Abstract
This research aimed to investigate the composition and diversity of endophytic bacterial community in seeds of four hybrid maize and their parental lines, which was used to reveal the potential relationship and association of endophytic bacteria between maize genotypes and their genetic relevance. High-throughput sequencing (HTS) technology showed that a total of 1419 OTUs (46.6%) were parental lines unique and 1052 OTUs (34.5%) were hybrid varieties unique, with only 575 core OTUs revealed in all the samples. Most OTUs belonged to Proteobacteria. Enterobacter (23.2%), Shigella (21.2%), Pseudomonas (15.8%) and Achromobacter (10.1%) were the major genera; the bacterial community composition and diversity of endophytic bacteria were inconsistent among different seed genotypes. Based on principal component analysis (PCA), the results referred that the endophytic composition of hybrid sample showed obvious correlation with their female parental lines, and in ‘Jingke968’ and ‘MC738’ with the same female line the endophytic community was more similar than other hybrid samples. This was the first ever use of HTS technology for investigating the endophytic bacterial diversity and community structures in seeds of genetically related maize genotypes, it was shown that, there were core microbes shared among all genotypes of seed samples, and the female parental line was more significant to impact on the composition of their hybrid seeds than male parental line. This study would provide scientific clues for the future research on the vertical transmission of endophytes among maize generations.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-2034-8) contains supplementary material, which is available to authorized users.
Keywords: Maize, Seeds, Endophytic bacteria, Diversity, HTS
Introduction
Maize is known as the queen of cereals which is the major source of carbohydrates, it is one of the significant crops around the globe (Zhao and Wang 2013; Bodhankar et al. 2017; Liu et al. 2017a). Seed is the basic source of reproduction in plants. It does not only preserve and transmit genetic information, but it also keeps ensured adaptive strategy for plants to reproduce under stress (Guan 2009). Further, it also carries many useful bacteria and other pathogenic organisms. According to many theorists, there exist many microbial communities over the surface and even inside the seeds (Nelson 2004; Liu et al. 2013). Studies on the physiology and pathology of seeds have confirmed that when the microbial community in a plant seed has a special relationship with the seed, it has a significant impact on plant health and soil fertility. (Bacilio-Jiméne et al. 2001; Cottyn et al. 2001; Barea et al. 2005).
Plant endophytes are microbes colonizing plant internal tissues and ubiquitously associated with plants; they play key role in plant growth and health, and subsequently establish a harmonious relationship with the host plant (Kloepper and Beauchamp 1992; Yang et al. 2017). Endophytes can directly or indirectly affect the physiology and anatomy of plant, may do impact on other plant growth promoting activities (Liu et al. 2013). The endophytic bacteria in hybrid offsprings could be inherited from their parental lines through the vertical transmission, and also affected by other factors during the breeding and growth period. There have been reports that, there are two major sources of endophytes in plants, one is the external environment of the surface of plants, and the other is the seed (Hardoim et al. 2008; Mano and Morisaki 2008; Liu et al. 2018).
In recent years, with the rapid development in plant microecology and microbiology research, the biological functions and mechanism of endophytic bacteria on plant growth and development have become a research hotspot in agricultural biology. Deciphering the interaction between plants and their endophytes is also a hot topic in plant sciences, agronomy and ecology (Vandenkoornhuyse et al. 2015). At present, there are many reports on the integration of microorganisms with host plant seeds using culture-dependent method (Mastretta et al. 2009; Liu et al. 2009; Jiang et al. 2013), 16S rDNA clone library method (Liu et al. 2011, 2012a, 2013; Zou et al. 2012), denaturing gradient gel electrophoresis (DGGE) (Hardoim et al. 2012) and high-throughput sequencing (HTS) method (Liu et al. 2014, 2017a, 2018; Huang et al. 2016; Qin et al. 2016; Zhang et al. 2018). The subject and research progress on plant micro-ecology is gradually developing (Liu et al. 2017a). Seed microbes may play an important role in plant productivity through influencing the primary composition of the plant microbiota. Nevertheless, the diversity and structure of seed-associated microorganisms have often been ignored, especially seed endophytes are still not well understood (Shade et al. 2017; Zhang et al. 2018).
Besides that, seeds serve as the initial inoculum for plant microbiota, transmitting microorganisms vertically from generation to another (Shade et al. 2017). The seed microbes transmitted across generations have profound impacts on plant health, quality, productivity and micro-ecology (Truyens et al. 2015; Nelson 2017). Although three main pathways of transmission between plant generations have been well described but the necessity and importance of vertical transmission remains unclear (Vandenkoornhuyse et al. 2015; Shade et al. 2017).
In this study, the seeds of four hybrid maize combinations and their parental lines independently developed and cultivated by China were used as research plant materials. To understand the composition and diversity of endophytic bacterial community of different seed genotypes and the vertical genetic correlation on endophytic bacteria among maize parental lines and their hybrid combination, high-throughput sequencing (HTS) technology based on Illumina Hiseq 2500 platform was used to study the community structure and diversity of their endophytic bacteria in hybrid combinations and their parental lines. This study provides a seed-source microbial ecological basis for the analysis of high quality and high yielding mechanism of hybrid maize, and supplies a theoretical basis for promoting the application of the idea of “plant and microbial symbiotic breeding (Wang et al. 2015; Liu et al. 2018)” in major and essential agricultural crops.
Materials and methods
Sample collection
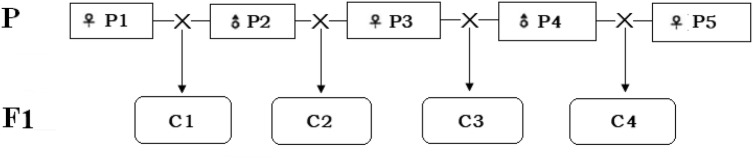
Seeds were collected from four varieties of hybrid maize combinations (Zea mays L.) (Jingke665, Jingke968, MC738, Jingnongke728) and their parental lines (Jing 725, Jing 92, Jing 724, Jing 2416, MC01), which are self-developed and extensively planted in China, supplied by Professor Jiuran Zhao at Beijing Academy of Agriculture and Forestry Sciences (BAAFS). Among them, the variety ‘MC01’ is a unique low-heritability variety proven from previous breeding and genetics study. The detailed information about the maize seeds and the genetic relationships among the samples are shown in Fig. 1. All of the samples were collected from the scientific research base of BAAFS in Sanya, Hainan (18.36774333340131 N, 109.18169975280762 E, southern China) in March 2018; each seed sample was randomly collected from ten plants located in five sites and the base and the weight of each sample was approximately 1000 g; and the seed samples were stored at 4 °C in aseptic bags for 2 weeks prior to conduction of experiment.
