Abstract
Background.
Kenya introduced 10-valent pneumococcal conjugate vaccine (PCV10) among children <1 year in 2011 with catch-up vaccination among children 1–4 years in some areas. We assessed changes in pneumococcal carriage and antibiotic susceptibility patterns in children <5 years and adults.
Methods.
During 2009–2013, we performed annual cross-sectional pneumococcal carriage surveys in 2 sites: Kibera (children <5 years) and Lwak (children <5 years, adults). Only Lwak had catch-up vaccination. Nasopharyngeal and oropharyngeal (adults only) swabs underwent culture for pneumococci; isolates were serotyped. Antibiotic susceptibility testing was performed on isolates from 2009 and 2013; penicillin nonsusceptible pneumococci (PNSP) was defined as penicillin-intermediate or -resistant. Changes in pneumococcal carriage by age (<1 year, 1–4 years, adults), site, and human immunodeficiency virus (HIV) status (adults only) were calculated using modified Poisson regression, with 2009–2010 as baseline.
Results.
We enrolled 2962 children (2073 in Kibera, 889 in Lwak) and 2590 adults (2028 HIV+, 562 HIV−). In 2013, PCV10-type carriage was 10.3% (Lwak) to 14.6% (Kibera) in children <1 year and 13.8% (Lwak) to 18.7% (Kibera) in children 1–4 years. This represents reductions of 60% and 63% among children <1 year and 52% and 60% among children 1–4 years in Kibera and Lwak, respectively. In adults, PCV10-type carriage decreased from 12.9% to 2.8% (HIV+) and from 11.8% to 0.7% (HIV−). Approximately 80% of isolates were PNSP, both in 2009 and 2013.
Conclusions.
PCV10-type carriage declined in children <5 years and adults post–PCV10 introduction. However, PCV10-type and PNSP carriage persisted in children regardless of catch-up vaccination.
Keywords: Streptococcus pneumoniae, 10-valent pneumococcal conjugate vaccine, pneumococcal carriage, catch-up vaccination, antibiotic nonsusceptibility
Pneumonia remains the leading cause of deaths in children aged less than 5 years worldwide; and in 2015, Streptococcus pneumoniae was the most common etiology of pneumonia-related deaths [1]. The World Health Organization (WHO) recommends inclusion of pneumococcal conjugate vaccine (PCV) in childhood immunization programs [2]. With support of Gavi, the Vaccine Alliance, 33 out of 37 Gavi-supported countries in Africa introduced PCV by 2015 [3].
Kenya introduced 10-valent PCV (PCV10) into the routine national immunization program in February 2011 using a 3-primary-doses-without-a-booster (3 + 0) schedule. During the first year of introduction, all children aged less than 1 year were targeted to receive 3 doses. Catch-up vaccination campaigns took place in selected sites, where children aged 1–4 years received up to 2 doses of PCV10. After the first year of introduction, PCV10 was only offered following the routine immunization schedule, with doses given to infants at 6, 10, and 14 weeks. The impact of PCV introduction against pneumococcal carriage and invasive pneumococcal disease (IPD) has been well documented [4–7]; however, as one of the first countries in Africa to introduce PCV10, it was unclear whether the significant impact seen in other settings would be observed in Kenya, given the differences in pneumococcal carriage, serotype distribution, and factors associated with carriage (eg, household settings, prevalence of underlying conditions). Therefore, annual cross-sectional pneumococcal carriage surveys were conducted pre– and post–PCV10 introduction in sites with and without catch-up vaccination among children aged 1–4 years. The primary objective was to assess the impact of PCV10 introduction in Kenya on pneumococcal carriage among children aged less than 5 years targeted for vaccination and adults in the same household who were not targeted for vaccination. Our secondary objectives were as follows: 1) to compare the impact of different PCV10 introduction strategies on carriage, 2) to evaluate differences in pneumococcal carriage prevalence among adults living with and without human immunodeficiency virus (HIV) infection, and 3) to evaluate changes in the proportion of antibiotic-nonsusceptible pneumococci among carried strains.
METHODS
Study Setting
We utilized 2 ongoing surveillance platforms to conduct annual pneumococcal carriage surveys: the Population-based Infectious Disease Surveillance (PBIDS) and the Western Kenya Health and Demographic Surveillance System (HDSS). PBIDS is conducted in 2 geographically distinct regions, Kibera and Lwak [8, 9]. Catch-up vaccination was implemented in Lwak but not in Kibera. HDSS has been in place in western Kenya; and since 2005, residents of 33 of the HDSS villages have also been enrolled in Lwak PBIDS [10] (see Supplementary Methods).
Cross-sectional Survey in Children
Annual cross-sectional pneumococcal carriage surveys were performed in Kibera and Lwak among children aged less than 5 years during 2009–2013, October–December. Children aged less than 5 years were sampled differently in the 2 sites: in Lwak (with catch-up vaccination targeting children aged 1–4 years), children aged less than 5 years were randomly selected; in Kibera (no catch-up vaccination), age-stratified (<1 year and 1–4 years) random sampling was performed (see Supplementary Figure). Survey methods are described in detail elsewhere [11]. Upon enrollment, trained surveyors collected information on household characteristics, respiratory illness, and antibiotic use using standardized forms. PCV10 vaccination history was obtained for children enrolled during 2011–2013. Surveyors were instructed to verify each reported vaccine dose with the child’s vaccination card. Vaccination history was validated through PBIDS and HDSS records whenever possible.
Cross-sectional Survey in Adults
In Lwak, pneumococcal carriage surveys among adults living with children aged less than 5 years were conducted in 2009 and annually from 2011 to 2013 during October–December. Details of the survey methods were previously described [10]. Briefly, HDSS records were used to identify compounds where at least 1 HIV-positive parent of a child aged less than 5 years resided. HDSS records are linked to home-based counseling and testing for HIV that occurred widely in the area during 2008–2009 [12]. To maintain confidentiality of HIV testing status, all adults with children aged less than 5 years living in the same compound were invited to participate, regardless of HIV status. For each adult enrolled, trained survey staff used a standardized questionnaire to collect information [10].
Laboratory Methods
Calcium alginate swabs were used for nasopharyngeal specimen (children and adults) oropharyngeal specimen (adults only) collection. Processed specimens were transported to the Kenya Medical Research Institute laboratory in Kisumu, Kenya, for pneumococcal isolation [13]. Pneumococcal isolates recovered from specimens collected from 2009 survey participants were serotyped by Quellung reaction. Isolates recovered from participants enrolled during 2010–2013 were serotyped by multiplex polymerase chain reaction–based testing. Antibiotic susceptibility testing was performed on pneumococcal isolates from 2009 and 2013 by broth microdilution (Trek Diagnostics). Antibiotic susceptibility was determined using the 2012 Clinical and Laboratory Standards Institute criteria for minimum inhibitory concentrations [11, 14, 15]. Intermediate and resistant isolates were considered nonsusceptible to the antibiotic tested (see Supplementary Methods).
Data Management and Analysis
We performed descriptive analyses of participants by site, year, and age group (<1 year, 1–4 years, and adults). We calculated unadjusted prevalence ratios using classic methods for estimation of risk ratios with 2009–2010 (2009 only in adults) as the reference period. Potential confounders associated with pneumococcal carriage identified through previous studies were explored, and all models were adjusted for respiratory illness within the past 30 days, antibiotic use within 7 days, and area used for cooking [10, 16–18]. Adjusted prevalence ratios (aPRs) were calculated using Poisson regression with robust error variance [19]. Changes in carriage prevalence by serotype were compared between 2009 and 2013 for children aged less than 5 years and for adults. Changes in the proportion (among pneumococcal isolates) and carriage prevalence (among survey participants) of antibiotic-nonsusceptible pneumococci between 2009 and 2013 were tested. Chi-square test or Fisher’s exact test was used to compare categorical variables and Wilcoxon rank-sum test was used for continuous variables. Analyses were performed using SAS software (version 9.4; SAS Institute) (see Supplementary Methods).
Ethical Considerations
The study was approved by the ethics committees at the Kenya Medical Research Institute and the Centers for Disease Control and Prevention. Written informed consent was obtained from all adult participants and the parent or guardian of all participating children.
