Abstract
Purpose:
5-fluorouracil/leucovorin, irinotecan, and nab-paclitaxel are all active agents in gastrointestinal cancers; the combination, FOLFIRABRAX, has not been previously evaluated. UDP Glucuronosyltransferase 1A1 (UGT1A1) clears SN-38, the active metabolite of irinotecan. UGT1A1*28 polymorphism reduces UGT1A1 enzymatic activity and predisposes to toxicity. We performed a trial to assess the safety and tolerability of FOLFIRABRAX with UGT1A1 genotype-guided dosing of irinotecan.
Experimental Design:
Patients with previously untreated, advanced gastrointestinal cancers received FOLFIRABRAX with prophylactic pegfilgrastim every 14 days. UGT1A1 *1/*1, *1/*28, and *28/*28 patients received initial irinotecan doses of 180, 135, and 90 mg/m2, respectively. 5-FU 2400 mg/m2 over 46 hours, leucovorin 400 mg/m2, and nab-paclitaxel 125 mg/m2 were administered. Doses were deemed tolerable if the dose limiting toxicity (DLT) rate during cycle 1 was ≤35% in each genotype group. DLTs were monitored using a sequential procedure.
Results:
Fifty patients enrolled: 30 pancreatic, 9 biliary tract, 6 gastroesophageal, and 5 others. DLTs occurred in 5/23 (22%) *1/*1 patients, 1/19 (5%) *1/*28 patients, and 0/7 *28/*28 patients. DLTs were all grade 3: diarrhea (3 patients), nausea (2 patients), and febrile neutropenia (1 patient). The overall response rate was 31%. Response rates in pancreatic, gastroesophageal, and biliary tract cancers were 34%, 50%, and 11%, respectively. Eighteen patients (36%) received therapy for at least 24 weeks.
Conclusion:
FOLFIRABRAX with genotype-guided dosing of irinotecan is tolerable in patients with advanced gastrointestinal cancer and UGT1A1*1*1 or UGT1A1*1*28 genotypes. Too few *28/*28 patients were enrolled to provide conclusive results. Responses occurred across multiple tumor types.
Keywords: UGT1A1, Pancreatic cancer, Biliary tract cancer, Gastric Cancer, Irinotecan, Nab-paclitaxel, 5-fluorouracil
INTRODUCTION
The combination of nab-paclitaxel and gemcitabine (125 mg/m2 and 1000 mg/m2, respectively, on days 1, 8, and 15 of a 4 week cycle) improves survival compared to gemcitabine in metastatic pancreatic cancer; however, with moderate grade 3-5 adverse effects (AEs) including neutropenia (38%), febrile neutropenia (3%), and peripheral neuropathy (17%).(1) FOLFIRINOX (5-Fluorouracil (5-FU) 400 mg/m2, infusional 5-FU 2400 mg/m2 over 46 hours, leucovorin 400 mg/m2, irinotecan 180 mg/m2, and oxaliplatin 85 mg/m2 given every 14 days) also improves survival compared to gemcitabine in metastatic pancreatic cancer.(2) However, this regimen also produces grade 3-4 toxicities including neutropenia (45.7%), febrile neutropenia (5.4%), diarrhea (12.7%), and sensory neuropathy (9.0%).(2) A number of trials have been conducted with modified FOLFIRINOX (mFOLFIRINOX) in which 5-FU, irinotecan and/or oxaliplatin have been dose-reduced to decrease AEs.(3–7) Even with these dose adjustments, FOLFIRINOX remains a toxic regimen.
Taxanes have previously been combined with fluoropyrimidines, such as for the treatment of gastric cancer.(8,9) Taxanes have also been combined with irinotecan. The combination of docetaxel and irinotecan was explored in a phase I study in patients with advanced solid tumors with safety established and antitumor activity observed at all dose levels.(10) The combination of paclitaxel and irinotecan administered every two weeks was explored in a phase I/II study in patients with advanced non-small cell lung cancer and was found to be effective, with 19% of patients developing Grade ¾ myelosuppression.(11) To our knowledge, triple combination therapies with taxanes, fluoropyrimidines, and irinotecan have not previously been studied.
The UGT1A1 gene encodes the enzyme UDP-glucuronosyltransferase 1 family polypeptide A1, which glucuronidates SN-38, the active metabolite of irinotecan. There are germline polymorphisms in the UGT1A1 gene based on the number of TA repeats in the promoter region. There are six TA repeats in the wild-type allele (UGT1A1*1); the most frequent variant allele (UGT1A1*28) has seven TA repeats. The UGT1A1*28 polymorphism leads to decreased enzyme activity, resulting in reduced clearance of SN-38 and increased toxicity. Patients with the UGT1A1*28/*28 genotype have a dose-dependent greater risk of grade 3-4 hematologic toxicity and diarrhea.(12–14) The product label for irinotecan suggests that patients with the UGT1A1*28*28 genotype receive a lower dose but does not make a specific dose recommendation.(15) We recently reported a study of UGT1A1 genotype-guided dosing of mFOLFIRINOX in advanced gastrointestinal malignancies.(7) Tolerability could not be established in pancreatic and biliary tract cancers based upon prespecified criteria, however the regimen was effective. Although tolerability of a UGT1A1 genotype-guided dosing was not demonstrated with mFOLFIRINOX, the trial used conservative definitions of DLT and tolerability that were not applied in the original FOLFIRINOX trial.(16) The assumption is that genotype-guided dose reduction of irinotecan can only reduce toxicity and therefore we wanted to investigate this approach with FOLFIRABRAX.
