Abstract
Purpose:
To report the 5-year overall survival (OS) landmark and the long-term safety profile of vemurafenib plus cobimetinib (BRAF plus MEK inhibition, respectively) in the BRIM7 study.
Patients and Methods:
This phase 1b, dose-finding and expansion study evaluated combination treatment with vemurafenib and cobimetinib in two cohorts of patients with advanced BRAFV600-mutated melanoma: patients who were BRAF inhibitor (BRAFi)-naive (n = 63) or patients who had progressed on prior treatment with BRAFi monotherapy (vemurafenib monotherapy–progressive disease [PD]) (n = 66). Patients in the dose-escalation phase received vemurafenib at 720 or 960 mg twice daily in combination with cobimetinib at 60, 80, or 100 mg/d for 14 days on/14 days off; 21 days on/7 days off; or continuously. Two regimens were selected for expansion: vemurafenib (720 and 960 mg twice daily) and cobimetinib (60 mg/d 21/7).
Results:
Median OS was 31.8 months (95% CI: 24.5–not estimable) in the BRAFi-naive cohort. The landmark OS rate plateaued at 39.2% at years 4 and 5 of follow-up. In the vemurafenib monotherapy–PD cohort, median OS was 8.5 months (95% CI: 6.7–11.1) and the landmark OS rate plateaued at 14.0% from 3 years of follow-up. No increase was observed in the frequency and severity of adverse events with long-term follow-up. No new toxicities were detected and there was no increase in the frequency of symptomatic MEK inhibitor class-effect adverse events.
Conclusion:
A subset of patients with advanced BRAFV600-mutated melanoma treated with a combination regimen of vemurafenib and cobimetinib achieve favorable long-term outcomes.
INTRODUCTION
The reactivation of the mitogen-activated protein kinase (MAPK) signaling pathway through MEK is the most common core pathway mechanism of resistance to single-agent BRAF inhibitor therapy [1, 2]. Therefore, preventing MAPK-driven acquired resistance mechanisms by using combined therapy with a BRAF inhibitor and a MEK inhibitor may result in increased clinical responses when given upfront, and also potentially provide a therapeutic benefit to patients who have progressed on prior treatment with BRAF inhibitor monotherapy, which was the only available treatment option for of BRAFV600-mutated melanoma when this study began [3]. Over the past 5 years, results from four large randomized clinical trials demonstrated that the combination of a BRAF inhibitor and a MEK inhibitor improves objective responses, progression-free survival (PFS), and overall survival (OS) compared with BRAF inhibitor monotherapy, becoming the new standard of care therapy for patients with BRAFV600-mutated metastatic melanoma [4-7]. Furthermore, by inhibiting paradoxical MAPK activation, the combination of BRAF inhibitors plus MEK inhibitors decreases some of the toxicities related to BRAF inhibitor monotherapy, as MAPK activation is the mechanistic basis for secondary squamous cell carcinomas and other skin and musculoskeletal toxicities associated with BRAF inhibitor monotherapy [4-7].
In 2014, we reported the initial results of BRIM7 [3], a phase 1b trial that enrolled 129 patients treated with 10 dosing regimens combining vemurafenib and cobimetinib. The maximum tolerated dose (MTD) was established at vemurafenib 960 mg twice daily continuously administered combined with cobimetinib 60 mg daily on a 21 days on/7 days off schedule. Confirmed objective responses were observed in 55/63 (87%) of patients naive to BRAF inhibitors, including 6/63 (9%) patients attaining complete responses; median PFS was 13.7 months (95% CI: 10.1–17.5). For patients who entered the study after progression on prior vemurafenib monotherapy, 10/66 (15%) achieved a confirmed response with a median PFS of 2.8 months (95% CI: 2.6–3.4). We concluded that the combination of vemurafenib and cobimetinib was safe and tolerable and could be administered at their respective MTDs, warranting further clinical development in a randomized controlled trial to investigate the potential to establish it as a standard of care for patients with BRAFV600-mutated melanoma.
