pHs corresponding to very high pCO2 (pH = 7.2–7.3, pCO2 = 2,000 ppm CO2; Drake et al., 2018; Zoccola et al., 2016). Although upregulation of STPCA2 under reduced pH conditions may compensate for its lower activity, allowing maintenance of biomineralization under moderately acidified conditions by continuously supplying bicarbonate to the calcifying medium (Chen et al., 2018; reviewed in Bertucci et al., 2013), its role is dramatically diminished under strong ocean acidification conditions (Zoccola et al., 2016).
Further coral SOM proteins that remain to be functionally characterized include a very large protocadherin, mucin, vitellogenin, and proteins containing MAM and LDL receptor, zona pellucida, and EGF and laminin G domains (Drake et al., 2013; Ramos-Silva et al., 2013; Takeuchi et al., 2016). The function of galaxin, the first fully sequenced coral SOM protein, also remains to be described (Conci, Wörheide, & Vargas, 2019; Fukuda et al., 2003; Reyes-Bermudez, Lin, Hayward, Miller, & Ball, 2009; Watanabe, 2003; Wirshing & Baker, 2014). The shared skeletal proteins comprise a portion of the coral biomineralization protein “toolkit” (Drake, Mass, & Falkowski, 2014; Marin, Bundeleva, Takeuchi, Immel, & Medakovic, 2016; Ramos-Silva et al., 2013).
In addition to the acidic amino acids, coral SOM acidity is increased bythe presence of polysaccharides and the sulfation and glycosylation of SOM proteins. Staining has revealed that epidermally derived mucus contains polysaccharides (Goreau, 1956), as does the extracellular matrix secreted by coral cell cultures (Helman et al., 2008). Saccharide moieties similar to the polysaccharides, hyaluronan (Goldberg, 2001a) and chitin (Adamiano et al., 2014), and a variety of monosaccharides (Naggi et al., 2018; Ramos-Silva et al., 2014; Takeuchi et al., 2018), as well as modifications by sulfation (Cuif & Dauphin, 1998; Cuif et al., 2003; Dauphin & Cuif, 1997; Goldberg, 2001a) have been observed in coral SOM.
While the coral SOM lipid fraction has not been well characterized, lipids may make up a significant fraction of the organic component of skeleton (Adamiano et al., 2014; Falini et al., 2013; Farre, Cuif, & Dauphin, 2010; Goffredo et al., 2011). Phospholipids likely function in the vesicular transport of mineral precursors to the calcifying space (Akiva et al., 2018; Goffredo et al., 2011) and may stabilize amorphous calcium carbonate (Goffredo et al., 2011). It is also established that some skeletal phospholipids bind Ca2+ directly (Isa & Okazaki, 1987).
When added to a solution containing CaCl2 and bicarbonate or unamended seawater, macromolecules of the SOM complex interact with the growing mineral, often in a species-specific manner (e.g., Falini et al., 2013; Goffredo et al., 2011; Mass et al., 2013; Sancho-Tomás et al., 2014). In vitro experiments have highlighted the effects of the SOM complex as combining with the aragonite saturation state of the calcifying milieu to distinguish various growth patterns such as early mineralization zones (Njegic et al., 2019).
4.3. Cellular biochemical processes in coral skeleton formation
Coral skeletal aragonite is produced within a semienclosed extracellular compartment, termed the extracellular calcifying medium (ECM), proposed to be of a few nano- to micrometer thickness between the skeleton and the calicoblastic epithelium (Allemand, Tambutté, Zoccola, & Tambutté, 2011; Mass, Giuffre, et al., 2017; Sevilgen et al., 2019; Tambutté et al., 2007; Venn, Tambutté, Holcomb, Allemand, & Tambutté, 2011; Figure 6). Together with the three overlaying tissue layers, the calicoblastic epithelium spatially separates the ECM from direct contact with the surrounding seawater, which is the main source of ions to the ECM (reviewed in Allemand et al., 2011). Therefore, in order to precipitate CaCO3 the coral must transport Ca2+ and dissolved inorganic carbon (DIC) from the seawater or intracellular fluid, respectively, to the ECM. Furthermore, in addition to the import of necessary ions, there are suggestions that corals must use additional means to raise the aragonite saturation state of the calcifying fluid to obtain rapid rates of calcification (Cohen & McConnaughey, 2003).
Ca2+ can be transported from seawater to the calcifying space through transcellular (i.e., diffusion or active transport out of the calicoblastic ectodermal cells; Cai et al., 2016; Sevilgen et al., 2019) or paracellular (i.e., between cells at junctions) processes (Allemand et al., 2011), or a combination of the two (Allemand et al., 2011; Ohno et al., 2017; Tambutté et al., 2011, 2012). A plasma membrane calcium pump (PMCA) in corals functions in the same manner as is known in mammals, where Ca2+ ATPases transport calicoblastic ectodermal cytosolic Ca2+ to the calcifying space (Zoccola et al., 2004). stpPMCA immunolocalizes to the calicoblastic ectoderm (Barott, Perez, Linsmayer, & Tresguerres, 2015; Zoccola et al., 2004) and is upregulated in coral nubbins grown at very low pH (7.2; Vidal-Dupiol et al., 2013). Combined with its added function of exchanging H+ and Ca2+ across the calicoblastic ectodermal membrane (Salvador, Inesi, Rigaud, & Mata, 1998), this upregulation of the gene likely allows corals to increase the saturation state of the calcifying fluid to maintain biomineralization under suboptimal ocean conditions. Additional Ca2+ transport from the calicoblastic ectodermal cells to the calcifying space can be achieved by voltage-dependent Ca2+ channels (Tambutté, Allemand, Mueller, & Jaubert, 1996), one of which has been identified and localized to the calicoblastic ectoderm in S. pistillata (Zoccola et al., 1999).
It has been suggested that corals biologically control the composition of the ECM by increasing pH and DIC concentrations above that of the surrounding seawater (Al-Horani et al., 2003; Allison, Cohen, Finch, Erez, & Tudhope, 2014; Cai et al., 2016; McCulloch, Falter, Trotter, & Montagna, 2012; Venn et al., 2011). This allows the formation of aragonite, which is thought to constitute a crucial step in the coral biomineralization process (Lowenstam, 1981). Recent studies conducted using microscope-guided microsensors, B stable isotopes, and B/Ca ratios to measure or calculate pH, [Ca2+], [CO32-], or [DIC] in the ECM, and Ωarag, showed that all parameters were elevated with respect to the surrounding seawater (Schoepf, Jury, Toonen, & McCulloch, 2017; Sevilgen et al., 2019). pH elevation is achieved through PMCA activity, exchanging H+ and Ca2+ as described above. Most of the inorganic carbon for biomineralization comes from an internal pool (Erez, 1978; Furla, Galgani, Durand, & Allemand, 2000) and is delivered to the site of calcification either by CO2 diffusion or by transporters (Tambutté et al., 1996). It has also been suggested that elevation of ECM DIC occurs via a carbon concentration mechanism in the calicoblastic cells to favor calcification (Allison, Cohen, Finch, Erez, & Tudhope, 2014; Comeau et al., 2017; McCulloch et al., 2012). Metabolic CO2 can then diffuse from the coral tissue to the ECM (Erez et al., 2011; Furla et al., 2000; Hohn & Merico, 2012). Alternatively, a bicarbonate transporter in the SLC4γ family, which has been immunolocalized to the calicoblastic layer (Barott et al., 2015; Zoccola et al., 2015), introduces bicarbonate anions from the cytosol of the calicoblastic cells to the ECM and so may both provide ECM DIC and raise ECM pH (Bhattacharya et al., 2016; Zoccola et al., 2015). Furthermore, a sensory mechanism has been identified that enables the coral to sense the internal pH via the enzyme soluble adenylyl cyclase (sAC) that is activated by bicarbonate ions to produce the secondary messenger molecule cyclic AMP (cAMP; Barott, Barron, & Tresguerres, 2017; Tresguerres, Barron, Barott, Ho, & Roa, 2013).
Although the macroscopic coral skeleton is clearly an extracellular feature, there are increasing suggestions that some part of the process of its formation may commence intracellularly. Early coral research of the mechanism of ion transport to the site of calcification suggested that the initial site of mineral formation is intracellular based on the observation of vesicles and calcareous elements in the calicoblastic cells (Bourne, 1899; Fowler, 1885; Hayes & Goreau, 1977; Kawaguti & Sato, 1968; Ogilvie, 1896; Von Heider, 1881). While this model was later replaced by the concept of transcellular and paracellular pathways, recent studies have revisited this component of biomineralization, indicating a strong biological control of skeletal formation via an intracellular pathway. There is evidence that high concentrations of calcium can be transported via vesicles to the site of calcification in S. pistillata (Barott et al., 2015; Mass, Drake, et al., 2017). Indeed, calicoblastic cells in rapidly calcifying portions of the calicodermis contain a higher concentration of such types of vesicles (Isa & Yamazato, 1981). Some intracellular vesicles, particularly those in calicoblastic cells, also contain a Na+/Ca2+ exchanger (Barron et al., 2018). Furthermore, Nano-SIMS reveals that intracellular sites of concentrated calcium colocate with aspartic acid-rich regions of the cell, such as those contained in highly acidic proteins later retained in coral skeleton (Mass, Drake, et al., 2017).
In addition to the potential for intracellular aggregations of Ca2+ and/or mineral precursors, it has been revealed that the ECM of an intact coral is not a prerequisite for aragonite formation. Isolated coral cells in culture on bare Petri dishes re-aggregate into protopolyps and precipitate aragonite both on the exterior of these protopolyps (Mass et al., 2012) and on the extracellular matrix surrounding individual cells (Mass, Drake, et al., 2017; Figure 7). In these cultures, cells killed with azide do not form aragonite (Mass et al., 2012) suggesting that such precipitation is actively controlled by the live cells. The three-dimensional structure of a coral should not be taken for granted, however, as it is precisely this which directs the aggregation of aragonite bundles into species-specific corallite patterns.
Figure 7.
CaCO3 crystal bundle growing from the extracellular calcifying medium (ECM) surrounding Stylophora pistillata cells aggregated into protopolyps. XRD analysis has previously determined that these crystal bundles are made of aragonite (Mass et al., 2012) and that they do not occur on coral cells killed with azide
Further evidence that the precipitation of calcium carbonate is controlled biologically comes from the form of the crystal. It has been frequently suggested that major shifts in skeletal mineralogy of calcifying organisms, including corals, were driven by ocean Mg/Ca ratios (Morse, Wang, & Tsio, 1997; Porter, 2007; Stanley & Hardie, 1998). During periods with high Mg/Ca ratios, aragonite-forming organisms dominated, whereas low Mg/Ca seawater ratios promoted diversification of calcite-secreting organisms, in line with the control of abiotically produced polymorphs by Mg/Ca ratios (Berner, 1975). The Mg/Ca ratio of seawater is modulated by a number of processes, one of which is sea floor spreading rates with high spreading rates leading to the removal of Mg by hydrothermal processes and a release of Ca. Inflated mid-ocean ridges will also lead to higher sea levels and higher CO2 in the atmosphere (Tolstoy, 2015). Although, experimental and fossil record examples show that occasionally the Mg/Ca seawater ratio may indeed have direct influence on coral skeletal mineralogy (Higuchi et al., 2014; Stolarski, Meibom, Przeniosło, & Mazur, 2007; Webb & Sorauf, 2002), the main body of evidence suggests a remarkable evolutionary stability of coral skeletal mineralogy despite the major Mg/Ca fluctuations (Figure 3). For example, acroporid corals continued to form aragonitic skeletons from the Paleocene to the present, even as the seawater Mg/Ca increased from ca. 1.5 to 5.2 mol/mol (Stolarski et al., 2016) and aragonite is still detected among calcite in modern Acropora spp. when reared at Mg/Ca = 0.5 mol/mol (Higuchi et al., 2014, 2017; Yuyama & Higuchi, 2019). Furthermore, calcite-promoting conditions in the Cretaceous seas (the lowest Mg/Ca in the Phanerozoic) did not dictate the CaCO3 polymorph change in skeletons of corals that remained aragonitic through their entire fossil record (Janiszewska, Mazur, Escrig, Meibom, & Stolarski, 2017). These observations suggest that the skeletal formation process in corals is strongly physiologically controlled and these organisms are capable of biomineralization using the same mineral in geochemically altered sea conditions.
