Abstract
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate approved for use in the treatment of human epidermal growth factor receptor 2-positive metastatic breast cancer. Here, we present the cases of two patients with metastatic breast cancer who received T-DM1 monotherapy and developed noncirrhotic portal hypertension (NCPH). Patient 1 presented with ruptured gastric varices at 2 years and 5 months after T-DM1 treatment. Patient 2 presented with intrahepatic portal-hepatic venous shunt at 2 years and 6 months and portal-systemic shunt encephalopathy at 4 years and 11 months after T-DM1 treatment. In both the patients, liver biopsies revealed sinusoidal obstruction syndrome (SOS). T-DM1-induced hepatotoxicity can result from SOS. In long-term administration of T-DM1 the unfavorable events associated with chronic liver circulatory disorder due to SOS, such as NCPH, are concerning.
Keywords: Trastuzumab emtansine, Sinusoidal obstruction syndrome, Breast cancer, Portal hypertension, Gastric varices, Portal-systemic shunt
Introduction
The antibody–drug conjugate trastuzumab emtansine (T-DM1) is approved for use in the treatment of human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. T-DM1 comprises three components: trastuzumab (Tmab), HER2 monoclonal antibody; thioether linker; and maytansinoid [derivative maytansine 1 (DM1)]. They are designed to selectively deliver cytotoxic DM1 to tumor cells where HER2 is overexpressed on the cell surface, thereby minimizing systemic toxicity. DM1 is a potent microtubule polymerization inhibitor that induces mitotic arrest and kills tumor cells at the subnanomolar concentration [1]. Recently, a large phase III open label study of trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer [EMILIA] reported an improved progression-free and overall survival with T-DM1 compared with the combination treatment of lapatinib and capecitabine. In this study, the T-DM1 arm experienced adverse events of elevated aspartate aminotransferase (AST) levels of 22.4%, elevated alanine aminotransferase levels of 16.9%, and thrombocytopenia of 28.0%, on the other hand demonstrated that T-DM1 is well tolerated and can improve patients’ quality of life [2]. However, the safety of long-term administration is unknown. Herein we present the cases of two patients with noncirrhotic portal hypertension (NCPH) due to sinusoidal obstruction syndrome (SOS) believed to be caused by T-DM1.
Presentation of case
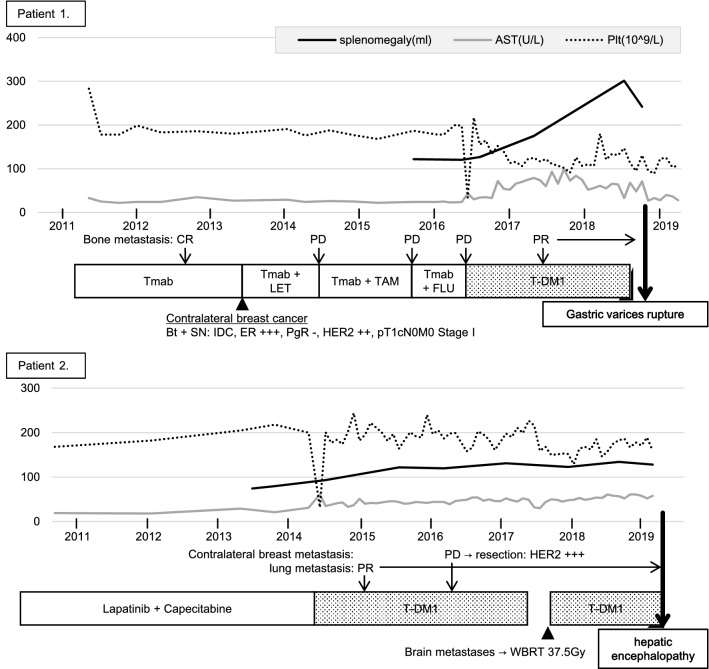
Patient 1 was a 68-year-old woman who underwent mastectomy for the right breast cancer in 1993 and was diagnosed with bone metastasis and chest wall recurrence at our hospital in 2011. Core needle biopsy from the chest wall recurrence was performed, and an estrogen receptor (ER)-negative, progesterone receptor (PgR)-negative, and HER2-positive invasive ductal carcinoma was diagnosed. The patient underwent multiple systemic therapies until June 2016 (Fig. 1). Subsequently, 3.6 mg/kg of T-DM1 was intravenously administered once every 3 weeks for the occurrence of new bone metastasis. Positron emission tomography-computed tomography (PET-CT) was consistently performed to evaluate the activity of bone lesions, which revealed a normal-appearing liver with no evidence of portal hypertension just before T-DM1 administration (Fig. 2a). T-DM1 treatment was continued until August 2018. During this time, laboratory examinations had been showing mild chronic elevation of AST and reduction of platelet count (Fig. 1); however, these findings were was not enough to withdraw the treatment.
