Abstract
Rationale and Objectives:
Use of preoperative breast MRI (pMRI) to evaluate ductal carcinoma in situ (DCIS) extent is controversial due to limited data on its impact on surgical management. We sought to evaluate the effect of pMRI on surgical management of women with core needle biopsy (CNB)-diagnosed pure DCIS at a multidisciplinary academic institution.
Materials and Methods:
This retrospective study included all women with CNB-diagnosed DCIS (1/2004—12/2013) without prior ipsilateral breast cancer and who underwent surgery within 180 days of diagnosis. Patient features, number of CNBs and surgeries, and single successful breast conserving surgery (BCS) rate were compared between pMRI and no-pMRI cohorts. Number of surgeries and single BCS success rates were also compared to published US (SEER) and Danish National Registry data.
Results:
Among the 373 women included, no clinical differences were identified between the pMRI (n=332) and no-pMRI (n= 41) cohorts (p>0.05). The pMRI group experienced a higher additional CNB rate (30% vs. 7%, p=0.002) but fewer total surgeries (mean=1.2 vs. 1.5, p<0.001) than the no-pMRI group. Among the 245 women for whom BCS was attempted, the pMRI cohort underwent fewer mean surgeries (1.3 vs. 1.7, p<0.001) with a greater single successful BCS rate (77% vs. 43%, p<0.001). Compared to published data, women with pMRI who underwent BCS experienced fewer surgeries (difference(Δ)=−0.22 vs. −0.17, p<0.001) with a higher single successful BCS rate (Δ=+20% vs. +14%, p<0.001).
Conclusion:
pMRI may improve surgical management of DCIS at multidisciplinary centers with breast cancer specialists.
Keywords: Preoperative breast MRI, ductal carcinoma in situ (DCIS), core needle biopsy, surgical management, surgical outcomes
Introduction
Ductal carcinoma in situ (DCIS) diagnosis rates have increased dramatically with screening mammography utilization, accounting for an estimated 20% of new breast cancer cases in 2017(1). Central to its management is surgical excision of the entire span of DCIS, either by breast conserving surgery (BCS) or mastectomy, with only 4% of patients undergoing non-surgical management(2-4). The decision to pursue BCS versus mastectomy is multi-faceted and based on extent of DCIS as well as patient and surgeon preferences(5). Because over 90% of DCIS lesions are clinically asymptomatic, appropriate management options are reliant on accurate determination of DCIS extent on imaging.
Preoperative breast MRI (pMRI) can provide more sensitive and accurate assessments of DCIS disease extent compared to mammography alone, which theoretically could assist with determining the optimal surgical approach and allow for improved BCS outcomes(6-10). On dynamic contrast-enhanced (DCE) MRI, most DCIS lesions exhibit suspicious enhancement, which is proposed to better reflect biology and extent than mammographic depiction of calcifications(11, 12). However, the practical clinical benefit of using pMRI to determine DCIS extent is unclear, with several studies reporting that pMRI contributes to increases in false-positive core needle biopsies (CNBs), delays in surgical treatment, and a rise in mastectomy rates without lower re-operation rates(13-18).
One challenge of many studies examining the impact of pMRI on DCIS surgeries is the wide variability in MRI technique, radiologist experience, and how information from an MRI informs surgical approach(19, 20). Furthermore, the few randomized controlled trials on pre-operative MRI included generally low numbers of subjects, in particular those with DCIS(20-22). Finally, the lack of observed pMRI benefit in patients diagnosed with DCIS in the prior studies may in part be due to differences in important patient factors between women who underwent pMRI and those who did not(16, 23). Accordingly, we sought to determine the impact of pMRI on the number and type of surgeries as well as BCS success rates for DCIS treatment at a center where pMRI is routinely obtained and interpreted by breast imaging fellowship-trained radiologists. Furthermore, we compared the number of surgeries and BCS success rates to published Surveillance, Epidemiology, and End Results (SEER) and Danish National Registry data to better assess the impact of pMRI at our institution versus benchmarks reported in other US cities and in Europe.
Patients and Methods
This was a retrospective review of a prospectively collected clinical database, which was approved by our institutional review board for which the requirement for informed consent was waived. Our study cohort was obtained by querying a solid tumor clinical research database [BLINDED]. This database uses data sources including our institutional pathology database, prospectively recorded breast MRI data forms, and a [BLINDED-regional tumor registry]. All women ≥18 years old without a personal history of ipsilateral breast cancer diagnosed with pure DCIS (no associated invasive disease) on CNB between January 1, 2004 and December 31, 2013 and underwent surgical management at our institution within 180 days of CNB date were identified. The first instance of DCIS histology from CNB was considered the index event. All included patients underwent pre-treatment evaluation at our institution with a multidisciplinary approach, including imaging reviewed by breast imaging fellowship-trained radiologists and pathology evaluated by sub-specialized breast pathologists. Surgeries were performed by surgeons (7 total) specialized in breast surgical oncology with initial approach (e.g. BCS vs. mastectomy) determined through shared decision-making with the patient and in consultation with sub-specialized radiation oncologists and medical oncologists.