The clinical efficacy of nab-paclitaxel in metastatic pancreatic cancer has raised questions about whether it might also have efficacy in other gastrointestinal malignancies and whether gemcitabine is the best chemotherapy drug to use in combination with nab-paclitaxel. While the combination of 5-FU, irinotecan and oxaliplatin is effective in advanced pancreatic cancer, neuropathy often limits its use and we hypothesized that nab-paclitaxel may result in less neuropathy when substituted for oxaliplatin. In this study, we sought to assess the safety and tolerability of nab-paclitaxel in combination with 5-FU, leucovorin, and irinotecan – together known as FOLFIRABRAX – with UGT1A1 genotype-guided dosing of irinotecan in patients with advanced gastrointestinal malignancies.
PATIENTS AND METHODS
Patient Population
This study enrolled patients with untreated locally advanced or metastatic pancreatic cancer, gastroesophageal adenocarcinoma, biliary tract cancers (including gallbladder adenocarcinoma, ampullary adenocarcinoma, and cholangiocarcinoma), adenocarcinoma of unclear primary (with a gastrointestinal primary suspected), or any other primary gastrointestinal malignancy felt to be appropriate by the treating physician. Age ≥18 years and Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 were required. Adequate organ function was required as defined by absolute neutrophil count (ANC) ≥1500/μL, hemoglobin >9g/dL, platelets >100,000/μL, total bilirubin <1.25x upper limit of normal (ULN), creatinine ≤1.5mg/dL, AST and ALT ≤2.5x ULN, and alkaline phosphatase ≤2.5x ULN unless bone metastasis was present in the absence of liver metastasis. Patients taking substrates, inhibitors, or inducers of CYP3A4 were encouraged to switch to alternative drugs if possible, given the potential for drug-drug interactions with irinotecan. Patients were excluded if they had received prior chemotherapy or radiation therapy for any cancer; diarrhea ≥ grade 1; neuropathy ≥ grade 2, as per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE)(17); or any UGT1A1 polymorphism other than *1 or *28. Additional enrollment criteria are described in the Supplementary Methods.
Study Design
This was a multicenter prospective trial conducted at eight sites. Institutional review boards approved this study and all participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki. The primary objective was to determine the dose-limiting toxicity (DLT) rate in cycle 1 in the two most common UGT1A1 genotype groups (*1/*1, *1/*28), using genotype-guided dosing of irinotecan in FOLFIRABRAX. Patients with the less common *28/*28 genotype were also enrolled so that exploratory results could be obtained in this population. This study design of creating cohorts by genotype was similar to our previous study with mFOLFIRINOX.(7) Secondary objectives were to determine the cumulative dose intensity of irinotecan in each genotype group (Supplemental Methods) and the objective response rate (ORR) by response evaluation criteria in solid tumors (RECIST) version 1.1 by tumor type.
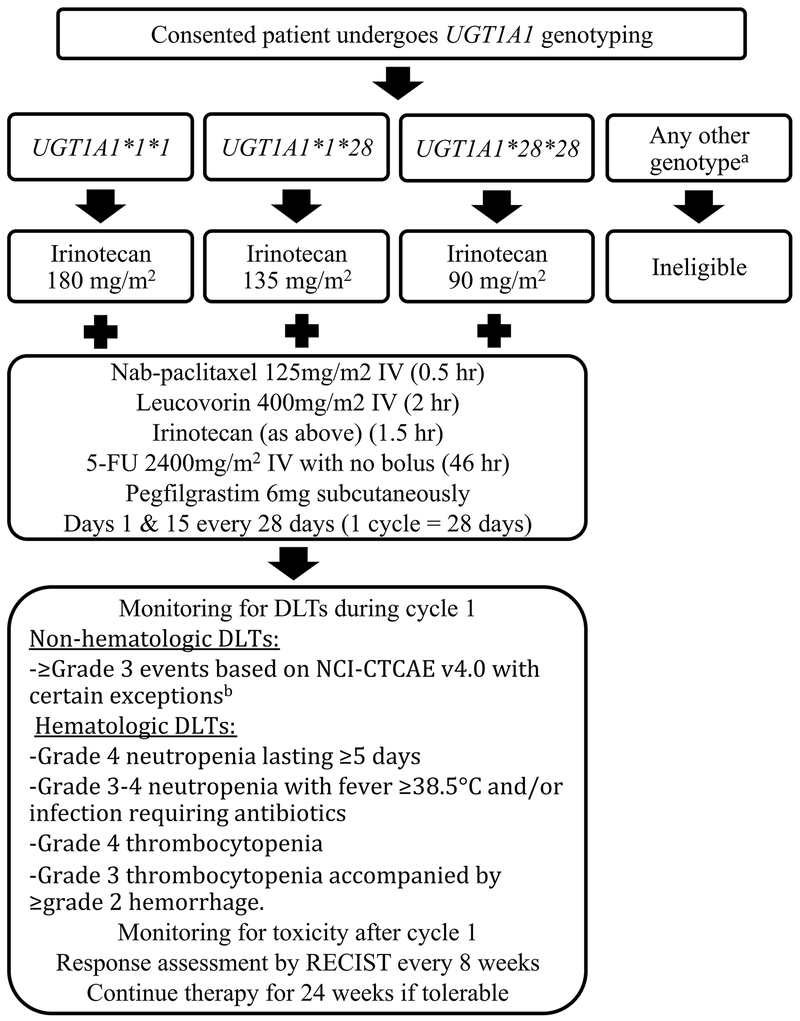
Consented patients underwent UGT1A1 genotyping at The University of Chicago Genetic Services Laboratory (Clinical Laboratory Improvement Amendments certified) as described previously.(18) Based on the results, patients were assigned to the UGT1A1*1/*1, UGT1A1*1/*28, or UGT1A1*28/*28 groups. The treatment schema is shown in Figure 1. All patients received FOLFIRABRAX with irinotecan dose based on UGT1A1 genotype: UGT1A1*1/*1: 180mg/m2; UGT1A1*1/*28: 135 mg/m2; UGT1A1*28/*28: 90 mg/m2. Previous pharmacokinetic data showed that exposure to SN-38 was approximately double in *28/*28 patients compared to *1/*1 patients, with an intermediate exposure in *1/*28 patients.