Fig. 1.
Genetic relationships among the four hybrid combinations. P1, Jing 725; P2, Jing 92; P3, Jing 724; P4, Jing 2416; P5, MC01; C1, Jingke665; C2, Jingke968; C3, MC738; C4, Jingnongke728
Surface sterilization of samples
Firstly, the husks of each maize seed sample were removed by a small sheller. Then, under aseptic conditions the following operations were performed in the order listed: husked seeds were washed three times with prepared sterile water; 2.5 g of seeds were placed in a clean and sterile 50-mL tube containing 25 mL of phosphate buffer (Zhang et al. 2018), and the seeds were sonicated twice by an Ultrasonic Processor Scientz-IID sonicator (Ningbo Scientz Biotechnology Co., Ltd, China) at low power (237.5 W; 950 W × 25%) in an ice bath for 5 min (alternating thirty 2-s bursts and thirty 2-s rests) (Lundberg et al. 2012; Zhang et al. 2018). To validate that the surface was sterilized, sterile tweezers were used to press surface-sterilized seeds into LB medium (LUQIAO), and the seeds were incubated at 30 °C for 72 h (Bodhankar et al. 2017; Zhang et al. (2018) and after incubation, there was no colony formed on LB medium, so the result of surface sterilization of samples met the requirements for subsequent experiments.
DNA extraction, 16S rRNA gene amplification and sequencing
DNA was extracted using the FastDNA® SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA) according to the manufacturer’s protocol after grounding into a fine powder in liquid nitrogen. Then 799F (AACAGGATTAGATACCCTG) and 1492R (GGTTACCTTGTTACGACTT) were used for the first-round amplification of 16S rRNA gene. Then the 750 bp fragment amplified from endophytic bacteria was cut and used as the template for the second-round amplification for V6-V8 regions (968F: AACGCGAAGAACCTTAC and 1378R: CGGTGTGTACAAGGCCCGGGAACG). The amplified products were purified and then sequenced on MiSeq instrument using the MiSeq® Reagent Kit v3 (600 cycle) (Illumina).
Bioinformatics and statistical analysis
The assembled paired FASTQ files were performed by Mothur (version 1.39.0) (Schloss et al. 2011). Briefly, paired sequence reads were assembled after removing raw reads with low quality, such as read length less than 50 base pairs, or reads without the valid index. The high-quality DNA sequences were grouped into OTUs (Operational taxonomic units) under the threshold of 97% identity in comparison to SILVA reference database (V119) (Quast et al. 2013). Community richness, evenness and diversity analysis (Shannon, Simpsonenven, ACE, Chao and Good’s coverage) were performed using Mothur. The t-test (with 95% confidence intervals) was used to determine whether the means of evaluation indices were statistically different and p values < 0.05 were considered as significant standard. Taxonomy was assigned using the online software RDP classifier (Wang et al. 2007) at default parameter (80% threshold) based on the Ribosomal Database Project (Cole et al. 2009). Genera and family abundance differences among samples were analyzed by Metastats (White et al. 2009). Spearman correlation coefficient between two variables was calculated using the R command ‘cor.test’. The sequence data were submitted to NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/) with BioProject and accession number.
Results
Sequencing data for diversity analysis
The raw high-throughput sequencing data was submitted to the NCBI database with BioProject number PRJNA510484 and accession number SRR8357070-SRR8357096. Nine seeds samples containing three repetitions for each were separately collected from five parental lines and four hybrid varieties (Fig. 1). A total of 2,513,380 (24,042—197,606) 16S rRNA genes were obtained by high-throughput DNA sequencing, and a minimum of 24,042 reads was applied as the criteria for data normalization in this study. Operational taxonomic unit (OTU), the group of 16S rRNA genes, was set at 97% similarity to estimate the diversity and richness of bacterial community, using a normalized read number 24,042. A total of 3046 OTUs (1994 of parents and 1627 of varieties) were obtained. The number of OTUs in the nine samples was roughly the same, indicating that the endophytic bacteria have some similarity at the host species level. The Good’s coverage was both 99.4% for parental lines and hybrid varieties (Supplementary Table 1), that meant the sequencing depth was sufficient for microbiota investigation.
Endophytic bacterial populations in maize seeds
A total of 1419 OTUs (46.6%) were parental lines unique while 1052 OTUs (34.5%) were hybrid varieties unique (Supplementary Fig. 1a), with only 575 OTUs (core OTUs) existing in both groups. However, over 98% of sequenced 16S rRNA genes were contained in the core OTUs (Supplementary Fig, 1b), and the average reads number was 2.4 in each parental line unique OTUs and 2.9 in each variety unique OTUs. It can be seen from the experimental results that most of the core OTUs in hybrid offsprings are consistent with their parental lines, and it can be concluded that the core OTUs are inherited by the parental lines. However, the existence of a small number of unique OTUs may be affected by other factors such as reproduction and growth period.