Patient characteristics, including use of pMRI, age, menopausal status, family history, breast density, and DCIS pathology subtype (hormone receptor status, presence of comedonecrosis and grade) were extracted from the electronic medical record. Use of pMRI was defined as either an internally performed MRI at our institution or an MRI performed at an outside facility but reviewed by an internal radiologist for the multidisciplinary conference. MRI utilization rate comparison was performed between the first and second half of the study period (2004-2008 and 2009-2013). All MRIs were performed with the patients in prone position, with imaging sequences including one non-contrast enhanced and at least two contrast-enhanced T1-weighted fat-suppressed 3D fast spoiled gradient recall acquisitions in accordance with the American College of Radiology (ACR) Breast MRI Accreditation Program(24). Mammographic breast density was defined using the ACR Breast Imaging Reporting and Data System (BI-RADS), and patients with heterogeneously or extremely dense assessments were categorized as having “dense breasts”(25). The total number of CNBs after initial CNB but before initial surgery, surgeon performing the DCIS treatment operation(s), total number and type of surgeries, reason for additional surgeries, and time from CNB diagnosis to first surgery were obtained from the electronic medical record. Additional surgeries were included in the study if they were within 180 days of the initial surgery. A successful BCS was defined as no mastectomy performed, regardless of whether the patient required re-excisions after primary BCS attempt.
Biopsy data after index CNB was categorized as benign or malignant (DCIS and invasive breast cancer). Biopsy events were counted per breast, per day, with multiple CNBs performed in the same breast in a single day counted as a single biopsy event. In cases of multiple CNBs in a single breast, the worst outcome was selected for that biopsy event as follows: invasive malignancy > DCIS > benign pathology.
To further assess the value of pMRI for BCS for treating pure DCIS, outcomes from our institution were also compared to published outcomes from two previous large studies, using the U.S. Surveillance, Epidemiology, and End Results (SEER) database for Los Angeles, CA and Detroit, MI(26) and the Danish National Registry(27), respectively. The first study included BCSs performed between 2005 and 2007 and the second included BCSs performed between 2010 and 2013. The use of pMRI was not part of the published protocols of these studies. For both studies, the number of surgeries (BCSs and subsequent mastectomy if performed), rate of successful BCS, and rate of single operation for successful BCS were extracted from the published manuscripts.
Clinical characteristics and surgical outcomes were compared between the pMRI and no-pMRI groups using the Wilcoxon rank-sum test and Fisher’s exact test, as appropriate. The mean and median times to initial surgery from the index CNB were compared between groups using the t-test and a median test(28) respectively. The positive predictive value of all biopsies performed as a result of a pMRI finding (PPV3) was calculated at the examination level. Uncertainty in the differences of mean and median time to initial surgery between the pMRI and no-pMRI groups was assessed using 95% confidence intervals (CIs). The number of surgeries from the present pMRI group in the BCS-attempted cohort was compared to the published number of surgeries reported from the SEER database and Danish National Registry using the Wilcoxon rank-sum test. The rates of successful BCS and single operation for successful BCS (“single successful BCS”) were compared between the present and previously published studies using Fisher’s exact test. All statistical calculations were conducted with the statistical computing language R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-sided tests were used, with statistical significance defined as p<0.05.
Results
Patient Characteristics
A total of 373 women (332 with pMRI, 41 without pMRI) diagnosed with DCIS on CNB were included in the study. Two-hundred ninety-five of the 332 pMRIs (89%) were performed at our institution, with the remaining 37 pMRIs performed at outside facilities with images reviewed by our internal breast radiologists. The mean age of all patients was 55.5±11.3 years, and the clinical and surgeon characteristics of the pMRI and no-pMRI cohorts are shown in Table 1. There were no significant differences in the clinical (age, menopausal status, family history, or breast density) or DCIS pathology features (hormone receptor status, presence of comedonecrosis or grade) between the pMRI and no-pMRI groups (p>0.05 for all comparisons). Reasons documented in clinical notes for no-pMRI included contraindication or inability to undergo an MRI (e.g. claustrophobia, body habitus, contrast allergy) (N=6), advanced age (generally over 75 years old) with other comorbidities (N=4), planned mastectomy (N=13), and not documented (N=18). Overall there were no significant differences in the proportion of patients who underwent pMRI between the seven surgeons (85-92% across all surgeons, p=0.53, Table 1). There was also no significant difference in the utilization of pMRI between the first and second halves of the study period (159/176 [90%] vs. 173/197 [88%], p=0.51, Table 1).