(14) Given that the standard starting dose of irinotecan in FOLFIRINOX is 180 mg/m2, a dose of 90 mg/m2 in *28/*28 patients and 135 mg/m2 in *1/*28 patients would result in similar exposure to SN-38 across genotypes. Patients also received nab-paclitaxel 125mg/m2, leucovorin 400mg/m2, and 5-FU 2400mg/m2 over 46 hours (no bolus). FOLFIRABRAX was given every 14 days, on days 1 and 15 of each 28-day cycle. Prophylactic pegfilgrastim 6 mg subcutaneously was given on days 4 and 18 of each cycle. Treatment was continued until progressive disease, death, unacceptable toxicity, or withdrawal of patient consent. Guidelines were in place for treatment delays and dose reductions (Supplemental Table 1).
Figure 1:
Treatment schema based on UGT1A1 genotype.
aPatients were also tested for the UGT1A1*6 variant
bThe following did not constitute a DLT: Grade 3 nausea, vomiting or diarrhea occurring without optimal medical management; grade 3 or 4 nausea, vomiting or diarrhea that resolves (grade </= 2) within 48 hours; elevated amylase or lipase in the absence of clinical signs or symptoms of pancreatitis, grade 3 hyperglycemia, abnormalities of liver function tests clearly related to malignant biliary obstruction, or other grade 3 AEs deemed clinically insignificant (such as laboratory abnormalities not requiring therapeutic intervention or change in management).
DLTs were recorded during the initial 28 days (first cycle) of treatment. Non-hematologic DLTs were ≥grade 3 events based on NCI-CTCAE v. 4.0 with the exceptions of nausea, vomiting or diarrhea occurring without optimal medical management; grade 3 or 4 nausea, vomiting or diarrhea that resolves (≤grade 2) within 48 hours; elevated amylase or lipase in the absence of clinical signs or symptoms of pancreatitis; grade 3 hyperglycemia; abnormalities of liver function tests clearly related to malignant biliary obstruction; or other grade 3 AEs deemed clinically insignificant (such as laboratory abnormalities not requiring therapeutic intervention or change in management). Hematologic DLTs were grade 4 neutropenia lasting ≥5 days; grade 3-4 neutropenia with fever ≥38.5 degrees Celsius and/or infection requiring antibiotics; grade 4 thrombocytopenia; and grade 3 thrombocytopenia accompanied by ≥grade 2 hemorrhage. Delay of cycle 1 day 15 dose of mFOLFIRINOX by more than 14 days constituted a DLT if the reason was attributable to study treatment. CT was performed every 8 weeks and response was evaluated according to RECIST version 1.1.(19)
Statistical analysis
The primary endpoint was the DLT rate in cycle 1 for each of the three genotype groups (*1/*1, *1/*28, *28/*28). The regimen was defined as tolerable if the DLT rate was less than or equal to 35%, which is comparable to the standard 3+3 phase I design with less than 2 out of 6 patients experiencing a DLT. To monitor toxicity, we employed the sequential design described by Goldman.(20) Specifically, letting π denote the true DLT rate, we tested the null hypothesis H0: π = π0 vs. HA: π= πA, where π0 and πA are acceptable and unacceptable rates of toxicity. Here we chose π0=0.15 and πA=0.35. A design that achieved an alpha level of 0.05 and 80% power was to enroll a maximum of 17 patients. If DLTs were observed in 4 of the first 4 patients, 5 of the first 8, 6 of the first 13, or 7 of 17 patients, the null hypothesis was to be rejected and the dose considered too toxic. Otherwise, if 6 or fewer of 17 patients (<=35%) experienced a DLT, the dose was considered acceptable. The maximum sample size necessary was 51 patients (17 per genotype group).