Definitions
See the Supplementary Methods for definitions on PCV10-type, 13-valent PCV (PCV13)-unique-type, nonvaccine-type (NVT), and antibiotic susceptibility determinations.
RESULTS
Overall Characteristics in Children
During 2009–2013, 2073 children in Kibera and 889 children in Lwak were enrolled (Table 1). Kibera children were more likely to sleep in a crowded room (median number of people sleeping in the same room, 5 vs 3; P < .001) and report recent (≤30 days of the survey) respiratory illness than Lwak children (67.3% vs 56.7%, P < .001). Kibera children were less likely than Lwak children to be exposed to tobacco smoke at home (10.2% vs 15.8%, P < .001) and have recent (≤7 days before the survey) antibiotic use (18.3% vs 22.4%, P = .01). In both sites, about one-third of participants reported taking antibiotics within 30 days of the survey; cotrimoxazole and penicillin/amoxicillin were the most frequently reported antibiotics. Differences in types of fuel used and area used for cooking were also noted between the sites. Characteristics of children by survey year are summarized in Supplementary Tables 1 and 2.
Table 1.
Characteristics of Survey Participants Aged <5 Years in Kibera and Lwak, Kenya: 2009–2013
| Kibera (N = 2073) | Lwak (N = 889) | P Value | |
|---|---|---|---|
| Age, n (%) | |||
| <1 year | 623 (30.1) | 111 (12.5) | <.001 |
| 1–4 years | 1450 (69.9) | 778 (87.5) | |
| Females, n (%) | 1053 (50.8) | 438 (49.3) | .446 |
| Number of people sleeping in the same room as the child, median (IQR) | 5 (4–6) | 3 (3–4) | <.001 |
| Number of children in the household attending school or daycare, n (%) | |||
| 0 | 370 (17.9) | 118 (13.3) | .002 |
| ≥1 | 1700 (82.1) | 771 (86.7) | |
| Cough, fever, fast breathing, or pneumonia within 30 days of the survey, n (%) | 1394 (67.3) | 504 (56.7) | <.001 |
| Tobacco smoke at home, n (%) | 212 (10.2) | 140 (15.8) | <.001 |
| Types of fuels used for cooking, n (%) | |||
| Fuelwood | 22 (1.1) | 862 (97.0) | <.001 |
| Charcoal | 1968 (94.9) | 353 (39.7) | <.001 |
| Kerosene | 1421 (68.6) | 32 (3.6) | <.001 |
| Others | 67 (3.2) | 22 (2.5) | .269 |
| Area used for cooking, n (%) | |||
| Same area where you live or sleep | 1923 (92.8) | 244 (27.5) | <.001 |
| All other sites | 150 (7.2) | 645 (72.6) | |
| Any antibiotic use, n (%) | |||
| Current use | 132 (6.4) | 77 (8.7) | .026 |
| Use within 7 days of the survey | 379 (18.3) | 199 (22.4) | .010 |
| Use within 30 days of the survey | 780 (37.6) | 332 (37.4) | .885 |
| Use of cotrimoxazole, n (%) | |||
| Current usea | 62 (3.0) | 54 (6.1) | <.001 |
| Use within 7 days of the surveyb | 162 (7.8) | 148 (16.7) | <.001 |
| Use within 30 days of the surveyc | 290 (14.1) | 190 (21.6) | <.001 |
| Use of penicillin or amoxicillin, n (%) | |||
| Current used | 65 (3.1) | 14 (1.6) | .017 |
| Use within 7 days of the surveye | 219 (10.6) | 49 (5.5) | <.001 |
| Use within 30 days of the surveyf | 502 (24.3) | 102 (11.6) | <.001 |
Abbreviation: IQR, interquartile range.
Excluded 8 who responded “don’t know.”
Excluded 8 who responded “don’t know.”
Excluded 21 who responded “don’t know.”
Excluded 9 who responded “don’t know.”
Excluded 7 who responded “don’t know.”
Excluded 16 who responded “don’t know.”
Overall Characteristics in Adults
In 2009 and during 2011–2013, a total of 3547 adults were recruited in Lwak. Of those, 2590 (73.0%) had known HIV status (2028 [78.3%] HIV positive and 562 [21.7%] HIV negative) and were further analyzed (Table 2). Overall, median age was 33 years (interquartile range, 28–38 years) and 67.0% were female. HIV-positive adults were older than HIV-negative adults (median age, 34 vs 30 years; P < .001) and more likely to report antibiotic use within 30 days of the survey, particularly cotrimoxazole (68.7% vs 15.5%, P < .001). Characteristics of HIV-positive adults by survey year are summarized in Supplementary Table 3.
Table 2.
Characteristics of Adult Participants in Lwak by Human Immunodeficiency Virus Status: 2009, 2011–2013
| Total (N = 2590) | HIV Positive (n = 2028) | HIV Negative (n = 562) | P Value (HIV Positive vs HIV Negative) | |
|---|---|---|---|---|
| Females, n (%) | 1734 (67.0) | 1349 (66.5) | 385 (68.5) | .375 |
| Median age (IQR), y | 33 (28–38) | 34 (29–39) | 30 (25–37) | <.001 |
| Aged <30 years, n (%) | 838 (32.4) | 581 (28.7) | 257 (45.7) | <.001 |
| Aged 30–39 years, n (%) | 1224 (47.3) | 1013 (50.0) | 211 (37.5) | |
| Aged ≥40 years, n (%) | 527 (30.4) | 433 (21.4) | 94 (16.7) | |
| Employed, n (%) | 429 (16.0) | 342 (16.9) | 87 (15.5) | .425 |
| Number of children aged <5 years in the household, median (range) | 1 (1–6) | 1 (1–6) | 1 (1–4) | <.001 |
| Cough, fever, fast breathing, or pneumonia within 30 days, n (%) | 1233 (47.6) | 984 (48.5) | 249 (44.3) | .077 |
| Tobacco smoker,a n (%) | 127 (4.9) | 92 (4.6) | 35 (6.2) | .098 |
| Tobacco smoke at home,b n (%) | 341 (13.2) | 244 (12.1) | 97 (17.3) | .001 |
| Any antibiotic use, n (%) | ||||
| Current use | 998 (38.5) | 966 (47.6) | 32 (5.7) | <.001 |
| Use within 7 days of the survey | 1499 (57.9) | 1405 (69.3) | 94 (16.7) | <.001 |
| Use within 30 days of the survey | 1658 (64.0) | 1508 (74.4) | 150 (26.7) | <.001 |
| Use of cotrimoxazole, n (%) | ||||
| Current usec | 960 (37.1) | 936 (46.2) | 24 (4.3) | <.001 |
| Use within 7 days of the surveyd | 1404 (54.2) | 1349 (66.5) | 55 (9.8) | <.001 |
| Use within 30 days of the surveye | 1481 (57.2) | 1394 (68.7) | 87 (15.5) | <.001 |
| Use of penicillin or amoxicillin, n (%) | ||||
| Current usef | 31 (1.2) | 28 (1.4) | 3 (0.5) | .103 |
| Use within 7 days of the surveyg | 130 (5.0) | 109 (5.4) | 21 (3.7) | .114 |
| Use within 30 days of the surveyh | 240 (9.3) | 201 (9.9) | 39 (6.9) | .030 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
Excluded 13 who responded “don’t know” or had a missing response.
Excluded 8 who responded “don’t know” or had a missing response.
Excluded 5 who responded “don’t know.”
Excluded 1 who responded “don’t know.”
Excluded 6 who responded “don’t know.”
Excluded 17 who responded “don’t know.”
Excluded 7 who responded “don’t’ know.”
Excluded 15 who responded “don’t know.”
Vaccine Coverage in Children
Vaccination history was available for 96.0% (1734 of 1806) of children enrolled during 2011–2013 (Supplementary Tables 1 and 2), of which 60.8% (1055 of 1734) was validated. Thus, we considered any vaccine dose given to children prior to the survey as valid regardless of when it was administered. Among children aged less than 1 year, coverage for 2 or more PCV10 doses increased from 87% (2011) to 95% (2013) in Kibera and from 77% (2011) to 100% (2013) in Lwak (Table 3). Among children aged 1–4 years, only 10% in Kibera had received 2 or more PCV10 doses in the 2011 survey compared with 59% in Lwak (Table 3). By 2013, the proportion who received 2 or more PCV10 doses increased to 61% and 82% in Kibera and Lwak, respectively.