Fig. 1.
Clinical course of patients 1 and 2. Tmab trastuzumab, LET letrozole, TAM tamoxifen, FLU fulvestrant, T-DM1 trastuzumab emtansine, ER estrogen receptor, PgR progesterone receptor, HER2 human epidermal growth factor receptor 2, IDC invasive ductal carcinoma, WBRT whole-brain radiotherapy, CR complete response, PR partial response, PD progressive disease
Fig. 2.
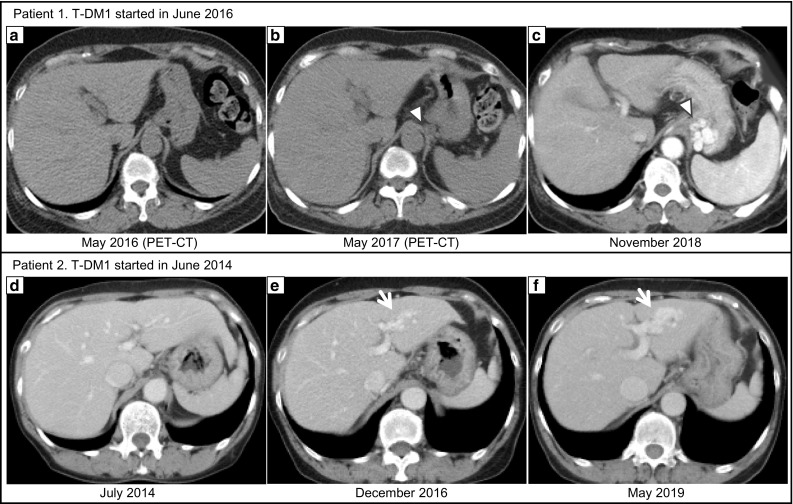
In patient 1, PET-CT performed just before T-DM1 administration revealed a normal-appearing liver with no evidence of portal hypertension (a). At 2 years and 5 months after initiating T-DM1 treatment, when gastric varices ruptured, splenomegaly and gastroesophageal varices were suggested (c arrowhead). Retrospectively, the development of the collateral (b: arrowhead) and splenomegaly was confirmed at 1 year after initiating T-DM1 treatment. In patient 2, at 2 years and 6 months after initiating T-DM1 treatment, the intrahepatic portal-hepatic venous shunt was pointed out, which was gradually growing (e, f: arrow)
In November 2018, hematemesis occurred, contrast-enhanced CT revealed splenomegaly and gastrorenal shunt (Fig. 2c), and upper gastrointestinal endoscopy revealed esophageal varices with Form 1 and gastric varices with Form 3 and red plug. Endoscopic variceal ligation of the ruptured gastric varices and balloon-occluded retrograde transvenous obliteration were emergently performed with 5% ethanolamine oleate and iopamidol. The hematemesis elapsed without rebleeding, and gastric varices were confirmed to be reduced. Serologic tests for chronic hepatitis were negative, such as for virus, antinuclear antibody, antimitochondrial antibody, immunoglobulins, and ceruloplasmin. The liver biopsy revealed sinusoidal dilatation and congestion with collagen fiber growth. In the portal vein area, interface hepatitis with lymphocyte infiltration was observed, but cirrhosis was not observed. Silver impregnation stain revealed hepatic plate disturbances (Fig. 3a–d). These findings suggested drug-induced chronic hepatitis, and the patient was diagnosed with SOS. Retrospectively, splenomegaly (≥ 50%) occurred approximately 12 months after initiating T-DM1 treatment, with development of collaterals (Fig. 2b). Eight months after the termination of T-DM1 treatment, the spleen size and AST levels tended to improve.
