Abstract
MMR-deficient colorectal cancers (dMMR CRC) are characterized by the expression of highly-immunogenic neoantigen peptides, which stimulate lymphocytic infiltration as well as up-regulation of inflammatory cytokines. These features are key to understanding why immunotherapy (specifically PD-1 and/or CTLA-4 checkpoint blockade) has proved to be highly effective for the treatment of patients with advanced dMMR CRC. Importantly, pre-clinical studies also suggest that this correlation between potent tumor neoantigens and the immune microenvironment is present in early (pre-malignant) stages of dMMR colorectal tumorigenesis as well, even in the absence of a high somatic mutation burden. Here, we discuss recent efforts to characterize how neoantigens and the tumor immune microenvironment co-evolve throughout the dMMR adenoma-to-carcinoma pathway. We further highlight how this pre-clinical evidence forms the rational basis for developing novel immunotherapy-based CRC prevention strategies for patients with Lynch syndrome.
Keywords: Lynch syndrome, Mismatch Repair Deficiency, Neoantigens, Mutational rate, Checkpoint inhibitors, Immunoprevention, Microsatellite Instability, Colorectal cancer
Introduction
Over the past decade, DNA mismatch repair (MMR) deficiency has emerged as a critically-important biomarker with implications for the management of both early- and advanced-stage colorectal cancer (CRC) (1). Approximately 10–15% of CRC exhibit MMR deficiency, which is characterized by a propensity for accumulating single-nucleotide mutations and insertion-deletion loops (indels) in the somatic genome, particularly within short repetitive sequences such microsatellites (2,3). MMR-deficient tumors often exhibit a high mutation burden and may express neoantigens generated by frameshift mutations in coding microsatellites, such as the 10-adenine mononucleotide repeat in the TGFBR2 gene (4).
As part of the standard molecular workup for CRC, MMR deficiency can be assessed on the basis of microsatellite instability (MSI) and/or loss-of-expression of MMR proteins in bulk tumor tissue specimens (5). MMR-deficiency in the tumor is often secondary to Lynch syndrome, an autosomal dominant hereditary cancer syndrome caused by monoallelic pathogenic germline mutations in MMR pathway genes (MLH1, MSH2, MSH6, PMS2, EPCAM) (6). More frequently, MMR deficiency occurs as a sporadic (non-hereditary) process characterized by a distinctive hyper-proliferative, serrated morphology, DNA methylation abnormalities including MLH1 epigenetic silencing (CpG Island Methylator Phenotype, CIMP), and elevated frequency of activating BRAF mutations (7–9).
Altogether, dMMR CRC represents a unique molecular sub-type of this disease with distinctive histopathologic features and clinical outcomes. One of the most prominent features is the enrichment of tumor stroma with infiltrating lymphocytes, and overexpression of prostaglandins and inflammatory cytokines in dMMR tumors (10–16). This inflammatory microenvironment is thought to be driven by recognition of the high burden of tumor neoantigens on Major Histocompatibility Complex (MHC) class I alleles by the adaptive immune system (Figure 1, later stages). This model not only helps explain the favorable prognostic implications of MMR-deficiency in CRC, but also supports the rationale for immunotherapy-based treatment strategies such as with checkpoint inhibition. In this regard, pivotal examples can be found in the setting of metastatic CRC (17,18). In particular, the phase II study CheckMate-142 (clinicaltrials.gov ID NCT02060188) recently demonstrated the safety and durable efficacy of nivolumab (anti-programmed cell death protein 1 [PD-1]) given with or without low-dose ipilimumab (cytotoxic T lymphocyte associated antigen 4 [CTLA-4]) as second-line therapy for patients with advanced dMMR CRC (19,20). Similar benefit was reported by the phase II study Keynote-164 (NCT02460198) in patients treated with single-agent pembrolizumab (anti-PD-1) (19). These breakthrough results have amplified interest in the potential applications of novel immunotherapy agents not only in the adjuvant therapy setting for dMMR CRC, but also in primary prevention for patients with Lynch syndrome.
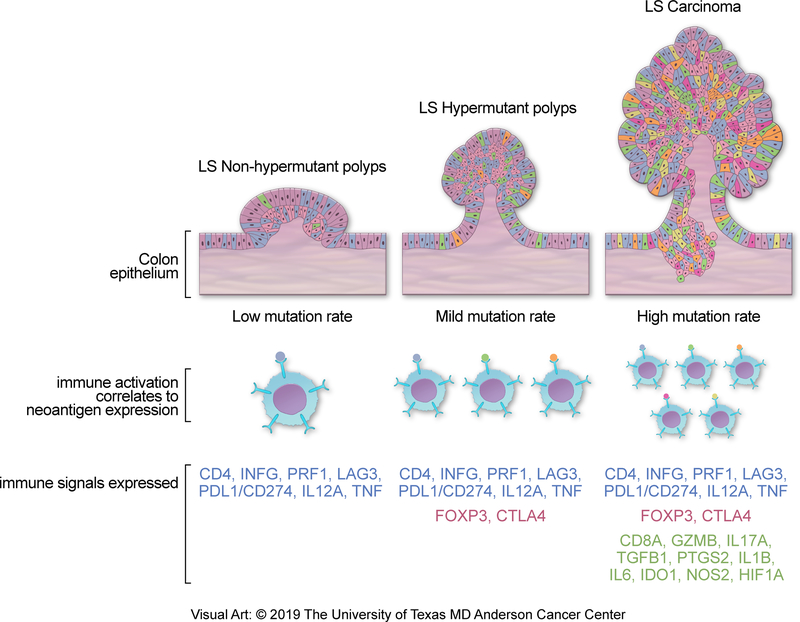
Fig. 1.