Table 3.
Changes in Pneumococcal Carriage by Year, Participant Group, and 10-valent Pneumococcal Conjugate Vaccine Dose: Kibera and Lwak, 2009–2013
| Pneumococcal Carriage | |||||
|---|---|---|---|---|---|
| Site, Participant Group, and Year | Coverage of ≥2 PCV10 Doses, % | n/N | % | Crude PR (95% CI) | Adjusted PR (95% CI)a |
| Pneumococcal carriage, all participantsb | |||||
| Kibera, aged <1 year | |||||
| 2009–2010 | … | 193/207 | 93.2 | Ref | Ref |
| 2011 | 87.3 | 119/137 | 86.9 | 0.93 (.86, 1.00) | 0.93 (.87, 1.01) |
| 2012 | 91.1 | 121/128 | 94.5 | 1.01 (.96, 1.07) | 1.01 (.96, 1.07) |
| 2013 | 94.6 | 135/151 | 89.4 | 0.96 (.90, 1.02) | 0.97 (.91, 1.04) |
| Lwak, aged <1 year | |||||
| 2009–2010 | … | 38/40 | 95.0 | Ref | Ref |
| 2011 | 76.5 | 15/17 | 88.2 | 0.93 (.77, 1.12) | 0.93 (.77, 1.14) |
| 2012 | 70.8 | 20/25 | 80.0 | 0.84 (.68, 1.04) | 0.84 (.68, 1.04) |
| 2013 | 100 | 26/29 | 89.7 | 0.94 (.82, 1.09) | 0.94 (.82, 1.09) |
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 535/588 | 91.0 | Ref | Ref |
| 2011 | 10.0 | 244/265 | 92.1 | 1.01 (.97, 1.06) | 1.01 (.97, 1.06) |
| 2012 | 35.0 | 266/286 | 93.0 | 1.02 (.98, 1.06) | 1.02 (.98, 1.06) |
| 2013 | 60.7 | 294/311 | 94.5 | 1.04 (1.00, 1.08) | 1.04 (1.00, 1.08) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 280/321 | 87.2 | Ref | Ref |
| 2011 | 59.2 | 105/131 | 80.2 | 0.92 (.84, 1.01) | 0.92 (.84, 1.02) |
| 2012 | 66.7 | 138/152 | 90.8 | 1.04 (.97, 1.11) | 1.04 (.98, 1.11) |
| 2013 | 82.3 | 148/174 | 85.1 | 0.98 (.90, 1.05) | 0.98 (.91, 1.05) |
| HIV-positive adults | |||||
| 2009 | … | 237/549 | 43.2 | Ref | Ref |
| 2011 | … | 167/423 | 39.5 | 0.91 (.79, 1.06) | 0.95 (.81, 1.11) |
| 2012 | … | 246/526 | 46.8 | 1.08 (.95, 1.24) | 1.10 (.96, 1.26) |
| 2013 | … | 149/530 | 28.1 | 0.65 (.55, .77) | 0.66 (.56, .78) |
| HIV-negative adults | |||||
| 2009 | … | 41/153 | 26.8 | Ref | Ref |
| 2011 | … | 58/143 | 40.6 | 1.51 (1.09, 2.10) | 1.52 (1.09, 2.12) |
| 2012 | … | 45/131 | 34.4 | 1.28 (.90, 1.82) | 1.31 (.92, 1.87) |
| 2013 | … | 29/135 | 21.5 | 0.80 (.53, 1.21) | 0.82 (.54, 1.24) |
| PCV10-type carriage, all participantsb | |||||
| Kibera, aged <1 year | |||||
| 2009–2010 | … | 79/207 | 38.2 | Ref | Ref |
| 2011 | 87.3 | 31/137 | 22.6 | 0.59 (.42, .85) | 0.59 (.41, .85) |
| 2012 | 91.1 | 23/128 | 18.0 | 0.47 (.31, .71) | 0.47 (.31, .71) |
| 2013 | 94.6 | 22/151 | 14.6 | 0.38 (.25, .58) | 0.40 (.26, .62) |
| Lwak, aged <1 year | |||||
| 2009–2010 | … | 12/40 | 30.0 | Ref | Ref |
| 2011 | 76.5 | 3/17 | 17.7 | 0.59 (.19, 1.82) | 0.73 (.26, 2.02) |
| 2012 | 70.8 | 4/25 | 16.0 | 0.53 (.19, 1.47) | 0.58 (0.22, 1.50) |
| 2013 | 100 | 3/29 | 10.3 | 0.34 (.11, 1.11) | 0.37 (.11, 1.24) |
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 227/588 | 38.6 | Ref | Ref |
| 2011 | 10.0 | 91/265 | 34.3 | 0.89 (.73, 1.08) | 0.88 (.73, 1.08) |
| 2012 | 35.0 | 51/286 | 17.8 | 0.46 (.35, .60) | 0.47 (.36, .61) |
| 2013 | 60.7 | 58/311 | 18.7 | 0.48 (.37, .62) | 0.48 (.37, .62) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 110/321 | 34.3 | Ref | Ref |
| 2011 | 59.2 | 36/131 | 27.5 | 0.80 (.58, 1.10) | 0.81 (.59, 1.12) |
| 2012 | 66.7 | 26/152 | 17.1 | 0.50 (.34, .73) | 0.50 (.34, .74) |
| 2013 | 82.3 | 24/174 | 13.8 | 0.40 (.27, .60) | 0.40 (.27, .60) |
| HIV-positive adults | |||||
| 2009 | … | 71/549 | 12.9 | Ref | Ref |
| 2011 | … | 33/423 | 7.8 | 0.60 (.41, .89) | 0.65 (.44, .96) |
| 2012 | … | 34/526 | 6.5 | 0.50 (.34, .74) | 0.54 (.36, .80) |
| 2013 | … | 15/530 | 2.8 | 0.22 (.13, .38) | 0.24 (.14, .41) |
| HIV-negative adults | |||||
| 2009 | … | 18/153 | 11.8 | Ref | Ref |
| 2011 | … | 18/143 | 12.6 | 1.07 (.58, 1.97) | 1.11 (.60, 2.06) |
| 2012 | … | 9/131 | 6.9 | 0.58 (.27, 1.26) | 0.63 (.30, 1.34) |
| 2013 | … | 1/135 | 0.7 | 0.06 (.01, .47) | 0.07 (.01, .51) |
| PCV10-type carriage, ≤1 PCV10 doseb | |||||
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 227/588 | 38.6 | Ref | Ref |
| 2011 | … | 83/239 | 34.7 | 0.90 (.74, 1.10) | 0.90 (.73, 1.10) |
| 2012 | … | 37/190 | 19.5 | 0.50 (.37, .69) | 0.51 (.38, .70) |
| 2013 | … | 29/138 | 21.0 | 0.54 (.39, .76) | 0.54 (.38, .76) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 110/321 | 34.3 | Ref | Ref |
| 2011 | … | 19/54 | 35.2 | 1.03 (.69, 1.52) | 1.01 (.68, 1.52) |
| 2012 | … | 15/54 | 27.8 | 0.81 (.51, 1.28) | 0.81 (.51, 1.28) |
| 2013 | … | 6/39 | 15.4 | 0.45 (.21, .95) | 0.45 (.21, .96) |
| PCV10-type carriage, ≥2 PCV10 dosesb | |||||
| Kibera, aged <1 year | |||||
| 2009–2010 | … | 79/207 | 38.2 | Ref | Ref |
| 2011 | … | 29/117 | 24.8 | 0.65 (.45, .93) | 0.66 (.46, .95) |
| 2012 | … | 22/113 | 19.5 | 0.51 (.34, .77) | 0.51 (.35, .77) |
| 2013 | … | 22/139 | 15.8 | 0.41 (.27, .63) | 0.44 (.29, .67) |