4.4. Effects of photosynthetic symbionts
Photosynthesis in shallow-water scleractinians is widespread but is neither a necessary nor sufficient process leading to biomineralization. Deep-water Scleractinia calcify, but are totally heterotrophic, while many “soft” corals have photosynthetic symbionts but do not produce an extracellular skeleton. In the shallow-water species that have photosynthetic symbionts, the alga is an intracellular dinoflagellate, formerly of genus Symbiodinium (Berkelmans & van Oppen, 2006; Blank & Trench, 1985; Schoenberg & Trench, 1980). There are many species within the family Symbiodiniaceae (LaJeunesse et al., 2018), commonly called zooxanthellae. The relationship between reef-building scleractinians and members of Symbiodiniaceae has been extensively described (e.g., Kirk & Weis, 2016; Roth, 2014).
Recent molecular clock recalibration suggests that members of family Symbiodiniaceae diversified during the Jurassic period (LaJeunesse et al., 2018), coincident with the radiation of shallow-water scleractinians (Park et al., 2012; Stanley, 2003). There are several lines of evidence for photosymbiosis of fossil corals, determined from modern symbiotic stony corals and observed in the fossil record. Skeletal growth band spacing is regular in symbiotic corals and irregular in asymbiotic corals (Frankowiak et al., 2016). Additionally, while δ13C of skeletal organic matter is not significantly influenced by symbiotic status, δ15N is significantly depleted (Muscatine et al., 2005; Tornabene, Martindale, Wang, & Schaller, 2017) and skeletal U/Ca exhibits a “clear and systematic decrease” (Inoue et al., 2018) in symbiotic versus nonsymbiotic corals. Differences in δ18O and δ13C have also been used to support the hypothesis of photosymbiosis in fossil corals (Frankowiak et al., 2016).
Photosymbiosis benefits stony corals, and specifically calcification in a variety of ways. The most obvious is the in-house provision of fixed carbon provided by the dinoflagellate to the animal host (Falkowski, Dubinsky, Muscatine, & Porter, 1984; Muscatine, McCloskey, & Marian, 1981). While there is recent evidence that the animals themselves may respond to light of specific wavelengths with increased calcification rates (Cohen, Dubinsky, & Erez, 2016), historically the increase in calcification rate by symbiotic corals during the day, termed “light-enhanced calcification” is attributed to photosynthesis and subsequent translocation of photosynthate to the coral host (Chalker & Taylor, 1975; Goreau, 1959; Goreau & Goreau, 1959; Kawaguti & Sakumoto, 1948), with higher rates of calcification observed in some coral species attributed to the assimilation of the fixed carbon by the animal (Gattuso, Allemand, & Frankignoulle, 1999; Moya et al., 2006) or by increased O2 production (Chalker & Taylor, 1975; Galli & Solidoro, 2018; Rinkevich & Loya, 1984). The process is further enhanced by the reflective and refractive properties of the coral skeleton itself, although it may also increase susceptibility to “bleaching,” a phenomenon that leads to the expulsion of alga from the host animal (Enríquez, Méndez, Hoegh-Guldberg, & Iglesias-Prieto, 2017; Swain et al., 2018).
4.5. Skeleton micromorphology
The relatively simple anatomy of scleractinian corals facilitates the study of early stages of coral biomineralization by analyzing the skeletal surface from intact coral branches and sectioning of various skeletal structures (typically perpendicularly to the growth direction; Barnes, 1970; Cuif & Dauphin, 1998; Cuif & Dauphin, 2005b; DeCarlo et al., 2019; Goldberg, 2001b; Hidaka, 1991). Traditionally, based on such observations, a two-step model was proposed that emphasized differences in the time of formation of “centers of calcification” (first step) and the fibers (second step; Cuif et al., 2003). However, studies of longitudinal sections of the skeleton (thus showing ontogenetic succession of skeletal deposition; Stolarski, 2003) and combined Nano-SIMS and SEM observations of isotopically labeled skeleton (86Sr) reveal that the skeleton is formed continuously although skeletal growth dynamics are different in various regions (Brahmi, Domart-Coulon, et al., 2012; Domart-Coulon et al., 2014; Houlbreque et al., 2009). The distal-most regions of septa, columella, and thecae form significantly faster than lateral skeletal layers (Brahmi, Kopp, Domart-Coulon, Stolarski, & Meibom, 2012). The fast-deposited regions are called rapid accretion deposits (RADs), whereas slower growing regions are termed thickening deposits (TDs).
Initially, the mineral deposits at the growing edges of skeletal structures were characterized as “randomly oriented fusiform crystals” (Gladfelter, 1982; Hidaka, 1991) “patches of microcrystals” (Stolarski, 2003), “tiny isodiametric crystals” (Cuif & Dauphin, 2005a) or “granular nanocrystals” (Clode & Marshall, 2003b; Figure 8). The use of scanning helium ion microscopy (SHIM), which bridges the gap between SEM and transmission electron microscopy in terms of spatial resolution, provides ultrahigh-resolution three-dimensional images of RADs (Figure 8o; Von Euw et al., 2017) These are in the form of randomly arranged, spherical, nano-sized CaCO3 particles ca. 100 nm in diameter, which are characteristics typical of ACC nanoparticles as observed in in vitro experiments (Gal et al., 2014). Recently, a more complete picture of the earliest phase of biomineralization has been revealed using X-ray photoemission electron spectromicroscopy (X-PEEM). ACC has been detected in very new skeletal growth—minutes to hours old—of recently terminated S. pistillata; the newest material is hydrated ACC that dehydrates and then crystalizes as aragonite (Mass, Giuffre, et al., 2017), similar to noncrystalline units identified in sea urchin embryonic spicules (Politi et al., 2008). Similar ACC phases have also been detected within “centers of calcification” (COCs, sometimes alternatively called RADs) in the skeleton of Madracis sp. (DeVol et al., 2015) using X-PEEM. Furthermore, Mg-ACC (Akiva et al., 2018) and Mg-calcite (Neder et al., 2019) were detected in the initial mineral deposits of primary polyps of pocilloporid corals, which may explain the rapid production of skeleton during initial development and settlement.
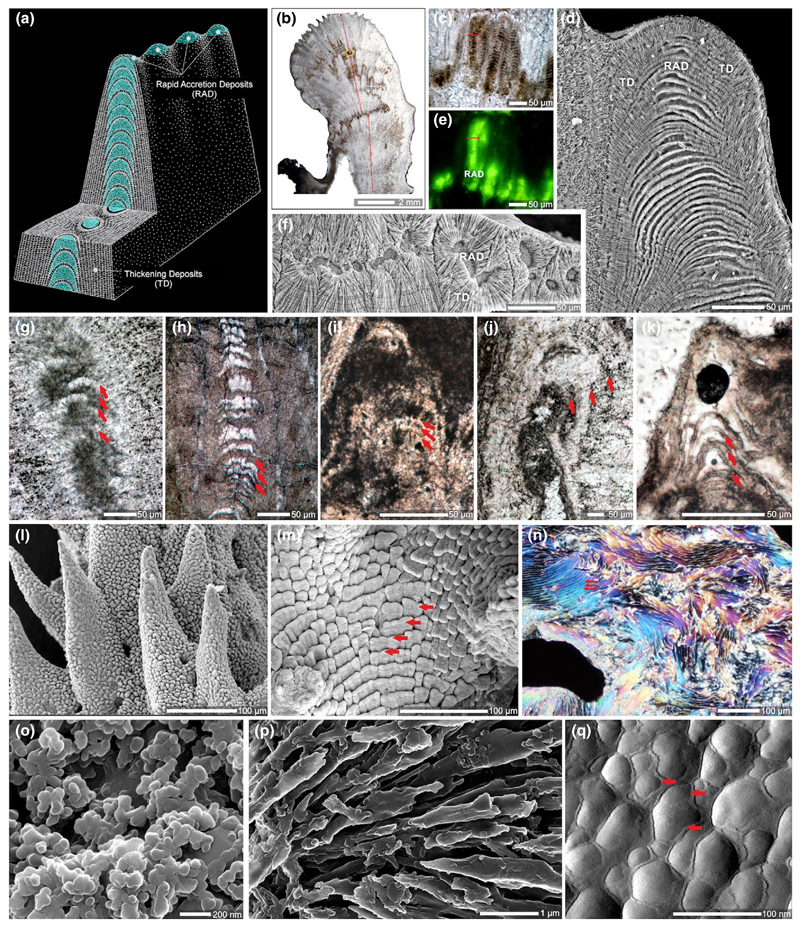
Figure 8.
Hierarchical structure of the coral skeleton. Skeletal structures of calcifying corals consist of two main regions: (1) skeletal tips which are composed of Rapid Accretion Deposits (RADs, called also Centers of Calcification [COCs]) and (2) lateral sides of skeleton composed of thickening deposits (TDs, called also “fibers”); (a) schematic drawing with RADs and TDs in longitudinal and transverse sections, (b–d) RADs are cyclically deposited and show alteration of organic-enriched/depleted layers (b, c, optical microscope image of longitudinally sectioned septum; e, epifluorescence microscope image highlighting organic-enriched regions). Individual skeletal layers are often continuous between RADs and TDs (d) pointing to different growth dynamics between two regions but not different timings of their formation; in transverse sections (f) that intersect layers of different growth stage this aspect is not observable. (g–k) Regular alteration of layers analogous to organic-enriched/depleted layers in modern corals can be found in skeletons of Paleozoic (e.g, (g) rugose calcite corals) and Triassic (h), Jurassic (i), Cretaceous (j, calcite Coelosmilia), and Cenozoic (k) scleractinian corals. Distinct patterns of TD arrangement are shared between phylogenetically related taxa (e.g., (l) tuberculate pattern in pocilloporiids (Seriatopora), and (m, n) shingled (red arrows) pattern in acroporiids (Acropora); l, m SEM; n section in polarized light. (o) RADs are composed of well-differentiated nanograins (ca. 100 nm in diameter); (p) TDs show larger, fibrous units that exhibit nanocomposite structure under AFM (q, amplitude image with arrows pointing to a different (?organic) phase of grain envelope)
Together, SHIM (Figure 4o) and X-PEEM (Mass, Giuffre, et al., 2017) observations provide strong evidence that ACC nanoparticles are initially deposited and then transform into aragonite crystals. Such a pathway to crystallization during which an amorphous solid phase is initially deposited seems to be a widespread strategy in the formation of biogenic crystals, including phosphates (Beniash, Metzler, Lam, & Gilbert, 2009; Mahamid, Sharir, Addadi, & Weiner, 2008), oxalates (Evan et al., 2007; Ihli et al., 2015; Nakata, 2003), and carbonates (Beniash, Aizenberg, Addadi, & Weiner, 1997; DeVol et al., 2015; Griesshaber et al., 2009; Politi, Arad, Klein, Weiner, & Addadi, 2004; Politi et al., 2008; Weiss, Tuross, Addadi, & Weiner, 2002), and is likely achieved through Mg/Ca ratio modifications as well as inclusion of highly acidic proteins in the calcifying milieu (Evans, Webb, Penkman, Kröger, & Allison, 2019).