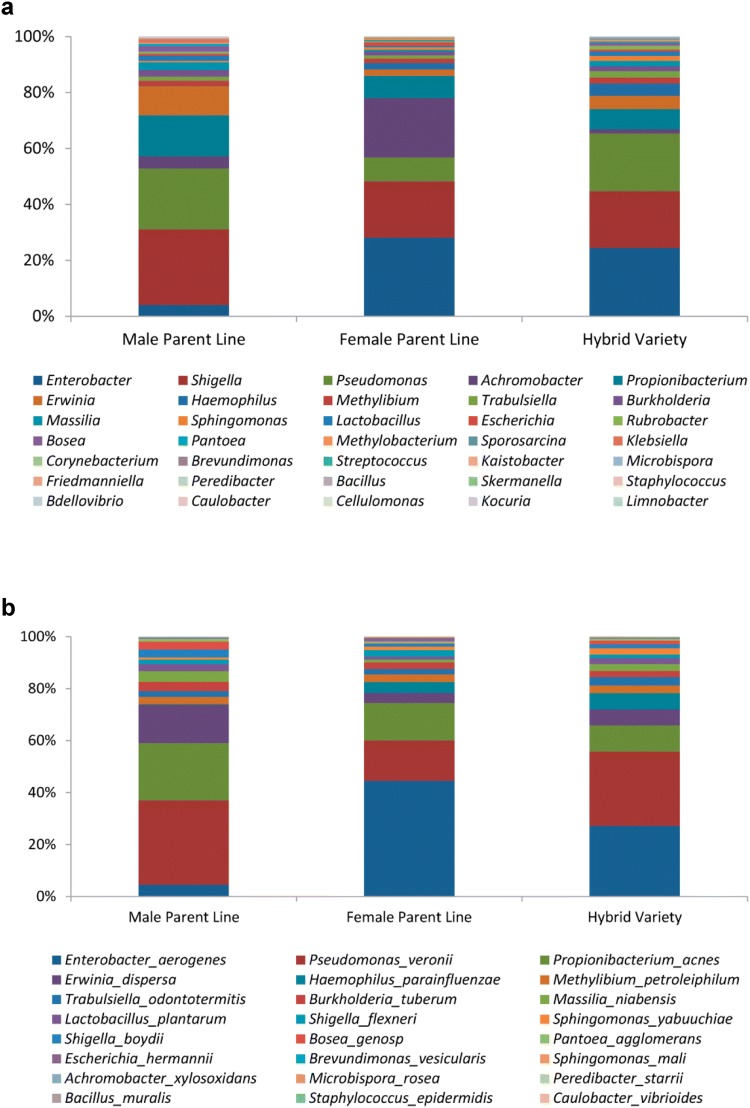
Low quality and potentially contaminated DNA sequences were effectively removed though quality control and testing. At the phylum level, 544 out of the 575 core OTUs could be classified with 511 in Proteobacteria, 23 in Firmicutes and ten in Actinobacteria. While at the genus level, 35 genera were revealed, with Enterobacter (23.2%), Shigella (21.2%), Pseudomonas (15.8%) and Achromobacter (10.1%) as major microbiota. Besides these major microbiota, the proportion of other microbiota such as Propionibacterium, Enwinia, Haemophilus etc. was also similar to that of the female parent lines in the hybrid offspring (Fig. 2a). In addition, 34 OTUs were classified into species level (24 species), with Enterobacter aerogenes, Pseudomonas veronii, Propionibacterium acnes and Erwinia dispersa as most abundant (Fig. 2b). While on genus and species level, the bacterial community composition and group abundance of endophytic bacteria were obviously inconsistent from nine different seed genotypes, and the core seed microbiome was shared among different plant genotypes. There was a certain similarity among generations, and more emphasis was placed on the parental lines.
Fig. 2.
Endophytic bacterial distribution in different maize seeds. a The composition distribution and relative abundance on genus-level. b The composition distribution and relative abundance on species-level
The correlation of endophytic composition between parents and hybrid varieties
Among the 575 core OTUs, the abundance of 11 OTUs showed significant difference (p value < 0.05) between male and female parents, while 17 OTUs showed significant richness difference between male parent line and hybrid variety, with only nine OTUs showing significant richness difference between female parent line and hybrid variety (Supplementary Fig. 2). The difference between hybrid offspring and female parent line was less than that of male parent line.
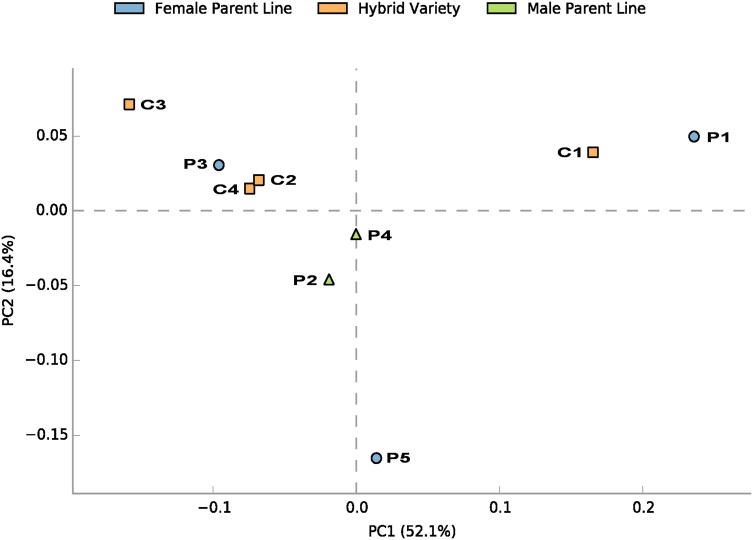
Principal component analysis (PCA) was performed to investigate whether the endophytic bacteria community was similar between parental lines and their hybrid varieties (Fig. 3 and Supplementary Fig. 3). Female parental line sample P3 showed similar bacterial composition with its two hybrid varieties sample C2 and C3, and female parent sample P1 located near its hybrid variety sample C1. The endophytic composition of hybrid sample C1, C2, C3 showed obvious correlation with their female parental lines. In particular, endophytic bacterial structure and diversity also showed differences among offspring with common parents. The samples C2 and C3 with the same female parent were more similar than samples C1 and C2, and samples C3 and C4 with the common male parent. However, the female parent sample P5 contained different endophytic bacterial composition from its variety sample C4, which may be potentially due to the characteristic of low heritability of sample P5.
Fig. 3.
Principal component analysis (PCA) analysis with Bray–Curtis dissimilarity based on OTUs. Points represent female parent lines, triangles represent male parent lines, and squares represent hybrid variety
In addition, the hierarchical clustering heatmap (Fig. 4) also supported the above results that each variety had more similar bacteria community with its female parental line. Then the correlation coefficients were calculated among different samples using the Spearman test (Table 1) and it showed that except for sample C4, all the other three hybrid varieties showed the highest correlation with their female parental lines. From the results mentioned above, it is indicated that the composition of female parental lines might have a higher influence on the composition of hybrid progenies than the ones from male parental, and the heritability of the maize parental line would be a potential factor to influence on the vertical transmission of endophytes among maize generations.