Correlation between immune activation (lower track) and neoantigen burden (middle track) along the adenoma-to-carcinoma pathway (top track) in Lynch syndrome-related CRC. The colonic epithelium is shown as confluent cells with an admixture of different colors to represent intralesional mutation and/or neoantigen diversity. Early colorectal adenomas (left column) display markers of immunoreactivity even in the absence of high somatic mutation or neoantigen burden. As the lesions progress to advanced adenomas (middle column) and carcinomas (right column), there is a corresponding rise in mutation/neoantigen burden and markers of immune tolerance. LS, Lynch syndrome. Redrawn with permission; copyright The University of Texas MD Anderson Cancer Center.
A rationale for immunotherapy-based prevention (hereafter referred to as immunoprevention) strategies in Lynch syndrome is supported by multiple lines of evidence, including the identification MMR-deficient histologically normal appearing colon crypts as the earliest definable abnormality in pre-neoplastic colorectal epithelium in Lynch syndrome (13). With respect to existing immunomodulatory agents, non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclo-oxygenase 2 (COX-2) and the downstream production of pro-tumorigenic prostaglandins that promote local inflammation. Prior work has shown that NSAIDs (21), more specifically aspirin (22,23), are associated with a modest but reliable chemopreventive benefit to reduce the risk of Lynch syndrome-related CRC (and perhaps other sites) after a continuous exposure of at least two years of duration. Recent pre-clinical work has highlighted that naproxen sodium may have greater chemopreventive efficacy than aspirin (24), although the mechanism is not yet well delineated.
Towards the goal of further improving Lynch syndrome-related cancer mortality, we propose that novel prevention strategies can be developed by elucidating the sequence of events that relate acquisition of MMR deficiency to accumulation of somatic mutations, generation of neoantigens, tumorigenesis and immune recognition, and characterizing the immune cells in the microenvironment of pre-neoplastic lesions (25). Such strategies would include novel immunomodulatory agents, tumor vaccines (26–29), and even low-dose immune checkpoint inhibitors. Importantly, given the unique challenges of drug development in the prevention setting, each strategy needs focused re-examination of the risks and benefits. For example, while anti-PD-1/PD-L1 antibodies may increase immune surveillance, they are also associated with significant rates of severe adverse events. These include immune-related lung, hepatic, skin, neurologic, gastrointestinal, and endocrine toxicities, some of which are fatal (30–44). Thus, while the risk:benefit ratio of PD-1/PD-L1 blockade is acceptable for patients with metastatic tumors and poor prognosis, it is almost certainly not acceptable in the setting of healthy asymptomatic Lynch syndrome patients for cancer prevention, where the tolerance for side effects is very low. PD-1/PD-L1 inhibitors also do not have clear dose response, which makes giving lower doses of these drugs for cancer prevention problematic (30–44).
Here, we will briefly review the molecular basis of neoantigen generation and immune activation as it pertains to MMR-deficient colorectal tumorigenesis. We focus particularly on the pre-cancer state in order to shed light on possible rationales for the development of novel immunoprevention strategies.
Functional implications of MMR Deficiency in CRC carcinogenesis
The highly conserved MMR system facilitates repair of two important types of errors that arise during DNA replication: base pair mismatches and indels (45). Base pair mismatches occur when incorrect nucleotides are inserted into the newly synthesized strand and escape the proofreading function of DNA polymerases. Indel loops usually arise in the context of microsatellites, which are highly polymorphic short repetitive DNA sequences found throughout both prokaryotic and eukaryotic genomes (46–48). At microsatellites, the template and primer strands are prone to slippage (i.e. dissociation and re-annealing) during replication. This generates a loop structure and, most importantly, a discordant number of repeated units between the template and newly synthesized strand (49). In humans, the repair process begins with binding of the MSH2/MSH6 heterodimer to the DNA defect. This is followed by recruitment of the MLH1/PMS2 heterodimer, formation of a sliding clamp structure, and then activation of exonuclease 1 (EXO1) to remove the error-laden DNA segment. The resulting gap is filled in by DNA polymerases, PCNA and ligases (45). Failure to repair base mismatches or indels leads to propagation of single-nucleotide mutations or MSI, respectively.