Although stable ACC solid phases have been observed in vivo (Akiva-Tal et al., 2011; Raz, Testeniere, Hecker, Weiner, & Luquet, 2002), more frequently occurring metastable ACC solid phases, or “transient amorphous precursor phases,” are replaced by more stable phases (i.e., commonly, their crystalline counterparts such as aragonite and calcite) at a later stage of the biomineralization process (Addadi, Raz, & Weiner, 2003; Radha, Forbes, Killian, Gilbert, & Navrotsky, 2010). In coral, a close look at the RAD (or COC) in direct comparison to TD (sometimes referred to as fibers) regions in skeletal cross sections sheds light on the crystal growth process (Benzerara et al., 2011; Cuif & Dauphin, 2005b; Stolarski, 2003; van de Locht et al., 2013; Von Euw et al., 2017). In RAD skeletal regions, fast skeletal deposition incorporates a relatively high amount of organic matrix (Cuif & Dauphin, 2005b; Mass et al., 2014; Stolarski, 2003); therefore, the nano-sized spheres are easily distinguished well after initial deposition (even in well-preserved fossil corals; Stolarski & Mazur, 2005), and are not randomly arranged but are instead crystallographically aligned (Benzerara et al., 2011). In contrast, the TD regions consist of elongated fibers and have a much more compact structure which is an outcome of a significantly smaller amount of organics involved in their formation. Consequently, transformation of ACC to a crystalline phase occurs in a much more discreet way. Nonetheless, because the formation of RAD and TDs occurs via an amorphous precursor phase, even fully crystalline aragonite TDs, the principal “building blocks” of the coral skeleton, show a highly textured surface (Figure 8). They are clearly distinct from the classical image of an inorganic crystal exhibiting a smooth, faceted surface derived from different crystallographic planes (Figure 8). Such a nanoparticulate texture has been observed across biomineralizing taxa (Gal et al., 2014; Gal, Weiner, & Addadi, 2015) and is associated with crystallization by particle attachment (De Yoreo et al., 2015), particularly in invertebrates such as at the surface of mollusk nacre aragonite crystals (Dauphin, 2001; DeVol et al., 2015; Macías-Sánchez, Willinger, Pina, & Checa, 2017).
Combining all lines of evidence (Brahmi, Domart-Coulon, et al., 2012; Brahmi, Kopp, et al., 2012; Domart-Coulon et al., 2014; Mass, Giuffre, et al., 2017; Neder et al., 2019; Stolarski, 2003; Stolarski et al., 2016; Von Euw et al., 2017), the coral skeleton deposition can be described as follows: (a) a transient amorphous precursor phase in the form of ACC nanoparticles is initially deposited in actively formed skeletal regions (in RADs, the individual nanograins are embedded in significantly higher amounts of organic matrix than in slower growing TD regions); (b) the ACC nanoparticles subsequently attach to one another so that (c) nascent aragonite crystals start to grow by the accretion of amorphous nanoparticles; and this process progressively results in the formation of (d) acicular aragonite crystals following the crystallization of their ACC nanoparticulate building blocks. The full process by which ions are brought to the ECM by the paracellular pathway remains to be settled; this is an exciting resolution that we look forward to in the coming years.
5. Conclusions
This overview of the evolutionary history of stony corals and their biomineralization process clearly shows that, although these organisms have experienced several major mass extinction events over geological time, reef-forming corals have continued to survive these events, as well as periods, such as the PETM, when atmospheric CO2 increased rapidly and markedly and was accompanied by a decrease in ocean pH. The biomineralization process in corals is highly regulated by the host animal cells at a molecular level. While we do not understand the entire process in detail, much has been elucidated over the past century or more. It is clear, for example, that coral biomineralization is not simply a function of the aragonite saturation state. Over geological time, natural selection facilitated the adaptation to changing ocean conditions, such that, although coral species inevitably change, the group as a whole has survived. While coral cover is likely to decline further in the coming centuries due directly or indirectly to human activities (Hoegh-Guldberg et al., 2019), that corals, in some form, have survived multiple traumas over the past 540 million years, strongly suggests that they will almost certainly survive our disruption of this planet.
Acknowledgements
JLD was supported by a National Science Foundation (United States) (Award #1611943). PGF acknowledges the National Science Foundation (United States) (Award #EF-1416785). TM acknowledges the Israel Science Foundation (Grant 312/15) and the European Research Council (ERC; Grant #755876). JS acknowledges the National Science Centre (Poland) (Grant #2017/25/B/ST10/002221). SVE was supported by the European Commission under the Marie Sklodowska-Curie grant (Agreement #793861).
Funding information
National Science Foundation (United States), Grant/Award Number: 1611943; National Science Foundation (United States), Grant/Award Number: EF-1416785; Israel Science Foundation, Grant/Award Number: 312/15; European Research Council, Grant/Award Number: 755876; National Science Centre (Poland), Grant/Award Number: 2017/25/B/ST10/02221; European Commission, Grant/Award Number: 793861
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
ORCID
Jeana L. Drake https://orcid.org/0000-0002-9851-379X
Tali Mass https://orcid.org/0000-0002-7298-290X
Jarosław Stolarski https://orcid.org/0000-0003-0994-6823
Stanislas Von Euw https://orcid.org/0000-0001-8954-962X
Bas van de Schootbrugge https://orcid.org/0000-0003-2270-6285
Paul G. Falkowski https://orcid.org/0000-0002-2353-1969
References
- Adamiano A, Goffredo S, Dubinsky Z, Levy O, Fermani S, Fabbri D, Falini G. Analytical pyrolysis-based study on intra-skeletal organic matrices from Mediterranean corals. Analytical and Bioanalytical Chemistry. 2014;406(24):6021–6033. doi: 10.1007/s00216-014-7995-1. [DOI] [PubMed] [Google Scholar]
- Addadi L, Raz S, Weiner S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Advanced Materials. 2003;15(12):959–970. doi: 10.1002/chin.200333237. [DOI] [Google Scholar]
- Adkins JF, Boyle EA, Curry W, Lutringer A. Stable isotopes in deep-sea corals and a new mechanism for “vital effects”. Geochimica et Cosmochimica Acta. 2003;67(6):1129–1143. doi: 10.1016/s0016-7037(02)01203-6. [DOI] [Google Scholar]
- Akiva A, Neder M, Kahil K, Gavriel R, Pinkas I, Goobes G, Mass T. Minerals in the pre-settled coral Stylophora pistillata crystallize via protein and ion changes. Nature Communications. 2018;9(1):1880. doi: 10.1038/s41467-018-04285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiva-Tal A, Kababya S, Balazs YS, Glazer L, Berman A, Sagi A, Schmidt A. In situ molecular NMR picture of bioavailable calcium stabilized as amorphous CaCO3 biomineral in crayfish gastroliths. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14763–14768. doi: 10.1073/pnas.1102608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Horani FA, Al-Moghrabi SM, De Beer D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: Active internal carbon cycle. Journal of Experimental Marine Biology and Ecology. 2003;288(1):1–15. doi: 10.1016/s0022-0981(02)00578-6. [DOI] [Google Scholar]
- Allemand D, Tambutté É, Zoccola D, Tambutté S. Coral calcification, cells to reefs. In: Dubinsky Z, editor. Coral reefs: An ecosystem in transition. Berlin: Springer; 2011. pp. 119–150. [Google Scholar]
- Allison N, Cohen I, Finch AA, Erez J, Tudhope AW. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nature Communications. 2014;5:5741. doi: 10.1038/ncomms6741. [DOI] [PubMed] [Google Scholar]
- Allwood AC, Walter MR, Kamber BS, Marshall CP, Burch IW. Stromatolite reef from the Early Archaean era of Australia. Nature. 2006;441(7094):714–718. doi: 10.1038/nature04764. [DOI] [PubMed] [Google Scholar]
- Ammann D, Bührer T, Schefer U, Müller M, Simon W. Intracellular neutral carrier-based Ca2+ microelectrode with sub-nanomolar detection limit. Pfluegers Archiv. 1987;409(3):223–228. doi: 10.1007/bf00581922. [DOI] [PubMed] [Google Scholar]
- Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awramik SM. Ancient stromatolites and microbial mats. MBL Lectures in Biology, Microbial Mats: Storomatolites; New York, NY: Alan R. Liss Inc.; 1984. pp. 1–22. [Google Scholar]
- Baars C, Ghobadi Pour M, Atwood RC. The earliest rugose coral. Geological Magazine. 2012;150(2):371–380. doi: 10.1017/s0016756812000829. [DOI] [Google Scholar]
- Baliński A, Sun Y, Dzik J. 470-Million-year-old black corals from China. Naturwissenschaften. 2012;99(8):645–653. doi: 10.1007/s00114-012-0947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DJ. Coral skeletons: An explanation of their growth and structure. Science. 1970;170(3964):1305–1308. doi: 10.1126/science.170.3964.1305. [DOI] [PubMed] [Google Scholar]
- Barott KL, Barron ME, Tresguerres M. Identification of a molecular pH sensor in coral. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1866) doi: 10.1098/rspb.2017.1769. 20171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barott KL, Perez SO, Linsmayer LB, Tresguerres M. Differential localization of ion transporters suggests distinct cellular mechanisms for calcification and photosynthesis between two coral species. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2015;309(3):R235–R246. doi: 10.1152/ajpregu.00052.2015. [DOI] [PubMed] [Google Scholar]
- Barron ME, Thies AB, Espinoza JA, Barott KL, Hamdoun A, Tresguerres M. A vesicular Na+/Ca2+ exchanger in coral calcifying cells. PLoS ONE. 2018;13(10):e0205367. doi: 10.1371/journal.pone.0209734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniash E, Aizenberg J, Addadi L, Weiner S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proceedings of the Royal Society of London Series B Biological Sciences. 1997;264(1380):461–465. doi: 10.1098/rspb.1997.0066. [DOI] [Google Scholar]
- Beniash E, Metzler RA, Lam RS, Gilbert P. Transient amorphous calcium phosphate in forming enamel. Journal of Structural Biology. 2009;166(2):133–143. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzerara K, Menguy N, Obst M, Stolarski J, Mazur M, Tylisczak T, et al. Meibom A. Study of the crystallographic architecture of corals at the nanoscale by scanning transmission X-ray microscopy and transmission electron microscopy. Ultramicroscopy. 2011;111(8):1268–1275. doi: 10.1016/j.ultramic.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1599):2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner RA. The role of magnesium in the crystal growth of calcite and aragonite from sea water. Geochimica et Cosmochimica Acta. 1975;89:489–504. doi: 10.1016/0016-7037(75)90102-7. [DOI] [Google Scholar]