Table 1:
Clinical and Surgeon Characteristics in pMRI and no pMRI Cohorts
| Preoperative Breast MRI* | ||||
|---|---|---|---|---|
| Variable | Yes (N=332) |
No (N=41) |
P-value | |
| Age (Mean) | 55.7 ± 11.2 | 53.8 ± 11.8 | 0.29 | |
| Pre-Menopausal | 116 (34.9%) | 20 (48.8%) | 0.088 | |
| Family history† | 144 (44.2%) | 15 (38.5%) | 0.61 | |
| Dense breasts† | 227 (68.6%) | 25 (62.5%) | 0.47 | |
| DCIS ER positive† | 264 (83.8%) | 29 (85.3%) | >0.99 | |
| DCIS Necrosis present† | 273 (83.2%) | 32 (88.9%) | 0.48 | |
| DCIS Grade† | Low | 37 (11.1%) | 4 (10.0%) | 0.79 |
| Intermediate | 125 (37.7% | 13 (32.5%) | ||
| High | 170 (51.2%) | 23 (57.5%) | ||
| Year of index CNB | 2004-2008 | 159 (47.9%) | 17 (41.5%) | 0.51 |
| 2009-2013 | 173 (52.1%) | 24 (58.5%) | ||
| Surgeon | Surgeon 1 | 97 (29.2%) | 9 (22.0%) | 0.53 |
| Surgeon 2 | 71 (21.4%) | 10 (24.4%) | ||
| Surgeon 3 | 85 (25.6%) | 8 (19.5%) | ||
| Surgeon 4 | 62 (18.7%) | 11 (26.8%) | ||
| Others‡ | 17 (5.1%) | 3 (7.3%) | ||
CNB = core needle biopsy
Values are mean ± SD or no. (%);
Subjects were excluded from the summary due to missing values: family history (n=8), breast density (n=2), DCIS ER status (n=24), DCIS necrosis (n=9), and DCIS grade (n=1);
Three surgeons treated 20 patients total, so these three surgeons were grouped together to facilitate the statistical comparison between pMRI and no-pMRI groups.
Number and Pathology of Additional Core Needle Biopsies
Women who received pMRI were more likely to have additional CNBs than the no-pMRI cohort (100/332 [30%] vs. 3/41 [7%], p=0.002). Within the pMRI group, a total of 128 additional CNBs in 101 women were performed. CNBs revealing malignant pathology were seen in 43 CNBs in 40 of these 101 women (positive predictive value 3 [PPV3]=40%). Of the malignant pathologies, 14/128 [11%] were unsuspected contralateral disease and 29/128 [23%] were unsuspected ipsilateral disease. Contralateral invasive disease was seen in 7 additional biopsies in 6 patients (6/101 [2%] of patients), and additional DCIS was found in 36 CNBs in 34 women [34%] (7 contralateral and 29 ipsilateral). In these 34 women, there were 3 who were originally diagnosed with low grade DCIS on CNB, with the additional biopsy prompted by MRI revealing high grade DCIS in 1 woman. High-risk lesions, including atypical ductal hyperplasia, atypical lobular hyperplasia, LCIS, and flat epithelial atypia, were found in 37 additional CNB in 33 women (18 contralateral, 18 ipsilateral). The three additional CNBs in the no-pMRI patients were performed for unresolved suspicious findings seen either on the contralateral (n=2) or ipsilateral (n=l) breast on mammography prior to initial surgery, with all yielding malignancy.
Timing of Surgery
Of the 332 women who underwent pMRI, 42 (13%) received MRI for high-risk screening or problem solving indications, which resulted in the detection of the index DCIS lesion. As a result, the subjects’ pMRIs were performed prior to the index CNB and were excluded from surgical timing analysis since MRI could not have caused a surgical delay. Of the remaining 290 pMRI patients, the mean time to surgery from index CNB was 54.8±28.2 days, which was not significantly different than the no-pMRI group (48.3±27.3 days, difference(Δ)=6.8 days, 95% CI: −2.4 to 16.0 days, p=0.14). The median times to surgery were also similar at 48 days for both groups (Δ=0 days, 95% CI: −13.9 to 13.9, p>0.99), as was the proportion of women who underwent surgery >90 days after the index CNB (28/332[9.7%] vs. 4/41[9.8%], p>0.99).