The mutagenic process described above is observable not only at the population level, but also within specific individuals, particularly in the context of acquired MMR deficiency. In the case of CRC, MMR-deficiency may occur as an entirely sporadic process due to aberrant hypermethylation of MLH1 in the tumor, commonly associated with the BRAF V600E mutation. However, the hereditary counterpart of this process is Lynch syndrome, which affects more than 1.1 million people in the Unites States, serves as a disease model in which to understand the relevance of MMR deficiency across many cancer types (6). Deleterious germline mutations in MLH1, MSH2, MSH6, PMS2, or EPCAM render affected individuals with only one functional allele of the respective gene. This is accompanied by somatic inactivation of the second allele (e.g. through mutation or deletion) and thus a predisposition to developing MMR-deficient neoplasms. In general, Lynch syndrome is associated with higher life-time risks of not only colorectal cancer, but also endometrial, ovarian, gastric, small bowel, and urothelial cancers (6).
To place the MMR deficiency phenotype in context, it is helpful to consider it within the mutational ‘signature’ framework (50,51). Recent mutational profiling efforts have revealed at least three different signatures among pre-cancer lesions (adenomas) and carcinomas of the colorectum (52). The most common of these is ‘Signature 1b’, which is characterized by C>T transitions that are produced after spontaneous deamination of 5-methyl-cytosine. This signature is thought to represent a cumulative and age-related process, as it is also observed in histologically “normal” colonic mucosa that juxtaposes carcinomas with an intact MMR system (52). By contrast, sporadic and hereditary MMR deficiency are associated with ‘Signature 6’, in which tumors accumulate C>T transitions preferentially at NpCpG loci and indels at microsatellites. Colorectal tumors and pre-cancers with this signature are enriched for mutations in BRAF V600E (and under-enriched for alterations in APC, KRAS, and TP53) (53). ‘Signature 6’ is often referred to by the synonymous term “hypermutator”, which denotes a high tumor mutation burden (TMB) that is conventionally defined as more than 10 mutations per megabase (Mb) when measured by whole exome sequencing (54,55). In a related way, ‘Signature 10’ is observed in CRC tumors that harbor error-prone DNA polymerase activity due to somatic inactivation of POLE or germline defects in POLE or POLD1 (56). Signature 10 is often termed the ‘ultramutator’ phenotype, with a mutation rate on the order of 150 mutations per Mb (56).
We and others propose that the biological and clinical implications of MMR deficiency for immunoprevention are best understood not only with respect to overall mutation burden, but perhaps more importantly with respect to the specific functional impacts of MSI. A comprehensive analysis by Hause et al. demonstrated that MSI can be detected in the exomes of multiple different tumor types, albeit with varying frequency and affected loci (57). The study identified a common set of microsatellites, such as those in the coding regions of NIPBL, TCF4, and PTEN, that showed instability across many different tumor types (57). Yet, other microsatellite loci showed instability in only a specific tumor type, thus forming an instability signature. This work builds on prior efforts over the past twenty years to catalogue instable microsatellites that occur in colorectal adenomas and established carcinomas. For example, MSI has been detected in the coding regions of known oncogenes and tumor suppressor genes such as TGFβRII, BAX, MSH3, MSH6, IGFIIR and MRE11A (58–63), as well as others associated with immune surveillance and B2M (which is part of the neoantigen presentation machinery) in particular (64). A large set of intergenic (non-coding) microsatellite targets has also been identified (65).
MMR-deficiency not only promotes the development and progression of CRC, but also contributes to the generation of tumor-specific neoantigens. Specifically, neoantigens are created when indels arise within coding microsatellites, leading to an erroneous reading frame (15). Upon translation, the frame-shifted peptide is often truncated and non-functional. In addition to altering downstream functions of the protein, the frameshift creates a new and foreign-appearing amino acid sequence that serves as a substrate for antigen processing and presentation via MHC class I and class II (27,29,66).
Taken together, these findings have contributed to the rational basis for novel primary and/or secondary immunoprevention strategies for dMMR CRC. In particular, while colorectal adenomas may lack the high TMB typically found at later stages of MMR-deficient tumorigenesis, the presence of robust and tissue-specific neoantigens indicates an opportunity to leverage the immune microenvironment to block progression into carcinomas.
Neoantigen-mediated Immune Activation in MMR-deficient Colorectal Tissue
Lynch syndrome serves as a disease model in which to understand how MMR deficiency and the immune microenvironment co-evolve during tumorigenesis. At the earliest stage, comprehensive work by Kloor et al (13) and Shia et al (67) showed that MMR-deficiency is present amongst a large proportion of non-neoplastic intestinal crypts in patients with Lynch syndrome. This observation, which is based on loss of MMR protein staining, may be explained by clonal expansion of histologically-normal appearing crypts that acquired inactivating mutations in the remaining MMR gene allele. Furthermore, CD8+ intra-epithelial lymphocytes were more abundant in these affected crypts, suggesting recognition of microsatellite-derived neoantigens in the normal crypt cells (68). Although such a hypothesis has not been definitively tested in experimental models, striking evidence comes from the observation that neoantigen-specific T cells and antibodies can be detected in the peripheral blood of Lynch syndrome without malignancy (which is more pronounced in patients with advanced MMR-deficient tumors that have higher TMB) (69,70).