- Bertucci A, Moya A, Tambutté S, Allemand D, Supuran CT, Zoccola D. Carbonic anhydrases in anthozoan corals—A review. Bioorganic & Medicinal Chemistry. 2013;21(6):1437–1450. doi: 10.1016/j.bmc.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Bertucci A, Tambutté S, Supuran C, Allemand D, Zoccola D. A new coral carbonic anhydrase in Stylophora pistillata. Marine Biotechnology. 2011;13(5):992–1002. doi: 10.1007/s10126-011-9363-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Agrawal S, Aranda M, Baumgarten S, Belcaid M, Drake JL, et al. Falkowski P. Comparative genomics explains the evolutionary success of reef-forming corals. Elife. 2016;5:e13288. doi: 10.7554/eLife.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank RJ, Trench RK. Speciation and symbiotic dinoflagellates. Science. 1985;229(4714):656–658. doi: 10.1126/science.229.4714.656. [DOI] [PubMed] [Google Scholar]
- Bosellini A, Gianolla P, Stefani M. Geology of the dolomites. Episodes. 2003;26:181–185. doi: 10.18814/epiiugs/2003/v26i3/005. [DOI] [Google Scholar]
- Bourne GC. Memoirs: Studies on the structure and formation of the calcareous skeleton of the anthozoa. Journal of Cell Science. 1899;2(164):499–547. [Google Scholar]
- Brahmi C, Domart-Coulon I, Rougée L, Pyle D, Stolarski J, Mahoney J, et al. Meibom A. Pulsed 86Sr-labeling and NanoSIMS imaging to study coral biomineralization at ultra-structural length scales. Coral Reefs. 2012;1–12 doi: 10.1007/s00338-012-0890-3. [DOI] [Google Scholar]
- Brahmi C, Kopp C, Domart-Coulon I, Stolarski J, Meibom A. Skeletal growth dynamics linked to trace-element composition in the scleractinian coral Pocillopora damicornis. Geochimica et Cosmochimica Acta. 2012;99:146–158. doi: 10.1016/j.gca.2012.09.031. [DOI] [Google Scholar]
- Cai W-J, Ma Y, Hopkinson BM, Grottoli AG, Warner ME, Ding Q, et al. Wang Y. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nature Communications. 2016;7 doi: 10.1038/ncomms11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Xiao S, Li G, Hua H. Diverse biomineralizing animals in the terminal Ediacaran Period herald the Cambrian explosion. Geology. 2019;47(4):380–384. doi: 10.1130/g45949.1. [DOI] [Google Scholar]
- Cairns SD. Deep-water corals: An overview with special reference to diversity and distribution of deep-water scleractinian corals. Bulletin of Marine Science. 2007;81(3):311–322. [Google Scholar]
- Chalker BE, Taylor DL. Light-enhanced calcification, and the role of oxidative phosphorylation in calcification of the coral Acropora cervicornis. Proceedings of the Royal Society of London. B. Biological Science. 1975;190:323–331. doi: 10.1098/rspb.1975.0096. [DOI] [PubMed] [Google Scholar]
- Chen S, Gagnon AC, Adkins JF. Carbonic anhydrase, coral calcification and a new model of stable isotope vital effects. Geochimica et Cosmochimica Acta. 2018;236:179–197. [Google Scholar]
- Clode PL, Lema K, Saunders M, Weiner S. Skeletal mineralogy of newly settling Acropora millepora (Scleractinia) coral recruits. Coral Reefs. 2011;30:1–8. doi: 10.1007/s00338-010-0673-7. [DOI] [Google Scholar]
- Clode PL, Marshall AT. Low temperature FESEM of the calcifying interface of a scleractinian coral. Tissue and Cell. 2002;34(3):187–198. doi: 10.1016/s0040-8166(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Clode PL, Marshall AT. Calcium associated with a fibrillar organic matrix in the scleractinian coral Galaxea fascicularis. Protoplasma. 2003a;220(3):153–161. doi: 10.1007/s00709-002-0046-3. [DOI] [PubMed] [Google Scholar]
- Clode PL, Marshall AT. Skeletal microstructure of Galaxea fascicularis exsert septa: A high-resolution SEM study. The Biological Bulletin. 2003b;204(2):146–154. doi: 10.2307/1543550. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Holcomb M. Why corals care about ocean acidification: Uncovering the mechanism. Oceanography. 2009;22(4):118–127. doi: 10.5670/oceanog.2009.102. [DOI] [Google Scholar]
- Cohen AL, McConnaughey TA. Geochemical perspectives on coral mineralization. In: Dove PM, Weiner S, DeYoreo J, editors. Biomineralization. Vol. 54. Washington, DC: Mineralogical Society of America; 2003. pp. 151–187. [Google Scholar]
- Cohen I, Dubinsky Z, Erez J. Light enhanced calcification in hermatypic corals: New insights from light spectral responses. Frontiers in Marine Science. 2016;2:122. doi: 10.3389/fmars.2015.00122. [DOI] [Google Scholar]
- Collins AG. Phylogeny of Medusozoa and the evolution of cnidarian life cycles. Journal of Evolutionary Biology. 2002;15:418–432. doi: 10.1046/j.1420-9101.2002.00403.x. [DOI] [Google Scholar]
- Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnology and Oceanography. 2013;58(1):388–398. doi: 10.4319/lo.2013.58.1.0388. [DOI] [Google Scholar]
- Comeau S, Tambutté E, Carpenter R, Edmunds P, Evensen N, Allemand D, et al. Venn A. Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proceedings of the Royal Society B. 2017;284(1847) doi: 10.1098/rspb.2016.1669. 20161669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conci N, Wörheide G, Vargas S. New non-bilaterian transcriptomes provide novel insights into the evolution of coral skeletomes. bioRxiv. 2019 doi: 10.1093/gbe/evz199. 594242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. LOGBOOK of Lieut. James Cook in the "Endeavour" during part of his first voyage round the world. 1770 Sep; 12 Feb-23. Retrieved from https://www.gutenberg.org/files/8106/8106-h/8106-h.htm.
- Copper P. Evolution, radiations, and extinctions in Proterozoic to mid-Palaeozoic reefs. In: Stanley GD, editor. The history and sedimentology of ancient reef systems. Vol. 17. Boston, MA: Springer; 2001. pp. 89–119. (Topics in Geobiology). [Google Scholar]
- Corrège T. Sea surface temperature and salinity reconstruction from coral geochemical tracers. Palaeogeography, Palaeoclimatology, Palaeoecology. 2006;232(2–4):408–428. doi: 10.1016/j.palaeo.2005.10.014. [DOI] [Google Scholar]
- Crook E, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A. Calcifying coral abundance near low-pH springs: Implications for future ocean acidification. Coral Reefs. 2012;31(1):239–245. doi: 10.1007/s00338-011-0839-y. [DOI] [Google Scholar]
- Cuif J-P, Dauphin Y. Microstructural and physico-chemical characterization of ‘centers of calcification’ in septa of some recent scleractinian corals. Paläontologische Zeitschrift. 1998;72(3–4):257–269. doi: 10.1007/BF02988357. [DOI] [Google Scholar]
- Cuif J, Dauphin Y. The Environment Recording Unit in coral skeletons–A synthesis of structural and chemical evidences for a biochemically driven, stepping-growth process in fibres. Biogeosciences. 2005a;2(1):61–73. [Google Scholar]
- Cuif J-P, Dauphin Y. The two-step mode of growth in the scleractinian coral skeletons from the micrometre to the overall scale. Journal of Structural Biology. 2005b;150(3):319–331. doi: 10.1016/j.jsb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Cuif J-P, Dauphin Y, Doucet J, Salome M, Susini J. XANES mapping of organic sulfate in three scleractinian coral skeletons. Geochimica et Cosmochimica Acta. 2003;67(1):75–83. doi: 10.1016/S0016-7037(02)01041-4. [DOI] [Google Scholar]
- Cusack M, England J, Dalbeck P, Tudhope AW, Fallick AE, Allison N. Electron backscatter diffraction (EBSD) as a tool for detection of coral diagenesis. Coral Reefs. 2008;27(4):905. [Google Scholar]
- Dalbeck P, Cusack M, Dobson PS, Allison N, Fallick AE, Tudhope AW. Identification and composition of secondary meniscus calcite in fossil coral and the effect on predicted sea surface temperature. Chemical Geology. 2011;280(3–4):314–322. [Google Scholar]
- Darwin C. The structure and distribution of coral reefs: Being the first part of the Geology of the Voyage of the Beage, under the command of Capt. Fitzroy, R.N. London, UK: Smith, Elder, and Company; 1842. [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. London, UK: John Murray; 1859. [Google Scholar]
- Dauphin Y. Nanostructures de la nacre des tests de céphalopodes actuels. PalZ. 2001;75(1):113–122. doi: 10.1007/bf03022601. [DOI] [Google Scholar]
- Dauphin Y, Cuif J-P. Isoelectric properties of the soluble matrices in relation to the chemical composition of some Scleractinian skeletons. Electrophoresis. 1997;18(7):1180–1183. doi: 10.1002/elps.1150180726. [DOI] [PubMed] [Google Scholar]
- de Beer D, Kühl M, Stambler N, Vaki L. A microsensor study of light enhanced Ca2+ uptake and photosynthesis in the reef-building hermatypic coral Favia sp. Marine Ecology Progress Series. 2000;194:75–85. doi: 10.3354/meps194075. [DOI] [Google Scholar]
- De Yoreo JJ, Gilbert PU, Sommerdijk NA, Penn RL, Whitelam S, Joester D, et al. Banfield JF. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science. 2015;349(6247) doi: 10.1126/science.aaa6760. aaa6760. [DOI] [PubMed] [Google Scholar]
- DeCarlo TM, Comeau S, Cornwall CE, Gajdzik L, Guagliardo P, Sadekov A, et al. McCulloch MT. Investigating marine bio-calcification mechanisms in a changing ocean with in vivo and high-resolution ex vivo Raman spectroscopy. Global Change Biology. 2019;25(5):1877–1888. doi: 10.1111/gcb.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVol RT, Sun C-Y, Marcus MA, Coppersmith SN, Myneni SC, Gilbert PU. Nanoscale transforming mineral phases in fresh nacre. Journal of the American Chemical Society. 2015;137(41):13325–13333. doi: 10.1021/jacs.5b07931. [DOI] [PubMed] [Google Scholar]
- Domart-Coulon I, Stolarski J, Brahmi C, Gutner-Hoch E, Janiszewska K, Shemesh A, Meibom A. Simultaneous extension of both basic microstructural components in scleractinian coral skeleton during night and daytime, visualized by in situ 86Sr pulse labeling. Journal of Structural Biology. 2014;185(1):79–88. doi: 10.1016/j.jsb.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annual Review of Marine Science. 2009;1(1):169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Drake JL, Mass T, Falkowski PG. The evolution and future of carbonate precipitation in marine invertebrates: Witnessing extinction or documenting resilience in the Anthropocene? Elementa Science of the Anthropocene. 2014;2(1) doi: 10.12952/journal.elementa.000026. 000026. [DOI] [Google Scholar]
- Drake JL, Mass T, Haramaty L, Zelzion E, Bhattacharya D, et al. Falkowski PG. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):3788–3793. doi: 10.1073/pnas.1305081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JL, Schaller MF, Mass T, Godfrey L, Fu A, Sherrell RM, Falkowski PG. Molecular and geochemical perspectives on the influence of CO2 on calcification in coral cell cultures. Limnology and Oceanography. 2018;63(1):107–121. doi: 10.1002/lno.10617. [DOI] [Google Scholar]
- Drenkard EJ, Cohen AL, McCorkle DC, Putron SJ, Starczak VR, Zicht AE. Calcification by juvenile corals under heterotrophy and elevated CO2. Coral Reefs. 2013;32:727–735. doi: 10.1007/s00338-013-1021-5. [DOI] [Google Scholar]
- Duerden JE. The coral Siderastrea radians and its postlarval development. Washington, DC: Carnegie Institution publication No. 20; 1904. [Google Scholar]
