Abstract
Introduction: Providing for patients’ comfort and reducing their pain is one of the important tasks of health care professionals in the Intensive Care Unit (ICU). The current study was conducted to determine the effect of a protocol using a Richmond Agitation-Sedation Scale (RASS) on some clinical outcomes of patients under mechanical ventilation (MV) in 2017.
Methods: This single-blind clinical trial was conducted on 79 traumatic patients in the ICU who were randomly allocated into the intervention (N=40) and the control groups (N=39). The sedation was achieved, using a sedation protocol in the intervention group and the routine care in the control group. The clinical outcomes of the patients (duration of MV, length of staying in ICU, final outcome) were measured. As the participants had different lengths of MV and staying in ICU, the data were restructured, and were analyzed, using proper statistical methods.
Results: The patients’ level of sedation in the intervention group was significantly closer to the ideal score of RASS (-1 to +1). The duration of MV was significantly reduced in the intervention group, and the length of stay in the ICU was also significantly shorter. There was no difference in terms of final outcome. The ICU cost in the control group was twice as high as the cost in of the intervention group.
Conclusion: The applied sedation protocol in this study would provide better sedation and could consequently lead to significantly better clinical outcomes, and the cost of caring as a result.
Keywords: Clinical trial, Mechanical ventilation, Intensive care unit, Sedation
Introduction
According to US statistics, 55,000 patients are hospitalized daily in the ICU for various reasons.1 Mechanical Ventilation (MV) is required in over 90% of adult patients with critical illness in ICUs.2 Although it saves the lives of tens of thousands of patients with oxygenation problems, it simultaneously causes many complications in these patients and is associated with a high mortality rate.3 The Long-term MV refers to MV more than 3 days, which can increase health care costs, such as MV related costs and mortality.4
Not only the late weaning of a patient from the MV causes complications, but also the early weaning also can lead to the unplanned intubation and re-intubation.
Research support that re-intubation increases the rate of hospital pneumonia by 8 times and the risk of death by 6 to 12 times compared to the first time.5 Unplanned extubating is a major complication of the endotracheal intubation.6 resulting in the long-term MV, long term length of stay in ICU, and even in some cases, mortality.7
This can be due to patient's agitation and lack of cooperation, or accidental rupture of the endotracheal tube cuff, cough, or other causes. Evidence supports the point that the unplanned extubation can be used as an index for quality of nursing and intensive medical care in ICUs.6
Sedation for mechanically ventilated patients is medically necessary and should be managed according to individual assessment and patient needs.8 Desirable sedation is affected by some factors such as the type of disease, underlying illnesses, severity of the disease, and necessary and supportive interventions.9 Pain and sedation management using a protocol, could reduce the MV time and length of stay in the ICU, and subsequently the length of stay in the hospital, mortality rate, and health care costs in mechanically ventilated patients.4,10
Inappropriate sedation could also affect the patient's clinical outcomes and costs.9 Providing the patient's comfort is the target level of sedation management in ICU, so that the patient can easily wake up in the normal sleep-awake course. However, some patients may need a deeper level of sedation to facilitate MV.8
As purposeful pain management and sedation, using sedation protocols and scoring systems lead to earlier achievement of spontaneous breathing in the patient, early weaning from the ventilator, and reducing the length of stay in the ICU and the hospital, and thereby reducing costs; the use of sedation protocols and scoring systems is very important to achieve better sedation.11 Different clinical scoring systems such as Ramsey, Ricker, Richmond are used to determine the levels of sedation-agitation.11
The RASS is one of the recommended measures to determine the level of sedation in the ICU, as it is an appropriate tool for measuring the sedation state among Persian speaking care givers with an appropriate agreement coefficient.12 Validity and reliability of the RASS have already been confirmed by Ely13 and Sessler14 and its validity has been proven in Iranian population in 2009, too.15
In 1997, it was reported that the implementation of the sedation protocol by nurses could reduce the need for MV and tracheostomy.16 Since then, a large body of studies have examined the effect of sedation protocol on MV,17,18 the time of weaning from the ventilator,19 length of stay in the ICU,17,20 length of stay in the hospital,19,20 the frequency of self-extubating,21 the rate of reintubation,21 and other variables separately. The use of sedation protocol can provide proper control of agitation, relieve pain, increase the level of patient’s consciousness, facilitate the patient’s control by the nurse, find the causes of agitation before the injection of the any drug, and prevent over-sedation.12
Assessing the nurses’ performance in the ICU indicates that they do not tend to use pain monitoring tools for patients who are not able to speak, and have little knowledge about pain control guideline which could negatively affect their performance on pain.22
Based upon existing knowledge, there is a contradictory finding about the effects of using sedation protocol on the length of MV, the length of stay in the ICU, and other clinical outcomes. On the other hand the former studies had some limitations such as the applying the sedation protocol for short time (24 to 48 hours), or low interval of assessment (e.g. each 4 or 6 hours). To our knowledge, this study is the first to conduct the sedation protocol using the RASS in total duration of MV and a short interval of assessment of the sedation condition (hourly) in head trauma patients in ICU. In addition, in this study, it was tried to design a different sedation protocol to evaluate the patient's level of sedation hourly for longer time in ICU and consequently to increase the non-drug interventions, and to reduce the overdose of sedative medications. The results of this study will add to the existing knowledge by increasing the duration of protocol application (total duration of mechanical ventilation), the study of different clinical outcomes, and measuring the amount of costs imposed on patients. Moreover, the limitations of the previous studies would be covered to some extent.