Whether MMR-deficient intestinal crypts give rise to some, or all, MMR-deficient adenomas and carcinomas remains a subject of debate (15,67,68,71), as some data suggests that MMR-deficiency can also appear at a later step in tumorigenesis (72). Addressing this question has important implications for CRC prevention in Lynch syndrome. In particular, the prevalence of pre-cancers (particularly adenomas) in Lynch syndrome is age- and gene mutation-dependent and ranges from 10.6% to 33% (73,74), but only around 50% of these adenomas display MMR-deficiency (75,76). By contrast, histologically-normal crypts with MMR-deficiency are relatively abundant in the mucosa of healthy Lynch syndrome patients. This discrepancy raises the possibilities that either MMR-deficient adenomas develop from a different precursor lesion, or that a significant number of MMR-deficient crypts undergo “immunoediting” prior to transforming into adenomas.
Immunoediting is the process by which aberrant cell growth is halted and regressed by T-cell mediated immunity (77–79). In cases where the lesion is not fully eradicated, immunoediting is followed by equilibrium and ultimately immune escape phases, where the remaining cells are able to evade detection by the immune system. It is therefore important to understand which intrinsic or extrinsic factors permit the formation of MMR-deficient adenomas despite early immune engagement. Notably, MMR-deficient adenomas tend to harbor significantly fewer mutations compared to carcinomas (80,81) and yet infiltrating T-cells directed against microsatellite-derived neoantigens are detectable at this stage as well (Figure 1, pre-cancer stage) (70,82). These observations suggested that neither having a low mutation burden nor a relatively low abundance of neoantigens fully explains immune evasion in colorectal pre-cancers. Indeed, recent work by our group provided evidence of a robust immune activation signature in Lynch syndrome adenomas regardless of their mutation burden (81). By further characterizing the immune signature of Lynch syndrome adenomas, we also revealed global enrichment for CD4+ T cells and enrichment for FOXP3+ regulatory T cells in the subset with high mutation burdens (Figure 1, advanced pre-cancer stage). Additionally, there was up-regulation of both pro-inflammatory cytokines (IL12A) and checkpoint blockade (IFNG, CD274/PD1 and, LAG3).
These findings correlate well with the known biology and clinical significance of immune activation in carcinomas. A high density of CD3+ cells in CRC is associated with longer cancer-specific survival (12,83,84). Similarly, the presence of CD45RO, CD8+ and CD4+ cells is associated with lower rates of metastasis, vascular or perineural invasion respectively (85). On the contrary, the presence of FOXP3-positive regulatory T cells in normal mucosa of patients with CRC portends a poorer prognosis (85).
Perhaps the best correlation may be found in the setting of advanced dMMR CRC, where treatment with single or dual-checkpoint blockade now plays a pivotal role. Recent work by Turajlic et al. showed up-regulation of multiple checkpoints in CRC tumors, including PD-1, CTLA-4, and lymphocyte activation gene 3 (LAG-3) (86–89). As noted above, checkpoint blockade is effective for a plurality of patients with advanced dMMR CRC (response rates 30–40%) and leads to durable disease control (19,20).
Opportunities and Challenges for Novel Immunoprevention Strategies
Based on the evidence outlined above, at least two novel strategies for the prevention of Lynch syndrome-related CRC are currently under investigation (Table 1). First, the implication of adaptive immune resistance (PD-1, LAG3, CTLA-4) in MMR-deficient colorectal adenomas (81) raises a key question of whether checkpoint blockade could halt the progression of such adenomas into carcinomas. The complete spectrum of factors that regulate the adaptive immune response in adenomas is yet unknown. However, given the availability of inhibitors already on the drug market and known efficacy for patients with advanced MMR-deficient CRC, the PD-1/PD-L1 axis is an especially completing target. Across multiple disease settings and cancer types, the safety profile of single- or dual-checkpoint blockade is relatively well established, as are common practice guidelines for management of immune-mediated toxicities (90). Nonetheless, clearly the safety and efficacy of such agents in the preventative setting requires thorough and specific evaluation. Towards this end, a phase II single-arm study was recently opened in which adults with Lynch syndrome with MLH1 and MSH2 germline mutations (and therefore with maximum life-time risks for colorectal cancer development) and a history of partial colectomy due to advanced adenomas or CRC will receive nivolumab infusion every 3 months for up to 8 doses (clinicaltrials.gov ID NCT03631641) (84). As a secondary prevention study, its primary objective is to determine the incidence of secondary adenomas and CRC among Lynch syndrome patients treated with anti-PD-1.
Table 1.