- Duerden JE. Recent results on the morphology and development of coral polyps. Smithsonian miscellaneous collections. 1905;47:93–111. Retrieved from https://repository.si.edu/bitstream/handle/10088/23246/SMC_47_Duerden_1905_6_93-111.pdf. [Google Scholar]
- Dunbar RB, Wellington GM, Colgan MW, Glynn PW. Eastern Pacific sea surface temperature since 1600 AD: The δ18O record of climate variability in Galápagos corals. Paleoceanography. 1994;9(2):291–315. doi: 10.1029/93pa03501. [DOI] [Google Scholar]
- Enmar R, Stein M, Bar-Matthews M, Sass E, Katz A, Lazar B. Diagenesis in live corals from the Gulf of Aqaba. I. The effect on paleo-oceanography tracers. Geochimica et Cosmochimica Acta. 2000;64(18):3123–3132. doi: 10.1016/S0016-7037(00)00417-8. [DOI] [Google Scholar]
- Enríquez S, Méndez ER, Hoegh-Guldberg O, Iglesias-Prieto R. Key functional role of the optical properties of coral skeletons in coral ecology and evolution. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1853) doi: 10.1098/rspb.2016.1667. 20161667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez J. Vital effect on stable-isotope composition seen in foraminifera and coral skeletons. Nature. 1978;273(5659):199–202. doi: 10.1038/273199a0. [DOI] [Google Scholar]
- Erez J, Reynaud S, Silverman J, Schneider K, Allemand D. Coral calcification under ocean acidification and global change. In: Dubinsky Z, Stambler N, editors. Coral reefs: An ecosystem in transition. Dordrecht, the Netherlands: Springer; 2011. pp. 151–176. [Google Scholar]
- Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, et al. Worcester EM. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2007;290(10):1315–1323. doi: 10.1002/ar.20580. [DOI] [PubMed] [Google Scholar]
- Evans D, Webb PB, Penkman K, Kröger R, Allison N. The characteristics and biological relevance of inorganic amorphous calcium carbonate (ACC) precipitated from seawater. Crystal Growth & Design. 2019;19(8):4300–4313. doi: 10.1021/acs.cgd.9b00003. [DOI] [Google Scholar]
- Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De'ath G, et al. Lough JM. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change. 2011;1:165–169. doi: 10.3410/f.11817956.12931055. [DOI] [Google Scholar]
- Falini G, Reggi M, Fermani S, Sparla F, Goffredo S, Dubinsky Z, et al. Cuif J-P. Control of aragonite deposition in colonial corals by intra-skeletal macromolecules. Journal of Structural Biology. 2013;183(2):226–238. doi: 10.1016/j.jsb.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. Light and the bioenergetics of a symbiotic coral. BioScience. 1984;34(11):705–709. doi: 10.2307/1309663. [DOI] [Google Scholar]
- Farre B, Cuif J-P, Dauphin Y. Occurrence and diversity of lipids in modern coral skeletons. Zoology. 2010;113(4):250–257. doi: 10.1016/j.zool.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Flugel E, Senowbari-Daryan B. Triassic reefs of the Tethys. In: Stanley GD, editor. The history and sedimentology of ancient reef systems. Vol. 17. Boston, MA: Springer; 2001. pp. 217–249. (Topics in Geobiology). [Google Scholar]
- Foster T, Clode P. Skeletal mineralogy of coral recruits under high temperature and pCO2. Biogeosciences. 2016;13(5):1717–1722. doi: 10.5194/bg-13-1717-2016. [DOI] [Google Scholar]
- Fowler GH. Memoirs: The anatomy of the madreporaria: I. Journal of Cell Science. 1885;2(100):577–597. [Google Scholar]
- Frankowiak K, Mazur M, Gothmann AM, Stolarski J. Diagenetic alteration of Triassic coral from the aragonite Konservat-Lagerstatte in Alakir Çay, Turkey: Implications for geochemical measurements. Palaios. 2013;28(6):333–342. doi: 10.2110/palo.2012.p12-116r. [DOI] [Google Scholar]
- Frankowiak K, Wang XT, Sigman DM, Gothmann AM, Kitahara MV, Mazur M, et al. Stolarski J. Photosymbiosis and the expansion of shallow-water corals. Science Advances. 2016;2(11):e1601122. doi: 10.1126/sciadv.1601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda I, Ooki S, Fujita T, Murayama E, Nagasawa H, Isa Y, Watanabe T. Molecular cloning of a cDNA encoding a soluble protein in the coral exoskeleton. Biochemical and Biophysical Research Communications. 2003;304(1):11–17. doi: 10.1016/S0006-291X(03)00527-8. [DOI] [PubMed] [Google Scholar]
- Furla P, Galgani I, Durand E, Allemand D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. Journal of Experimental Biology. 2000;203:3445–3457. doi: 10.1007/s00338-013-1032-2. [DOI] [PubMed] [Google Scholar]
- Gagnon AC, Adkins JF, Erez J. Seawater transport during coral biomineralization. Earth and Planetary Science Letters. 2012;329–330:150–161. doi: 10.1016/j.epsl.2012.03.005. [DOI] [Google Scholar]
- Gal A, Kahil K, Vidavsky N, DeVol RT, Gilbert PUPA, Fratzl P, et al. Addadi L. Particle accretion mechanism underlies biological crystal growth from an amorphous precursor phase. Advanced Functional Materials. 2014;24(34):5420–5426. doi: 10.1002/adfm.201400676. [DOI] [Google Scholar]
- Gal A, Weiner S, Addadi L. A perspective on underlying crystal growth mechanisms in biomineralization: Solution mediated growth versus nanosphere particle accretion. CrystEngComm. 2015;17(13):2606–2615. doi: 10.1039/C4CE01474J. [DOI] [Google Scholar]
- Galli G, Solidoro C. ATP supply may contribute to light-enhanced calcification in corals more than abiotic mechanisms. Frontiers in Marine Science. 2018;5:68. doi: 10.3389/fmars.2018.00068. [DOI] [Google Scholar]
- Gattuso J-P, Allemand D, Frankignoulle M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. American Zoologist. 1999;39(1):160–183. doi: 10.1093/icb/39.1.160. [DOI] [Google Scholar]
- Gehling JG. A cnidarian of actinian-grade from the Ediacaran Pound Subgroup, South Australia. Alcheringa. 1988;12(4):299–314. doi: 10.1080/03115518808619129. [DOI] [Google Scholar]
- Gibbs SJ, Stoll HM, Bown PR, Bralower TJ. Ocean acidification and surface water carbonate production across the Paleocene-Eocene thermal maximum. Earth and Planetary Science Letters. 2010;295(3–4):583–592. doi: 10.1016/j.epsl.2010.04.044. [DOI] [Google Scholar]
- Giri SJ, Swart PK, Devlin QB. The effect of changing sea-water Ca and Mg concentrations upon the distribution coefficients of Mg and Sr in the skeletons of the scleractinian coral Pocillopora damicornis. Geochimica et Cosmochimica Acta. 2018;222:535–549. doi: 10.1016/j.gca.2017.11.011. [DOI] [Google Scholar]
- Gladfelter E. Skeletal development in Acropora cervicornis: I. Patterns of calcium carbonate accretion in the axial corallite. Coral Reefs. 1982;1(1):45–51. doi: 10.1007/bf00286539. [DOI] [Google Scholar]
- Gladfelter E. Skeletal development in Acropora cervicornis II: Diel patterns of calcium carbonate accretion. Coral Reefs. 1983;2:91–100. doi: 10.1007/bf00286539. [DOI] [Google Scholar]
- Goffredo S, Vergni P, Reggi M, Caroselli E, Sparla F, Levy O, et al. Falini G. The skeletal organic matrix from Mediterranean coral Balanophyllia europaea influences calcium carbonate precipitation. PLoS ONE. 2011;6(7):e22338. doi: 10.1371/journal.pone.0022338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg WM. Acid polysaccharides in the skeletal matrix and calicoblastic epithelium of the stony coral Mycetophyllia reesi. Tissue and Cell. 2001a;33(4):376–387. doi: 10.1054/tice.2001.0191. [DOI] [PubMed] [Google Scholar]
- Goldberg WM. Desmocytes in the calicoblastic epithelium of the stony coral Mycetophyllia reesi and their attachment to the skeleton. Tissue and Cell. 2001b;33(4):388–394. doi: 10.1054/tice.2001.0192. [DOI] [PubMed] [Google Scholar]
- Goreau T. Histochemistry of mucopolysaccharide-like substances and alkaline phosphatase in Madreporaria. Nature. 1956;177(4518):1029–1030. doi: 10.1038/1771029a0. [DOI] [Google Scholar]
- Goreau TF. The physiology of skeleton formation in corals. I. A method for measuring the rate of calcium deposition by corals under different conditions. Biological Bulletin. 1959;116:59–75. doi: 10.2307/1539156. [DOI] [Google Scholar]
- Goreau TF, Goreau NI. The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. The Biological Bulletin. 1959;117(2):239–250. doi: 10.2307/1538903. [DOI] [Google Scholar]
- Greene SE, Martindale RC, Ritterbush KA, Bottjer DJ, Corsetti FA, Berelson WM. Recognising ocean acidification in deep time: An evaluation of the evidence for acidification across the Triassic-Jurassic boundary. Earth Science Reviews. 2012;113:72–93. doi: 10.1016/j.earscirev.2012.03.009. [DOI] [Google Scholar]
- Griesshaber E, Kelm K, Sehrbrock A, Mader W, Mutterlose J, Brand U, Schmahl WW. Amorphous calcium carbonate in the shell material of the brachiopod Megerliatruncata. European Journal of Mineralogy. 2009;21(4):715–723. doi: 10.1127/0935-1221/2009/0021-1950. [DOI] [Google Scholar]
- Gutjahr M, Ridgwell A, Sexton PF, Anagnostou E, Pearson PN, Pälike H, et al. Foster GL. Very large release of mostly volcanic carbon during the Palaeocene-Eocene Thermal Maximum. Nature. 2017;548(7669):573. doi: 10.1038/nature23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutner-Hoch E, Schneider K, Stolarski J, Domart-Coulon I, Yam R, Meibom A, et al. Levy O. Evidence for rhythmicity pacemaker in the calcification process of Scleractinian coral. Scientific Reports. 2016;6 doi: 10.1038/srep20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kubota S, Uchida HO, Stanley GD, Jr, Yao X, Shu D, et al. Yasui K. Tiny sea anemone from the Lower Cambrian of China. PLoS ONE. 2010;5(10):e13276. doi: 10.1371/journal.pone.0013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes RL, Goreau NI. Intracellular crystal-bearing vesicles in the epidermis of Scleractinian corals, Astrangia danae (Agassiz) and Porites porites (Pallas) The Biological Bulletin. 1977;152(1):26–40. doi: 10.2307/1540724. [DOI] [PubMed] [Google Scholar]
- Helman Y, Natale F, Sherrell RM, LaVigne M, Starovoytov V, Gorbunov MY, Falkowski PG. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):54–58. doi: 10.1073/pnas.0710604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henehan MJ, Ridgwell A, Thomas E, Zhang S, Alegret L, Schmidt DN, et al. Hull PM. Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(45):22500–22504. doi: 10.1073/pnas.1905989116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M. Deposition of fusiform crystals without apparent diurnal rhythm at the growing edge of septa of the coral Galaxea fascicularis. Coral Reefs. 1991;10(1):41–45. [Google Scholar]
- Higuchi T, Fujimura H, Yuyama I, Harii S, Agostini S, Oomori T. Biotic control of skeletal growth by scleractinian corals in aragonite–calcite seas. PLoS ONE. 2014;9(3):e91021. doi: 10.1371/journal.pone.0091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Shirai K, Mezaki T, Yuyama I. Temperature dependence of aragonite and calcite skeleton formation by a scleractinian coral in low mMg/Ca seawater. Geology. 2017;45(12):1087–1090. doi: 10.1130/g39516.1. [DOI] [Google Scholar]
- Hoegh-Guldberg O, Jacob D, Taylor M, Bolaños TG, Bindi M, Brown S, et al. Ebi K. The human imperative of stabilizing global climate change at 1.5°C. Science. 2019;365(6459) doi: 10.1016/b978-1-78548-051-5.50007-3. eaaw6974. [DOI] [PubMed] [Google Scholar]