| Lwak, aged <1 year | |||||
| 2009–2010 | … | 12/40 | 30.0 | Ref | Ref |
| 2011 | … | 2/13 | 15.3 | 0.51 (.13, 2.00) | 0.66 (.18, 2.40) |
| 2012 | … | 3/17 | 17.7 | 0.59 (.19, 1.82) | 0.75 (.25, 2.19) |
| 2013 | … | 3/29 | 10.3 | 0.34 (.11, 1.11) | 0.38 (.12, 1.25) |
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 227/588 | 38.6 | Ref | Ref |
| 2011 | … | 8/26 | 30.8 | 0.80 (.44, 1.43) | 0.81 (.45, 1.46) |
| 2012 | … | 14/96 | 14.6 | 0.38 (.23, .62) | 0.39 (.23, .63) |
| 2013 | … | 29/173 | 16.8 | 0.43 (.31, .61) | 0.43 (.30, .60) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 110/321 | 34.3 | Ref | Ref |
| 2011 | … | 17/77 | 22.1 | 0.64 (.41, 1.01) | 0.66 (.42, 1.03) |
| 2012 | … | 11/98 | 11.2 | 0.33 (.18, .58) | 0.33 (.18, .59) |
| 2013 | … | 18/135 | 13.3 | 0.39 (.25, .61) | 0.38 (.24, .59) |
| PCV13-unique type (serotypes 3, 6A, 19A) carriage, all participantsb | |||||
| Kibera, aged <1 year | |||||
| 2009–2010 | … | 26/207 | 12.6 | Ref | Ref |
| 2011 | 87.3 | 15/137 | 11.0 | 0.87 (.48, 1.58) | 0.86 (.48, 1.55) |
| 2012 | 91.1 | 18/128 | 14.1 | 1.12 (.64, 1.96) | 1.11 (.64, 1.93) |
| 2013 | 94.6 | 28/151 | 18.5 | 1.48 (.90, 2.41) | 1.38 (.84, 2.28) |
| Lwak, aged <1 year | |||||
| 2009–2010 | … | 7/40 | 17.5 | Ref | Ref |
| 2011 | 76.5 | 1/17 | 5.9 | 0.34 (.04, 2.53) | 0.24 (.03, 2.25) |
| 2012 | 70.8 | 3/25 | 12.0 | 0.69 (.20, 2.41) | 0.57 (.17, 1.89) |
| 2013 | 100 | 3/29 | 10.3 | 0.59 (.17, 2.09) | 0.52 (.14, 1.86) |
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 59/588 | 10.0 | Ref | Ref |
| 2011 | 10.0 | 32/265 | 12.1 | 1.20 (.80, 1.80) | 1.21 (.81, 1.82) |
| 2012 | 35.0 | 53/286 | 18.5 | 1.85 (1.31, 2.60) | 1.83 (1.30, 2.57) |
| 2013 | 60.7 | 52/311 | 16.7 | 1.67 (1.18, 2.36) | 1.70 (1.21, 2.41) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 43/321 | 13.4 | Ref | Ref |
| 2011 | 59.2 | 15/131 | 11.5 | 0.85 (.49, 1.48) | 0.93 (.53, 1.63) |
| 2012 | 66.7 | 15/152 | 9.9 | 0.74 (.42, 1.28) | 0.76 (.44, 1.32) |
| 2013 | 82.3 | 24/174 | 13.8 | 1.03 (.65, 1.64) | 1.03 (.65, 1.65) |
| HIV-positive adults | |||||
| 2009 | … | 37/549 | 6.7 | Ref | Ref |
| 2011 | … | 28/423 | 6.6 | 0.98 (.61, 1.58) | 1.05 (.65, 1.70) |
| 2012 | … | 52/526 | 9.9 | 1.47 (.98, 2.20) | 1.61 (1.06, 2.44) |
| 2013 | … | 36/530 | 6.8 | 1.01 (.65, 1.57) | 1.13 (.71, 1.79) |
| HIV-negative adults | |||||
| 2009 | … | 6/153 | 3.9 | Ref | Ref |
| 2011 | … | 5/143 | 3.5 | 0.89 (.28, 2.86) | 1.07 (.34, 3.38) |
| 2012 | … | 9/131 | 6.9 | 1.75 (.64, 4.79) | 1.89 (.67, 5.29) |
| 2013 | … | 5/135 | 3.7 | 0.94 (.29, 3.02) | 0.96 (.29, 3.21) |
| NVT carriage, all participantsb | |||||
| Kibera, aged <1 year | |||||
| 2009–2010 | … | 88/207 | 42.5 | Ref | Ref |
| 2011 | 87.3 | 73/137 | 53.3 | 1.25 (1.00, 1.57) | 1.26 (1.01, 1.58) |
| 2012 | 91.1 | 80/128 | 62.5 | 1.47 (1.19, 1.81) | 1.47 (1.19, 1.80) |
| 2013 | 94.6 | 85/151 | 56.3 | 1.32 (1.07, 1.64) | 1.35 (1.09, 1.67) |
| Lwak, aged <1 year | |||||
| 2009–2010 | … | 19/40 | 47.5 | Ref | Ref |
| 2011 | 76.5 | 11/17 | 64.7 | 1.36 (.84, 2.20) | 1.36 (.87, 2.15) |
| 2012 | 70.8 | 13/25 | 52.0 | 1.09 (.67, 1.80) | 1.10 (.68, 1.77) |
| 2013 | 100 | 20/29 | 69.0 | 1.45 (.97, 2.18) | 1.47 (.98, 2.19) |
| Kibera, aged 1–4 years | |||||
| 2009–2010 | … | 249/588 | 42.4 | Ref | Ref |
| 2011 | 10.0 | 121/265 | 45.7 | 1.08 (.92, 1.27) | 1.08 (.92, 1.27) |
| 2012 | 35.0 | 162/286 | 56.6 | 1.34 (1.16, 1.54) | 1.33 (1.16, 1.53) |
| 2013 | 60.7 | 184/311 | 59.2 | 1.40 (1.22, 1.59) | 1.40 (1.23, 1.60) |
| Lwak, aged 1–4 years | |||||
| 2009–2010 | … | 127/321 | 39.6 | Ref | Ref |
| 2011 | 59.2 | 54/131 | 41.2 | 1.04 (.82, 1.33) | 1.03 (.80, 1.32) |
| 2012 | 66.7 | 97/152 | 63.8 | 1.61 (1.35, 1.93) | 1.60 (1.34, 1.92) |
| 2013 | 82.3 | 100/174 | 57.5 | 1.45 (1.21, 1.75) | 1.46 (1.21, 1.76) |
| HIV-positive adults | |||||
| 2009 | … | 129/549 | 23.5 | Ref | Ref |
| 2011 | … | 106/423 | 25.1 | 1.07 (.85, 1.33) | 1.08 (.86, 1.36) |
| 2012 | … | 160/526 | 30.4 | 1.29 (1.06, 1.58) | 1.26 (1.03, 1.53) |
| 2013 | … | 98/530 | 18.5 | 0.79 (.62, .99) | 0.76 (.60, .96) |
| HIV-negative adults | |||||
| 2009 | … | 17/153 | 11.1 | Ref | Ref |
| 2011 | … | 35/143 | 24.5 | 2.20 (1.29, 3.75) | 2.10 (1.23, 3.58) |
| 2012 | … | 27/131 | 20.6 | 1.86 (1.06, 3.25) | 1.84 (1.05, 3.22) |
| 2013 | … | 23/135 | 17.0 | 1.53 (.86, 2.75) | 1.53 (.85, 2.72) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; NVT, nonvaccine type; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PR, prevalence ratio; Ref, reference.
Adjusted for respiratory illness within the past 30 days, antibiotic use within 7 days, and area used for cooking.
None of the children in 2009–2010 and none of the adults received pneumococcal conjugate vaccine.