Fig. 4.
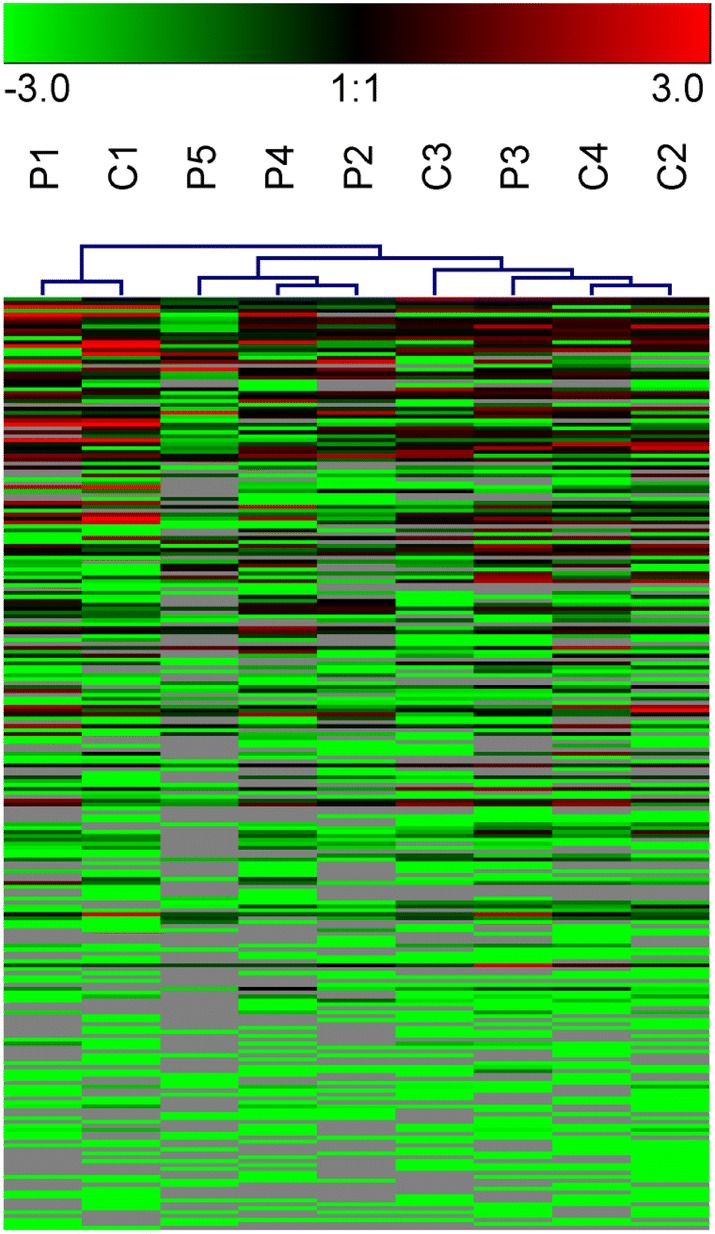
Heatmap of OTUs in nine seed samples constructed by hierarchical cluster analysis. The dendrogram at the top demonstrates the similarity of seed samples between parental lines (P) and hybrid varieties (C)
Table 1.
Spearman correlation coefficients (R2) between different samples
| Sample | C1 | C2 | C3 | C4 | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 1 | 0.129 | 0.1655 | 0.1227 | 0.4912 | 0.1328 | 0.1714 | 0.1551 | 0.1414 |
| C2 | 0.129 | 1 | 0.7434 | 0.6883 | 0.0663 | 0.5908 | 0.8582 | 0.7311 | 0.2644 |
| C3 | 0.1655 | 0.7434 | 1 | 0.7201 | 0.0386 | 0.5816 | 0.9384 | 0.5421 | 0.3272 |
| C4 | 0.1227 | 0.6883 | 0.7201 | 1 | 0.0603 | 0.6029 | 0.7354 | 0.5845 | 0.2772 |
| P1 | 0.4912 | 0.0663 | 0.0386 | 0.0603 | 1 | 0.0996 | 0.0554 | 0.1561 | 0.0505 |
| P2 | 0.1328 | 0.5908 | 0.5816 | 0.6029 | 0.0996 | 1 | 0.6235 | 0.6112 | 0.3713 |
| P3 | 0.1714 | 0.8582 | 0.9384 | 0.7354 | 0.0554 | 0.6235 | 1 | 0.6263 | 0.3543 |
| P4 | 0.1551 | 0.7311 | 0.5421 | 0.5845 | 0.1561 | 0.6112 | 0.6263 | 1 | 0.2638 |
| P5 | 0.1414 | 0.2644 | 0.3272 | 0.2772 | 0.0505 | 0.3713 | 0.3543 | 0.2638 | 1 |
Discussion
Compared with rice and soybean, in recent years, although investigations on bacteria associated with plants have been increased with each passing day (Liu et al. 2018). Research reports on endophytic bacteria in maize were relatively rare, and mainly concentrated in association with maize roots (Liu et al. 2013; Mehmood et al. 2018; Gao et al. 2018), while the existing research reports on endophytic bacteria in maize seeds are relatively limited (Liu et al. 2017a). Using culture-dependent method to investigate the endophytic bacterial communities in seeds of six hybrid maize varieties, Pseudomonas, Microbacterium, Enterobacter, Bacillus and Pantoea were isolated and identified from the seeds (Li et al. 2016). While Enterobacter, Burkholderia, Pseudomonas, Sphingomonas, and Limnobacter were detected by 16S rDNA clone library method from seeds of several hybrid maize and their parental lines (Liu et al. 2011, 2012a, b, 2013), and Paenibacillus sp. 5L8 isolated from the seeds of maize (Zea mays L., Jingke 968) had comprehensive and effective antagonistic activity against the common plant pathogens Fusarium graminearum, Bipolaris maydis, Bipolaris sorokiniana etc. (Liu et al. 2016a). High-throughput sequencing (HTS) contributes to gain more insight into rich endophytic bacteria in maize seeds, Pantoea, Enterobacter, Aeribacillus, Sphingomonas and Halomonas, were identified as the dominant species in different hybrid maize combinations (Chen et al. 2016; Liu et al. 2017a). It is worth mentioning that, there are also some new species of bacteria in maize seeds, Paenibacillus zeae sp. nov., Paenibacillus chinensis sp. nov. and Bacillus ciccensis sp. nov. were isolated from maize seeds and proposed as new species through microbial polyphasic taxonomic approach (Liu et al. 2015, 2016b, 2017b).