Therefore this study was conducted to determine the effect of sedation protocol, using RASS Scale on the improvement of patients' analgesia and sedation, clinical outcomes of patients (duration of MV, length of stay in the ICU, and the final outcome (transfer to general ward and death) in the ICU of Ayatollah Mousavi Hospital in Zanjan-Iran and its secondary effects on the treatment costs of patients in 2017.
Materials and methods
This study was a randomized single-blind clinical trial which was conducted on patients admitted to the ICU from January to June 2017 (patients and their legal guardians did not know about the method, but the evaluators and the researcher’s assistants were aware of the method and the study; the assistants, however, did not know about the process of selecting the control group and The clinical outcomes of the case).
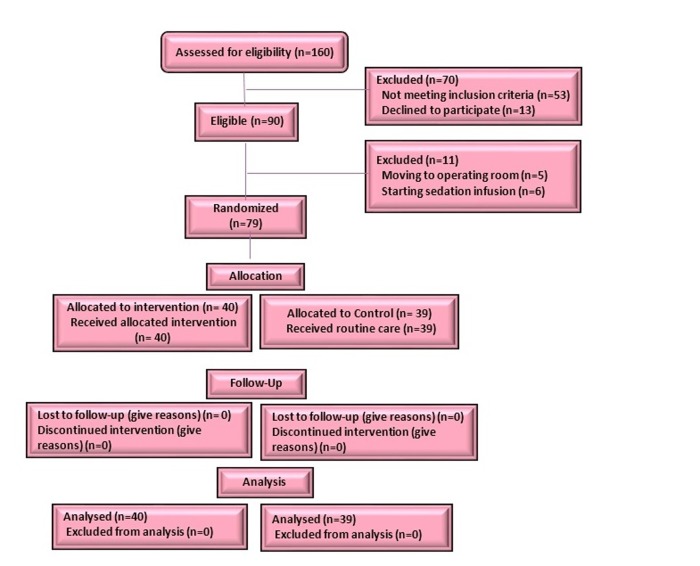
A total sample size of 90 patients was selected according to the results of other studies with power of 90% and β=0.1023,24 and 10% chance of loss to follow up for the main variable duration of MV. During the study, 11 participants were excluded due to exclusion criteria [initiation of continuous infusion of sedative (N=6); transferring to the operating room (N=5), and 79 patients (the intervention group: N=40, the controls group: N=39) were recruited into the study (Figure 1).
Figure 1.
Consort flow diagram of patients' recruitment in the study
Zanjan university of medical sciences (ZUMS.REC. 1395.215) and recorded in the clinical trials study database (IRCT2017010831824N1) was obtained, the newly admitted patients were selected, using simple randomized Poisson model. The goal of the study was explained to the patients' companions and legal guardians, and those who were willing to participate were recruited in the study after signing informed consent forms. The samples were randomly allocated into either an intervention or a control group using a blocking method (nine blocks of ten). The inclusion criteria of the study were: having an endotracheal tube, the need for MV with SIMV mode, having no addiction history (according to the patient's records and information from the patient's guardian), age of between 15-65 years, RASS score of higher than 3, Apache II score of between 10 and 20, and having a Glasgow level of consciousness between 5 and 13.
Exclusion criteria consisted of alertness and extubating for less than 24 hours, modifying a prescribed drug by the responsible physician, discontinuation of medication, and transferring to the operating room for surgery, reducing the patient’s level of consciousness in Glasgow criteria below 5, and starting the continuous infusion of the sedative.
Data gathering tools consisted of Glasgow Coma Scale (GCS), that has been widely used in various articles.,12,25 Richmond Agitation-Sedation Scale (RASS), and Apache II.