The results of BRIM7 led to the initiation of the coBRIM study [5], a randomized phase 3 clinical trial that compared the combination of vemurafenib and cobimetinib to treatment with vemurafenib monotherapy. The study enrolled 495 patients with unresectable locally advanced or metastatic treatment-naive BRAFV600 mutation–positive melanoma and met the primary end point of improved median PFS with combination treatment compared with vemurafenib monotherapy (9.9 vs 6.2 months; hazard ratio [HR], 0.51; P < 0.0001). Two other combinations of BRAF plus MEK inhibitors tested in three large randomized trials have also been demonstrated to be superior to BRAF inhibitor monotherapy in terms of both PFS and OS. The COMBI-v study compared the dabrafenib and trametinib combination with vemurafenib monotherapy, the COMBI-d study compared the dabrafenib and trametinib combination with dabrafenib monotherapy, and the COLUMBUS study compared the encorafenib and binimetinib combination with encorafenib or vemurafenib monotherapy [4, 6, 7]. Based on the findings of these studies, a combination of a BRAF inhibitor and a MEK inhibitor is the current standard of care in targeted therapy for patients with BRAFV600 mutation–positive advanced melanoma.
It is of great interest to understand the long-term effects of a combination of BRAF and MEK inhibitor therapy, in particular whether long-term use results in any increase in toxicities, and to assess the rate of long-term durable responses in this patient population. It is encouraging that the 5-year landmark analysis of patients with BRAFV600-mutant metastatic melanoma who received dabrafenib and trametinib within a phase 2 trial showed an apparent plateau in OS at 4 and 5 years (30% and 28%, respectively) and also a plateau in PFS of 13% at 4 and 5 years [8]. Patients who had a normal baseline lactate dehydrogenase (LDH) and fewer than three organ sites with metastasis had the best outcomes. In this analysis, we followed patients in BRIM7 for 5 years and herein report on their long-term outcomes after treatment with vemurafenib and cobimetinib.
METHODS
Study Design and Treatment
The design of the BRIM7 study has previously been reported in detail [3]. Briefly, BRIM7 was an open-label, multicenter, phase 1b dose-escalation study conducted in two stages (dose escalation and expansion). In the dose-escalation stage, patients received vemurafenib at 720 or 960 mg twice daily (BID) continuously combined with cobimetinib at 60, 80, or 100 mg/day for 14 days on/14 days off (14/14), 21 days on/7 days off (21/7), or continuously (28/0). Two dose levels were expanded: vemurafenib (720 and 960 mg twice daily) and cobimetinib (60 mg/day 21/7).
Key eligibility criteria were age ≥18 years; unresectable stage IIIc or stage IV melanoma; positive for the BRAFV600 mutation on real-time polymerase chain reaction assay (cobas 4800 BRAFV600 Mutation Test, Roche Molecular Systems, Branchburg, NJ, USA); measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; estimated life expectancy of ≥12 weeks; and adequate hematologic, hepatic, and renal function. Initially, only patients who had previously received and progressed on vemurafenib monotherapy (vemurafenib monotherapy–progressive disease [PD] cohort) were eligible, but the protocol was later amended on July 13, 2011, to include patients who had not received prior treatment with a BRAF inhibitor (BRAF inhibitor–naive cohort). Patients were analyzed in separate cohorts according to prior BRAF inhibitor therapy.
The study is registered with ClinicalTrials.gov () and was conducted according to the provisions of the Declaration of Helsinki guidelines for Good Clinical Practice. The study was approved by the local institutional review board, independent ethics committee, or research ethics board of all participating study sites. All study participants provided written informed consent. An independent data safety monitoring board monitored and evaluated safety data from the study.
Outcomes
The primary end points were the MTD, dose-limiting toxicity, tolerability, and pharmacokinetic profile of vemurafenib combined with cobimetinib, and the definition of the recommended dose and schedule of the combination for use in phase 2 and phase 3 trials. Antitumor activity, assessed according to RECIST version 1.1, duration of response, PFS, and OS were evaluated as secondary end points. Safety assessments included physical examination, electrocardiography, and laboratory evaluations that were conducted every week during the first two 28-day treatment cycles and every cycle thereafter. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI CTCAE v4.0).
Statistical Analysis
The data cutoff date for this analysis was May 25, 2018. PFS and OS were estimated using the Kaplan–Meier method in patients treated with the combination regimen. The safety population included all patients who received at least one dose of study drug.
RESULTS
Patient Characteristics
A total of 131 patients were enrolled in the BRIM7 trial between February 2011 and July 2013; data from two patients treated with cobimetinib monotherapy were excluded from these analyses. Of the 129 remaining patients included in this analysis, 63 patients were BRAF inhibitor–naive and 66 had progressed while on prior treatment with vemurafenib monotherapy (vemurafenib monotherapy–PD cohort) (Supplementary Figure 1). Baseline patient and disease characteristics were as previously reported for this study (Table 1) [3]. The relative proportion of patients with adverse prognostic factors in the BRAF inhibitor–naive cohort was lower, with 35% having an ECOG performance status of 1, 70% having stage IVc disease, and 46% having elevated LDH levels; the corresponding rates in the vemurafenib monotherapy–PD cohort were 65%, 82%, and 62%, respectively.