In this study, 35 genera were revealed with Enterobacter, Shigella, Pseudomonas and Achromobacter as major microbiota in nine seeds samples. Among them Enterobacter and Pseudomonas as endophytic dominant genera were frequently detected from seeds of several plants (Liu et al. 2009; Jiang et al. 2013; Sobolev et al. 2013; Zhang et al. 2018; Verma and White 2018). At present, there are some studies on the functions of endophytic bacteria (Ribeiro et al. 2018; Jai et al. 2019). Some species of Enterobacter and Pseudomonas genera have been previously found as the common plant growth-promoting bacteria (PGPB), which may directly or indirectly influence the growth, health and development of host plants (Liu et al. 2012a, b). Yang et al. (2017) showed inoculation with Enterobacter cloacae induces rice self-resistance and thus resisting infection by subsequent pathogens. Enterobacter sp. SA187 had been isolated from the indigenous desert plant, and could be able to provide abiotic stress tolerance to Arabidopsis thaliana (Andrés-Barrao et al. 2017). A novel Enterobacter cancerogenus MSA2 was isolated from the rhizosphere of Jatropha cucas which is potentially important biofuel and feed stock plant (Jha et al. 2012). Recent research demonstrated that Pseudomonas chlororaphis subsp. aureofaciens strain M71 exerts beneficial effects on plant metabolism and primes tolerance mechanisms against biotic stresses and water stress in tomatoes (Brilli et al. 2018). Pseudomonas fluorescens DUS1-27 could increase the growth of Brassica napus L. (canola) over that of uninoculated control plants in a soil-based system. Endophytic bacterium Pseudomonas fluorescens Sasm05 may be attributed to its ability to produce indole-3-acetic acid and enhance phytoremediation (Chen et al. 2017) and Pseudomonas spp. OFT5 can improve nutrient uptake and plant growth under moderate salt-affected conditions by reducing stress-related ethylene levels and enhance NaCl stress tolerance by reducing stress-related ethylene production, resulting in improving growth, photosynthetic performance, and ionic balance in tomato plants (Win et al. 2018).
Shigella and Achromobacter are common soil bacterial groups (Santamaría and Toranzos 2003; Adam et al. 2014; Farhadkhani et al. 2018; Egea et al. 2017; Furlan and Stehling 2017; Gunasekera et al. 2018; Thulasi et al. 2018; Garrido-Sanz et al. 2018; Fang et al. 2018), and they could colonize in plant tissues through plant roots (Liu et al. 2011). Pantoea, Bacillus, Burkholderia, Sphingomonas, Limnobacter, Methylobacterium and so on often separated or identified by high frequency from various plant seeds were also detected from this research (Mantica et al. 2010; Zhang et al. 2018), some species in these genera can also improve the plant growth and production (Liu et al. 2012a, 2018).
From the results of this study, the composition and diversity of endophytic bacterial community were obviously different among the seeds of four hybrid maize combinations and their parental lines. However, there was a certain similarity in endophytic diversity among genetically related varieties (Khalaf et al. 2016; Truyens et al. 2015), and the female parental line showed more affection on the endophytes of its hybrid variety. Although the endophytic diversity and group abundance were obviously different in each genotype, the great part of the seed microbiome was shared in the same plant. In recent years, during our research group excavating the endophytic microbial resources of maize seeds, progeny endophytic bacterial community diversity is affected not only by host genotype, but also by vertical transmission between generations (Liu et al. 2011, 2012a, b, 2013, 2017a, b; Frank et al. 2017). Besides, in this study it showed that the composition of female parental lines might have a higher influence on the composition of hybrid progenies than the ones from male parental, and several reports on the correlation of plant genotype and seed bacterial microbiome clearly achieved consistent research conclusions (Simon et al. 2001; Adams and Kloepper 2002; Sun et al. 2005; Picard and Bosco 2006; San et al. 2007; Chen et al. 2009; Zou et al. 2012; Liu et al. 2018; Zhang et al. 2018). This is the first time to select a unique low-heritability variety sample as a female parental line for hybridization, and it also reflected that the heritability of the maize parental line would be a potential factor to influence on the vertical transmission of endophytes among maize generations, and the related mechanisms need further research to be confirmed.
The 16S rDNA analysis using high-throughput sequencing first appeared in 2006, which applied to analysis deep-sea microbial communities (Sogin et al. 2006), and this technology has since been widely used in the field of microbial ecology. HTS technology could overcome the shortcomings of traditional molecular biology methods, and analyze microbial community structure from the level of genome, breaking through the technical bottleneck that many endophytic microorganisms cannot or difficult to be isolated and cultured, and efficiently detect low-abundance but important endophytic bacteria in plant (Chen et al. 2016). This is first ever use of HTS technology for investigating the composition and diversity of endophytic bacterial community in seeds of hybrid maize varieties and their parental lines, and to reveal the endophytic bacterial composition in maize seeds and the correlation between endophytic bacterial diversity with seed genotypes. From this research, it also indicated that female parental line is more important to impact on the composition of their hybrid seeds than male parental line, and the heritability of the maize parental line would be a potential factor to influence on the vertical transmission of endophytes among maize generations. This study laid a necessary theoretical foundation for the further study on the interaction between maize plant generations and endophytic microbes during the vertical transmission process, and also provided a basis of microbial ecological research and practical applications for high quality maize breeding.