RESULTS
Fifty patients enrolled from February 2015 to November 2017. The protocol was amended to allow for over-enrolment to the *1/*1 group (beyond 17 patients) until the *1/*28 group was fully accrued (17 evaluable patients). Given the low prevalence of the *28/*28 genotype, it was not feasible to accrue enough patients to this group to assess tolerability. The trial was closed after achieving accrual goals for the two more common genotypes. Thirty patients (60%) had pancreatic cancer, nine (18%) had biliary tract cancers, 6 (12%) had gastroesophageal cancer, three (6%) had unknown primary of suspected gastrointestinal source, one (2%) had a cecal neuroendocrine cancer, and one (2%) had hepatocellular carcinoma. Median age was 63 years (range 37-82 years). Twenty-three patients (46%) were in the UGT1A1 *1/*1 group, 20 (40%) in the *1/*28 group, and 7 (14%) in the *28/*28 group. Patient characteristics are summarized in Table 1.
Table 1:
Demographic and Clinical Characteristics
| All Patients (n=50) | UGT1A1 genotype group | |||
|---|---|---|---|---|
| *1/*1 (n=23) | *1/*28 (n=20) | *28/*28 (n=7) | ||
| Median Age (years, range) | 63 (37-82) | 63 (37-74) | 63 (45-82) | 65 (51-75) |
| Sex | ||||
| Female | 25 (50%) | 9 (39%) | 12 (60%) | 4 (57%) |
| Male | 25 (50%) | 14 (61%) | 8 (40%) | 3 |
| ECOG | ||||
| 0 | 20 (40%) | 10 (43%) | 9 (45%) | 1 (14%) |
| 1 | 30 (60%) | 13 (57%) | 11 (55%) | 6 (86%) |
| Race | ||||
| White | 43 (86%) | 21 (91%) | 15 (75%) | 7 (100%) |
| Black or African American | 7 (14%) | 2 (9%) | 5 (25%) | 0 |
| Diagnosis | ||||
| Pancreatic cancer | 30 (60%) | 13 (57%) | 12 (60%) | 5 (71%) |
| Biliary tract cancer | 9 (18%) | 5 (22%) | 3 (15%) | 1 (14%) |
| Gastroesophageal cancer | 6 (12%) | 2 (9%) | 3 (15%) | 1 (14%) |
| Unknown GI primary | 3 (6%) | 1 (4%) | 2 (10%) | 0 |
| Cecal neuroendocrine cancer | 1 (2%) | 1 (4%) | 0 | 0 |
| Hepatocellular carcinoma | 1 (2%) | 1 (4%) | 0 | 0 |
The DLT rate was 21.7% (5/23 patients, upper one-sided 90% confidence limit [CL]=36.6%) in the UGT1A1*1/*1 group, 5.3% (1/19, upper 90% CL=19.0%) in the UGT1A1*1/*28 group, and 0% (0/7, upper one-sided CL=28.0%) in the UGT1A1*28/*28 group. During sequential monitoring, none of the stopping boundaries was reached. Thus, FOLFIRABRAX was tolerable in the *1/*1 and *28/*28 groups, i.e., the observed DLT rates were all <35%, although the upper CL in the *1/*1 group did exceed 35%. DLTs were grade 3 diarrhea (3 patients, 6%), grade 3 nausea (2 patients, 4%), and grade 3 neutropenic fever (1 patient, 2%). Although there were not enough patients accrued to the *28/*28 group to declare tolerability per the prespecified protocol criteria, there were no DLTs in these 7 patients. One patient with pancreatic cancer in the UGT1A1*1/*28 group was not evaluable for DLT due to 5-FU pump malfunction, as the patient did not receive the full dose of 5-FU. Results are summarized in Table 2.
Table 2:
Dose-Limiting Toxicities and Response Rates by Genotype
| UGT1A1 genotype group | Overall (n=49) | |||
|---|---|---|---|---|
| *1/*1 (n=23) | *1/*28 (n=19) | *28/*28 (n=7) | ||
| # with DLTs (%) | 5 (22%) | 1 (5%) | 0 | 6 (12%) |
| DLT Description | Gr 3 diarrhea (3 pts) Gr 3 nausea (1 pt) Grade 3 FN (1 pt) |
Grade 3 nausea (1 pt) | Gr 3 diarrhea (3 pts) Gr 3 nausea (2 pts) Grade 3 FN (1 pt) |
|
| Complete Response | 1 (4%) | 0 | 0 | 1 (2%) |
| Partial Response | 6 (26%) | 6 (32%) | 2 (29%) | 14 (29%) |
| Stable Disease | 11 (48%) | 9 (47%) | 4 (57%) | 24 (49%) |
| Disease Control | 18 (78%) | 15 (79%) | 6 (86%) | 39 (80%) |
| Progressive Disease | 4 (17%) | 2 (11%) | 1 (14%) | 7 (14%) |
| Not Evaluable | 1 (4%) | 2 (11%) | 0 | 3 (6%) |
Abbreviations: DLT: Dose limiting toxicity; FN: Febrile neutropenia; Gr: Grade; pt: patient
Forty-nine patients were evaluable for best response per RECIST 1.1. In 23 patients with *1/*1 genotype, 1 (4%) had a complete response (CR), 6 (26%) had a partial response (PR), 11 (48%) had stable disease (SD), 4 (17%) had progressive disease (PD), and 1 (4%) was non-evaluable (NE) for response. In 19 patients with *1/*28 genotype, 6 (32%) had a PR, 9 (47%) had SD, 2 (11%) had PD, and 2 (11%) were NE for response. In 7 patients with *28/*28 genotype, 2 (29%) had a PR, 4 (57%) had SD, and 1 (14%) had PD. These data are summarized in Table 2.