Table 1.
Baseline characteristics
| BRAF inhibitor–naive cohort n = 63 |
Vemurafenib monotherapy–PD cohort n = 66 |
|
|---|---|---|
| Median age, years (range) | 56.0 (21–74) | 53.0 (19–88) |
| Male, n (%) | 35 (56) | 42 (64) |
| ECOG performance status, n (%) | ||
| 0 | 41 (65) | 23 (35) |
| 1 | 22 (35) | 43 (65) |
| Disease stage, n (%) | ||
| Unresectable stage IIc | 7 (11) | 3 (5) |
| Stage IVa | 3 (5) | 4 (6) |
| Stage IVb | 9 (14) | 5 (8) |
| Stage IVc | 44 (70) | 54 (82) |
| Elevated LDH, n (%) | 29 (46) | 39 (62)a |
| Treatment status | ||
| Previously untreated | 43 (68) | N/A |
| Previously treated, BRAF inhibitor–naive | 20 (32) | N/A |
| Median follow-up, months (range) | 28.0 (2.7–69.2) | 8.4 (1.6–76.4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PD, progressive disease.
Baseline data available for 63 patients.
At the time of data cutoff for the current analysis (May 25, 2018), median follow-up durations were 28.0 months (range, 2.7–69.2) for patients in the BRAF inhibitor–naive cohort and 8.4 months (range, 1.6–76.4) for patients in the vemurafenib monotherapy–PD cohort. Treatment had been discontinued in 62 (98.4%) patients in the BRAF inhibitor–naive cohort and 65 (98.5%) patients in the vemurafenib monotherapy–PD cohort; the most common reason for treatment discontinuation was PD in both cohorts (62.9% and 86.2%, respectively).
Updated Efficacy
At the time of data cutoff for the current analysis, the best overall response rate (BORR) was 87% (95% CI: 77%–94%) in the BRAF inhibitor–naive cohort and 15% (95% CI: 8%–25%) in the vemurafenib monotherapy–PD cohort. The updated BORR compared with the originally described BORR is detailed in Table 2. Late conversions to complete response were observed in six patients in the BRAF inhibitor–naive cohort and one patient in the vemurafenib monotherapy–PD cohort, respectively (Table 2).
Table 2.
Efficacy outcomes according to time of data cutoff
| Data cutoff October 1, 2013 [3] | Data cutoff May 25, 2018 | |||
|---|---|---|---|---|
| BRAF inhibitor– naive cohort n = 63 |
Vemurafenib monotherapy–PD cohort n = 66 |
BRAF inhibitor- naive cohort n = 63 |
Vemurafenib monotherapy–PD cohort n = 66 |
|
| Confirmed BORR, % (95% CI) | 87.3 (76.7–94.4) | 15.2 (7.5–25.5) | 87.3 (76.7–94.4) | 15.2 (7.5–25.5) |
| Best overall response, n (%) | ||||
| Complete response | 6 (9.5) | 0 | 12 (19.0) | 1 (1.5) |
| Partial response | 49 (77.8) | 10 (15.2) | 43 (68.3) | 9 (13.6) |
| Stable disease | 6 (9.5) | 28 (42.4) | 6 (9.5) | 28 (42.4) |
| Progressive disease | 2 (3.2) | 24 (36.4) | 2 (3.2) | 24 (36.4) |
| Not assessable/not done | 0 | 4 (6.0) | 0 | 4 (6.0) |
| Duration of first confirmed objective response | ||||
| Patients who progressed or died, n (%) | 55 | 10 | 55 | 10 |
| Patients who progressed or died, n (%) | 27 (49.1)a | 5 (50.0)a | 38 (69.1)a | 7 (70.0)a |
| Median response duration, months (95% CI) | 12.5 (9.7–NE) | 6.7 (4.9–NE) | 14.3 (9.7–23.1) | 6.8 (4.9–10.4) |
| Median PFS, months (95% CI) | 13.7 (10.1–17.5) | 2.8 (2.6–3.5) | 13.8 (10.8–20.6) | 2.8 (2.6–3.4) |
| Median OS, months (95% CI) | NE (NE–NE) | 8.3 (6.0–10.9) | 31.8 (24.5–NE) | 8.5 (6.7–11.1) |
| OS rate, % (95% CI) | ||||
| 1 year | 82.8 (73.0–92.6) | 32.0 (19.4–44.6) | 82.5 (73.2–91.9) | 36.1 (24.3–47.9) |
| 2 years | — | — | 63.9 (51.8–76.1) | 18.7 (8.7–28.7) |
| 3 years | — | — | 41.5 (28.1–54.8) | 14.0 (4.7–23.4) |
| 4 years | — | — | 39.2 (25.8–52.5) | 14.0 (4.7–23.4) |
| 5 years | — | — | 39.2 (25.8–52.5) | 14.0 (4.7–23.4) |
Abbreviations: BORR, best overall response rate; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
Bold text indicates change or new data since previous analysis; — indicates data not available at the time of the previous analysis.