Type and Number of Surgeries
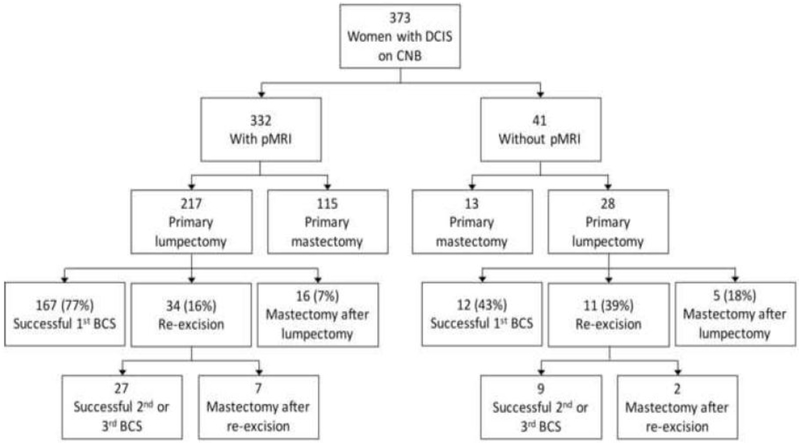
Patient distribution stratified by pMRI and no-pMRI is shown in Figure 1. The total number of surgeries per woman was significantly lower in the pMRI group than the no-pMRI group (mean=1.2 vs. 1.5, p<0.001), with 15% having more than one surgery in the pMRI group compared with 39% in the no-pMRI group (p<0.001, Table 2). All additional surgeries were performed because of close or positive margins, defined as <2 mm from inked surface. There were no significant differences between the pMRI vs. no-pMRI groups in rates of mastectomy as the initial (115/332[35%] vs. 13/42[31%], p=0.86) or final (138/332[42%] vs. 20/41[49%], p=0.41) surgery.
Figure 1. Distribution of surgeries in women stratified by pMRI versus no pMRI.
CNB = core needle biopsy
Table 2.
Outcomes of women who had pMRI or no pMRI
| Preoperative Breast MRI* | ||||
|---|---|---|---|---|
| Variable | Yes (N=332) |
No (N=41) |
P- value |
|
| Number of Additional Core Needle Biopsiesa | ||||
| Total number | 0 | 231 (69.6%) | 38 (90.5%) | 0.002 |
| 1 | 79 (23.8%) | 3 (7.3%) | ||
| 2 | 18 (5.4%) | 0 (0.0%) | ||
| 3 | 3 (0.9%) | 0 (0.0%) | ||
| 4 | 1 (0.3%) | 0 (0.0%) | ||
| Number with additional malignant findings | 0 | 266 (80.1) | 38 (92.7) | 0.096 |
| 1 | 55 (16.6) | 3 (7.3) | ||
| 2 | 9 (2.7) | 0 (0.0) | ||
| 3 | 2 (0.6) | 0 (0.0) | ||
| Timing of Surgery† | ||||
| Days between CNB and initial surgery | Median | 48 | 48 | >0.99 |
| Mean
± SD |
54.8 ± 28.2 | 48.0 ± 27.3 | 0.14 | |
| >90 days between CNB and initial surgery | 28 (9.7%) | 4 (9.8%) | 0.77 | |
| Type and Number of Surgeries | ||||
| Total number of surgeries | Mean
± SD |
1.2 ±0.4 | 1.5 ±0.7 | <0.001 |
| More than 1 surgery | 50 (15.1%) | 16 (39.0%) | <0.001 | |
| Initial surgery was a mastectomy | 115 (34.6%) | 13 (31.0%) | 0.86 | |
| Final surgery was a mastectomy | 138 (41.6%) | 20 (48.8%) | 0.41 | |
| Successful BCS (no mastectomy)‡ | 194 (89.4%) | 21 (75.0%) | 0.058 | |
| Single BCS‡ | 167 (77.0%) | 12 (42.9%) | <0.001 | |
| Total number of surgeries‡ | Mean
± SD |
1.3 ± 0.5 | 1.7 ± 0.7 | < 0.001 |
BCS = breast conserving surgery; CNB = core needle biopsy;
Values are mean ± SD, median, or n (%);
Based on a total of 331 women after excluding 42 women from the pMRI group who underwent MRI prior to the index CNB;
Based on a total of 245 women (217 with pMRI and 28 without) who underwent BCS as their first surgery
Of the 245 women who underwent BCS as their initial surgery, 217 had pMRI and 28 did not. Among these women who underwent BCS first, there was a trend towards more successful BCS in the pMRI group than the no-pMRI group, though not statistically significant (194/217[89%] vs. 21/28[75%], p=0.058). Those in the pMRI group had significantly fewer surgeries overall than those in the no-pMRI group (mean=1.3 vs. 1.7, p<0.001), with the pMRI group having a higher rate of a single successful BCS than the no-pMRI group (167/217[77%] vs. 12/28[43%], p<0.001). Case examples of women without and with pMRI are shown in Figures 2-3.