Fig. 3.
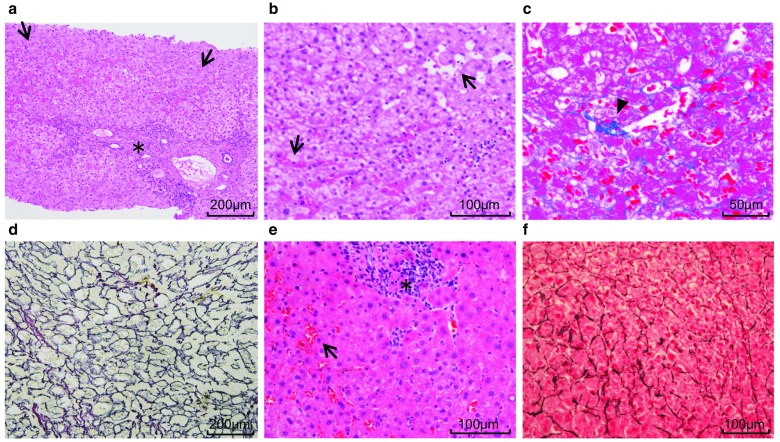
In both the patients 1 (a–d) and 2 (e, f), histopathological examination revealed interface hepatitis with lymphocyte infiltration in the portal vein area (asterisk) and sinusoidal dilatation and congestion (arrow). There were no cirrhosis findings. In patient 1, growth of collagen fibers with sinusoidal dilatation (arrowhead) was also observed. Silver impregnation staining (d, f) revealed hepatic plate disturbances
Patient 2 was a 69-year-old woman who was initially diagnosed with ER-negative, PgR-negative, and HER2-positive invasive ductal carcinoma of the left breast in 2009. She underwent neoadjuvant chemotherapy with FEC (fluorouracil, epirubicin, and cyclophosphamide), followed by Tmab + docetaxel. After a total mastectomy, she underwent adjuvant radiotherapy and Tmab sequentially. Subsequently, she was diagnosed with brain metastasis in 2010. Tumor resection was performed, and chemotherapy with lapatinib + capecitabine was administered until January 2014. The patient’s treatment regimen was changed to T-DM1 (3.6 mg/kg intravenously once every 3 weeks) in June 2014 because of the occurrence of contralateral breast and lung metastasis. Although she had maintained stable disease after the initiation of T-DM1 treatment, laboratory examinations had been showing mild chronic elevation of AST and reduction of platelets over time (Fig. 1), and an intrahepatic portal-hepatic venous shunt was pointed out in the surveillance CT in December 2016 (Fig. 2e). She was otherwise asymptomatic and thus, the treatment was not withdrawn.
However, in May 2019, the patient was urgently hospitalized for consciousness disorder; contrast-enhanced CT revealed a growth of intrahepatic portal-hepatic venous shunt (Fig. 2f). She was diagnosed with hepatic encephalopathy because of hyperammonemia (188 μg/dL) and the absence of other causes. Her consciousness was immediately normalized by branched chain amino acids supplementation but hyperammonemia persisted. Lactulose and levocarnitine medications were initiated with protein intake control. Serologic tests for chronic hepatitis were negative. The serum hyaluronic acid (HA) level was 818 ng/mL, which was abnormally high. The liver biopsy revealed sinusoidal dilatation and congestion, but growth of collagen fibers along with sinusoid or centrilobular vein was unclear in the Masson trichrome staining. Silver impregnation staining revealed hepatic plate disturbance (Fig. 3e–f). These findings were consistent with those of SOS; however, a definitive diagnosis was not made. Cirrhosis was not observed, and chronic portal-systemic shunt encephalopathy was presumed.