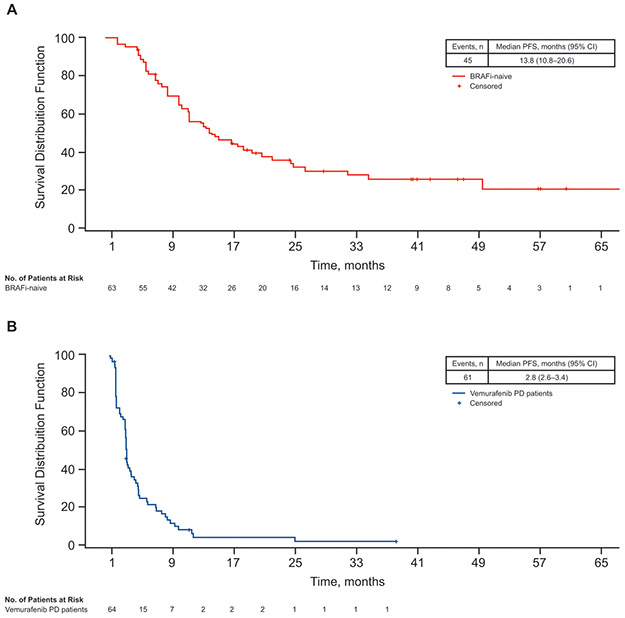
At the time of data cutoff, 45 of 63 patients (71%) in the BRAF inhibitor–naive cohort and 61 of 66 patients (92%) in the vemurafenib monotherapy–PD cohort had experienced progression or died. Median PFS was 13.8 months (95% CI: 10.8–20.6) in the BRAF inhibitor–naive cohort and 2.8 months (95% CI: 2.6–3.4) in the vemurafenib monotherapy–PD cohort (Figure 1). These median PFS durations with longer-term follow-up were not noticeably different compared with the previously reported PFS durations for the two cohorts (13.7 months and 2.8 months in the BRAF inhibitor–naive and vemurafenib monotherapy–PD cohorts, respectively) (Table 2) [3]. With the extended follow-up, the median response duration improved for patients in the BRAF inhibitor–naive cohort (ie, 14.3 months), whereas it remined the same in the vemurafenib monotherapy–PD cohort (6.8 months; Table 2). In the BRAF inhibitor–naive cohort, the plateau of long-term PFS was 20.3% (95% CI: 7.5%–33.1%) at 5 years. At the time of this analysis, 18 patients in the BRAF inhibitor–naive cohort were alive and progression-free. Of these 18 patients, 12 patients (67%) had normal serum LDH levels at baseline and the remaining 6 patients (33%) had elevated serum LDH levels. In contrast, among the 45 patients in the BRAF inhibitor–naive cohort who progressed or died, 22 patients (49%) had normal serum LDH levels at baseline and 23 patients (51%) had elevated serum LDH levels. A similar benefit was not observed in the vemurafenib monotherapy–PD cohort.
Figure 1.
Kaplan–Meier curves for PFS in (A) BRAFi-naive cohort and (B) vemurafenib monotherapy–PD cohort. Abbreviations: BRAFi, BRAF inhibitor; PD, progressive disease; PFS, progression-free survival.