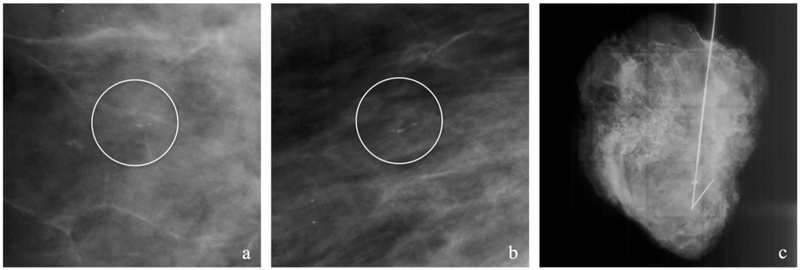
Figure 2. Case example of patient with no pMRI.
(a-c) Patient with biopsy proven intermediate grade DCIS with a 10 mm group of linear calcifications (circles) seen on cropped CC (a) and MLO (b) mammographic views. No pMRI was obtained and a wire-guided lumpectomy was performed with the surgical specimen (c) showing the targeted clip and wire. Pathology revealed DCIS spanning at least 22 mm with positive margins. The patient then underwent a skin-sparing mastectomy.
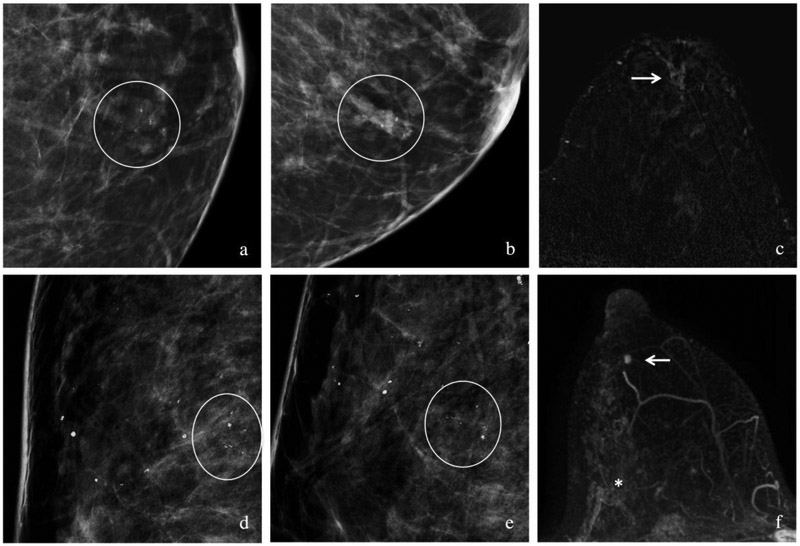
Figure 3. Case examples of patients with pMRI.
(a-c) Patient with biopsy proven intermediate grade DCIS with a focal asymmetry associated with amorphous calcifications on cropped CC (a) and MLO (b) views (circles) in the subareolar position. Preoperative MRI with post-contrast subtraction image (c) showed unifocal disease with non-mass enhancement (arrow) spanning 21 mm. The patient had a lumpectomy with negative margins.
(d-f) Patient with biopsy proven intermediate grade DCIS with grouped coarse heterogeneous calcifications in the upper outer quadrant on CC (d) and ML (e) spot-magnification views spanning 18 mm. A preoperative MRI with a maximum intensity projection image (f) showed non-mass enhancement spanning 54 mm in AP dimension, with the biopsy marker clip at the posterior (*) aspect of the non-mass enhancement. In addition, there was a separate mass at 6 o’clock (arrow). This was biopsied under MRI guidance and revealed an additional area of intermediate grade DCIS. A mastectomy was performed due to the patient’s multicentric disease which showed at least 52 mm of DCIS in the 9-12 o’clock region and at least 10 mm of DCIS at the 6 o’clock position.
Comparison of BCS Metrics in pMRI Cohort vs. Published Registry Data
Within the pMRI cohort, rate of successful BCS, rate of single successful BCS, and number of surgeries in women for whom BCS was attempted were compared to two prior studies by Morrow and colleagues (SEER data) and Langhans and colleagues (Danish National Registry data) (Table 3).(26, 27) No significant difference was identified in successful BCS rate between our cohort and DCIS cohorts from Morrow et al (1% difference, p=0.72) or Langhans et al (0% difference, p>0.99). However, patients in our pMRI cohort had a significantly higher rate of single successful BCS than that reported in Morrow et al (Δ=+20%, p<0.001) and Langhans et al (Δ=+14%, p<0.001). Our pMRI cohort also experienced a lower mean number of surgeries compared to Morrow et al (mean Δ=−0.22, p<0.001) and Langhans et al (mean Δ=− 0.17, p<0.001).
Table 3.