- Hohn S, Merico A. Modelling coral polyp calcification in relation to ocean acidification. Biogeosciences. 2012;9(11):4441–4454. doi: 10.5194/bg-9-4441-2012. [DOI] [Google Scholar]
- Hönisch B, Hemming NG, Grottoli AG, Amat A, Hanson GN, Bigma J. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochimica et Cosmochimica Acta. 2004;68(18):3675–3685. doi: 10.1016/j.gca.2004.03.002. [DOI] [Google Scholar]
- Hopkinson BM, Tansik AL, Fitt WK. Internal carbonic anhydrase activity in the tissue of scleractinian corals is sufficient to support proposed roles in photosynthesis and calcification. The Journal of Experimental Biology. 2015;218:2039–2048. doi: 10.1242/jeb.118182. [DOI] [PubMed] [Google Scholar]
- Houck JE, Buddemeier RW, Chave KE. Skeletal low-magnesium calcite in living scleractinian corals. Science. 1975;189(4207):997–999. doi: 10.1126/science.189.4207.997. [DOI] [PubMed] [Google Scholar]
- Houlbreque F, Meibom A, Cuif JP, Stolarski J, Marrocchi Y, Ferrier-Pagès C, et al. Dunbar RB. Strontium-86 labeling experiments show spatially heterogeneous skeletal formation in the scleractinian coral Porites porites. Geophysical Research Letters. 2009;364 doi: 10.1029/2008gl036782. [DOI] [Google Scholar]
- Ihli J, Wang Y-W, Cantaert B, Kim Y-Y, Green DC, Bomans PH, et al. Meldrum FC. Precipitation of amorphous calcium oxalate in aqueous solution. Chemistry of Materials. 2015;27(11):3999–4007. doi: 10.1029/2008gl036782. [DOI] [Google Scholar]
- Inoue M, Nakamura T, Tanaka Y, Suzuki A, Yokoyama Y, Kawahata H, et al. Gussone N. A simple role of coral-algal symbiosis in coral calcification based on multiple geochemical tracers. Geochimica et Cosmochimica Acta. 2018;235:76–88. doi: 10.1016/j.gca.2018.05.016. [DOI] [Google Scholar]
- Isa Y. An electron microscope study on the mineralization of the skeleton of the staghorn coral Acropora hebes. Marine Biology. 1986;93(1):91–101. doi: 10.1007/BF00428658. [DOI] [Google Scholar]
- Isa Y, Okazaki M. Some observations on the Ca2+ binding phospholipid from scleractinian coral skeletons. Comparative Biochemistry and Physiology Part B. 1987;87(3):507–512. doi: 10.1016/0305-0491(87)90045-9. [DOI] [Google Scholar]
- Isa Y, Yamazato K. The ultrastructure of calicoblast and related tissues in Acropora hebes (Dana). Paper presented at the Proceedings of the Fourth International Coral Reef Symposium; 1981. [Google Scholar]
- Ivantsov AY, Fedonkin MA. Conulariid–like fossil from the Vendian of Russia: A metazoan clade across the Proterozoic/Palaeozoic boundary. Palaeontology. 2002;45(6):1219–1229. doi: 10.1111/1475-4983.00283. [DOI] [Google Scholar]
- Janiszewska K, Mazur M, Escrig S, Meibom A, Stolarski J. Aragonitic scleractinian corals in the Cretaceous calcitic sea. Geology. 2017;45(4):319–322. doi: 10.1130/g38593.1. [DOI] [Google Scholar]
- Johnston IS. The ultrastructure of skeletogenesis in hermatypic corals. International Review of Cytology. 1980;67:171–214. doi: 10.1016/s0074-7696(08)62429-8. [DOI] [Google Scholar]
- Kawaguti S, Sakumoto D. The effect of light on the calcium deposition of corals. Bulletin of the Oceanograpaical Institute of Taiwan. 1948;4:65–70. [Google Scholar]
- Kawaguti S, Sato K. Electron microscopy on the polyp of staghorn corals with special reference to its skeleton formation. Biological Journal of Okayama University. 1968;14:87–98. [Google Scholar]
- Kayal E, Bentlage B, Pankey MS, Ohdera AH, Medina M, Plachetzki DC, et al. Ryan JF. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evolutionary Biology. 2018;18(1):68. doi: 10.1186/s12862-018-1142-0. [DOI] [Google Scholar]
- Kiessling W. Reef expansion during the Triassic: Spread of photosymbiosis balancing climatic cooling. Palaeogeography, Palaeoclimatology, Palaeoecology. 2010;290(1–4):11–19. doi: 10.1016/j.palaeo.2009.03.020. [DOI] [Google Scholar]
- Kiessling W, Baron-Szabo RC. Extinction and recovery patterns of scleractinian corals at the Cretaceous-Tertiary boundary. Palaeogeography Palaeoclimatology Palaeoecology. 2004;214:195–223. doi: 10.1016/s0031-0182(04)00421-3. [DOI] [Google Scholar]
- Kirch PV. On the road of the winds: An archaeological history of the Pacific Islands before European contact. Oakland, CA: University of California Press; 2017. [Google Scholar]
- Kirk NL, Weis VM. Animal-Symbiodinium symbioses: Foundations of coral reef ecosystems. In: Hurst C, editor. The mechanistic benefits of microbial symbionts. Advances in environmental microbiology. Vol. 2. Cham, Switzerland: Springer; 2016. pp. 269–294. [Google Scholar]
- Kitahara MV, Cairns SD, Stolarski J, Blair D, Miller DJ. A comprehensive phylogenetic analysis of the Scleractinia (Cnidaria, Anthozoa) based on mitochondrial CO1 sequence data. PLoS ONE. 2010;5(7):e11490. doi: 10.1371/journal.pone.0011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, Opdyke BN. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science. 1999;284(5411):118–120. doi: 10.1126/science.284.5411.118. [DOI] [PubMed] [Google Scholar]
- LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology. 2018;28(16):2570–2580.e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Lazareth CE, Soares-Pereira C, Douville E, Brahmi C, Dissard D, Le Cornec F, et al. Cabioch G. Intra-skeletal calcite in a live-collected Porites sp.: Impact on environmental proxies and potential formation process. Geochimica et Cosmochimica Acta. 2016;176:279–294. doi: 10.1016/j.gca.2015.12.020. [DOI] [Google Scholar]
- Le Goff C, Ganot P, Zoccola D, Caminiti-Segonds N, Allemand D, Tambutté S. Carbonic anhydrases in cnidarians: Novel perspectives from the ctocorallian Corallium rubrum. PLoS ONE. 2016;11(8):e0160368. doi: 10.1371/journal.pone.0160368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy N, Jackson DJ, Marie B, Ramos-Silva P, Marin F. The evolution of metazoan α-carbonic anhydrases and their roles in calcium carbonate biomineralization. Frontiers in Zoology. 2014;11(1):75. doi: 10.1186/s12983-014-0075-8. [DOI] [Google Scholar]
- Lowenstam HA. Minerals formed by organisms. Science. 1981;211(4487):1126–1131. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- Macías-Sánchez E, Willinger MG, Pina CM, Checa AG. Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-12673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre IG, Towe KM. Skeletal calcite in living scleractinian corals: Microboring fillings, not primary skeletal deposits. Science. 1976;193(4254):701–702. doi: 10.1126/science.193.4254.701. [DOI] [PubMed] [Google Scholar]
- Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin F, Bundeleva I, Takeuchi T, Immel F, Medakovic D. Organic matrices in metazoan calcium carbonate skeletons: Composition, functions, evolution. Journal of Structural Biology. 2016;196(2):98–106. doi: 10.1016/j.jsb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Mass T, Drake JL, Haramaty L, Kim JD, Zelzion E, Bhattacharya D, Falkowski PG. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Current Biology. 2013;23(12):1126–1131. doi: 10.1016/j.cub.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Mass T, Drake JL, Haramaty L, Rosenthal Y, Schofield OME, Sherrell RM, Falkowski PG. Aragonite precipitation by “proto-polyps” in coral cell cultures. PLoS ONE. 2012;7(4):e35049. doi: 10.1371/journal.pone.0035049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass T, Drake JL, Heddleston JM, Falkowski PG. Nanoscale visualization of biomineral formation in coral proto-polyps. Current Biology. 2017;27(20):3191–3196.e3. doi: 10.1016/j.cub.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Mass T, Drake JL, Peters EC, Jiang W, Falkowski PG. Immunolocalization of skeletal matrix proteins in tissue and mineral of the coral Stylophora pistillata. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(35):12728–12733. doi: 10.1073/pnas.1408621111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass T, Giuffre AJ, Sun C-Y, Stifler CA, Frazier MJ, Neder M, et al. Gilbert PUPA. Amorphous calcium carbonate particles form coral skeletons. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(37):E7670–E7678. doi: 10.1073/pnas.1707890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass T, Putnam HM, Drake JL, Zelzion E, Gates RD, Bhattacharya D, Falkowski PG. Temporal and spatial expression patterns of biomineralization proteins during early development in the stony coral Pocillopora damicornis. Proceedings of the Royal Society B: Biological Sciences. 2016;283(1829) doi: 10.1098/rspb.2016.0322. 20160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch MT, D'Olivo JP, Falter J, Georgiou L, Holcomb M, Montagna P, Trotter JA. Boron isotopic systematics in scleractinian corals and the role of pH up-regulation. In: Marschall H, Foster G, editors. Boron isotopes. Advances in isotope geochemistry. Cham, Switzerland: Springer; 2018. pp. 145–162. [Google Scholar]
- McCulloch M, Falter J, Trotter J, Montagna P. Coral resilience to ocean acidification and global warming through pH upregulation. Nature Climate Change. 2012;2:623–627. http://www.nature.com/nclimate/journal/vaop/ncurrent/abs/nclimate1473.html#supplementary-information. [Google Scholar]
- Mitsuguchi T, Matsumoto E, Abe O, Uchida T, Isdale PJ. Mg/Ca thermometry in coral skeletons. Science. 1996;274(5289):961–963. doi: 10.1126/science.274.5289.961. [DOI] [PubMed] [Google Scholar]
- Mitterer RM. Amino acid composition and metal binding capability of the skeletal protein of corals. Bulletin of Marine Science. 1978;28(1):173–180. doi: 10.1007/978-94-009-5307-9_23. [DOI] [Google Scholar]
- Morse JW, Wang Q, Tsio MY. Influences of temperature and Mg: Ca ratio on CaCO3 precipitates from seawater. Geology. 1997;25(1):85–87. doi: 10.1130/0091-7613(1997)025<0085:iotamc>2.3.co;2. [DOI] [Google Scholar]
- Moya A, Tambutté S, Tambutté E, Zoccola D, Caminiti N, Allemand D. Study of calcification during a daily cycle of the coral Stylophora pistillata: Implications for light-enhanced calcification'. Journal of Experimental Biology. 2006;209(17):3413–3419. doi: 10.1242/jeb.02382. [DOI] [PubMed] [Google Scholar]
- Muscatine L, Cernichiari E. Assimilation of photosynthetic products of zooxanthellae by a reef coral. Biological Bulletin. 1969;137:506–523. doi: 10.2307/1540172. [DOI] [PubMed] [Google Scholar]
- Muscatine L, Goiran C, Land L, Jaubert J, Cuif J-P, Allemand D. Stable isotopes (δ13C and δ15N) of organic matrix from coral skeleton. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1525–1530. doi: 10.1073/pnas.0408921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatine L, McCloskey L, Marian R. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnology and Oceanography. 1981;601 doi: 10.4319/lo.1981.26.4.0601. [DOI] [Google Scholar]
- Muscatine L, Tambutté E, Allemand D. Morphology of coral desmocytes, cells that anchor the calicoblastic epithelium to the skeleton. Coral Reefs. 1997;16(4):205–213. doi: 10.1007/s003380050075. [DOI] [Google Scholar]