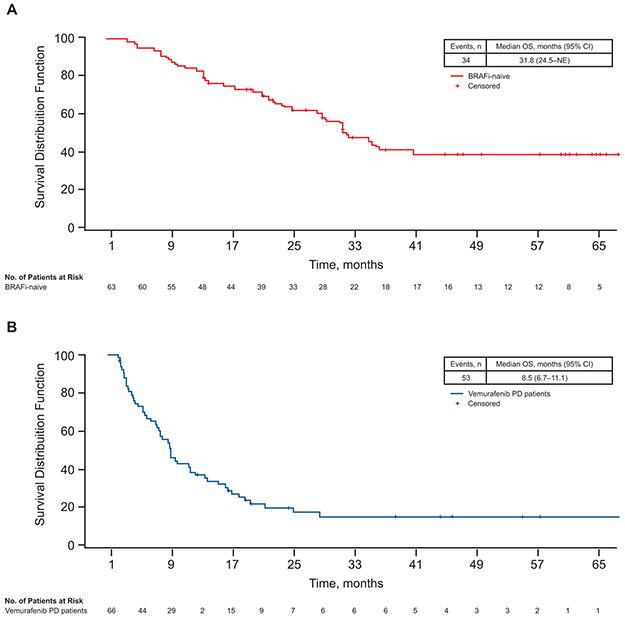
Thirty-four of 63 patients (54%) in the BRAF inhibitor–naive cohort and 53 of 66 patients (80%) in the vemurafenib monotherapy–PD cohort died. Median OS at the time of the previous analysis was not estimable (NE) in the BRAF inhibitor–naive cohort and 8.3 months in the vemurafenib monotherapy–PD cohort (Table 2) [3]. In this longer-term analysis, median OS was 31.8 months (95% CI: 24.5–not estimable) in the BRAF inhibitor–naive cohort and 8.5 months (95% CI: 6.7–11.1) in the vemurafenib monotherapy–PD cohort (Figure 2). It is notable that the survival plateaued at 4 and 5 years at 39% in the BRAF inhibitor–naive patients and at 14.0% from 3 years onwards in patients who had experienced progression on prior vemurafenib monotherapy (Figure 2 and Table 2). The median follow-up duration of patients who were still alive at the time of analysis was 47.0 months for patients in the BRAF inhibitor–naive cohort and 24.0 months for patients in the vemurafenib monotherapy–PD cohort.
Figure 2.
Kaplan–Meier curves for OS in (A) BRAFi-naive cohort and (B) vemurafenib monotherapy–PD cohort. BRAFi, BRAF inhibitor; NE, not estimable; OS, overall survival; PD, progressive disease.
Safety
At data cutoff, patients in the BRAF inhibitor–naive cohort had received a median of 16 cycles of vemurafenib plus cobimetinib over a median duration of ≥14.5 months; this represented an increase in drug exposure compared with the previous analysis [3]. Likewise, the median cumulative dose of each drug (709.4 g for vemurafenib and 16.6 g for cobimetinib) had increased since the previous analysis (534.7 g and 14.3 g, respectively). The mean dose intensity remained similar (Supplementary Table 1).
In contrast, patients in the vemurafenib monotherapy–PD cohort had received a median of just four cycles of each drug over a median duration of 3.3 months; this exposure was not noticeably different from the previous analysis (Supplementary Table 1). The median cumulative doses (162.8 g for vemurafenib and 4.5 g for cobimetinib) were also lower than those in the BRAF inhibitor–naive cohort and had not increased with longer follow-up.
The most frequently reported treatment-emergent AEs in each subgroup were generally similar to those reported at the earlier cutoff (October 1, 2013) [3] and often occurred at a higher frequency in the BRAF inhibitor–naive cohort compared with the vemurafenib monotherapy–PD cohort (Supplementary Table 2). The most common AEs, regardless of attribution to study drugs, were nonacneiform rash (89% and 38% in the BRAF inhibitor–naive and vemurafenib monotherapy-PD cohorts, respectively), diarrhea (83% and 47%), fatigue (73% and 27%), photosensitivity (70% and 18%), and elevations in liver function tests (70% and 33%).
Treatment-emergent AEs that increased by ≥5% in frequency over the longer-term follow-up are shown in Table 3. Of note, the frequency of photosensitivity reactions increased in both cohorts (+25% and +5% in the BRAF inhibitor–naive and vemurafenib monotherapy–PD cohorts, respectively), as did actinic keratitis (+20% and +2%), although these events were generally low grade (grade 1 and 2). The only grade ≥3 AEs that increased in more than one patient during the longer-term follow-up were anemia and hypophosphatemia in the BRAF inhibitor–naive cohort (two patients each). Rates of AEs of special interest (symptomatic MEK inhibitor class-effect AEs) were similar to those previously reported (Supplementary Table 3) [3].
Table 3.