Comparison of BCS results for pure DCIS resection in the present study within pMRI cohort versus prior studies.
| Prior Studies | Differences | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present Study |
Morrow JAMA 2009 |
Langhans JAMA 2017 |
Present study vs. Morrow JAMA 2009 |
Present study vs. Langhans JAMA 2017 |
||||||||
| Variable | (N=217) | (N=359) | (N=727) | Value* | (95% Cl) |
p- value |
Value* | (95% CI) |
p- value |
|||
| Years | 2004-2013 | 2005-2007 | 2010-2013 | |||||||||
| Source | Institutional Database | SEER Registry | Danish National Registry | |||||||||
| Successful BCS | 194 (89.4%) | 318 (88.0%†) | 650 (89.4%) | 1% | (−4, 7%) | 0.69 | 0% | (−5, 5%) | >0.99 | |||
| Single successful BCS | 167 (77.0%) | 218 (57.3%†) | 456 (62.7%) | 20% | (11, 27%) | <0.001 | 14% | (7, 21%) | <0.001 | |||
| Number of surgeries | 1.3 ± 0.5 | 1.5 ± 0.6 | 1.4 ± 0.6 | −0.22 | (− 0.31, −0.12) | <0.001 | −0.17 | (− 0.25, 0.08) | <0.001 | |||
Values are the calculated as the difference in percentage or mean number of surgeries (present study minus the prior study);
Percentages are weighted to account for the survey design
Discussion
In this single-site retrospective study of women diagnosed with pure DCIS on CNB, we found that women who underwent pMRI had fewer total surgeries on average than women without pMRI. In the group of women whose initial surgery was lumpectomy, there was a higher rate of single successful BCS compared to women without pMRI. pMRI was associated little to no delay in surgery after DCIS diagnosis and there was no significant difference in the initial mastectomy rate. We found additional contralateral invasive disease in 2% of our patients and additional sites of DCIS in 34% of our patients with pMRI. Finally, we found higher rates of single successful BCS and lower number of total surgeries in the subset of our pMRI cohort for whom BCS was attempted when compared to published data using SEER and Danish National Registry data. Our single successful BCS rate is of 77.0% is also similar to the 78.5% rate reported in a recently published multi-centered trial which evaluated the use of pMRI in women with newly diagnosed DCIS who initially qualified for BCS on conventional imaging (mammography and ultrasound).(29) These results support our current use of pMRI for surgical management of DCIS at an academic center with a specialized multidisciplinary approach to breast cancer care.
Our results are in contrast to previous reports of no differences in total number of operations and increased mastectomy rates in patients newly diagnosed with DCIS with and without pMRI(16, 30). A recent meta-analysis of nine studies comparing pMRI and no-pMRI in the setting of DCIS concluded that pMRI had no impact on the number of operations while increasing the initial mastectomy rate, though final mastectomy rates were not significantly different(31). Limitations of this meta-analysis included limited outcomes data, lack of consistency in the quality of imaging or radiologist breast MRI experience, no prescribed requirement of image-guided CNB of additional findings on pMRI prior to surgical treatment, and lack of information on differences in patient and DCIS characteristics that may have impacted surgical approach.
At our institution, pMRI has been obtained routinely in patients newly diagnosed with DCIS or invasive breast cancer, and any pMRI-finding that would potentially impact surgical approach typically undergoes image-guided CNB. Accordingly, it is not surprising that women with pMRI were more likely to have an additional biopsy, in service of the primary goal of single successful breast cancer surgery without prompting overly extensive surgeries. The likelihood of such a CNB revealing malignancy was similar to that reported as a benchmark at the exam level for diagnostic breast MRI (PPV3 in this study was 40% vs. published MRI performance of 38%)(32). It is likely that pMRI has greater benefit when pMRI results are incorporated into a multidisciplinary approach to breast cancer treatment.
It has been suggested that a substantial drawback of pMRI is that it can lead to delays in surgery(14, 34). We estimated a mean delay of 6.8 days between index CNB and initial surgery for patients with pMRI relative with no-pMRI. However, this difference was not statistically significant and the upper bound of the 95% CI was approximately 2 weeks. Furthermore, the observed difference in median time to surgery between the two groups was zero days, and both groups had a similar proportion of women whose initial surgery was >90 days after the index CNB. Taken together, this suggests that if there was a delay in surgery due to pMRI in this study, it was relatively small. At our institution, MRI guided biopsy is readily available and there is a 48-72 hour turnaround time from CNB to pathology assessment, suggesting access to this advanced biopsy technique is an important consideration for a pMRI program. Our findings are similar to those of Pogathnik et al., who also demonstrated no delay in time from CNB to surgery between pMRI and no-pMRI groups at a single academic university hospital(35).