Conclusion
In this study, 575 core OTUs were revealed in all seed samples which assigned to the phylum Proteobacteria, Firmicutes and Actinobacteria; and Proteobacteria, and Enterobacter (23.2%), Shigella (21.2%), Pseudomonas (15.8%) and Achromobacter (10.1%) were the major endophytic genus. The results also referred that the endophytic composition of hybrid sample ‘Jingke665’, ‘Jingke968’, ‘MC738’ showed obvious correlation with their female parental lines, in particular the endophytic community structure and diversity of sample ‘Jingke968’ and ‘MC738’ with the same female parent were more similar than sample ‘Jingke665’ and ‘Jingke968’, and sample ‘MC738’ and ‘Jingnongke728’ with the common male parent. Besides, a unique low-heritability variety sample ‘MC01’ was specially selected as a female parental line for hybridization, and unlike other varieties in this study, there was a significant difference of endophytic diversity shown between ‘MC01’ and its hybrid offspring ‘Jingnongke728’.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Shahbaz Ahmad at University of Science and Technology Beijing and Miss Xiaoxiao Cheng at New Mexico State University (USA) for assistance with English language and grammatical editing of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Beijing, China (no. 6182008), the Fundamental Research Funds for Central Non-profit Scientific Institution (no. 1610132017041) and the Fundamental Research Funds for the Central Universities (no. FRF-TP-18-012A1; FRF-BR-18-009B).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Contributor Information
Yang Liu, Email: liuyang@ustb.edu.cn, Email: ly81150@163.com.
Huajun Zheng, Email: zhenghj@chgc.sh.cn.
Jiuran Zhao, Email: maizezhao@126.com.
References
- Adam M, Westphal A, Hallmann J, Heuer H. Specific microbial attachment to root knot nematodes in suppressive soil. Appl Environ Microbiol. 2014;80(9):2679–2686. doi: 10.1128/AEM.03905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Kloepper JW. Effect of host genotype on indigenous bacterial endophytes of cotton (Gossypium hirsutum L.) Plant Soil. 2002;240(1):181–189. [Google Scholar]
- Andrés-Barrao C, Lafi FF, Alam I, Zélicourt A, Eida AA, Bokhari A, Alzubaidy H, Bajic VB, Hirt H, Saad MM. Complete genome sequence analysis of Enterobacter sp. SA187, a plant multi-stress tolerance promoting endophytic bacterium. Front Microbiol. 2017;8:2023. doi: 10.3389/fmicb.2017.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacilio-Jiméne M, Aguilar-Flores S, del Valle MV, Pérez A, Zepeda A, Zenteno E. Endophytic bacteria in rice seeds inhibit early colonization of roots by Azospirillum brasilense. Soil Biol Biochem. 2001;33:167–172. [Google Scholar]
- Barea JM, Pozo MJ, Azcon R, Azcon-Aguilar C. Microbial co-operation in the rhizosphere. J Exp Bot. 2005;56(417):1761–1778. doi: 10.1093/jxb/eri197. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Grover M, Hemanth S, Reddy G, Rasul S, Yadav SK, Desai S, Mallappa M, Mandapaka M, Srinivasarao C. Maize seed endophytic bacteria: dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech. 2017;7(4):232. doi: 10.1007/s13205-017-0860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Pollastri S, Raio A, Baraldi R, Neri L, Bartolini P, Podda A, Loreto F, Maserti BE, Balestrini R. Root colonization by Pseudomonas chlororaphis primes tomato (Lycopersicum esculentum) plants for enhanced tolerance to water stress. J Plant Physiol. 2018;232:82–93. doi: 10.1016/j.jplph.2018.10.029. [DOI] [PubMed] [Google Scholar]
- Chen G, Fan HW, Mao ZW, Liu CG. Breeding and popularization of new maize variety Jingdan 28. Bull Agric Sci Technol. 2009;6:125–127. [Google Scholar]
- Chen ZB, Li B, Wang DK, Yu L, Xu SG, Ren Z, Jin S, Dai LJ. Study on the diversity of endophytic bacteria in maize using Illumina MiSeq high-throughput sequencing system. Mod Food Sci Technol. 2016;32(2):113–120. [Google Scholar]
- Chen B, Luo S, Wu Y, Ye J, Wang Q, Xu X, Pan F, Khan KY, Feng Y, Yang X. The effects of the endophytic bacterium Pseudomonas fluorescens Sasm05 and IAA on the plant growth and cadmium uptake of Sedum alfredii Hance. Front Microbiol. 2017;8:2538. doi: 10.3389/fmicb.2017.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottyn B, Regalado E, Lanoot B, De Cleene M, Mew TW, Swings J. Bacterial populations associated with rice seed in the tropical environment. Phytopathology. 2001;91:282–292. doi: 10.1094/PHYTO.2001.91.3.282. [DOI] [PubMed] [Google Scholar]
- Egea TC, da Silva R, Boscolo M, Rigonato J, Monteiro DA, Grünig D, da Silva H, van der Wielen F, Helmus R, Parsons JR, Gomes E. Diuron degradation by bacteria from soil of sugarcane crops. Heliyon. 2017;3(12):e00471. doi: 10.1016/j.heliyon.2017.e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Han L, Zhang H, Long Z, Cai L, Yu Y. Dissemination of antibiotic resistance genes and human pathogenic bacteria from a pig feedlot to the surrounding stream and agricultural soils. Hazard Mater. 2018;357:53–62. doi: 10.1016/j.jhazmat.2018.05.066. [DOI] [PubMed] [Google Scholar]
- Farhadkhani M, Nikaeen M, Yadegarfar G, Hatamzadeh M, Pourmohammadbagher H, Sahbaei Z, Rahmani HR. Effects of irrigation with secondary treated wastewater on physicochemical and microbial properties of soil and produce safety in a semi-arid area. Water Res. 2018;144:356–364. doi: 10.1016/j.watres.2018.07.047. [DOI] [PubMed] [Google Scholar]