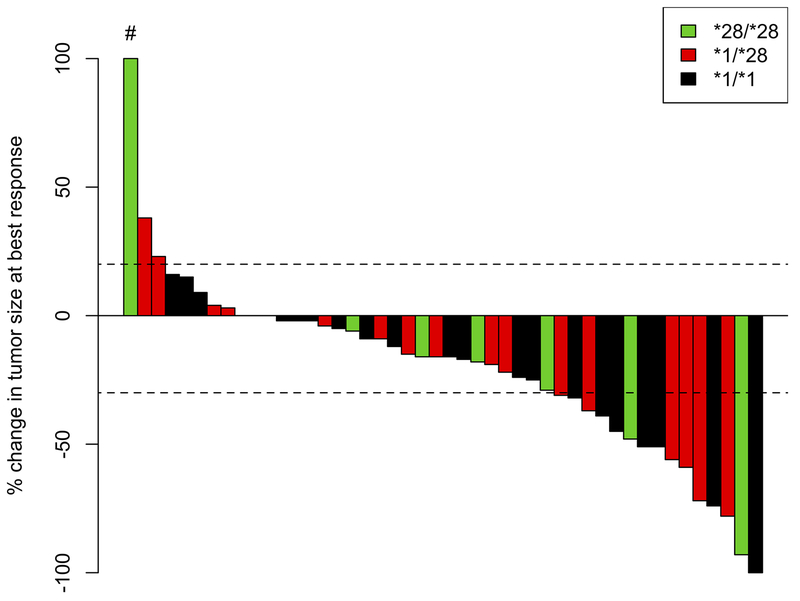
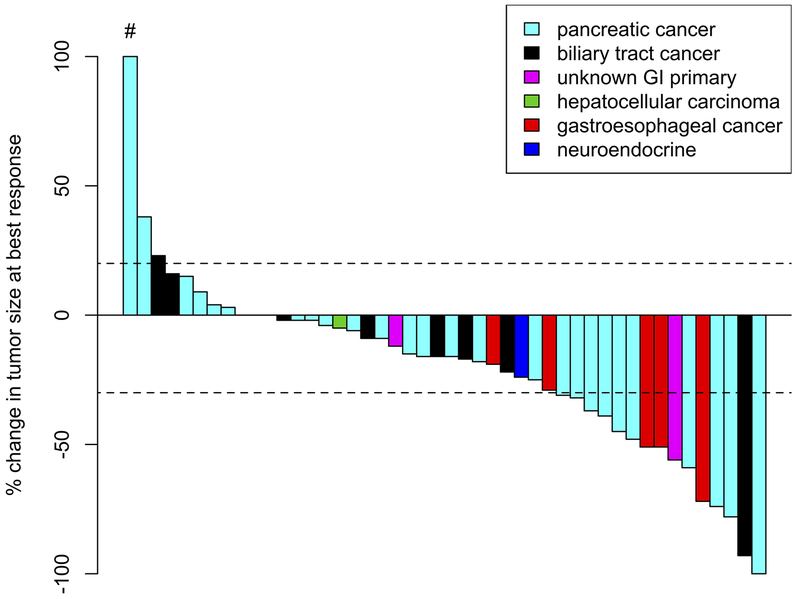
Response was also assessed by tumor type. In 29 patients with pancreatic cancer, 1 (3%) achieved a CR, 9 (31%) had a PR, 13 (45%) had SD, 4 (14%) had PD, and 2 (7%) were NE for response. In 9 patients with biliary tract cancer, 1 (11%) had PR, 5 (56%) had SD, and 3 (33%) had PD. In 6 patients with gastroesophageal cancer, 3 (50%) had PR, 2 (33%) had SD, and 1 (17%) was NE due to toxicity. In 3 patients with an unknown primary of suspected gastrointestinal origin, 1 (33%) had a PR and 2 (67%) had SD. These data are summarized in Table 3. A waterfall plot of evaluable patients and their best responses is shown in Figure 2 (by genotype) and Figure 3 (by tumor type).
Table 3:
Best Response by RECIST v1.1 By Tumor Type
| Best response | Pancreatic Cancer n=29 | Biliary Tract Cancer (n=9) | Gastroesophageal Cancer (n=6) | Unknown Primary of Suspected GI Origin (n=3) | Neuroendocrine Tumor (n=1) | HCC (n=1) | All Tumors (n=49) |
|---|---|---|---|---|---|---|---|
| Complete Response | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 1 (2%) |
| Partial Response | 9 (31%) | 1 (11%) | 3 (50%) | 1 (33%) | 0 | 0 | 14 (29%) |
| Stable Disease | 13 (45%) | 5 (56%) | 2 (33%) | 2 (67%) | 1 (100%) | 1 (100%) | 24 (49%) |
| Disease Control | 23 (79%) | 6 (67%) | 5 (83%) | 3 (100%) | 1 (100%) | 1 (100%) | 39 (80%) |
| Progressive Disease | 4 (14%) | 3 (33%) | 0 | 0 | 0 | 0 | 7 (14%) |
| Not evaluable | 2 (7%) | 0 | 1 (17%) | 0 | 0 | 0 | 3 (6%) |
Figure 2:
Waterfall plot of best response by genotype.
#This patient had a 264% increase in tumor size
Figure 3:
Waterfall plot of best response by tumor type.