Immunoprevention studies for Lynch syndrome-related and/or sporadic dMMR CRC.
| Study ID | Study type / design | Location of study | Population | Intervention | Primary endpoint(s) | Status |
|---|---|---|---|---|---|---|
| NCT01461148 | Phase I/II | Germany | Adults (18 year and older) Surgically resected stage III and IV colorectal cancers with MSI-H |
Biological FSP peptides TAF1B(−1), HT001(−1) and AIM2(−1) weekly for 4 consecutive weeks and repeated every four weeks up to a total of 3 cycles. |
Safety Immunogenicity |
Completed |
| NCT0363164 | Phase II open-label, single-arm | Ohio, United States Multi-site |
Adults (18 years or older) Lynch syndrome secondary to germline MLH1 or MSH2 mutation History of hemicolectomy for advanced adenoma or CRC |
Checkpoint blockade: nivolumab every 3 months for up to 8 cycles | Incidence rate of adenomas, high-risk adenomas, CRC, and non-CRC at 3 years | Active (recruiting) |
| NOUS-209 | Phase I | Rome, Italy Multi-site |
Adults (18 years and older) Stage IV MSI-H tumors |
Checkpoint blockade combined with Vaccine of virally-encoded library of 209 neoantigen peptides shared in MSI tumors | Safety Immunogenicity |
Not yet active (planned 2019–2020) |
| NCT01885702 | Phase I/II open-label, multi-arm | Nijmegen, Gelderland, Netherlands | Adults (18 years or older) with Lynch syndrome and no history of CRC; or adults with a history of MSI CRC | Vaccine: monocyte-derived peptide-loaded dendritic cells targeting MSI-specific neoantigens and tumor-associated antigen carcinoembryonic antigen (CEA) | Safety | Active (not recruiting) |
| OncoPeptVAC | Pre-clinical | India | Adults with Lynch syndrome secondary to germline MLH1 mutation | In silico prediction of tumor-derived neoantigen peptides | Immunogenicity | Completed |
Second, anti-cancer vaccines hold significant promise not only in hereditary colorectal cancer, but in other solid tumors as well (91). For patients with Lynch syndrome, instability within coding and non-coding microsatellites yields a robust signature of tumor/tissue-specific neoantigens that may be targeted by pre-designed vaccine libraries. In fact, this concept started to be explored in early 2000 triggered by meticulous efforts to catalogue the presence of instability in coding microsatellites using computational approaches coupled with labor intensive validation via PCR-based methods (92) that led to early-phase clinical trials using peptides identified as immunogenic. This approach has now recovered interest thanks to the development of improved pipelines for neoantigen identification that also incorporates immunogenicity predictions for both HLA-I and HLA-II presentation and the access to a wealth of genomic information from tumors (93–96). An example is the recent report from Scarselli and colleagues at NousCom (Rome, Italy) on the identification of 209 frameshift peptide neoantigens shared across colorectal, gastric, and endometrial MSI tumors. Using a viral vector-based delivery system, the investigators observed strong immunogenicity of vaccine in mouse models (26,97). These efforts are resulting in upcoming phase I clinical trials that are awaiting implementation and development in the following months (26,77,98,99). The results of these investigations will be critical for defining the technical feasibility and safety of preventative vaccines for patients with Lynch syndrome.
Conclusion
The up-regulation of immune checkpoints in Lynch syndrome-associated pre-cancers despite a relatively low mutation burden suggests that neoantigen peptides are potent targets. Checkpoint blockade in the adjuvant setting may prove to be highly effective for secondary prevention in patients with Lynch syndrome or sporadic MMR deficient CRCs. However, the benefit-to-risk ratio will need to be clarified given the adverse events associated with PD-1 blockade. Neoantigen vaccination is another approach that is being used for advanced melanoma, glioblastoma and other cancers, and repurposing this approach for primary prevention of MMR deficient cancers in Lynch syndrome patients may be promising. We propose that there is also a rationale for combining vaccine therapy and checkpoint blockade under the hypothesis that a more specific and durable response could be generated to prevent malignant transformation of adenomas, thereby reducing risk of CRC recurrence and increasing cancer-specific survival.
Acknowledgments
This work was supported by grant Research Training in Academic Medical Oncology T32 Award CA009666-23 (U.S. National Institutes of Health/National Cancer Institute) to J. Willis.; Cancer Prevention Educational Award R25T CA057730 (U.S. National Institutes of Health/National Cancer Institute) to K. Chang.; R01 CA219463 (US National Institutes of Health/National Cancer Institute) and a gift from the Feinberg Family to E. Vilar.; U01 CA233056 (US National Institutes of Health/National Cancer Institute) to S. Lipkin and E. Vilar.; and P30 CA016672 (US National Institutes of Health/National Cancer Institute) to the University of Texas Anderson Cancer Center Core Support Grant.
Abbreviations
- CRC
Colorectal cancer
- MMR
Mismatch Repair
- indels
insertion-deletion loops
- NGS
Next-Generation Sequencing
- MCH
Major histocompatibility complex
- CTL4
T lymphocyte associated antigen 4
- PD1
Programmed Cell Death molecule 1
- LAG3
Lymphocyte Activation Gene 3
Footnotes
Conflict of Interest Statement: Dr. Vilar has a consulting role with Janssen Research and Development. No other disclosures are reported.