Changes in Overall Pneumococcal Carriage Prevalence in Children and Adults
In children, overall pneumococcal carriage prevalence at baseline (2009–2010) was 87.2% (Lwak, aged 1–4 years) to 95.0% (Lwak, aged <1 years), and no significant changes were noted after vaccine introduction (Table 3). In adults, overall pneumococcal carriage prevalence decreased significantly in HIV-positive adults (43.2% to 28.1%; aPR, 0.66; 95% confidence interval [CI], 0.56–0.78) but not in HIV-negative adults (26.8% to 21.5%; aPR, 0.82; 95% CI, 0.54–1.24; Table 3).
Changes in PCV10-Type Pneumococcal Carriage Prevalence in Children and Adults
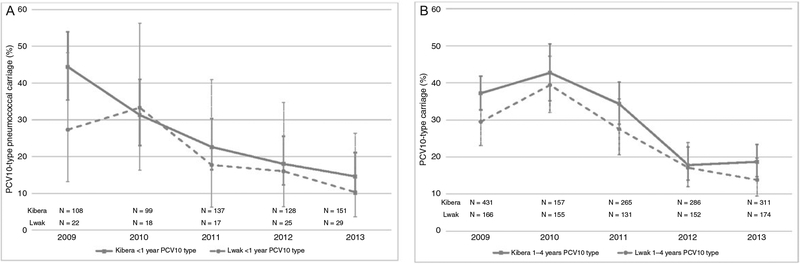
At baseline, PCV10-type carriage prevalences in children aged less than 1 year in Kibera and Lwak were 38.2% and 30.0%, respectively, and declined to 14.6% and 10.3% in 2013 (aPR in Kibera, 0.40; 95% CI, 0.26–0.62; aPR in Lwak, 0.37; 95% CI, 0.11–1.24) (Table 3, Figure 1A). For children aged 1–4 years, baseline PCV10-type carriage prevalences in Kibera and Lwak were 38.6% and 34.3%, respectively, and declined to 18.7% and 13.8% in 2013. The decline was somewhat larger in Lwak, but the CIs for changes in the 2 sites overlapped (aPR in Kibera, 0.48; 95% CI, 0.37–0.62; aPR in Lwak, 0.40; 95% CI, 0.27–0.60). There were no significant differences between sites in change in PCV10-type carriage when stratified by 1 or less PCV10 dose versus 2 or more PCV10 doses (Table 3). Unlike Kibera, carriage prevalence in children aged 1–4 years in Lwak appeared to decrease further between 2012 and 2013, but the changes were not significant (aPR, 0.84; 95% CI, 0.51–1.39) (Figure 1B). In adults, a significant reduction in 2013 compared with baseline was noted in both HIV-positive (12.9% to 2.8%; aPR, 0.24; 95% CI, 0.14–0.41) and HIV-negative (11.8% to 0.7%; aPR, 0.07; 95% CI, 0.01–0.51) adults.
Figure 1.
Trends in PCV10-type pneumococcal carriage prevalence, Kenya 2009–2013. Children aged <1 year in Kibera and Lwak (A) and children aged 1–4 years in Kibera and Lwak (B). Abbreviation: PCV10, 10-valent pneumococcal conjugate vaccine.
Changes in PCV13-Unique Type and NVT Pneumococcal Carriage Prevalence in Children and Adults
Compared with baseline, PCV13-unique type (serotypes contained in PCV13 but not in PCV10—3, 6A, 19A) carriage prevalence in 2013 increased in children in Kibera and HIV-positive adults, but the change was significant only in Kibera children aged 1–4 years (aPR, 1.70; 95% CI, 1.21–2.41) (Table 3). NVT carriage prevalence increased in all groups of children, although was nonsignificant in Lwak children aged less than 1 year. In contrast, NVT carriage prevalence decreased in HIV-positive adults (aPR, 0.76; 95% CI, 0.60–0.96) (Table 3).
Changes in Serotype Distribution, 2009–2013
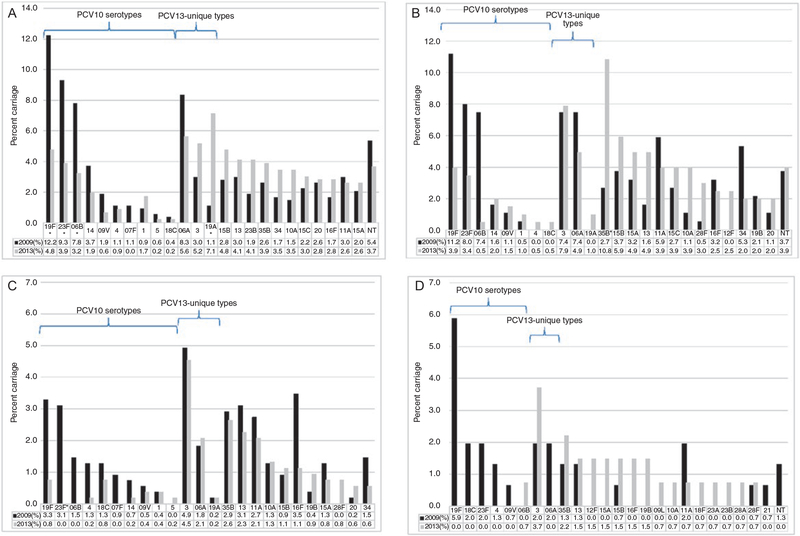
Among Kibera children aged less than 5 years, serotypes 19F, 23F, and 6B (PCV10 types) decreased significantly, while serotype 19A (PCV13-unique type) increased from 1.1% to 7.1% (P < .001) (Figure 2A). Among Lwak children, the decline was only significant for serotype 6B among the individual PCV10 types (Figure 2B). There was no significant change in the individual PCV13-unique types. Among NVT, serotype 35B increased significantly and was the predominant serotype in 2013.
Figure 2.
Pneumococcal carriage prevalence by serotype before (2009) and after (2013) PCV10 introduction. A, Children in Kibera aged <5 years, 2009 (N = 539) and 2013 (N = 462). B, Children in Lwak aged <5 years, 2009 (N = 188) and 2013 (N = 203). C, HIV-positive adults, 2009 (N = 549) and 2013 (N = 530). D, HIV-negative adults, 2009 (N = 153) and 2013 (N = 135). Abbreviations: HIV, human immunodeficiency virus; NT, non-typeable; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine. *P < .002.
In adults, the predominant serotypes in 2009 included PCV10 serotypes 19F and 23F along with PCV13-unique types 3 and 6A, similar to Lwak children (Figure 2C, D). In 2013, some of the PCV10 types were not detected in HIV-positive or HIV-negative adults. In HIV-positive adults, serotype 3 (PCV13-unique type) carriage remained stable and was the most commonly identified serotype in both years (4.9% vs 4.5%, P = .76) (Figure 2C); the increase in serotype 3 was not significant among HIV-negative adults (2.0% vs 3.7%, P = .37).
Changes in the Proportion of Antibiotic Nonsusceptible Pneumococcal Isolates
Antimicrobial susceptibility testing results were available from a total of 1637 out of 1714 (95.5%) pneumococcal isolates collected in 2009 and 2013 (944 from Kibera, 344 from Lwak children, and 349 from HIV-positive adults) (Table 4). In both sites, age groups, and years, more than 95% of pneumococcal isolates were nonsusceptible to cotrimoxazole, and approximately 80% were penicillin nonsusceptible; the majority of penicillin-nonsusceptible isolates had minimum inhibitory concentrations in the intermediate range. Ceftriaxone-intermediate isolates, not detected in 2009, were observed among 7 isolates (5 were serotype 3, one each of serotypes 19F and 35B) from Kibera children in 2013. PCV10-type penicillin-intermediate S. pneumoniae (PISP) carriage prevalence declined significantly in children and HIV-positive adults (Table 5). The reduction was offset by a significant increase in PISP among NVT in children but not in HIV-positive adults.
Table 4.