#This patient had a 264% increase in tumor size
Among evaluable patients who did not discontinue treatment due to DLT, 47 patients received a mean of 94.2% of the expected irinotecan dose over a mean of 10.8 doses. Patients with *1/*1, *1/*28, and *28/*28 genotypes received 94.2%, 94.8%, and 94.7% of the expected irinotecan dose, respectively, over 10.8 (range 2-27), 10.7 (range 2-45), and 11.0 (range 2-24) doses, respectively.
Notably, 18 out of 49 patients (37%) have remained on therapy for at least 24 weeks (12 doses of FOLFIRABRAX) and 7 of 49 patients (14%) were treated for at least 48 weeks (24 doses of FOLFIRABRAX). One patient received FOLFIRABRAX for 24 weeks, and then discontinued nab-paclitaxel but continued on 5-FU and irinotecan for at least another 24 weeks. One patient with hilar cholangiocarcinoma was initially deemed unresectable. She received 27 doses (approximately 13 months) of FOLFIRABRAX and was deemed to have SD. She ultimately underwent extended left hepatic lobectomy and removal of a portal lymph node. Pathology revealed ypT2N0 cholangiocarcinoma with grade 3 treatment effect. She received adjuvant gemcitabine and oxaliplatin and remains free of disease 17 months after surgery.
The most common ≥grade 2 adverse events related to treatment and occurring in >5% of 49 evaluable patients were diarrhea (16 patients, 33%), fatigue (14, 29%), nausea (13, 27%), anorexia (10, 20%), alopecia (9, 18%), decreased neutrophil count (7, 14%), anemia (5, 10%), decreased white blood cells (5, 10%), and vomiting (5, 10%) (Table 4). Notably, only 1 patient (2%) had grade 2 peripheral sensory neuropathy and 1 patient (2%) had grade 3 peripheral sensory neuropathy. The latter patient discontinued nab-paclitaxel after 24 weeks (12 doses of FOLFIRABRAX) and continued on 5-FU, leucovorin and irinotecan for >64 weeks. There were no deaths attributed to therapy.
Table 4:
Grade 2 or Higher Adverse Eventsa Occurring in >5% of Patients (N=49)
| Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|
| Diarrhea | 9 (18%) | 7 (14%) | 0 | 16 (33%) |
| Fatigue | 12 (25%) | 2 (4%) | 0 | 14 (29%) |
| Nausea | 8 (16%) | 5 (10%) | 0 | 13 (27%) |
| Anorexia | 9 (18%) | 1 (2%) | 0 | 10 (20%) |
| Alopecia | 9 (18%) | 0 | 0 | 9 (18%) |
| Neutrophil count decreased | 2 (4%) | 3 (6%) | 2 (4%) | 7 (14%) |
| Anemia | 5 (10%) | 0 | 0 | 5 (10%) |
| White blood cell decreased | 3 (6%) | 1 (2%) | 1 (2%) | 5 (10%) |
| Vomiting | 4 (8%) | 1 (2%) | 0 | 5 (10%) |
| Constipation | 3 (6%) | 1 (2%) | 0 | 4 (8%) |
| Abdominal pain | 1 (2%) | 2 (4%) | 0 | 3 (6%) |
| Alkaline phosphatase increased | 2 (4%) | 1 (2%) | 0 | 3 (6%) |
| Dysgeusia | 3 (6%) | 0 | 0 | 3 (6%) |
| Infusion related reaction | 3 (6%) | 0 | 0 | 3 (6%) |
| Platelet count decreased | 3 (6%) | 0 | 0 | 3 (6%) |
According to NCI-CTCAE v. 4.0
AEs attribution was definitely, probable, possible, or unknown/blank to study drug. AEs where attribution was not related or unlikely were not included.
DISCUSSION
In this study we observed that FOLFIRABRAX with genotype-guided dosing of irinotecan is tolerable in patients with advanced GI cancers and UGT1A1*1*1 or UGT1A1*1*28 genotypes. Exploratory analysis also suggests tolerability in the UGT1A1*28*28 genotype. Responses were observed in pancreatic cancer, gastroesophageal cancer, biliary tract cancer, and cancer of unknown primary with suspected GI origin.
This is the first study to combine 5-FU/leucovorin, irinotecan, and nab-paclitaxel in the treatment of any malignancy. We hypothesized that the substitution of nab-paclitaxel for oxaliplatin could improve tolerability while maintaining efficacy. Given the prevalence of the UGT1A1*28 allele and the potential for DLTs related to the overlapping toxicities of irinotecan and nab-paclitaxel, we attempted to optimize tolerability of this regimen by using UGT1A1 genotype-guided dosing of irinotecan. There is considerable variability in the frequency of the *28/*28 genotype, with reports noting 16% prevalence in Hispanic patients, 9-17% in patients of European descent, 0-33% in patients of African descent, and 1-4% in patients of Japanese or Chinese descent.(21–23) Prior pharmacokinetic studies established the rationale for examining genotype-guided dosing of irinotecan. A dose-finding study of irinotecan in *1/*1, *1/*28, and *28/*28 patients demonstrated a linear relationship between exposure to SN-38 and UGT1A1*28 status with maximal exposures in *28/*28 patients.(24) A trial investigating genotype-guided dosing of irinotecan in FOLFIRI found that when *28/*28 patients were excluded, patients with *1/*1 and *1/*28 genotypes could tolerate greater doses of irinotecan.(25)
In our previous genotype-guided mFOLFIRINOX trial(7), we used a rather conservative definition of tolerability (requiring that the upper confidence level be <33%) given that the regimen was not a novel combination of drugs and we wanted to show improved tolerability compared to historical controls with FOLFIRINOX. However, in this current trial we used a less conservative definition of tolerability that required the observed DLT rate be ≤35%. This DLT cut-off was based on the traditional 3+3 design of phase I trials in which dose escalation continues until at least two patients in a cohort of three to six patients experience a DLT. The observed DLT rates were well below 35% for both the *1/*1 and *1/*28 cohorts, although the upper CL in the *1/*1 group exceeded 35%. Only 1 patient (5%) in the *1/*28 group and no patients in the *28/*28 group experienced a DLT, raising the question of whether the dose reductions for irinotecan in these cohorts may have been more than necessary. However, additional studies would be needed to explore the safety of a smaller dose reduction.