References
- 1.Sinicrope FA. Lynch Syndrome–Associated Colorectal Cancer. New England Journal of Medicine 2018;379(8):764–73. [DOI] [PubMed] [Google Scholar]
- 2.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363(6429):558–61. [DOI] [PubMed] [Google Scholar]
- 3.Goel A, Arnold CN, Niedzwiecki D, Chang DK, Ricciardiello L, Carethers JM, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer research 2003;63(7):1608–14. [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013;45(10):1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov 2013;3(5):502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nature reviews Cancer 2015;15(3):181–94. [DOI] [PubMed] [Google Scholar]
- 7.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol 2010;7(3):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131(5):1400–7. [DOI] [PubMed] [Google Scholar]
- 9.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38(7):787–93. [DOI] [PubMed] [Google Scholar]
- 10.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001;91(12):2417–22. [PubMed] [Google Scholar]
- 11.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, et al. Tumor-Infiltrating Lymphocytes, Crohn’s-Like Lymphoid Reaction, and Survival From Colorectal Cancer. J Natl Cancer Inst 2016;108(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloor M, Huth C, Voigt AY, Benner A, Schirmacher P, von Knebel Doeberitz M, et al. Prevalence of mismatch repair-deficient crypt foci in Lynch syndrome: a pathological study. Lancet Oncol 2012;13(6):598–606. [DOI] [PubMed] [Google Scholar]
- 14.Chan TA. Prostaglandins and the colon cancer connection. Trends Mol Med 2006;12(6):240–4. [DOI] [PubMed] [Google Scholar]
- 15.Kloor M, von Knebel Doeberitz M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer 2016;2(3):121–33. [DOI] [PubMed] [Google Scholar]
- 16.Kloor M, von Knebel Doeberitz M. The Immune Biology of Microsatellite Unstable Cancer In: Valle L, Gruber SB, Capellá G, editors. Hereditary Colorectal Cancer: Genetic Basis and Clinical Implications. Cham: Springer International Publishing; 2018. p 367–84. [Google Scholar]
- 17.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overman MJ, Lonardi S, Wong KYM, Lenz H-J, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair–Deficient/Microsatellite Instability–High Metastatic Colorectal Cancer. Journal of Clinical Oncology 2018;36(8):773–9. [DOI] [PubMed] [Google Scholar]
- 20.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz H-J, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. The Lancet Oncology 2017;18(9):1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012;379(9826):1602–12. [DOI] [PubMed] [Google Scholar]
- 22.Burn J, Bishop DT, Mecklin JP, Macrae F, Moslein G, Olschwang S, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med 2008;359(24):2567–78. [DOI] [PubMed] [Google Scholar]
- 23.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378(9809):2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Lopez J, Gasparini P, Coombes K, Croce CM, Boivin GP, Fishel R. Mutation of TGFbeta-RII eliminates NSAID cancer chemoprevention. Oncotarget 2018;9(16):12554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spira A, Yurgelun MB, Alexandrov L, Rao A, Bejar R, Polyak K, et al. Precancer Atlas to Drive Precision Prevention Trials. Cancer Research 2017;77(7):1510–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leoni G, D’Alise AM, Cotugno G, Mori F, Catanese MT, Langone F, et al. A viral vectored vaccine based on shared tumor neoantigens for prevention and treatment of microsatellite instable (MSI) cancers. Journal for ImmunoTherapy of Cancer 2017;5((Suppl 2):86):P139. [Google Scholar]
- 27.von Knebel Doeberitz M, Kloor M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Fam Cancer 2013;12(2):307–12. [DOI] [PubMed] [Google Scholar]
- 28.Maby P, Tougeron D, Hamieh M, Mlecnik B, Kora H, Bindea G, et al. Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. Cancer Research 2015;75(17):3446–55. [DOI] [PubMed] [Google Scholar]
- 29.Townsend A, Ohlen C, Rogers M, Edwards J, Mukherjee S, Bastin J. Source of unique tumour antigens. Nature 1994;371(6499):662. [DOI] [PubMed] [Google Scholar]
- 30.Voudouri D, Nikolaou V, Laschos K, Charpidou A, Soupos N, Triantafyllopoulou I, et al. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer 2017;41(6):407–12. [DOI] [PubMed] [Google Scholar]
- 31.Zeng MF, Chen LL, Ye HY, Gong W, Zhou LN, Li YM, et al. Primary hypothyroidism and isolated ACTH deficiency induced by nivolumab therapy: Case report and review. Medicine (Baltimore) 2017;96(44):e8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajmir-Riahi A, Bergmann T, Schmid M, Agaimy A, Schuler G, Heinzerling L. Life-threatening Autoimmune Cardiomyopathy Reproducibly Induced in a Patient by Checkpoint Inhibitor Therapy. J Immunother 2018;41(1):35–8. [DOI] [PubMed] [Google Scholar]
- 33.Tchapyjnikov D, Borst AJ. Immune-related Neurological Symptoms in an Adolescent Patient Receiving the Checkpoint Inhibitor Nivolumab. J Immunother 2017;40(7):286–8. [DOI] [PubMed] [Google Scholar]
- 34.Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer 2017;25(10):3017–30. [DOI] [PubMed] [Google Scholar]
- 35.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 2017;22(4):470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Burel S, Champiat S, Mateus C, Marabelle A, Michot JM, Robert C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34–44. [DOI] [PubMed] [Google Scholar]