Richmond Agitation-Sedation Scale is one of the recommended scales for measuring the level of sedation in the ICU,26 which is a continuum of 10 scores ranging from 5 to 4 with three levels. In this instrument, 5 negative scores is assigned to the level of tranquility (-1 = drowsy, -2 = light sedation, -3 = moderate sedation, -4 = deep sedation, 5- = unarguable), 0 score for being alert and calm, and 4 positive scores are assigned to the level of agitation (+1 = restless, +2 = agitated, +3 = very agitated, 4 = combative). The validity of this tool has already been confirmed by Ely13 and Sessler.27 In one study in Iran, the inter-rater agreement coefficient and Cronbach's alpha coefficient was 0.95 and 0.86 respectively.12 another tool used in this research was Apache II scale that has been frequently used in many articles..28
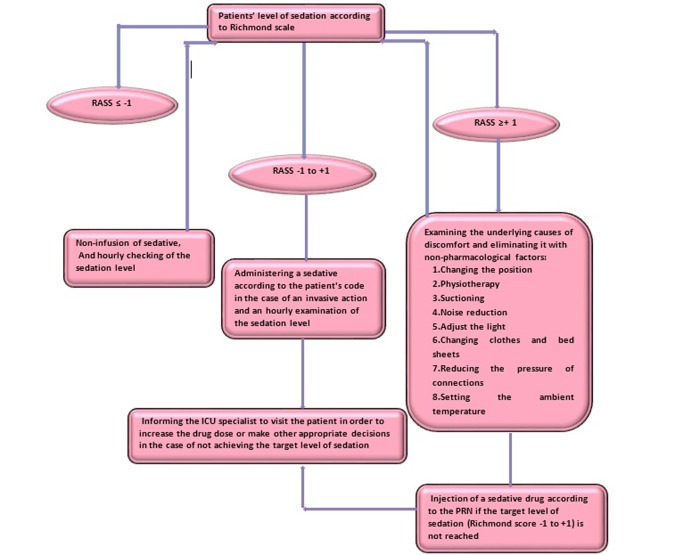
This intervention was designed to examine the effect of sedation protocol using the RASS on some clinical outcomes. To this end, the relative protocol was originally prepared (Figure 2). The content and formal validity methods were used to obtain scientific credentials.
Figure 2.
Steps to assess the patients’ level of sedation according to the RASS (Richmond Agitation-Sedation Scale) sedation protocol-consort diagram
Therefore, the proposed protocol was presented to 10 academic faculty members and expertise (including ICU physicians, the head nurse, and ICU nurses, and faculty members) of Zanjan University of Medical Sciences, and their comments were incorporated into the protocol. The application and evaluation of the sedation protocol using RASS Scale and the infusion of sedative drugs were priory instructed to research assistants on various shifts individually by the researcher. To investigate the agreement between the researcher and the research assistants (4 people aged 30-40 years old with more than 3 years of work experience in the ICU), following the individual training, each assistant researcher assessed 3 patients according to the protocol. The necessary steps were taken, based on the protocol and the inter-rater agreement coefficient between the researcher and researcher assistants was calculated 0.78. The level of consciousness and sedation of all patients in the intervention and control groups were measured at the time of recruitment, using GCS and RASS and recorded on the specific checklists. Apache II criteria were used to estimate the severity of the disease and control the confounding factors. The ICU physician monitored the implementation of the study and the clinical status of the patients at all stages of the study. In the control group, care management was carried out routinely based on physiological responses of patients, clinical judgment of nurses in the unit, and injection of sedative drugs according to the previous order of the ICU specialist that included ICU intravenous injection of fentanyl, midazolam, thiopental, haloperidol, morphine, and methadone. Control of pain and sedation in the intervention group were undertaken using a protocol designed by the researchers (Figure 2). It should be noted that the type of drugs were the same for both groups. If the sedation score was between -1 to 1, no special action was taken and only if an invasive action was needed, a sedative drug was injected as order. The pharmacological measures included injecting drugs: Fentanyl, Methadone, Midazolam, Haloperidol, Morphine and Thiopental, which were significantly the same for patients in two groups. The doses of infusion and the type of sedatives were recorded for each patient every day. Then, the frequency of prescribed injections was compared in the two groups.
The level of sedation was checked hourly at the time of recording the vital signs of the patients and recorded on specific forms. If the patient's level of sedation was greater than or equal to +1, the patient's discomfort was relieved using non-pharmacological factors, such as changing the position of the patient, reducing the pressure on the patient's lip tube or nose, reducing the ambient noise, etc.