AEs with ≥5% increase in frequency over time in any subgroup, regardless of attribution to study drugs
| BRAF inhibitor-naive cohort n = 63 |
Vemurafenib monotherapy–PD cohort n = 66 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Any Grade n (%) |
Grade ≥3 n (%) |
% change from previous data |
Any Grade n (%) |
Grade ≥3 n (%) |
% change from previous data |
|||
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |||||
| Photosensitivitya | 44 (70) | 1 (2) | +22% | +1% | 12 (18) | 1 (2) | +3% | 0% |
| Vomiting | 30 (48) | 0 | +5% | 0% | 14 (21) | 1 (2) | +1% | 0% |
| CPK level elevation | 30 (48) | 2 (3) | +5% | 0% | 10 (15) | 1 (2) | 0% | 0% |
| Anemia | 23 (37) | 7 (11) | +5% | +3% | 11 (17) | 5 (8) | +2% | +2% |
| Myalgia | 20 (32) | 1 (2) | +9% | 0% | 4 (6) | 0 | 0% | 0% |
| Actinic keratosis | 19 (30) | 0 | +20% | 0% | 3 (5) | 0 | +2% | 0% |
| Hypokalemia | 16 (25) | 3 (5) | +9% | 0% | 4 (6) | 0 | 0% | 0% |
| Seborrheic keratosis | 15 (24) | 1 (2) | +7% | 0% | 1 (2) | 0 | 0% | 0% |
| Skin papilloma | 15 (24) | 0 | +5% | 0% | 0 | 0 | 0% | 0% |
| Constipation | 13 (21) | 0 | +5% | 0% | 10 (15) | 1 (2) | +1% | 0% |
| Hypophosphatemia | 13 (21) | 6 (10) | +7% | +4% | 3 (5) | 3 (5) | 0% | +2% |
Abbreviations: AE, adverse event; CPK, creatine phosphokinase; PD, progressive disease.
AEs with an increase of ≥5% from the previous analysis [3] in either cohort are reported; CPK.
Grouped terms.
Treatment modifications due to AEs also remained similar to those previously reported [3], with the proportion of dose interruptions or reductions in the BRAF inhibitor–naive cohort remaining higher than in the vemurafenib monotherapy–PD cohort (Supplementary Table 4). In the BRAF inhibitor–naive cohort, the dose of vemurafenib, cobimetinib, or both agents was modified or interrupted because of AEs in 77.8%, 68.3%, or 65.1% of patients, respectively. Treatment with vemurafenib, cobimetinib, or both agents was discontinued permanently because of AEs in 11.1%, 11.1%, or 7.9% of patients, respectively. In the vemurafenib monotherapy–PD cohort, the dose of vemurafenib, cobimetinib, or both agents was modified or interrupted because of AEs in 27.3%, 21.2%, or 19.7% of patients, respectively. Treatment with vemurafenib, cobimetinib, or both agents was discontinued permanently because of AEs in 4.5%, 1.5%, or 1.5% of patients, respectively.
DISCUSSION
In the present analysis, patients who received vemurafenib plus cobimetinib in BRIM7, a phase 1 trial for the treatment of BRAFV600-mutated metastatic melanoma, were followed long-term for 5 years. We observed a plateau in survival, with a landmark OS rate of approximately 40% at years 4 and 5 of follow-up in those patients who had not received prior treatment with a BRAF inhibitor before entering the study. Of note, with longer follow-up there was an increase in patients who had a complete response to therapy, highlighting that late conversions from partial to complete response can be achieved with continued therapy. Patients who entered the study after experiencing progression on prior therapy with vemurafenib monotherapy had lower response, PFS, and OS rates than patients who were BRAF inhibitor–naive and a correspondingly lower 5-year landmark OS rate. However, there was also evidence of a plateau in the long-term survival curve at 14.0% beginning at 3 years, even among patients who had progressed on prior BRAF inhibitor therapy.
A steady increase has been observed in the long-term survival of patients enrolled in clinical trials for the treatment of advanced melanoma. The Korn meta-analysis of patients enrolled onto 42 phase 2 trials from the US cooperative groups treated between 1975 and 2005 showed 3-year OS rates in the range of 10% [9]. No single treatment, among the 70 included in this meta-analysis, showed evidence of higher long-term survival, and all cohorts appeared to have experienced similar outcomes compared with the previous standard of care therapy (single-agent dacarbazine). The 22% OS rate beyond 5 years in the pooled analysis of 1,861 patients with advanced melanoma treated with ipilimumab was considered a new hallmark in the treatment of patients with melanoma [7].