- 37.Kostine M, Chiche L, Lazaro E, Halfon P, Charpin C, Arniaud D, et al. Opportunistic autoimmunity secondary to cancer immunotherapy (OASI): An emerging challenge. Rev Med Interne 2017;38(8):513–25. [DOI] [PubMed] [Google Scholar]
- 38.Komaki Y, Komaki F, Yamada A, Micic D, Ido A, Sakuraba A. Meta-Analysis of the Risk of Immune-Related Adverse Events With Anticytotoxic T-Lymphocyte-Associated Antigen 4 and Antiprogrammed Death 1 Therapies. Clin Pharmacol Ther 2018;103(2):318–31. [DOI] [PubMed] [Google Scholar]
- 39.Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol 2017;74(10):1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer 2017;73:1–8. [DOI] [PubMed] [Google Scholar]
- 41.Cousin S, Seneschal J, Italiano A. Toxicity profiles of immunotherapy. Pharmacol Ther 2018;181:91–100. [DOI] [PubMed] [Google Scholar]
- 42.Costa R, Carneiro BA, Agulnik M, Rademaker AW, Pai SG, Villaflor VM, et al. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget 2017;8(5):8910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciccarese C, Iacovelli R, Bria E, Modena A, Massari F, Brunelli M, et al. The incidence and relative risk of pulmonary toxicity in patients treated with anti-PD1/PD-L1 therapy for solid tumors: a meta-analysis of current studies. Immunotherapy 2017;9(7):579–87. [DOI] [PubMed] [Google Scholar]
- 44.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13(4):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiricny J The multifaceted mismatch-repair system. Nature reviews Molecular cell biology 2006;7(5):335–46. [DOI] [PubMed] [Google Scholar]
- 46.Field D, Wills C. Long, polymorphic microsatellites in simple organisms. Proc Biol Sci 1996;263(1367):209–15. [DOI] [PubMed] [Google Scholar]
- 47.Li YC, Korol AB, Fahima T, Beiles A, Nevo E. Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Mol Ecol 2002;11(12):2453–65. [DOI] [PubMed] [Google Scholar]
- 48.Payseur BA, Jing P, Haasl RJ. A Genomic Portrait of Human Microsatellite Variation. Molecular Biology and Evolution 2011;28(1):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunkel TA. Slippery DNA and diseases. Nature 1993;365:207. [DOI] [PubMed] [Google Scholar]
- 50.Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nature reviews Cancer 2014;14(12):786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500(7463):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borras E, San Lucas FA, Chang K, Zhou R, Masand G, Fowler J, et al. Genomic Landscape of Colorectal Mucosa and Adenomas. Cancer prevention research 2016;9(6):417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekine S, Mori T, Ogawa R, Tanaka M, Yoshida H, Taniguchi H, et al. Mismatch repair deficiency commonly precedes adenoma formation in Lynch Syndrome-Associated colorectal tumorigenesis. Mod Pathol 2017;30(8):1144–51. [DOI] [PubMed] [Google Scholar]
- 54.The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487(7407):330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2016;34(18):2141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 2013;45(2):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nature medicine 2016;22(11):1342–50. [DOI] [PubMed] [Google Scholar]
- 58.Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, et al. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res 1995;55(23):5548–50. [PubMed] [Google Scholar]
- 59.Woerner SM, Kloor M, Mueller A, Rueschoff J, Friedrichs N, Buettner R, et al. Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene 2005;24(15):2525–35. [DOI] [PubMed] [Google Scholar]
- 60.Woerner SM, Benner A, Sutter C, Schiller M, Yuan YP, Keller G, et al. Pathogenesis of DNA repair-deficient cancers: a statistical meta-analysis of putative Real Common Target genes. Oncogene 2003;22(15):2226–35. [DOI] [PubMed] [Google Scholar]
- 61.Duval A, Rolland S, Compoint A, Tubacher E, Iacopetta B, Thomas G, et al. Evolution of instability at coding and non-coding repeat sequences in human MSI-H colorectal cancers. Human molecular genetics 2001;10(5):513–8. [DOI] [PubMed] [Google Scholar]
- 62.Woerner SM, Gebert J, Yuan YP, Sutter C, Ridder R, Bork P, et al. Systematic identification of genes with coding microsatellites mutated in DNA mismatch repair‐deficient cancer cells. International journal of cancer 2001;93(1):12–9. [DOI] [PubMed] [Google Scholar]
- 63.Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomarkers 2006;2(1–2):69–86. [DOI] [PubMed] [Google Scholar]
- 64.Kloor M, Michel S, Buckowitz B, Ruschoff J, Buttner R, Holinski-Feder E, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer 2007;121(2):454–8. [DOI] [PubMed] [Google Scholar]
- 65.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138(6):2073–87 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology 2017;18(8):1009–21. [DOI] [PubMed] [Google Scholar]
- 67.Shia J, Stadler ZK, Weiser MR, Vakiani E, Mendelsohn R, Markowitz AJ, et al. Mismatch repair deficient-crypts in non-neoplastic colonic mucosa in Lynch syndrome: insights from an illustrative case. Familial Cancer 2015;14(1):61–8. [DOI] [PubMed] [Google Scholar]