Additional strengths of our study include the similarity between pMRI and no-pMRI groups, with no statistically significant differences in patient or DCIS characteristics. Multiple prior studies have shown that younger, premenopausal women with dense breasts were more likely to receive pMRI and elect for more aggressive surgical approaches(20). We observed little difference between surgeons in their referral patterns for pMRI, suggesting that potential surgeon bias was not a confounder in our analysis. Finally, we found a lower number of surgeries for BCS and higher rate of single successful BCS in our pMRI patient cohort when compared to both U.S. and Danish National Registries.
Our study also has several important limitations. This was a retrospective audit of surgical outcomes in patients diagnosed with DCIS at an academic center with highly specialized breast cancer clinicians. Furthermore, pMRI is routinely obtained at our institution for most patients with a new diagnosis of either DCIS, resulting in a disproportionate number of patients in the pMRI group relative to the no-pMRI group. Consequently the no-pMRI group had a relatively small sample size limiting our ability to perform meaningful multivariate analysis. While this was mitigated in part by a lack of statistically significant differences in important clinical and pathologic features, there may have been selection bias as to which patients were in the no-pMRI group that was not detectable by our study. We also were only able to measure the number and types of surgeries and not the reasons behind initial surgical approach. Given the many factors known to impact shared decision-making between surgeons and patients when determining optimal DCIS treatment, it would be ideal to control for these factors between the pMRI and no-pMRI groups when assessing MRI’s impact on DCIS surgical treatment. Neither of the two prior publications used as references to confirm the clinical benefit of pMRI in our cohort reported pMRI rates, although pMRI utilization for the two SEER sites surveyed in the Morrow et al. study for newly diagnosed DCIS was about 24% in Los Angeles and 9% in Detroit (35) - much less than in our cohort. The mixture of women who did and did not have a pMRI in these two publications could also partially explain why our no-pMRI group had a lower single successful BCS compared to the reported numbers for Morrow et al. and Langhans et al. In addition, it is also possible that given the higher pMRI utilization at our site when compared to other sites, our surgeons plan and approach BCS differently when pMRI was obtained when compared to it was not obtained.
As our center also receives a number of referrals from outside institutions for second opinions, 37 of our 332 women with pMRI were from outside institutions. Although these were internally reviewed by our breast radiologists and all pMRIs were in accordance to the ACR Breast MRI accreditation program there may have been differences in original interpretation confounding patient and surgeon preference for initial surgery. Furthermore, patients were more likely to receive pMRI for DCIS if they were younger, have a larger size (>2cm) of known disease, and have no known comorbidities recently diagnosed(35). Finally, we were unable to assess whether pMRI is associated with longer-term benefits of improved disease-free survival given the relatively recent use of breast MRI.
Conclusion
pMRI may improve the surgical management of DCIS at an academic institution that routinely performs pMRI for new breast cancer diagnoses, leading to higher success rates of first BCS and lower re-excision rates, without causing a rise in mastectomies or delays in surgical treatment. Further studies are needed to understand the impact of pMRI on both short and long-term outcomes across a variety of practice types.
Acknowledgements
Funding: This study was supported by the 2014–2016 RSNA Research Scholar Grant (HR) and National Institutes of Health R01 [Grant Number CA203883] (HR, SCP, AK, DSH).
Abbreviations List:
- BCS
breast conserving surgery
- pMRI
preoperative breast MRI
- CNB
core needle biopsy
Footnotes
Disclosures: JML and CDL have a consulting agreement with GE Healthcare. DLL, SCP, AK, DSH, CDL, JML, and HR have received grant funding from GE Healthcare. DSH has also received grant funding from Philips Healthcare, Toshiba America Medical Systems, and Siemens Medical Solutions USA. JS and SHJ have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. Cancer: A Cancer Journal for Clinicians. 2017; 67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project experience. Seminars in Oncology. 2004; 28(4):400–18. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Pérez C, Turnbull AK, Ekatah GE, et al. Current treatment trends and the need for better predictive tools in the management of ductal carcinoma in situ of the breast. Cancer Treatment Reviews. 2017; 55:163–72. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2015. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html.