Changes in Antibiotic Susceptibility Among Pneumococcal Strains Identified From Carriers Before (2009) and After (2013) 10-Valent Pneumococcal Conjugate Vaccine Introduction
| Susceptible | Intermediate | Resistant | |||||
|---|---|---|---|---|---|---|---|
| Antibiotic and Year | Break Point (μg/mL) | n (%) | Break Point (μg/mL) | n (%) | Break Point (μg/mL) | n (%) | P Value (2009 vs 2013) |
| Pneumococcal isolates from Kibera children aged <5 years (N = 499 in 2009, N = 445 in 2013) | |||||||
| Penicillin | |||||||
| 2009 | ≤0.06 | 100 (20.0) | 0.12–1 | 387 (77.6) | ≥2 | 12 (2.4) | .618 |
| 2013 | … | 100 (22.5) | … | 333 (74.8) | … | 12 (2.7) | … |
| Chloramphenicol | |||||||
| 2009 | ≤4 | 490 (98.2) | NA | … | ≥8 | 9 (1.8) | .182 |
| 2013 | … | 431 (96.9) | … | … | … | 14 (3.2) | … |
| Levofloxacin | |||||||
| 2009 | ≤2 | 499 (100) | 4 | 0 | ≥8 | 0 | NA |
| 2013 | … | 445 (100) | … | 0 | … | 0 | … |
| Erythromycin | |||||||
| 2009 | ≤0.25 | 490 (98.2) | 0.5 | 1 (0.2) | ≥1 | 8 (1.6) | .042 |
| 2013 | … | 428 (96.2) | … | 0 | … | 17 (3.8) | … |
| Ceftriaxone | |||||||
| 2009 | ≤1 | 499 (100) | 2 | 0 | ≥4 | 0 | .005 |
| 2013 | … | 438 (98.4) | … | 7 (1.6) | … | 0 | … |
| Tetracycline | |||||||
| 2009 | ≤2 | 401 (80.4) | 4 | 13 (2.6) | ≥8 | 85 (17.0) | .187 |
| 2013 | … | 377 (84.7) | … | 11 (2.5) | … | 57 (12.8) | … |
| Cotrimoxazole | |||||||
| 2009 | ≤0.5/9.5 | 15 (3.0) | 1/19–2/38 | 54 (10.8) | ≥4/76 | 430 (86.2) | .029 |
| 2013 | … | 17 (3.8) | … | 27 (6.1) | … | 401 (90.1) | … |
| Clindamycin | |||||||
| 2009 | ≤0.25 | 498 (99.8) | 0.5 | 0 | ≥1 | 1 (0.2) | .001 |
| 2013 | … | 433 (97.3) | … | 0 | … | 12 (2.7) | … |
| Pneumococcal isolates from Lwak children aged <5 years (N = 163 in 2009, N = 181 in 2013) | |||||||
| Penicillin | |||||||
| 2009 | ≤0.06 | 27 (16.6) | 0.12–1 | 133 (81.6) | ≥2 | 3 (1.8) | .235 |
| 2013 | … | 31 (17.1) | … | 150 (82.3) | … | 0 | … |
| Chloramphenicola | |||||||
| 2009 | ≤4 | 153 (97.5) | N/A | … | ≥8 | 4 (2.6) | .757 |
| 2013 | … | 175 (96.7) | … | … | … | 6 (3.3) | … |
| Levofloxacina | |||||||
| 2009 | ≤2 | 157 (100) | 4 | 0 | ≥8 | 0 | NA |
| 2013 | … | 181 (100) | … | 0 | … | 0 | … |
| Erythromycin | |||||||
| 2009 | ≤0.25 | 162 (99.4) | 0.5 | 0 | ≥1 | 1 (0.6) | .474 |
| 2013 | … | 181 (100) | … | 0 | … | 0 | … |
| Ceftriaxone | |||||||
| 2009 | ≤1 | 163 (100) | 2 | 0 | ≥4 | 0 | NA |
| 2013 | … | 181 (100) | … | 0 | … | 0 | … |
| Tetracycline | |||||||
| 2009 | ≤2 | 131 (80.4) | 4 | 2 (1.2) | ≥8 | 30 (18.4) | .001 |
| 2013 | … | 143 (79.0) | … | 17 (9.4) | … | 21 (11.6) | … |
| Cotrimoxazoleb | |||||||
| 2009 | ≤0.5/9.5 | 0 | 1/19–2/38 | 9 (5.6) | ≥4/76 | 152 (94.4) | 1.000 |
| 2013 | … | 0 | … | 10 (5.5) | … | 171 (94.5) | … |
| Clindamycin | |||||||
| 2009 | ≤0.25 | 163 (100) | 0.5 | 0 | ≥1 | 0 | NA |
| 2013 | … | 181 (100) | … | 0 | … | 0 | … |
| Pneumococcal isolates from HIV-positive adults (N = 199 in 2009, N = 150 in 2013) | |||||||
| Penicillin | |||||||
| 2009 | ≤0.06 | 33 (16.6) | 0.12–1 | 162 (81.4) | ≥2 | 4 (2.0) | .549 |
| 2013 | … | 32 (21.3) | … | 116 (77.3) | … | 2 (1.3) | … |
| Chloramphenicol | |||||||
| 2009 | ≤4 | 196 (98.5) | N/A | … | ≥8 | 3 (1.5) | .181 |
| 2013 | … | 144 (96.0) | … | … | … | 6 (4.0) | … |
| Levofloxacin | |||||||
| 2009 | ≤2 | 199 (100) | 4 | 0 | ≥8 | 0 | NA |
| 2013 | … | 150 (100) | … | 0 | … | 0 | … |
| Erythromycin | |||||||
| 2009 | ≤0.25 | 197 (99.0) | 0.5 | 0 | ≥1 | 2 (1.0) | 1.000 |
| 2013 | … | 148 (98.7) | … | 0 | … | 2 (1.3) | … |
| Ceftriaxone | |||||||
| 2009 | ≤1 | 199 (100) | 2 | 0 | ≥4 | 0 | NA |
| 2013 | … | 150 (100) | … | 0 | … | 0 | … |
| Tetracyclinec | |||||||
| 2009 | ≤2 | 150 (75.8) | 4 | 8 (4.0) | ≥8 | 40 (20.2) | .534 |
| 2013 | … | 109 (72.7) | … | 10 (6.7) | … | 31 (20.7) | … |
| Cotrimoxazolec | |||||||
| 2009 | ≤0.5/9.5 | 1 (0.5) | 1/19–2/38 | 6 (3.1) | ≥4/76 | 190 (96.5) | .474 |
| 2013 | … | 0 | … | 2 (1.3) | … | 148 (98.7) | … |
| Clindamycin | |||||||
| 2009 | ≤0.25 | 198 (99.5) | 0.5 | 0 | ≥1 | 1 (0.5) | .579 |
| 2013 | … | 148 (98.7) | … | 0 | … | 2 (1.3) | … |
Abbreviations: HIV, human immunodeficiency virus; NA, not applicable.
Six isolates in 2009 were missing susceptibility testing results for both chloramphenicol and levofloxacin.
Two isolates in 2009 were missing susceptibility testing results for cotrimoxazole only.
One isolate from 2009 was missing susceptibility testing results for both tetracycline and cotrimoxazole; 1 isolate from 2009 was missing susceptibility testing results for cotromixazole only.
Table 5.
Changes in Carriage Prevalence of Penicillin-nonsusceptible Pneumococci in 2009 Versus 2013 by Serotype Groups
| Children in Kibera Aged <5 Years | Children in Lwak Aged <5 Years | HIV-positive Adults | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype | 2009 (N = 521a), n (%) | 2013 (N = 459b), n (%) | P Value | 2009 (N = 184c), n (%) | 2013 (N = 201d), n (%) | P Value | 2009 (N = 506e), n (%) | 2013 (N = 529f), n (%) | P Value |
| Intermediate susceptibility to penicillin | |||||||||
| All PISP | 366 (70.2) | 312 (68.0) | .441 | 125 (67.9) | 141 (70.1) | .639 | 156 (30.8) | 114 (21.6) | <.001 |
| PCV10 type | 174 (33.4) | 72 (15.7) | <.001 | 46 (25.0) | 22 (10.9) | <.001 | 50 (9.9) | 8 (1.5) | <.001 |
| PCV13-unique | 57 (10.9) | 55 (12.0) | .609 | 23 (12.5) | 25 (12.4) | .985 | 28 (5.5) | 28 (5.3) | .864 |
| NVT | 141 (27.1) | 185 (40.3) | <.001 | 64 (34.8) | 102 (50.7) | .002 | 79 (15.6) | 78 (14.7) | .697 |
| Resistant to penicillin | |||||||||
| All PRSP | 11 (2.1) | 12 (2.6) | .604 | 3 (1.6) | 0 | .108 | 4 (0.8) | 2 (0.4) | .442 |
| PCV10 type | 8 (1.5) | 2 (0.4) | .115 | 1 (0.5) | 0 | .478 | 1 (0.2) | 0 | .489 |
| PCV13-unique | 1 (0.2) | 11 (2.4) | .002 | 1 (0.5) | 0 | .478 | 1 (0.2) | 2 (0.4) | 1.000 |
| NVT | 2 (0.4) | 0 | .502 | 1 (0.5) | 0 | .478 | 2 (0.4) | 0 | .239 |
| Total | 377 (72.4) | 324 (70.6) | .540 | 128 (69.6) | 141 (70.1) | .901 | 160 (31.6) | 116 (21.9) | <.001 |
Abbreviations: HIV, human immunodeficiency virus; NVT, nonvaccine type; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13-unique, 3 serotypes contained in 13-valent pneumococcal conjugate vaccine but not in PCV10; PISP, penicillin-intermediate Streptococcus pneumoniae; PRSP, penicillin-resistant Streptococcus pneumoniae.