The long-term outcomes of patients with metastatic melanoma have markedly improved with the advent of BRAF plus MEK inhibitor therapies and with anti–programmed cell death-1 (PD-1) immunotherapies. As mentioned in the last paragraph of the Introduction, the long-term follow-up with the treatment of dabrafenib and trametinib in 54 patients with BRAFV600-mutated metastatic melanoma who had not previously received therapy with a BRAF inhibitor had an apparent survival plateau at 30% at 4 years and 28% at 5 years [8]. Several factors have been reported to favorably impact clinical outcomes in patients with BRAFV600-mutated melanoma treated with the combination of BRAF and MEK inhibitors [11, 12]. These include the number of disease sites (<3 vs ≥3), ECOG performance status, and serum LDH levels. In a pooled analysis of 617 patients treated with dabrafenib and trametinib, patients initiating treatment with a normal LDH level and <3 organ sites with metastatic disease had a 3-year survival of 70%, whereas in patients with a baseline LDH level ≥2x upper limit of normal (ULN) the 3-year survival rate was <10% [13]. Similarly, in a pooled analysis of patients treated with vemurafenib plus cobimetinib, those with favorable prognostic criteria (normal LDH and tumor diameter ≤45 mm) had a 3-year survival rate of 53% while patients with LDH >2x ULN had a 3-year survival rate of 9% [11]. Therefore, differences in prognostic factors in the treated population are likely to have a substantial effect on the long-term survival of patients receiving targeted therapy with BRAF and MEK inhibitors, making it difficult to perform direct comparisons between the outcomes of different clinical trials.
With the advent of PD-1 blockade therapies, it is possible that some patients may have received nivolumab or pembrolizumab after experiencing progression during the BRIM7 trial and benefitted from this therapy long-term. Indeed, patients progressing on BRAF inhibitor–based therapy were eligible for clinical trials with anti–PD-1 antibodies before they were approved by regulatory agencies (starting in 2014 in the United States and later in other countries). However, the response rate to anti–PD-1 therapy in patients who had progression on prior BRAF inhibitor–based therapy is lower than in patients who had previously received other immunotherapies or were treatment-naive at the start of therapy [14]. For example, in the pembrolizumab phase 1 trial, the response rate among patients who were treatment-naive was 38.6%, whereas it was 30.1% in patients progressing on other immunotherapies (mostly those who had previously received anti–cytotoxic T-lymphocyte antigen-4 antibody [eg, ipilimumab]) and 23.7% in patients who had previously received a BRAF or MEK inhibitor [15]. Therefore, although it is possible that the long-term benefit may be mediated by the treatment with vemurafenib plus cobimetinib, subsequent PD-1 blockade therapy likely also contributed to the plateau of the survival curve.
With the extended follow-up in the present analysis, an increased rate of certain toxicities was observed. The increased frequency of photosensitivity and actinic keratosis is likely due to the increased exposure time, which in turn leads to an increase in the accumulated skin toxicity events related to vemurafenib use. There was also an increase, albeit of lower magnitude, in myalgias, hypokalemia, and hypophosphatemia, which may reflect electrolyte imbalances and chronic toxicities that are more likely due to the MEK inhibitor cobimetinib.
In conclusion, combination therapy with vemurafenib and cobimetinib can result in a significant rate of long-term responses and a plateau in the survival curve of patients with BRAFV600-mutated metastatic melanoma. Long-term treatment achieved late conversions from partial to complete responses, and it did not result in unexpected long-term toxicities.
Supplementary Material
Statement of Translational Relevance.
This report evaluated the safety and long-term benefit of combined BRAF and MEK inhibition with vemurafenib and cobimetinib, respectively, and assessed their ability to improve survival outcomes in patients with BRAFV600-mutated metastatic melanoma who were treatment-naive or who had progressed on prior vemurafenib (BRAF inhibitor) monotherapy. The results suggested that long-term treatment with the combination of vemurafenib and cobimetinib achieved late conversions from partial to complete responses and that a subset of patients (~40%) achieved extended survival at 4–5 years after study entry. It is of interest to further understand the prognostic characteristics of this patient population.
ACKNOWLEDGMENTS
This study was funded by F. Hoffmann-La Roche Ltd. Medical editorial assistance was provided by Melanie Sweetlove, MSc (ApotheCom, San Francisco, CA), and Lucy Smithers, PhD (ApotheCom, London, UK), and was funded by F. Hoffmann-La Roche Ltd.
Research support: This study was funded by F. Hoffmann-La Roche Ltd.
Footnotes
Erica Park was employed by Genentech at the time the study was conducted.