- Frank AC, Saldierna Guzmán JP, Shay JE. Transmission of bacterial endophytes. Microorganisms. 2017;5(4):70. doi: 10.3390/microorganisms5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan JPR, Stehling EG. High-level of resistance to β-lactam and presence of β-lactamases encoding genes in Ochrobactrum sp. and Achromobacter sp. isolated from soil. J Glob Antimicrob Resist. 2017;11:133–137. doi: 10.1016/j.jgar.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Gao JL, Sun P, Sun XH, Tong S, Yan H, Han ML, Mao XJ, Sun JG. Caulobacter zeae sp. nov. and Caulobacter radicis sp. nov., novel endophytic bacteria isolated from maize root (Zea mays L.) Syst Appl Microbiol. 2018;41(6):604–610. doi: 10.1016/j.syapm.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Garrido-Sanz D, Manzano J, Martín M, Redondo-Nieto M, Rivilla R. Metagenomic analysis of a biphenyl-degrading soil bacterial consortium reveals the metabolic roles of specific populations. Front Microbiol. 2018;9:232. doi: 10.3389/fmicb.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL. Seed physiological ecology. Beijing: China Agriculture Press; 2009. [Google Scholar]
- Gunasekera TS, Radwan O, Bowen LL, Brown LM, Ruiz ON. Draft genome sequence of Achromobacter spanius strain 6, a soil bacterium isolated from a hydrocarbon-degrading microcosm. Microbiol Resour Announc. 2018;7(11):e01124–e1218. doi: 10.1128/MRA.01124-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoim PR, van Overbeek LS, van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;16(10):463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Hardoim PR, Hardoim CC, van Overbeek LS, van Elsas JD. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE. 2012;7(2):e30438. doi: 10.1371/journal.pone.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kuang Z, Wang W, Cao L. Exploring potential bacterial and fungal biocontrol agents transmitted from seeds to sprouts of wheat. Biol Control. 2016;98:27–33. [Google Scholar]
- Jai P, Naveen KA. Phosphate-solubilizing Bacillus sp. enhances growth, phosphorus uptake and oil yield of Mentha arvensis L. 3 Biotech. 2019;9:126. doi: 10.1007/s13205-019-1660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha CK, Patel B, Saraf M. Stimulation of the growth of Jatropha curcas by the plant growth promoting bacterium Enterobacter cancerogenus MSA2. World J Microbiol Biotechnol. 2012;28(3):891–899. doi: 10.1007/s11274-011-0886-0. [DOI] [PubMed] [Google Scholar]
- Jiang XY, Gao JS, Xu FH, Cao YH, Tang X, Zhang XX. Diversity of endophytic bacteria in rice seeds and their secretion of indole acetic acid. Acta Microbiologica Sinica. 2013;53:269–275. [PubMed] [Google Scholar]
- Khalaf EM, Raizada MN. Taxonomic and functional diversity of cultured seed associated microbes of the cucurbit family. BMC Microbiol. 2016;16(1):131. doi: 10.1186/s12866-016-0743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepper JW, Beauchamp CJ. A review of issues related to measuring colonization of plant roots by bacteria. Can J Microbiol. 1992;38:1219–1232. [Google Scholar]
- Li NN, Liu Y, Zhao R, Wang RH, Xiao M, Zhao JR, Cheng C. Diversity of endophytic bacteria in beijing high-quality hybrid maize (Zea mays L.) seed. J Food Sci Technol. 2016;34(5):55–63. [Google Scholar]
- Liu L, Liu Y, Song W. Indigenous bacterial community diversity in hybrid rice (Oryza sativa L.) seed. Biotechnol Bull. 2009;1:95–111. [Google Scholar]
- Liu Y, Zuo S, Xu LW, Zou YY, Song W. Diversity of endophytic bacterial communities in seeds of hybrid maize (Zea mays L., Nongda108) and their parental lines. Scientia Agricultura Sinica. 2011;44(23):4763–4771. [Google Scholar]
- Liu Y, Zuo S, Xu LW, Zou YY, Song W. Study on diversity of endophytic bacterial communities in seeds of hybrid maize and their parental lines. Arch Microbiol. 2012;194:1001–1012. doi: 10.1007/s00203-012-0836-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zuo S, Zou YY, Wang JH, Song W. Investigation on diversity and population succession dynamics of indigenous bacteria of the maize spermosphere. World J Microbiol Biotechnol. 2012;28:391–396. doi: 10.1007/s11274-011-0822-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zuo S, Zou YY, Wang JH, Song W. Investigation on diversity and population succession dynamics of endophytic bacteria from seeds of maize (Zea mays L., Nongda108) at different growth stages. Ann Microbiol. 2013;63:71–79. [Google Scholar]
- Liu Y, Yao S, Xu PP, Cao YH, Li JX, Wang JM, Tan WQ, Cheng C. Composition and diversity of endophytic bacterial communities in noni (Morinda citrifolia L.) seeds. Int J Agric Pol Res. 2014;2(3):98–104. [Google Scholar]
- Liu Y, Zhai L, Wang RH, Zhao R, Zhang X, Chen CY, Cao Y, Cao YH, Xu TJ, Ge YY, Zhao JR, Cheng C. Paenibacillus zeae sp. nov., isolated from maize (Zea mays L.) seeds. Int J Syst Evol Microbiol. 2015;65:4533–4538. doi: 10.1099/ijsem.0.000608. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang RH, Cao YH, Chen CY, Bai FR, Xu TJ, Zhao R, Zhang X, Zhao JR, Cheng C. Identification and antagonistic activity of endophytic bacterial strain Paenibacillus sp. 5L8 isolated from the seeds of maize (Zea mays L., Jingke 968) Ann Microbiol. 2016;66:653–660. [Google Scholar]
- Liu Y, Zhao R, Wang RH, Yao S, Zhai L, Zhang X, Chen CY, Cao YH, Xu TJ, Ge YY, Zhao JR, Cheng C. Paenibacillus chinensis sp. nov., isolated from maize (Zea mays L.) seeds. Antonie Van Leeuwenhoek. 2016;109:207–213. doi: 10.1007/s10482-015-0622-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li NN, Eom MK, Schumann P, Zhang X, Cao YH, Ge YY, Xiao M, Zhao JR, Cheng C, Kim SG. Bacillus ciccensis sp. nov., isolated from maize (Zea mays L.) seeds. Int J Syst Evol Microbiol. 2017;67:4606–4611. doi: 10.1099/ijsem.0.002341. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang RH, Li YH, Cao YH, Chen CY, Qiu CZ, Bai FR, Xu TJ, Zhang X, Dai WK, Zhao JR, Cheng C. High-throughput sequencing-based analysis of the composition and diversity of endophytic bacterial community in seeds of hybrid maize planted in China. Plant Growth Regul. 2017;81:317–324. [Google Scholar]
- Liu Y, Xu PP, Yang FZ, Li M, Yan H, Li N, Zhang XX, Wang WP. Composition and diversity of endophytic bacterial community in seeds of super hybrid rice ‘Shenliangyou 5814’ (Oryza sativa L.) and its parental lines. Plant Growth Regul. 2018 doi: 10.1007/s10725-018-0467-4. [DOI] [Google Scholar]
- Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Ri TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Morisaki H. Endophytic bacteria in the rice plant. Microbes Environ. 2008;23(2):109–117. doi: 10.1264/jsme2.23.109. [DOI] [PubMed] [Google Scholar]
- Mantica P, Tala T, Ferreira JS, Peeters AG, Salmi A, Strintzi D, Weiland J, Brix M, Giroud C, Corrigan G, Zastrow KD, Naulin V, Tardini G. Perturbative studies of toroidal momentum transport using neutral beam injection modulation in the Joint European Torus: experimental results, analysis methodology, and first principles modeling. Phys Plasmas. 2010;17(9):112–134. [Google Scholar]
- Mastretta C, Taghavi S, van der Lelie D, Mengoni A, Galardi F, Gonnelli C, Braca T, Boulet J, Weyens N, Vangronsveld J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int J Phytoremediation. 2009;11:251–267. [Google Scholar]
- Mehmood A, Hussain A, Irshad M, Hamayun M, Iqbal A, Rahman H, Tawab A, Ahmad A, Ayaz S. Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant Physiol Biochem. 2018;135:61–68. doi: 10.1016/j.plaphy.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Nelson EB. Microbial dynamics and interactions in the spermosphere. Annu Rev Phytipathol. 2004;42:271–309. doi: 10.1146/annurev.phyto.42.121603.131041. [DOI] [PubMed] [Google Scholar]
- Nelson EB. The seed microbiome: origins, interactions, and impacts. Plant Soil. 2017;422:7–34. [Google Scholar]
- Picard C, Bosco M. Heterozygosis drives maize hybrids to select elite 2, 4-diacethylphloroglucinol-producing Pseudomonas strains among resident soil populations. FEMS Microbiol Ecol. 2006;58(2):193–204. doi: 10.1111/j.1574-6941.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- Qin Y, Pan X, Yuan Z. Seed endophytic microbiota in a coastal plant and phytobeneficial properties of the fungus Cladosporium cladosporioides. Fungal Ecol. 2016;24:53–60. [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro VP, Marriel IE, Sousa SM, Lana UGP, Mattos BB, Oliveira CA, Gomes EA. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz J Microbiol. 2018;49(Suppl 1):40–46. doi: 10.1016/j.bjm.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San XC, Sun KZ, Liu JB. Cultivation of hybrid maize, Zhengdan 958. Mod Agric Sci Technol. 2007;2:73. [Google Scholar]
- Santamaría J, Toranzos GA. Enteric pathogens and soil: a short review. Int Microbiol. 2003;6(1):5–9. doi: 10.1007/s10123-003-0096-1. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE. 2011;6(12):e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade A, Jacques MA, Barret M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr Opin Microbiol. 2017;37:15–22. doi: 10.1016/j.mib.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Simon HM, Smith KP, Dodsworth JA, Guenthner B, Handelsman J, Goodman RM. Influence of tomato genotype on growth of inoculated and indigenous bacteria in the spermosphere. Appl Environ Microbiol. 2001;67(2):514–520. doi: 10.1128/AEM.67.2.514-520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolev VS, Orner VA, Arias RS. Distribution of bacterial endophytes in peanut seeds obtained from axenic and control plant material under field conditions. Plant Soil. 2013;371:367–376. [Google Scholar]
- Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci USA. 2006;103(32):12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HC, Wan JH, Niu YF. Breeding and popularization of maize variety Yuyu 23 with high-yield, good-quality, and multiresistant. J Maize Sci. 2005;13:95–96. [Google Scholar]
- Thulasi K, Jayakumar A, Balakrishna Pillai A, Gopalakrishnapillai Sankaramangalam VK, Kumarapillai H. Efficient methanol-degrading aerobic bacteria isolated from a wetland ecosystem. Arch Microbiol. 2018;200(5):829–833. doi: 10.1007/s00203-018-1509-z. [DOI] [PubMed] [Google Scholar]
- Truyens S, Weyens N, Cuypers A, Vangronsveld J. Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ Microbiol Rep. 2015;7:40–50. [Google Scholar]
- Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A. The importance of the microbiome of the plant holobiont. New Phytol. 2015;206:1196–1206. doi: 10.1111/nph.13312. [DOI] [PubMed] [Google Scholar]
- Verma SK, White JF. Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in browntop millet (Urochloa ramosa L.) J Appl Microbiol. 2018;124(3):764–778. doi: 10.1111/jam.13673. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Ji YL, Chen YG. Studies and biological significances of plant endophytes. Microbiol China. 2015;42(2):349–363. [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4):e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win KT, Tanaka F, Okazaki K, Ohwaki Y. The ACC deaminase expressing endophyte Pseudomonas spp. Enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol Biochem. 2018;127:599–607. doi: 10.1016/j.plaphy.2018.04.038. [DOI] [PubMed] [Google Scholar]
- Yang L, Danzberger J, Schöler A, Schröder P, Schloter M, Radl V. Dominant groups of potentially active bacteria shared by barley seeds become less abundant in root associated microbiome. Front Plant Sci. 2017;8:1005. doi: 10.3389/fpls.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang CW, Yang J, Zhang RJ, Gao JS, Zhao X, Zhao JJ, Zhao DF, Zhang XX. Insights into endophytic bacterial community structures of seeds among various Oryza sativa L. rice genotypes. J Plant Growth Regul. 2018 doi: 10.1007/s00344-018-9812-0. [DOI] [Google Scholar]
- Zhao JR, Wang RH. Development process, problem and countermeasure of maize production in China. J Agric Sci Technol. 2013;15(3):1–6. [Google Scholar]
- Zou YY, Liu L, Liu Y, Zhao L, Deng QY, Wu J, Zhuang W, Song W. Diversity of indigenous bacterial communities in Oryza sativa seeds of different varieties. Chin J Plant Ecol. 2012;36:880–890. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.