Excludes 18 children with pneumococcal carriage who are missing penicillin susceptibility test results from all 539 enrolled children.
Excludes 3 children with pneumococcal carriage who are missing penicillin susceptibility test results from all 462 enrolled children.
Excludes 4 children with pneumococcal carriage who are missing penicillin susceptibility test results from all 188 enrolled children.
Excludes 2 children with pneumococcal carriage who are missing penicillin susceptibility test results from all 203 enrolled children.
Excludes 43 adults with pneumococcal carriage who are missing penicillin susceptibility test results from all 549 enrolled adults.
Excludes 1 adult with pneumococcal carriage who are missing penicillin susceptibility test results from all 530 enrolled adults.
DISCUSSION
In children, we observed a 52% (Kibera, aged 1–4 years) to 60% (Kibera, aged <1 year; Lwak, aged 1–4 years) reduction in PCV10-type pneumococcal carriage prevalence approximately 2 years after PCV10 introduction, similar to the report from Kilifi, Kenya (site with catch-up vaccination targeting children aged 1–4 years) [4]. Reductions were also observed among adults, including those who were HIV positive. Despite reductions, PCV10-type carriage remained common in children in 2013 (15–19% in Kibera, 10–14% in Lwak). These figures are consistent with the results from Kilifi, Kenya [4], but higher than from countries that used a PCV10 schedule with 3 primary doses plus a booster (3 + 1) [20–22]. No significant reduction in penicillin nonsusceptible pneumococci (PNSP) carriage was observed except in HIV-positive adults. WHO currently recommends both the 3 + 0 and 2 primary doses and a booster (2 + 1) vaccination schedule for PCV administration for infants [23]. Although the 3 + 0 schedule is widely used in sub-Saharan Africa, data from carriage [24] and IPD [25] studies suggest that the booster dose in the 2 + 1 schedule might be required to achieve a sustained reduction in vaccine-type colonization. While this might explain the residual PCV10-type carriage that we observed in children, the long-term significance of this finding is unknown. In Kilifi, PCV10-type carriage in children aged less than 5 years remained at 6% 6 years after PCV10 introduction [26], yet a significant reduction in PCV10-type IPD in all age groups including unvaccinated adults was observed (92% reduction in children aged <5 years, 74% in children aged 5–14 years, and 81% in those aged ≥15 years) [27]. Whether this impact was blunted due to persistent circulation of PCV10-type pneumococci or waning mucosal immunity due to lack of a booster PCV dose is unknown. Of note, our results showed that PCV10-type carriage in 2013 was close to elimination in HIV-negative adults.
Of the PCV13-unique types, we observed a significant increase in serotype 19A carriage prevalence post–PCV10 introduction in Kibera. Although the serotype 19F antigen contained in both the 7-valent PCV (PCV7) and PCV10 was thought to provide some cross-protection against serotype 19A [28], cross-protection has not been proven and increases in serotype 19A IPD incidence post–PCV7 introduction were reported in multiple countries [29–31]. In our study, the increase in carriage of 19A was nonsignificant among children in Lwak and was not observed in adults. Carriage studies conducted in Kilifi [4] and Brazil [20] within 3 years of PCV10 introduction (both with catch-up vaccination targeting the studied age groups) also reported nonsignificant increases in serotype 19A carriage post–PCV10 introduction. Although Brazil reported an increase in IPD due to non-PCV10 serotypes (3, 6C, and 19A) 5 years after PCV10 introduction, results from Kilifi showed nonsignificant results [27]; and so far, longer-term impact of PCV10 against serotype 19A has been undetermined [32, 33]. Since our study was not powered to assess changes in individual serotypes, follow-up and correlation with IPD trends are needed.
As previously described [11], a high proportion of pneumococcal carriage isolates were nonsusceptible to penicillin and cotrimoxazole, and the proportion was essentially unchanged after PCV10 introduction. PISP carriage prevalence among PCV10 types declined significantly, but this decline was balanced by a significant increase in PISP NVT carriage in children. Similar findings were reported both in US children [7, 34] and adults [35] post–PCV7 introduction. However, isolates with intermediate susceptibility to ceftriaxone were detected for the first time in the post-PCV10 period, most of which were serotype 3. In the United States, IPD caused by NVT antibiotic-nonsusceptible S. pneumoniae remained below the pre-PCV period [36, 37]. In Kenya, however, the higher proportion of overall pneumococcal carriage and antibiotic use in the community might result in more sustained transmission of antibiotic-nonsusceptible S. pneumoniae. Although antibiotic susceptibility results were not available, data from Kilifi showed a nonsignificant increase in NVT-IPD in all age groups aged 2 or more months, despite significant reductions in overall and PCV10-type IPD incidence [27].
We observed a reduction in overall pneumococcal carriage prevalence and PNSP carriage prevalence among HIV-positive adults post–PCV10 introduction. This is likely due to the significant reduction in NVT carriage prevalence observed in 2013, but not in 2011 or 2012, compared with baseline. Similar findings have been reported [38] and attributed to different dynamics in pneumococcal carriage (HIV-positive mothers are more likely to carry vaccine-type pneumococci) [39], changes in HIV management over time, and widespread use of antibiotic prophylaxis [40, 41]. Despite the observed reductions, the high proportion of serotype 3 carriage, a serotype known to be associated with severe outcomes [42], and the higher carriage prevalence compared with HIV-negative adults indicate that HIV-positive adults will continue to be at a higher risk of IPD than HIV-negative adults, as seen in other countries [40, 43].
Our study is subject to several limitations. First, we were not able to assess differences in indirect effects between Lwak and Kibera due to the small number of unvaccinated Lwak children aged 1–4 years. We used children who received 1 or less PCV10 dose as a proxy for unvaccinated children, but the number of children in this group was also small. Since adults were only recruited in Lwak, we could not compare indirect effects among adults between the 2 sites. Second, although we were not able to confirm PCV10 vaccination dates for all children, we considered any vaccine dose reported prior to the survey regardless of the timing; therefore, some reported doses might not have been valid. Third, misclassification of HIV-negative adults might have occurred since some adults had a test date more than 1 year before the survey. Last, we did not have data on CD4 counts or HIV treatment history when assessing the impact of PCV10 among HIV-positive adults.
Despite these limitations, our study addresses key questions related to PCV10 introduction in sub-Saharan Africa. We observed significant declines in PCV10-type carriage in children and adults approximately 2 years after PCV10 introduction in Kenya. Although not statistically significant, there was a greater reduction in PCV10-type carriage in the site with catch-up vaccination targeting older children compared with the site without catch-up vaccination. However, as additional birth cohorts receive vaccination and as vaccinated children age, the differences between the 2 sites might be less noticeable. We did not observe changes in the proportion of isolates that were PNSP in children post-PCV10; this is likely due to rapid replacement of PCV10-type isolates by NVT PNSP. Very few studies have comprehensively addressed the impact of PCV10 introduction in sub-Saharan Africa, where 3 + 0 is widely used. The most recent WHO position statement on PCV states the potential benefits of the 2 + 1 schedule over the 3 + 0 schedule in providing longer protection, although supporting data are currently limited [23]. Continued monitoring of PCV10-type pneumococcal carriage in children and antibiotic-nonsusceptible pneumococci and their association with disease rates could help inform pneumococcal vaccination policies.