- 5.Bleicher RJ, Morrow M. MRI and breast cancer: role in detection, diagnosis, and staging. Oncology (Williston Park, NY). 2007; 21(12):1521–8, 30; discussion 30, 32-3. [PubMed] [Google Scholar]
- 6.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003; 10(4):381–8. [DOI] [PubMed] [Google Scholar]
- 7.Doyle AJ, Prakash S, Wang K, Cranshaw I, Taylor E, Oldfield R. DCIS of the breast: The value of preoperative MRI. J Med Imaging Radiat Oncol. 2016; 60(2):194–8. [DOI] [PubMed] [Google Scholar]
- 8.Lehman CD. Magnetic Resonance Imaging in the Evaluation of Ductal Carcinoma In Situ. Journal of the National Cancer Institute Monographs. 2010; 2010(41):150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baur A, Bahrs SD, Speck S, et al. Breast MRI of pure ductal carcinoma in situ: sensitivity of diagnosis and influence of lesion characteristics. Eur J Radiol. 2013; 82(10):1731–7. [DOI] [PubMed] [Google Scholar]
- 10.Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005; 11(6):382–90. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology. 2009; 253(2):281–3. [DOI] [PubMed] [Google Scholar]
- 12.Esserman LJ, Kumar AS, Herrera AF, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006; 24(28):4603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis KL, Barth RJ Jr., Gui J, Dann E, Eisenberg B, Rosenkranz K. Use of MRI in preoperative planning for women with newly diagnosed DCIS: risk or benefit? Ann Surg Oncol. 2012; 19(10):3270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallemand M, Barron M, Bingham J, Mosier A, Hardin M, Sohn V. The true impact of breast magnetic resonance imaging on the management of in situ disease: more is not better. Am J Surg. 2017; 213(1):127–31. [DOI] [PubMed] [Google Scholar]
- 15.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009; 27(25):4082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itakura K, Lessing J, Sakata T, et al. The Impact of Preoperative Magnetic Resonance Imaging on Surgical Treatment and Outcomes for Ductal Carcinoma In Situ. Clinical breast cancer. 2011; ll(l):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013; 257(2):249–55. [DOI] [PubMed] [Google Scholar]
- 18.Pilewskie M, King TA. Magnetic resonance imaging in patients with newly diagnosed breast cancer: a review of the literature. Cancer. 2014; 120(14):2080–9. [DOI] [PubMed] [Google Scholar]
- 19.Morris EA. Should we dispense with preoperative breast MRI? Lancet. 2010; 375(9714):528–30. [DOI] [PubMed] [Google Scholar]
- 20.Rahbar H, Lehman CD. Rethinking Preoperative Breast Magnetic Resonance Imaging. JAMA Oncol. 2015; l(9):1226–7. [DOI] [PubMed] [Google Scholar]
- 21.Peters NH, van Esser S, van den Bosch MA, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. European Journal of Cancer. 2011; 47(6):879–86. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010; 375(9714):563–71. [DOI] [PubMed] [Google Scholar]
- 23.Kropcho LC, Steen ST, Chung AP, Sim MS, Kirsch DL, Giuliano AE. Preoperative breast MRI in the surgical treatment of ductal carcinoma in situ. Breast J. 2012; 18(2):151–6. [DOI] [PubMed] [Google Scholar]
- 24.American College of Radiology. ACR Breast MRI Accreditation. Updated Nov 09 2018; cited 2018 Nov 25; Available from: https://www.acraccreditation.org/Modalities/Breast-MRI.
- 25.D’Orsi CJ SE, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 26.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009; 302(14):1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhans L, Jensen MB, Talman MM, Vejborg I, Kroman N, Tvedskov TF. Reoperation Rates in Ductal Carcinoma In Situ vs Invasive Breast Cancer After Wire-Guided Breast-Conserving Surgery. JAMA Surg. 2017; 152(4):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonett DG, Price RM. Statistical inference for a linear function of medians: Confidence intervals, hypothesis testing, and sample size requirements. Psychological Methods. 2002; 7(3):370–83. [DOI] [PubMed] [Google Scholar]
- 29.Lehman CD, Gatsonis C, Romanoff J, et al. Association of Magnetic Resonance Imaging and a 12-Gene Expression Assay With Breast Ductal Carcinoma In Situ Treatment JAMA Oncol. 2019. January 17. doi: 10.1001/jamaoncol.2018.6269. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilewskie M, Kennedy C, Shappell C, et al. Effect of MRI on the management of ductal carcinoma in situ of the breast. Ann Surg Oncol. 2013; 20(5):1522–9. [DOI] [PubMed] [Google Scholar]
- 31.Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. The British Journal of Surgery. 2015; 102(8):883–93. [DOI] [PubMed] [Google Scholar]
- 32.Niell BL, Gavenonis SC, Motazedi T, et al. Auditing a Breast MRI Practice: Performance Measures for Screening and Diagnostic Breast MRI. Journal of the American College of Radiology. 2014; 11(9):883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. Journal of the American College of Surgeons. 2009; 209(2):180–7; 294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogatchnik B, Kuehn-Hajder J, Nelson M, Emory T. No Effect of Pretreatment Breast MRI on the Timing of Surgical Treatment of Newly Diagnosed Breast Cancer. Journal of the American College of Radiology. 2017; 14(10):1310–5. [DOI] [PubMed] [Google Scholar]
- 35.Henderson LM, Weiss J, Hubbard RA, et al. Factors Associated with Preoperative Magnetic Resonance Imaging Use among Medicare Beneficiaries with Nonmetastatic Breast Cancer. The Breast Journal. 2016; 22(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]