If the patient's discomfort was resolved, the patient's level of comfort was checked every hour according to the chart; otherwise, the sedation level was evaluated using RASS Scale and the sedative was infused according to the drug's prescription. The duration of the intervention for each patient was the total period of MV. As the duration of mechanical ventilation was different among the patients in the control and intervention groups, the duration of the intervention varied for individual patients. Therefore, the data restructuring method was used to test the research hypotheses and analyze the process evaluation of the intervention (frequency of the Richmond scores (+4 to -5), as well as drug and non-drug interventions). In this structure, the frequency of the total actions taken (Richmond scale assessment and drug and non- drug assessment) were pooled together and calculated per day in both the intervention and control groups (number of patients who were assessed per day * number of actions per hour * 24). In order to prevent bias, the researcher's assistants did not know about the recording of the clinical outcomes and all cases were recorded by the researcher.
The patients' treatment costs were calculated as a secondary objective, based on the cost of hospitalization in the ICU, and finally the clinical outcomes of the two groups were compared, using appropriate statistical tests.Kolmogorov-Smirnov test was used to determine the normal distribution of variables. Regarding the normal distribution of variables, parametric tests were used. The data were analyzed, using descriptive statistics, Chi-square test (and Fisher's exact test), independent t-test and survival analysis. To test the research hypotheses using systematic restructure in SPSS (version 13.0, Chicago, IL, USA), the participants’ scores on each shift were adjusted for each participant and then Chi-square test (and Fisher's exact test) and independent t-test were used. The significance level was considered 0.05. The R statistical software was used or the survival analysis.
Results
In this study, 79 trauma patients under MV were studied in two groups: intervention (N=40) and control (N=39).
The comparison of demographic characteristics (age, gender) and clinical conditions (Apache II score, level of consciousness based on Glasgow criteria, underlying diseases, and cause of hospitalization) showed that there was no significant difference between the two groups in terms of main confounding factors. As the number of days under MV was not uniform in the two groups, the restructuring as well as appropriate statistical tests were employed to compare the frequency of RASS scores during MV based on a day, morning, evening, and night shifts, duration of MV, length of ICU stay, and clinical outcomes. The results of Fisher's exact test suggested that the RASS scores of the intervention group who used the sedation protocol were significantly in the ideal range (-1, 0, and 1) (P<0.001). These results were repeated throughout the staying in the ICU, except for the tenth day (which was close to significant). To better understand the status of RASS scores during the intervention, the data were displayed in the form of two Figure of 3 (the first 5 days) and 4 (the second 5 days) (Figure 3 and 4).
Figure 3.
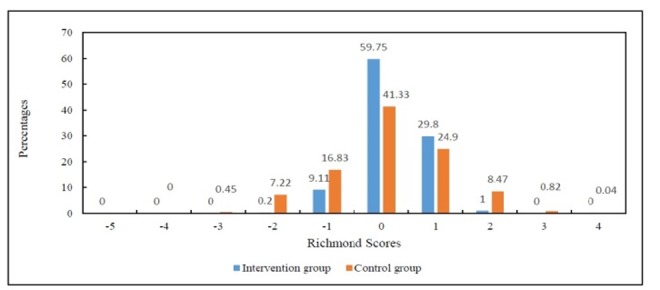
The percentage of desired Richmond score on the first five days of intervention
Figure 4.
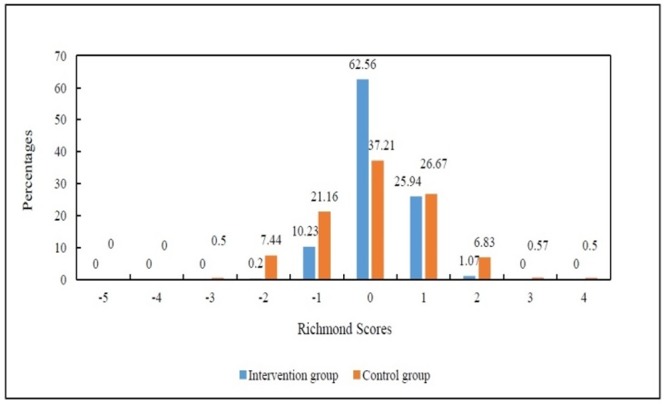
The percentage of desired Richmond score on the second five days of intervention.