- 68.Staffa L, Echterdiek F, Nelius N, Benner A, Werft W, Lahrmann B, et al. Mismatch repair-deficient crypt foci in Lynch syndrome--molecular alterations and association with clinical parameters. PLoS One 2015;10(3):e0121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reuschenbach M, Kloor M, Morak M, Wentzensen N, Germann A, Garbe Y, et al. Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Familial cancer 2010;9(2):173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008;134(4):988–97. [DOI] [PubMed] [Google Scholar]
- 71.Augenlicht LH, Richards C, Corner G, Pretlow TP. Evidence for genomic instability in human colonic aberrant crypt foci. Oncogene 1996;12(8):1767–72. [PubMed] [Google Scholar]
- 72.Giuffrè G, Müller A, Brodegger T, Bocker-Edmonston T, Gebert J, Kloor M, et al. Microsatellite analysis of hereditary nonpolyposis colorectal cancer-associated colorectal adenomas by laser-assisted microdissection: correlation with mismatch repair protein expression provides new insights in early steps of tumorigenesis. The Journal of Molecular Diagnostics 2005;7(2):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liljegren A, Barker G, Elliott F, Bertario L, Bisgaard ML, Eccles D, et al. Prevalence of adenomas and hyperplastic polyps in mismatch repair mutation carriers among CAPP2 participants: report by the colorectal adenoma/carcinoma prevention programme 2. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2008;26(20):3434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lanspa SJ, Lynch HT, Smyrk TC, Strayhorn P, Watson P, Lynch JF, et al. Colorectal adenomas in the Lynch syndromes. Results of a colonoscopy screening program. Gastroenterology 1990;98(5 Pt 1):1117–22. [DOI] [PubMed] [Google Scholar]
- 75.Stoffel EM, Turgeon DK, Stockwell DH, Zhao L, Normolle DP, Tuck MK, et al. Missed adenomas during colonoscopic surveillance in individuals with Lynch Syndrome (hereditary nonpolyposis colorectal cancer). Cancer prevention research 2008;1(6):470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yurgelun MB, Goel A, Hornick JL, Sen A, Turgeon DK, Ruffin MTt, et al. Microsatellite instability and DNA mismatch repair protein deficiency in Lynch syndrome colorectal polyps. Cancer prevention research 2012;5(4):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3(11):991–8. [DOI] [PubMed] [Google Scholar]
- 78.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 79.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012;482(7385):400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin SH, Raju GS, Huff C, Ye Y, Gu J, Chen JS, et al. The somatic mutation landscape of premalignant colorectal adenoma. Gut 2018;67(7):1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang K, Taggart MW, Reyes-Uribe L, et al. Immune profiling of premalignant lesions in patients with lynch syndrome. JAMA Oncology 2018;4(8):1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauer K, Nelius N, Reuschenbach M, Koch M, Weitz J, Steinert G, et al. T cell responses against microsatellite instability-induced frameshift peptides and influence of regulatory T cells in colorectal cancer. Cancer Immunology, Immunotherapy 2013;62(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011;24(5):671–82. [DOI] [PubMed] [Google Scholar]
- 84.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012;147(4):366–72. [DOI] [PubMed] [Google Scholar]
- 85.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353(25):2654–66. [DOI] [PubMed] [Google Scholar]
- 86.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol 2007;19(3):309–14. [DOI] [PubMed] [Google Scholar]
- 88.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991;174(3):561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017;276(1):80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2015;33(17):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol 2018;18(3):183–94. [DOI] [PubMed] [Google Scholar]
- 92.Woerner SM, Yuan YP, Benner A, Korff S, von Knebel Doeberitz M, Bork P. SelTarbase, a database of human mononucleotide-microsatellite mutations and their potential impact to tumorigenesis and immunology. Nucleic acids research 2010;38(Database issue):D682–D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hundal J, Carreno BM, Petti AA, Linette GP, Griffith OL, Mardis ER, et al. pVAC-Seq: A genome-guided in silico approach to identifying tumor neoantigens. Genome medicine 2016;8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nielsen M, Andreatta M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome medicine 2016;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018;154(3):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Majumder S, Shah R, Elias J, Manoharan M, Shah P, Kumari A, et al. A cancer vaccine approach for personalized treatment of Lynch Syndrome. Sci Rep 2018;8(1):12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Alise AM, Leoni G, Cotugno G, Troise F, Langone F, Fichera I, et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade. Nature Communications 2019;10(1):2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kloor M, Reuschenbach M, Karbach J, Rafiyan M-R, Al-Batran S-E, Pauligk C, et al. Vaccination of MSI-H colorectal cancer patients with frameshift peptide antigens: A phase I/IIa clinical trial. Journal of Clinical Oncology 2014;32(15_suppl):e14530–e. [Google Scholar]
- 99.de Goede AL, Figdor CG, Schreibelt G, Westdorp H, Nagtegaal ID, de Vries IJM, et al. Preventive dendritic cell vaccination in healthy Lynch syndrome mutation carriers. Annals of Oncology 2016;27(suppl_6) [Google Scholar]