Supplementary Material
Acknowledgments.
The authors thank the study participants, their caregivers, the communities, and the field study staff for their active participation. They gratefully acknowledge the support and advice from Bernard Beall, Deron Burton, Daniel Feikin, Barry Fields, Katherine Fleming-Dutra, Lee Hampton, Muthoni Junghae, Kayla Laserson, Joel Montgomery, Nong Shang, John Vulule, Chris Van Beneden, and Newton Wamola throughout the study.
Financial support. This work was supported by the Bill and Melinda Gates Foundation and the US Agency for International Development (to L. K. and C. G. W.).
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17:1133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Pneumococcal vaccines. WHO position paper—2012 recommendations. Vaccine 2012; 30:4717–8. [DOI] [PubMed] [Google Scholar]
- 3.Gavi The Vaccine Alliance. Annual progress report. 2016. Available at: https://www.gavi.org/library/publications/gavi-progress-reports/gavi-progress-report-2016/. Accessed May 26th, 2018.
- 4.Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014; 2:e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Gottberg A, de Gouveia L, Tempia S, et al. ; GERMS-SA Investigators. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med 2014; 371:1889–99. [DOI] [PubMed] [Google Scholar]
- 6.Whitney CG, Farley MM, Hadler J, et al. ; Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737–46. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 2005; 116:e408–13. [DOI] [PubMed] [Google Scholar]
- 8.Feikin DR, Olack B, Bigogo GM, et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One 2011; 6:e16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol 2010; 39:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin LM, Bigogo G, Jagero G, et al. High Streptococcus pneumoniae colonization prevalence among HIV-infected Kenyan parents in the year before pneumococcal conjugate vaccine introduction. BMC Infect Dis 2016; 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, Conklin LM, Bigogo G, et al. Pneumococcal carriage and antibiotic susceptibility patterns from two cross-sectional colonization surveys among children aged <5 years prior to the introduction of 10-valent pneumococcal conjugate vaccine—Kenya, 2009–2010. BMC Infect Dis 2017; 17: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalal W, Feikin DR, Amolloh M, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr 2013; 62:e47–54. [DOI] [PubMed] [Google Scholar]
- 13.da Gloria Carvalho M, Pimenta FC, Jackson D, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol 2010; 48:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement CLSI Document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute, 2012. [Google Scholar]
- 15.Conklin LM, Bigogo G, Jagero G, et al. High Streptococcus pneumoniae colonization prevalence among HIV-infected Kenyan parents in the year before pneumococcal conjugate vaccine introduction. BMC Infect Dis 2016; 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JA. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J 2008; 27:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullahi O, Karani A, Tigoi CC, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One 2012; 7:e30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verani JR, Massora S, Acácio S, et al. Nasopharyngeal carriage of Streptococcus pneumoniae among HIV-infected and -uninfected children <5 years of age before introduction of pneumococcal conjugate vaccine in Mozambique. PLoS One 2018; 13:e0191113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 20.Brandileone MC, Zanella RC, Almeida SCG, et al. ; Pneumococcal Carriage Study Group. Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in São Paulo, Brazil. Vaccine 2016; 34:5604–11. [DOI] [PubMed] [Google Scholar]
- 21.Sáez-Llorens X, Rowley S, Wong D, et al. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine against acute otitis media and nasopharyngeal carriage in Panamanian children—a randomized controlled trial. Hum Vaccin Immunother 2017; 13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch AATM, van Houten MA, Bruin JP, et al. Nasopharyngeal carriage of Streptococcus pneumoniae and other bacteria in the 7th year after implementation of the pneumococcal conjugate vaccine in the Netherlands. Vaccine 2016; 34:531–9. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Position paper on pneumococcal conjugate vaccines (PCV)—February 2019. Wkly Epidemiol Rec. No 8 2019; 94:85–104. [Google Scholar]
- 24.Swarthout TD, Lourenco J, Fronterre C, et al. Limited vaccine-induced control of pneumococcal carriage amongst children six years post-introduction of 13-valent pneumococcal conjugate vaccine in Malawi: impact of force-of-infection and natural immunity. In: 11th International Symposium on Pneumocicci and Pneumococcal Diseases (Melbourne, Australia), 2018. Abstract No. ISPPD-0228. Available at: https://isppd.kenes.com/2018/Documents/ISPPD-11%20Abstract%20Book.pdf [Google Scholar]
- 25.Jayasinghe S, Menzies R, Chiu C, et al. Long-term impact of a “3 + 0” schedule for 7- and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in Australia, 2002–2014. Clin Infect Dis 2017; 64:175–83. [DOI] [PubMed] [Google Scholar]
- 26.Otiti M, Akech D, Simiyu S, et al. Residual nasopharyngeal carriage of vaccine type pneumococci in a mature PCV10 immunisation programme in Kenya. In: 11th International Symposium on Pneumococci and Pneumococcal Diseases (Melbourne, Australia), 2018. Abstract No. ISPPD-0590. Available at: https://isppd.kenes.com/2018/Documents/ISPPD-11%20Abstract%20Book.pdf [Google Scholar]
- 27.Hammitt L, Etyang AO, Morpeth SC, et al. Impact of 10-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya. 2018: 369876. doi: 10.1101/369876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr 2010; 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilishvili T, Lexau C, Farley MM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 30.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760–8. [DOI] [PubMed] [Google Scholar]
- 31.Steens A, Bergsaker MA, Aaberge IS, Rønning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013; 31:6232–8. [DOI] [PubMed] [Google Scholar]
- 32.Vissers M, Wijmenga-Monsuur AJ, Knol MJ, et al. Increased carriage of nonvaccine serotypes with low invasive disease potential four years after switching to the 10-valent pneumococcal conjugate vaccine in The Netherlands. PLoS One 2018; 13:e0194823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinta-Kokko H, Palmu AA, Auranen K, et al. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine 2018; 36:1934–40. [DOI] [PubMed] [Google Scholar]
- 34.Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J 2008; 27:335–40. [DOI] [PubMed] [Google Scholar]
- 35.Hammitt LL, Bruden DL, Butler JC, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis 2006; 193:1487–94. [DOI] [PubMed] [Google Scholar]
- 36.Hampton LM, Farley MM, Schaffner W, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis 2012; 205:401–11. [DOI] [PubMed] [Google Scholar]
- 37.Tomczyk S, Bennett NM, Stoecker C, et al. ; Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 38.Nzenze SA, von Gottberg A, Shiri T, et al. Temporal changes in pneumococcal colonization in HIV-infected and HIV-uninfected mother-child pairs following transitioning from 7-valent to 13-valent pneumococcal conjugate vaccine, Soweto, South Africa. J Infect Dis 2015; 212:1082–92. [DOI] [PubMed] [Google Scholar]
- 39.Nunes MC, Shiri T, van Niekerk N, et al. Acquisition of Streptococcus pneumoniae in pneumococcal conjugate vaccine-naïve South African children and their mothers. Pediatr Infect Dis J 2013; 32:e192–205. [DOI] [PubMed] [Google Scholar]
- 40.von Gottberg A, Cohen C, de Gouveia L, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003–2008. Vaccine 2013; 31: 4200–8. [DOI] [PubMed] [Google Scholar]
- 41.Madhi SA, Nunes MC. The potential impact of pneumococcal conjugate vaccine in Africa: considerations and early lessons learned from the South African experience. Hum Vaccin Immunother 2016; 12:314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henriques B, Kalin M, Ortqvist A, et al. Molecular epidemiology of Streptococcus pneumoniae causing invasive disease in 5 countries. J Infect Dis 2000; 182:833–9. [DOI] [PubMed] [Google Scholar]
- 43.Cohen AL, Harrison LH, Farley MM, et al. ; Active Bacterial Core Surveillance Team. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS 2010; 24:2253–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.