In 29 patients with pancreatic cancer treated with FOLFIRABRAX, 79% had disease control (3% CR, 31% PR, 45% SD). This is comparable to historical controls with FOLFIRINOX, which has a 70% disease control rate (0.6% CR, 31% PR, 39% SD).(2) Recognizing the limitations of cross trial comparisons, our results suggest that FOLFIRABRAX does not compromise response rate in pancreatic cancer. Moreover, FOLFIRABRAX appears to be well tolerated with a low DLT rate and no grade 4-5 DLTs.
When compared to historical patients with metastatic pancreatic cancer who received FOLFIRINOX, in this study FOLFIRABRAX was associated with a lower rate of ≥grade 3 sensory neuropathy (2% in FOLFIRABRAX vs. 9% in FOLFIRINOX).(2) While definitive conclusions cannot be made with cross-trial comparisons, FOLFIRABRAX appears to be better tolerated than FOLFIRINOX with regards to neuropathy and without added toxicity. It is noteworthy that 14% of patients were able to continue on FOLFIRABRAX for at least 48 weeks, as it confirms that they did not develop dose-limiting neuropathy during that time.
Our data suggest that FOLFIRABRAX may be less neurotoxic than FOLFIRINOX, with similar response rates. We suggest that future studies should directly compare FOLFIRINOX to FOLFIRABRAX in a randomized trial in pancreatic cancer. Further development of this regimen could also be considered in gastroesophageal cancer, given the encouraging results in a small number of patients seen in this study.
Of note, only 1 of 9 patients (11%) with biliary tract cancer had an objective response and 5 (56%) had SD. In comparison, in our genotype-guided mFOLFIRINOX trial, 6 of 29 patients (21%) had PR, and 14 (48%) had SD. While sample sizes are small, these results suggest that nab-paclitaxel may not be a good substitute for oxaliplatin in biliary tract cancers. Given the small number of patients in each tumor subtype, data regarding response rates are exploratory and further research is needed to establish the efficacy of this regimen across tumor types.
There are several limitations to this study. First, there was a considerable amount of investigator discretion regarding when patients stopped therapy after the DLT window and there were no criteria in the protocol regarding when to stop therapy aside from DLTs or PD. Second, patients were also allowed to resume therapy after a break if they had not had disease progression prior to the break. Thus, patients, especially across institutions, may not have been treated homogenously and this may have affected the outcomes of our secondary endpoints. Third, our definition of tolerability was narrow and based upon the DLT rate in the first cycle only, while in practice, toxicities are cumulative over time. Fourth, aside from UGT1A1*6, we did not assess other polymorphisms of UGT1A1 or other UGT1A genes that may affect metabolism of irinotecan. Finally, we did not measure the pharmacokinetics of irinotecan and thus we were unable to demonstrate that SN38 exposure was similar across groups.
In conclusion, FOLFIRABRAX with genotype guided dosing of irinotecan is tolerable in patients with advanced gastrointestinal cancers and UGT1A1*1/*1 and UGT1A1*1/*28 genotypes. Limited data suggests tolerability of this regimen in the UGT1A1*28/*28 group as well. The response rate in pancreatic cancer was comparable to historical controls who received FOLFIRINOX; however, with less neuropathy. Further development of this regimen is warranted.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
FOLFIRINOX is an effective but toxic regimen for pancreatic cancer. Since neuropathy often limits its use, we hypothesized that nab-paclitaxel may result in less toxicity when substituted for oxaliplatin. The combination of 5-fluorouracil/leucovorin, irinotecan, and nab-paclitaxel (FOLFIRABRAX) has never been evaluated. Previous data have shown a relationship between UGT1A1 genotype and exposure to SN-38, the active metabolite of irinotecan. Based on this, we sought to assess the tolerability of UGT1A1 genotype-guided dosing of irinotecan in FOLFIRABRAX in patients with advanced gastrointestinal malignancies. We observed that this regimen is tolerable in patients with UGT1A1*1*1 and UGT1A1*1*28 genotypes. Exploratory analysis also suggests tolerability in the UGT1A1*28*28 genotype. Responses were observed in pancreatic cancer, gastroesophageal cancer, and biliary tract cancer. FOLFIRABRAX had a lower rate of ≥grade 3 neuropathy compared to historical patients treated with FOLFIRINOX. Randomized studies comparing FOLFIRINOX and FOLFIRABRAX with regards to both safety and efficacy are warranted.