- Naggi A, Torri G, Iacomini M, Colombo Castelli G, Reggi M, Fermani S, et al. Falini G. Structure and function of stony coral intraskeletal polysaccharides. ACS Omega. 2018;3(3):2895–2901. doi: 10.1021/acsomega.7b02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata PA. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science. 2003;164(6):901–909. doi: 10.1016/s0168-9452(03)00120-1. [DOI] [Google Scholar]
- Neder M, Laissue PP, Akiva A, Akkaynak D, Albéric M, Spaeker O, et al. Mass T. Mineral formation in the primary polyps of pocilloporoid corals. Acta Biomaterialia. 2019 doi: 10.1016/j.actbio.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njegic BD, Fermani S, Dubinsky Z, Goffredo S, Falini G, Kralj D. Chemistry. Weinheim an der Bergstrasse; Germany: 2019. In vitro coral biomineralization under relevant aragonite supersaturation conditions. [DOI] [PubMed] [Google Scholar]
- Nothdurft LD, Webb GE. Microstructure of common reef-building coral genera Acropora, Pocillopora, Goniastrea and Porites: Constraints on spatial resolution in geochemical sampling. Facies. 2007;53(1):1–26. doi: 10.1007/s10347-006-0090-0. [DOI] [Google Scholar]
- Nothdurft LD, Webb GE, Buster NA, Holmes CW, Sorauf JE, Kloprogge JT. Brucite microbialites in living coral skeletons: Indicators of extreme microenvironments in shallow-marine settings. Geology. 2005;33(3):169. doi: 10.1130/G20932.1. [DOI] [Google Scholar]
- Ogilvie MM. Microscopic and systematic study of madreporarian types of corals. Philosophical Transactions of the Royal Society B: Biological Sciences. 1896;187:83–345. doi: 10.1098/rstb.1896.0003. [DOI] [Google Scholar]
- Ohno Y, Iguchi A, Shinzato C, Gushi M, Inoue M, Suzuki A, et al. Nakamura T. Calcification process dynamics in coral primary polyps as observed using a calcein incubation method. Biochemistry and Biophysics Reports. 2017;9:289–294. doi: 10.1016/j.bbrep.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Hwang D-S, Lee J-S, Song J-I, Seo T-K, Won Y-J. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Molecular Phylogenetics and Evolution. 2012;62(1):329–345. doi: 10.1016/j.ympev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Pochon X, Stat M, Takabayashi M, Chasqui L, Chauka LJ, Logan DDK, Gates RD. Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. Journal of Phycology. 2010;46(1):53–65. doi: 10.1111/j.1529-8817.2009.00797.x. [DOI] [Google Scholar]
- Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science. 2004;306(5699):1161–1164. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- Politi Y, Metzler RA, Abrecht M, Gilbert B, Wilt FH, Sagi I, et al. Gilbert PUPA. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17362–17366. doi: 10.1073/pnas.0806604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SM. Seawater chemistry and early carbonate biomineralization. Science. 2007;316(5829):1302–1302. doi: 10.1126/science.1137284. [DOI] [PubMed] [Google Scholar]
- Puverel S, Tambutté E, Pereira-Mouries L, Zoccola D, Allemand D, Tambutté S. Soluble organic matrix of two Scleractinian corals: Partial and comparative analysis. Comparative Biochemistry and Physiology Part B. 2005;141:480–487. doi: 10.1016/j.cbpc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Puverel S, Tambutté E, Zoccola D, Dumart-Coulon I, Bouchot A, Lotto S, et al. Tambutté S. Antibodies against the organic matrix in scleractinians: A new tool to study coral biomineralization. Coral Reefs. 2005;24:149–156. doi: 10.1007/s00338-004-0456-0. [DOI] [Google Scholar]
- Radha AV, Forbes TZ, Killian CE, Gilbert PUPA, Navrotsky A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(38):16438–16443. doi: 10.1073/pnas.1009959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Silva P, Kaandorp J, Herbst F, Plasseraud L, Alcaraz G, Stern C, et al. Luquet G. The skeleton of the staghorn coral Acropora millepora: Molecular and structural characterization. PLoS ONE. 2014;9(6):e97454. doi: 10.1371/journal.pone.0097454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Silva P, Kaandorp J, Huisman L, Marie B, Zanella-Cleon I, Guichard N, et al. Marin F. The skeletal proteome of the coral Acropora millepora: The evolution of calcification by cooption and domain shuffling. Molecular Biology and Evolution. 2013;30(9):2099–2112. doi: 10.1093/molbev/mst109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raup DM, John Sepkoski J. Mass extinctions in the marine fossil record. Science. 1982;215:1501–1503. doi: 10.1126/science.215.4539.1501. [DOI] [PubMed] [Google Scholar]
- Raz S, Testeniere O, Hecker A, Weiner S, Luquet G. Stable amorphous calcium carbonate is the main component of the calcium storage structures of the crustacean Orchestia cavimana. The Biological Bulletin. 2002;203(3):269–274. doi: 10.2307/1543569. [DOI] [PubMed] [Google Scholar]
- Reyes-Bermudez A, Lin Z, Hayward DC, Miller DJ, Ball EE. Differential expression of three galaxin-related genes during settlement and metamorphosis in the scleractinian coral Acropora millepora. BMC Evolutionary Biology. 2009;9:178. doi: 10.1186/1471-2148-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riding R. Microbial carbonate abundance compared with fluctuations in metazoan diversity over geological time. Sedimentary Geology. 2006;185(3–4):229–238. doi: 10.1016/j.sedgeo.2005.12.015. [DOI] [Google Scholar]
- Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37(12):1131–1134. doi: 10.1130/g30210a.1. [DOI] [Google Scholar]
- Rinkevich B, Loya Y. Does light enhance calcification in hermatypic corals? Marine Biology. 1984;80(1):1–6. doi: 10.1007/bf00393120. [DOI] [Google Scholar]
- Rosenberg GD. The revolution in geology from the Renaissance to the Enlightenment. Vol. 203 Berkeley, CA: Geological Society of America, University of California Press; 2009. [Google Scholar]
- Roth MS. The engine of the reef: Photobiology of the coral–algal symbiosis. Frontiers in Microbiology. 2014;5:422. doi: 10.3389/fmicb.2014.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador JM, Inesi G, Rigaud J-L, Mata AM. Ca2+ transport by reconstituted synaptosomal ATPase is associated with H+ countertransport and net charge displacement. Journal of Biological Chemistry. 1998;273(29):18230–18234. doi: 10.1074/jbc.273.29.18230. [DOI] [PubMed] [Google Scholar]
- Sancho-Tomás M, Fermani S, Goffredo S, Dubinsky Z, García-Ruiz J, Gómez-Morales J, Falini G. Exploring coral biomineralization in gelling environments by means of a counter diffusion system. CrystEngComm. 2014;16(7):1257–1267. doi: 10.1039/c3ce41894d. [DOI] [Google Scholar]
- Savarese M, Mount JF, Sorauf JE, Bucklin L. Paleobiologic and paleoenvironmental context of coral-bearing Early Cambrian reefs: Implications for Phanerozoic reef development. Geology. 1993;21:917–920. doi: 10.1130/0091-7613(1993)021<0917:papcoc>2.3.co;2. [DOI] [Google Scholar]
- Schoenberg D, Trench R. Genetic variation in Symbiodinium (= Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. I. Isoenzyme and soluble protein patterns of axenic cultures of Symbiodinium microadriaticum. Proceedings of the Royal Society of London. Series B. Biological Sciences. 1980;207(1169):405–427. doi: 10.1098/rspb.1980.0033. [DOI] [Google Scholar]
- Schoepf V, Jury CP, Toonen RJ, McCulloch MT. Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proceedings of the Royal Society B: Biological Sciences. 2017;284(1868) doi: 10.1098/rspb.2017.2117. 20172117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton CT. The Palaeozoic corals, II: Structure, variation and palaeoecology. Proceedings of the Yorkshire Geological Society. 1998;52(1):1–57. doi: 10.1144/pygs.52.1.1. [DOI] [Google Scholar]
- Scrutton C. Palaeozoic corals: Their evolution and palaeoecology. Geology Today. 1999:184–193. doi: 10.1046/j.1365-2451.1999.1505005.x. [DOI] [Google Scholar]
- Scrutton CT, Clarkson EN. A new scleractinian-like coral from the Ordovician of the Southern Uplands, Scotland. Palaeontology. 1991;34(1):179–194. [Google Scholar]
- Sevilgen DS, Venn AA, Hu MY, Tambutté E, de Beer D, Planas-Bielsa V, Tambutté S. Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Science Advances. 2019;5(1) doi: 10.1126/sciadv.aau7447. eaau7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamberger KEF, Cohen AL, Golbuu Y, McCorkle DC, Lentz SJ, Barkley HC. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophysical Research Letters. 2014;41(2):499–504. doi: 10.1002/2013gl058489. [DOI] [Google Scholar]
- Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, et al. Satoh N. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476(7360):320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- Silliman B. On the chemical composition of the calcareous corals. American Journal of Science and Arts. 1846;1:189–199. doi: 10.1007/978-94-009-3581-5_29. [DOI] [Google Scholar]
- Silverman J, Lazar B, Cao L, Caldeira K, Erez J. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophysical Research Letters. 2009;36(5) doi: 10.1029/2008gl036282. [DOI] [Google Scholar]
- Sluijs A, Schouten S, Pagani M, Woltering M, Brinkhuis H, Damsté JSS, et al. Stein R. Subtropical Arctic Ocean temperatures during the Palaeocene/Eocene Thermal Maximum. Nature. 2006;441(7093):610. doi: 10.1038/nature04668. [DOI] [PubMed] [Google Scholar]
- Stanley GD., Jr The history of early Mesozoic reef communities: A three step process. Palaios. 1988;3:170–183. doi: 10.2307/3514528. [DOI] [Google Scholar]
- Stanley GD. The evolution of modern corals and their early history. Earth-Science Reviews. 2003;60(3–4):195–225. doi: 10.1016/S0012-8252(02)00104-6. [DOI] [Google Scholar]
- Stanley GD, Fautin DG. The origins of modern corals. Science. 2001;291(5510):1913–1914. doi: 10.1126/science.1056632. [DOI] [PubMed] [Google Scholar]
- Stanley GD, Jr, van de Schootbrugge B. The evolution of the coral-algal symbiosis. In: van Oppen MJH, Lough JM, editors. Coral bleaching. Berlin and Heidelberg, Germany: Springer; 2009. [Google Scholar]
- Stanley SM, Hardie LA. Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeography, Palaeoclimatology, Palaeoecology. 1998;144(1–2):3–19. doi: 10.1016/s0031-0182(98)00109-6. [DOI] [Google Scholar]
- Stolarski J. Three-dimensional micro- and nanostructural characteristics of the scleractinian coral skeleton: A biocalcification proxy. Acta Palaeontologica Polonica. 2003;48(4):497–530. [Google Scholar]
- Stolarski J, Bosellini FR, Wallace CC, Gothmann AM, Mazur M, Domart-Coulon I, et al. Shemesh A. A unique coral biomineralization pattern has resisted 40 million years of major ocean chemistry change. Scientific Reports. 2016;6 doi: 10.1038/srep27579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarski J, Kitahara MV, Miller DJ, Cairns SD, Mazur M, Meibom A. The ancient evolutionary origins of Scleractinia revealed by azooxanthellate corals. BMC Evolutionary Biology. 2011;11(1):316. doi: 10.1186/1471-2148-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarski J, Mazur M. Nanostructure of biogenic versus abiogenic calcium carbonate crystals. Acta Palaeontologica Polonica. 2005;50(4):847–865. [Google Scholar]