Disclosure of Potential Conflicts of Interest
A.R. reports institutional research grants from Genentech; honoraria for consultancy from Genentech, Roche, Chugai, Novartis, Amgen, Merck, and Bristol-Myers Squibb (BMS); and personal fees for advisory role from Arcus Biosciences, Bioncotech Therapeutics, Compugen, CytomX, Five Prime Therapeutics, FLX Bio, ImaginAb, Kite Pharma, Merus, Rgenix, Tango Therapeutics, and PACT Pharma. A.D. reports personal fees from Merck Sharp & Dohme, Incyte, Genentech/Roche, BMS, Alexion, EMD Serono, and Novartis. A.C.P. reports personal fees from BMS, Seattle Genetics, Genentech/Roche, Merck Sharp & Dohme, Novartis, and Amgen. R.G. reports personal fees from BMS, Incyte, Novartis, and Genentech/Roche. K.D.L. reports personal fees for consultancy from Roche. O.H. reports institutional research support from AstraZeneca, BMS, Celldex Therapeutics, Genentech/Roche, Immunocore, Incyte, Merck, Merck Serono, MedImmune, Novartis, Pfizer, and Rinat Neuroscience, and personal fees for advisory boards and/or speaker bureaus from Genentech/Roche, Amgen, Novartis, BMS, Merck, Array BioPharma, and Sanofi. T.F.G. reports personal fees from Incyte, Alkermes, BMS, Genentech/Roche, Janssen, Merck Sharp & Dohme, Pfizer, and Novartis. I.P. reports personal fees for consultancy from Amgen and Roche. M.W. reports employment with Genentech/Roche and reports stock ownership with Genentech/Roche and ARIAD Pharmaceuticals. I.R. and J.J.H. report employment with Genentech/Roche. Y.Y. and E.P. report employment and stock ownership with Genentech/Roche. G.A.M. reports institutional research grants from Genentech/Roche.
This study has been presented in part at the 37th Congress of the European Society for Medical Oncology, September 28 – October 2, 2012, Vienna, Austria; 9th congress of the European Association of Dermato Oncology; July 17–20, 2013, Hamburg Germany; 38th Congress of the European Society for Medical Oncology, September 27 – October 1, 2012, Amsterdam, Netherlands; 15th Congress of the Associazione Italiana di Oncologia Medica; October 11, 2013; Melanoma Bridge; December 5–8; 2013, Naples, Italy; 10th Congress of the European Association of Dermato-Oncology; May 7–10; 2014; Vilnius, Lithuania; 39th Congress of the European Society for Medical Oncology, September 26–30, 2014; Madrid, Spain; 51st Annual Meeting of the American Society of Clinical Oncology; May 29, 2015; Chicago, Illinois, USA; 10th Anniversary International Congress of the Society for Melanoma Research; November 17–20, 2013; Philadelphia, PA, USA; 13th International Congress of the Society for Melanoma Research; November 6–9, 2016; Boston, Massachusetts, USA; 52nd Annual Meeting of the American Society of Clinical Oncology; June 3, 2016; Chicago, Illinois, USA; 40th Congress of the European Society for Medical Oncology, September 25–29, 2015; Vienna, Austria; 11th EADO Congress and 8th World Meeting of Interdisciplinary Melanoma/Skin Centers; October 28–31, 2015, Marseille, France; Melanoma Bridge; November 30 – December 3; 2016; Naples, Italy; 12th Congress of the European Association of Dermato Oncology; August 31 – September 3, 2016; Wien, Germany; 13th International Congress of the Society for Melanoma Research; November 6–9, 2016; Boston, MA; and the 14th International Congress of the Society for Melanoma Research; October 18–21, 2017; Brisbane, Australia.
REFERENCES
- 1.Shi H, Hugo W, Kong X et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014; 4: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Allen EM, Wagle N, Sucker A et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014; 4: 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Gonzalez R, Pavlick A et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol 2014; 15: 954–965. [DOI] [PubMed] [Google Scholar]
- 4.Dummer R, Ascierto PA, Gogas HJ et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19: 603–615. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Ascierto PA, Dreno B et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Stroyakovskiy D, Gogas H et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386: 444–451. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Karaszewska B, Schachter J et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–39. [DOI] [PubMed] [Google Scholar]
- 8.Long GV, Eroglu Z, Infante J et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018; 36: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korn EL, Liu PY, Lee SJ et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008; 26: 527–534. [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A et al. 5-year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. J Clin Oncol 2018; 36: 9516–9516. [Google Scholar]
- 11.Hauschild A, Larkin J, Ribas A et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-mutated metastatic melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol 2018; 4: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schadendorf D, Long GV, Stroiakovski D et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017; 82: 45–55. [DOI] [PubMed] [Google Scholar]
- 13.Long GV, Grob JJ, Nathan P et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 17: 1743–1754. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman A, Klein O, McDermott DF et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 2014; 120: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 15.Ribas A, Hamid O, Daud A et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016; 315: 1600–1609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.