Financial Support/Acknowledgements:
This research was an investigator-initiated trial funded by Celgene. This research supported (in part) by the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant P30CA046592 through use of the Oncology Clinical Trials Support Unit (M Zalupski).
Conflict of interest disclosure: DVT: Consulting (Genentech/Roche, OncoPlex Diagnostics, Amgen), speakers’ bureau (Guardant Health, Foundation Medicine), honoraria (Genentech/Roche, Lilly, Amgen, OncoPlex Diagnostics, Foundation Medicine, Taiho Pharmaceutical, Guardant Health, Tempus, Merck, Bristol-Myers Squibb, Gritstone Oncology, Five Prime Therapeutics), research funding (Genentech). MMZ: Research funding (institution) (Merck, Halozyme/Parexel, MedImmune). MFK: Consulting (Genentech, AbbVie), speakers’ bureau (Genentech, AbbVie, Amgen, Merck). BNP: Consulting (AstraZeneca, Pfizer, Gerson Lehrman Group), research funding (Merck). HLK: Consulting (Aduro Biotech, Bayer, GlaxoSmithKline, AstraZeneca, Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Ipsen, Erytech Pharma, Five Prime Therapeutics, Paredox Therapeutics, Kyowa, Astellas), research funding (institution) (Aduro Biotech, AstraZeneca, Bayer, GlaxoSmithKline, Merck, MedImmune, Bristol-Myers Squibb, Lilly, Polaris, Deciphera, Genentech). MRS: Consulting (Ipsen, Bayer, Abbvie, Taiho Pharmaceuticals, Eisai, Exelixis). Remaining authors declare no conflicts of interest.
References:
- 1.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369(18):1691–703 doi 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364(19):1817–25 doi 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Ueno M, Ozaka M, Ishii H, Sato T, Ikeda M, Uesugi K, et al. Phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Journal of Clinical Oncology 2016;34(15_suppl):4111- doi 10.1200/JCO.2016.34.15_suppl.4111. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Ma T, Zhang Q, Chen YG, Guo CX, Shen YN, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer letters 2017;406:22–6 doi 10.1016/j.canlet.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114(7):737–43 doi 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42(8):1311–5 doi 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 7.Sharma MR, Joshi SS, Karrison TG, Allen K, Suh G, Marsh R, et al. A UGT1A1 genotype-guided dosing study of modified FOLFIRINOX in previously untreated patients with advanced gastrointestinal malignancies. Cancer 2019;125(10):1629–36 doi 10.1002/cncr.31938. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XT, Li J, Bai Y, Chu YP, Li J, Li Y, et al. A phase II study of triweekly paclitaxel and capecitabine combination therapy in patients with fluoropyrimidine-platinum-resistant metastatic gastric adenocarcinoma. Journal of cancer research and therapeutics 2013;9 Suppl:S153–7 doi 10.4103/0973-1482.122512. [DOI] [PubMed] [Google Scholar]
- 9.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393(10184):1948–57 doi 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 10.Adjei AA, Klein CE, Kastrissios H, Goldberg RM, Alberts SR, Pitot HC, et al. Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J Clin Oncol 2000;18(5):1116–23 doi 10.1200/jco.2000.18.5.1116. [DOI] [PubMed] [Google Scholar]
- 11.Stathopoulos GP, Dimitroulis J, Antoniou D, Katis C, Tsavdaridis D, Armenaki O, et al. Front-line paclitaxel and irinotecan combination chemotherapy in advanced non-small-cell lung cancer: a phase I-II trial. Br J Cancer 2005;93(10):1106–11 doi 10.1038/sj.bjc.6602827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 2007;99(17):1290–5 doi 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 13.Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 2000;60(24):6921–6. [PubMed] [Google Scholar]
- 14.Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004;22(8):1382–8 doi 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 15.Pfizer. Camptosar (irinotecan) prescribing information <https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf>.
- 16.Ychou M, Conroy T, Seitz JF, Gourgou S, Hua A, Mery-Mignard D, et al. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/ 5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol 2003;14(3):481–9. [DOI] [PubMed] [Google Scholar]
- 17.NCI. Common terminology criteria for adverse events (CTCAE) v4.0. <https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf>. [PubMed]
- 18.Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002;2(1):43–7. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Goldman AI. Issues in designing sequential stopping rules for monitoring side effects in clinical trials. Control Clin Trials 1987;8(4):327–37. [DOI] [PubMed] [Google Scholar]
- 21.Hall D, Ybazeta G, Destro-Bisol G, Petzl-Erler ML, Di Rienzo A. Variability at the uridine diphosphate glucuronosyltransferase 1A1 promoter in human populations and primates. Pharmacogenetics 1999;9(5):591–9. [PubMed] [Google Scholar]
- 22.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proceedings of the National Academy of Sciences of the United States of America 1998;95(14):8170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 1996;347(9001):578–81. [DOI] [PubMed] [Google Scholar]
- 24.Innocenti F, Schilsky RL, Ramirez J, Janisch L, Undevia S, House LK, et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 2014;32(22):2328–34 doi 10.1200/JCO.2014.55.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toffoli G, Cecchin E, Gasparini G, D’Andrea M, Azzarello G, Basso U, et al. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 2010;28(5):866–71 doi 10.1200/JCO.2009.23.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.