Discussion
In patients 1 and 2, the absence of competing etiologies with the close temporal association between splenomegaly or portosystemic shunt and laboratory abnormalities after the initiation of T-DM1 treatment suggests that T-DM1 was directly associated with hepatotoxicity and portal hypertension. A previous issue of Journal of Clinical Oncology has reported that two patients receiving T-DM1-based combination therapy developed NCPH due to nodular regenerative hyperplasia (NRH) [3]; however, the present study showed that T-DM1 monotherapy also causes NCPH. NRH is considered as a histopathological concept coexisting with SOS, and is the most severe form of SOS [4]. SOS is considered to result from severe toxicity of the sinusoidal endothelial cells. It is histologically characterized by sinusoidal dilatation and congestion, perisinusoidal hemorrhage and fibrosis, nodular regeneration, and loss of hepatocytes. Fibrosis can also affect the centrilobular vein and contribute to liver congestion, which causes its typical bluish appearance and may result in portal hypertension or sometimes fatal acute hepatic failure [5]. Although patient 2 was not definitely diagnosed with SOS during the pathological examination of the liver biopsy, the presence of sinusoidal dilatation and congestion suggested SOS with the careful consideration of no competing etiologies. Liver biopsy samples are limited considering that they evaluate only 0.002% of the liver area [6]. A previous case report has demonstrated that SOS was diagnosed by autopsy without being diagnosed in the liver biopsy, which showed sinusoidal dilatation and congestion only [7].
Hepatotoxicity is one of the main adverse events of T-DM1. The hepatotoxicity of T-DM1 has been observed in preclinical animal toxicology studies with a characteristic range of lesions including hepatocellular degeneration/necrosis, increased mitotic figures in hepatocytes, Kupffer cells and cholangiocytes, hypertrophy/vacuolation of Kupffer cells and increased sinusoidal leukocytes with elevations in liver enzymes. In addition, T-DM1 and DM1 safety profiles were similar and consistent with the mechanism of action of DM1 (i.e., microtubule arrest) [8]. Yan et al. revealed that T-DM1, once bound to HER2, was internalized into human hepatocytes. Following the endocytosis, T-DM1 induced apoptosis and inhibited the growth of hepatocytes. Moreover, they demonstrated that necrosis and inflammation were highlighted in the mouse liver tissue after the T-DM1 treatment, whereas Tmab induced only inflammation and no necrosis [9]. There is an only one pathological study that demonstrated Tmab-induced hepatotoxity, and the liver biopsy did not reveal sinusoid abnormality [10]. Thus, among the T-DM1 components, SOS is presumed to be mainly caused by DM1. The payload DM1 is a derivative maytansine, which is a potent microtubule polymerization inhibitor that induces mitotic arrest [1]. Although the development of maytansine had been discontinued because of its high toxicity and extremely narrow therapeutic range, to our knowledge, there have been no reports of adverse events suggesting SOS [11]. The pathophysiology of drug-induced SOS is not yet completely understood; however, the involvement of reactive oxygen, depletion of sinusoidal endothelial cell glutathione, and a release of matrix metalloproteinase (MMP)-2 and MMP-9 have been suggested [12, 13]. Hepatocyte necrosis or Kupffer cell damage may activate the release of cytokines and reactive oxygen that may contribute to sinusoidal endothelial cell injury and fibrin deposition, and to the occurrence of SOS. In contrast, Gorovits proposed that the mechanism of the off-target hepatotoxicities for antibody-drug conjugates is at least in part driven by their interactions with the mannose receptor, which is a known multi-domain lectin found on the surface of hepatic sinusoids, and resulting in glycan-derived cellular uptake of antibody-drug conjugate [14]. A targeted delivery of emtansine to hepatic sinusoids, via uptake by mannose receptor, can provide an alternate explanation to the hepatotoxicity. Emtansine contains saccharides and could potentially interact with cell surface lectins, including mannose receptor, similar to calicheamicin known to cause SOS.
In our hospital, we have experienced 15 cases that received T-DM1 for metastatic breast cancer from June 2014 to May 2019. The median administration of T-DM1 period was 424.1 days. All cases showed elevated AST levels and 9 of 15 cases showed splenomegaly (≥ 50%) measured by CT volumetry. Although only the presented two cases showed unfavorable events associated with portal hypertension, it is suggested that potential chronic liver circulatory disorder due to SOS might frequently occur. SOS occurs in up to 50% of patients receiving an L-OHP-based regimen for those with metastatic colorectal cancer [5]. In the post marketing report of T-DM1 (6 months of follow up period, 271 cases), adverse events of elevated AST levels of 31.2% and liver dysfunction of 8.0% were reported. The following were observed as symptoms of portal hypertension: one case (0.4%) of gastric varices and hyperammonemia, and two cases (0.8%) of esophageal varices. One case presented ruptured esophageal gastric varices and fatal hepatic failure [15]. One possible explanation for the frequent appearance of portal hypertension symptoms in our clinical data may be the difference in administration period.