- Stolarski J, Meibom A, Przenioslo R, Mazur M. A Cretaceous scleractinian coral with a calcitic skeleton. Science. 2007;318(5847):92–94. doi: 10.1126/science.1149237. [DOI] [PubMed] [Google Scholar]
- Strahl J, Stolz I, Uthicke S, Vogel N, Noonan SHC, Fabricius KE. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2015;184:179–186. doi: 10.1016/j.cbpa.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Swain TD, Lax S, Lake N, Grooms H, Backman V, Marcelino LA. Relating coral skeletal structures at different length scales to growth, light availability to Symbiodinium, and thermal bleaching. Frontiers in Marine Science. 2018;5:450. doi: 10.3389/fmars.2018.00450. [DOI] [Google Scholar]
- Takeuchi T, Plasseraud L, Ziegler-Devin I, Brosse N, Shinzato C, Satoh N, Marin F. Biochemical characterization of the skeletal matrix of the massive coral, Porites australiensis–The saccharide moieties and their localization. Journal of Structural Biology. 2018;203(3):219–229. doi: 10.1016/j.jsb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Yamada L, Shinzato C, Sawada H, Satoh N. Stepwise evolution of coral biomineralization revealed with genome-wide proteomics and transcriptomics. PLoS ONE. 2016;11(6):e0156424. doi: 10.1371/journal.pone.0156424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambutté E, Allemand D, Zoccola D, Meibom A, Lotto S, Caminiti N, Tambutté S. Observations of the tissue-skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs. 2007;26(3):517–529. doi: 10.1007/s00338-007-0263-5. [DOI] [Google Scholar]
- Tambutté E, Tambutté S, Segonds N, Zoccola D, Venn A, Erez J, Allemand D. Calcein labelling and electrophysiology: Insights on coral tissue permeability and calcification. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1726):19–27. doi: 10.1098/rspb.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté É, Zoccola D, Allemand D. Coral biomineralization: From the gene to the environment. Journal of Experimental Marine Biology and Ecology. 2011;408(1–2):58–78. doi: 10.1016/j.jembe.2011.07.026. [DOI] [Google Scholar]
- Tambutté É, Allemand D, Mueller ER, Jaubert JE. A compartmental approach to the mechanism of calcification in hermatypic corals. Journal of Experimental Biology. 1996;199(5):1029–1041. doi: 10.1242/jeb.199.5.1029. [DOI] [PubMed] [Google Scholar]
- Taubner I, Hu MY, Eisenhauer A, Bleich M. Electrophysiological evidence for light-activated cation transport in calcifying corals. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1896) doi: 10.1098/rspb.2018.2444. 20182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoy M. Mid-ocean ridge eruptions as a climate valve. Geophysical Research Letters. 2015;42(5):1346–1351. doi: 10.1002/2014gl063015. [DOI] [Google Scholar]
- Tornabene C, Martindale RC, Wang XT, Schaller MF. Detecting photosymbiosis in fossil scleractinian corals. Scientific Reports. 2017;7(1):9465. doi: 10.1038/s41598-017-09008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Barron ME, Barott KL, Ho J, Roa JN. Sensing acid/base conditions via soluble adenylyl cyclase in aquatic animals. The FASEB Journal. 2013;27(Suppl 1) 937.3. [Google Scholar]
- Valentine JW. Prelude to thecambrianexplosion. Annual Review of Earth and Planetary Sciences. 2002;30(1):285–306. doi: 10.1146/annurev.earth.30.082901.092917. [DOI] [Google Scholar]
- van de Locht R, Verch A, Saunders M, Dissard D, Rixen T, Moya A, Kröger R. Microstructural evolution and nanoscale crystallography in scleractinian coral spherulites. Journal of Structural Biology. 2013;183(1):57–65. doi: 10.1016/j.jsb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Vandermeulen JH. Studies on reef corals. III. Fine structural changes of calicoblast cells in Pocillopora damicornis during settling and calcification. Marine Biology. 1975;31(1):69–77. doi: 10.1007/BF00390649. [DOI] [Google Scholar]
- Venn A, Tambutté E, Holcomb M, Allemand D, Tambutté S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE. 2011;6(5):e20013. doi: 10.1371/journal.pone.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron JEN. Corals of the world. Monterey, CA: Sea Challengers; 2000. [Google Scholar]
- Vidal-Dupiol J, Zoccola D, Tambutté E, Grunau C, Cosseau C, Smith KM, et al. Tambutté S. Genes related to ion-transport and energy production are upregulated in response to CO2-driven pH decrease in corals: New insights from transcriptome analysis. PLoS ONE. 2013;8(3):e58652. doi: 10.1371/journal.pone.0058652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Euw S, Zhang Q, Manichev V, Murali N, Gross J, Feldman LC, et al. Falkowski PG. Biological control of aragonite formation in stony corals. Science. 2017;356(6341):933–938. doi: 10.1126/science.aam6371. [DOI] [PubMed] [Google Scholar]
- Von Heider A. Die Gattung Cladocora Ehrenb. Sitzungsberichte der Akademie der Wissenschaften mathematisch-naturwissen-schaftliche Klasse. 1881;84:634–667. [Google Scholar]
- Wainwright SA. Skeletal organization in the coral, Pocillopora damicornis. Quarterly Journal of Microscopical Science. 1963;3(66):169–183. [Google Scholar]
- Wang XT, Sigman DM, Cohen AL, Sinclair DJ, Sherrell RM, Cobb KM, et al. Ren H. Influence of open ocean nitrogen supply on the skeletal δ15N of modern shallow-water scleractinian corals. Earth and Planetary Science Letters. 2016;441:125–132. doi: 10.1016/j.epsl.2016.02.032. [DOI] [Google Scholar]
- Watanabe T. Molecular analyses of protein components of the organic matrix in the exoskeleton of two scleractinian coral species. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2003;136:767–774. doi: 10.1016/s1096-4959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Webb GE, Sorauf JE. Zigzag microstructure in rugose corals: A possible indicator of relative seawater Mg/Caratios. Geology. 2002;30(5):415–418. doi: 10.1130/0091-7613(2002)030<0415:zmirca>2.0.co;2. [DOI] [Google Scholar]
- Webby B. Global biogeography of Ordovician corals and stromatoporoids. In: Laurie JR, Webby DB, editors. Global perspectives on ordovician geology. Boca Raton: FL CRC Press, Taylor Francis Group; 1992. pp. 261–276. [Google Scholar]
- Weber JN. Incorporation of strontium into reef coral skeletal carbonate. Geochimica et Cosmochimica Acta. 1973;37(9):2173–2190. doi: 10.1016/0016-7037(73)90015-x. [DOI] [Google Scholar]
- Weiss IM, Tuross N, Addadi L, Weiner S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. Journal of Experimental Zoology. 2002;293(5):478–491. doi: 10.1002/jez.90004. [DOI] [PubMed] [Google Scholar]
- Wirshing HH, Baker AC. Molecular evolution of calcification genes in morphologically similar but phylogenetically unrelated scleractinian corals. Molecular Phylogenetics and Evolution. 2014;77:281–295. doi: 10.1016/j.ympev.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Wood R, Liu AG, Bowyer F, Wilby PR, Dunn FS, Kenchington CG, et al. Penny A. Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nature Ecology & Evolution. 2019;3(4):528–538. doi: 10.1038/s41559-019-0821-6. [DOI] [PubMed] [Google Scholar]
- Work TM, Aeby GS, Maragos JE. Phase shift from a coral to a corallimorph-dominated reef associated with a shipwreck on Palmyra Atoll. PLoS ONE. 2008;3(8):e2989. doi: 10.1371/journal.pone.0002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Laflamme M. On the eve of animal radiation: Phylogeny, ecology and evolution of the Ediacara biota. Trends in Ecology & Evolution. 2009;24(1):31–40. doi: 10.1016/j.tree.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Yamashiro H, Samata T. New type of organic matrix in corals formed at the decalcified site: Structure and composition. Comparative Biochemistry and Physiology Part A: Physiology. 1996;113(3):297–300. doi: 10.1016/0300-9629(95)02071-3. [DOI] [Google Scholar]
- Young SD. Organic material from scleractinian coral skeletons - I. Variation in composition between several species. Comparitive Biochemistry and Physiology. 1971;40B:113–120. doi: 10.1016/0305-0491(71)90067-8. [DOI] [Google Scholar]
- Young SD. Calcification and synthesis of skeletal organic material in the coral, Pocillopora damicornis (L.) (Astrocoeniidae, Scleractinia) Comparative Biochemistry and Physiology Part A: Physiology. 1973;44:669–672. doi: 10.1016/0300-9629(73)90520-3. [DOI] [Google Scholar]
- Young SD, O'Connor JD, Muscatine L. Organic material from scleractinian coral skeletons–II. Incorporation of 14C into protein, chitin and lipid. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1971;40(4):945–958. doi: 10.1016/0305-0491(71)90040-x. [DOI] [Google Scholar]
- Yuyama I, Higuchi T. Differential gene expression in skeletal organic matrix proteins of scleractinian corals associated with mixed aragonite/calcite skeletons under low mMg/Ca conditions. PeerJ. 2019 doi: 10.7287/peerj.7241v0.1/reviews/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos JC, Röhl U, Schellenberg SA, Sluijs A, Hodell DA, Kelly DC, et al. Kroon D. Rapid acidification of the ocean during the Paleocene-Eocene Thermal Maximum. Science. 2005;308(5728):1611–1615. doi: 10.1126/science.1109004. [DOI] [PubMed] [Google Scholar]
- Zachos JC, Wara MW, Bohaty S, Delaney ML, Petrizzo MR, Brill A, et al. Premoli-Silva I. A transient rise in tropical sea surface temperature during the Paleocene-Eocene Thermal Maximum. Science. 2003;302(5650):1551–1554. doi: 10.1126/science.1090110. [DOI] [PubMed] [Google Scholar]
- Zapalski MK, Berkowski B. The oldest species of ?Yavorskia (Tabulata) from the Upper Famennian of the Holy Cross Mountains (Poland) Acta Geologica Polonica. 2012;62(2):197–204. doi: 10.2478/v10263-012-0009-8. [DOI] [Google Scholar]
- Zeebe RE, Wolf-Gladrow D. CO2 in seawater: Equilibrium, kinetics, isotopes. Vol. 65 New York: Elsevier; 2001. [Google Scholar]
- Zeebe RE, Zachos JC, Dickens GR. Carbon dioxide forcing alone insufficient to explain Palaeocene-Eocene Thermal Maximum warming. Nature Geoscience. 2009;2(8):576. doi: 10.1038/ngeo578. [DOI] [Google Scholar]
- Zoccola D, Ganot P, Bertucci A, Caminiti-Segonds N, Techer N, Voolstra CR, et al. Casey JR. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Scientific Reports. 2015;5 doi: 10.1038/srep09983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola D, Innocenti A, Bertucci A, Tambutté E, Supuran CT, Tambutté S. Coral carbonic anhydrases: Regulation by ocean acidification. Marine Drugs. 2016;14(6):109. doi: 10.3390/md14060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola D, Tambutté E, Kulhanek E, Puverel S, Scimeca J-C, Allemand D, Tambutté S. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora pistillata. Biochimica et Biophysica Acta - Biomembranes. 2004;1663(1–2):117–126. doi: 10.1016/j.bbamem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zoccola D, Tambutté E, Sénégas-Balas F, Michiels J-F, Failla J-P, Jaubert J, Allemand D. Cloning of a calcium channel α1 subunit from the reef-building coral, Stylophora pistillata. Gene. 1999;227(2):157–167. doi: 10.1016/s0378-1119(98)00602-7. [DOI] [PubMed] [Google Scholar]