There is no biomarker to assess the development, presence, or severity of SOS. A biomarker that can prompt clinicians to adjust the chemotherapeutic regimen is warranted for the noninvasive diagnosis of SOS. Previous studies have demonstrated that the systemic HA level, splenomegaly (≥ 50%) and the AST to platelet ratio index score were significantly correlated with the severity of L-OHP-induced SOS [4, 16, 17]. HA degradation occurs almost exclusively in the sinusoidal endothelial cells, which comprised HA by receptor-mediated endocytosis. Thus, systemic HA levels are considered as a direct indicator of sinusoidal endothelial function [16]. However, systemic HA must be cautiously evaluated because it is affected in various ways, considering that HA is a major extracellular matrix component of various solid malignancies and its expression has been associated with disease progression [18]. Although systemic HA levels were abnormally high in the case of patient 2, the influence of portosystemic shunts should also be considered.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Human/animal rights statement
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Remillard S, Rebhun LI, Howie GA, et al. Antinitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189:1002–1005. doi: 10.1126/science.1241159. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Force J, Saxena R, Schneider BP, et al. Nodular regenerative hyperplasia after treatment with trastuzumab emtansine. J Clin Oncol. 2016;34:e9–e12. doi: 10.1200/JCO.2013.49.8543. [DOI] [PubMed] [Google Scholar]
- 4.Hubert C, Sempoux C, Humblet Y, et al. Sinusoidal obstruction syndrome (SOS) related to chemotherapy for colorectal liver metastases: factors predictive of severe SOS lesions and protective effect of bevacizumab. HPB. 2013;15:858–864. doi: 10.1111/hpb.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Sever hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 6.Rochy DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 7.Sakumura M, Tajiri K, Miwa S, et al. Hepatic sinusoidal obstruction syndrome induced by non-transplant chemotherapy for non-hodgkin lymphoma. Intern Med. 2017;56:395–400. doi: 10.2169/internalmedicine.56.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon KA, Flagella K, Beyer J, et al. Preclinical safety profile of trastuzumab emtansine (T-DM1): mechanism of action of its cytotoxic component retained with improved tolerability. Toxicol Appl Pharmacol. 2013;273:298–313. doi: 10.1016/j.taap.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Endo Y, Shen Y, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther. 2016;15:480–490. doi: 10.1158/1535-7163.MCT-15-0580. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Parsa V, Liu CY, et al. Trastuzumab-induced hepatotoxicity. Ann Pharmacother. 2008;42:1497–1501. doi: 10.1345/aph.1L217. [DOI] [PubMed] [Google Scholar]
- 11.Cassady JM, Chan KK, Floss HG, et al. Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull (Tokyo) 2004;52:1–26. doi: 10.1248/cpb.52.1. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology. 2000;31:428–434. doi: 10.1002/hep.510310224. [DOI] [PubMed] [Google Scholar]
- 13.Deleve LD, Wang X, Tsai J, et al. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882–890. doi: 10.1016/S0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- 14.Gorovits B, Krinos-Fiorotti C. Proposed mechanism of off-target toxicity for antibody-drug conjugates driven by mannose receptor uptake. Cancer Immunol Immunother. 2013;62:217–223. doi: 10.1007/s00262-012-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KADCYLA® 100 mg–160 mg final report of drug use surveillance (2018) Internal document. Chugai Pharmaceutical Co., Ltd.
- 16.van den Broek MA, Vreuls CP, Winstanley A, et al. Hyaluronic acid as a marker of hepatic sinusoidal obstruction syndrome secondary to oxaliplatin-based chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2013;20:1462–1469. doi: 10.1245/s10434-013-2915-8. [DOI] [PubMed] [Google Scholar]
- 17.Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 18.Lugli A, Zlobec I, Günthert U, et al. Overexpression of the receptor for hyaluronic acid mediated motility is an independent adverse prognostic factor in colorectal cancer. Mod Pathol. 2006;20:1462–1469. doi: 10.1038/modpathol.3800648. [DOI] [PubMed] [Google Scholar]