Figure 3. shows the frequency of ideal RASS scores (-1 to +1) in the intervention is significantly more than the control group, indicating the patients under sedation protocol are more likely to have a better RASS score and consequently better sedation in the first five days of the intervention. Figure 4. shows the frequency of ideal RASS scores (-1 to +1) in the intervention is significantly more than the control group, indicating the patients under the sedation protocol are more likely to have a better RASS score and consequently better sedation in the second five days of the intervention. The comparison of non-pharmacological measures showed that the frequency of these procedures were significantly higher in the intervention group (P<0.001). It was also revealed that the application of sedation protocol reduced the level of sedative drugs in the intervention group (P<0.001). A significant difference was found in the mean duration of MV between the intervention 5.00(2.00) and the control group 10.05 (7.00) (P<0.001). Accordingly, the average length of ICU stay in the intervention group 10.00 (4.00) days was significantly lower than that in the control group 19.00 (12.00) days (P <0.001) (Table 1).
Table 1. Comparison of the mean of MV duration and length of stay in the ICU in the intervention and control groups .
| Variable | Mean(SD) | Independent t- test |
P |
| MV duration | 3.807 | <0.001 | |
| Intervention | 5.00(2.00) | ||
| Control | 10.05(7.00) | ||
| Length of stay in the ICU | 4.225 | <0.001 | |
| Intervention | 10.00(4.00) | ||
| Control | 19.00(12.00) |
Indicating the patients under RASS protocol are more likely to have lower duration of MV and ICU stay. In order to compare the final outcomes (transfer to general ward and death) between the intervention and the control groups, using the Fisher’s exact test showed no significant difference between groups. Although 97.5% of participants in the intervention group (N=39) compared to 87.2% (N=34) in the control group were transferred to the ward. In other words, the use of sedation protocol had no effect on the final outcome of the patients (including death or transfer) (P= 0.083) (Table 2).
Table 2. Comparison of the final outcome of the patients in the intervention and control groups .
| Group | Transfer to unit N (%) |
Death N (%) |
Fisher's exact test | P value |
| Intervention | 39 (97.5) | 1(2.5) | 2.97 | P=0.083 |
| Control | 34 (87.2) | 5(12.8) | ||
| Total | 73 (92.40) | 6(7) |
To achieve a better understanding of the effects of using the RASS sedation protocol in this study, a survival analysis using the Cox relative risk model was conducted between the length of MV, and length of stay in ICU with the clinical outcomes (death and transfer to the ward). In this analysis, the score of Apache II, sex and age was considered as predicting factors of the length of MV, and stay in ICU. The survival analysis showed that the intervention group was three times more likely to survive MV compared to the control group (P<0.001) and for one unit increases in Apache score, the MV duration increases 0.77 day (P<0.001). No significant difference was found between age and sex and the survival rate of the patients (Table 3).
Table 3. The survival analysis for the duration of mechanical ventilation in the intervention and the control group .
| Group | B | Exp€ | SE€ | Z | P |
| Intervention | 1.08 | 2.96 | 0.27 | 3.89 | <0.001 |
| Apache II | -0.26 | 0.77 | 0.04 | -5.75 | <0.001 |
| Sex | -0.41 | 0.66 | 0.26 | -1.54 | 0.12 |
| Age | -0.00 | 0.99 | 0.01 | -0.48 | 0.62 |
€(Coef)
The survival analysis for length of stay in ICU showed that the survival rate in the intervention group is four times higher than that in the control group (P<0.001) and For one unit increases in Apache score, the ICU length of stay would increase by 0.82 day (P<0.0001).No significant difference was found between age and sex and the survival rate of the patient (Table 4).
Table 4. The survival analysis for the length of stay in ICU in the intervention and the control group .
| Group | B | Exp€ | SE€ | Z | P |
| Intervention | 1.38 | 3.99 | 0.27 | 4.97 | <0.001 |
| Apache II | -0.19 | 0.82 | 0.04 | -4.85 | <0.001 |
| Sex | -0.35 | 0.69 | 0.26 | -1.34 | 0.18 |
| Age | -0.02 | 0.98 | 0.01 | -1.75 | 0.079 |
€(Coef)
Self-extubation, re-intubation, and patient admission costs were studied as the secondary objectives of this study. The results showed that only 2.5% (1 patient) of the intervention group had self-extubation, while 10.3% (4 patients) in the control group had self-extubation. Despite the higher frequency of self-extubation in the control group, the Fisher's exact test did not show statistically significant difference between the two groups. The intervention group did not require any reintubation, while in the control group, 7 patients (17.9%) were re-intubated and Fisher's exact test showed a significant difference between the two groups (P= 0.005). The average cost of the treatment in the intervention group 6699000 (3179507) was significantly lower than that of the control group 12810769 (85664139). Using independent t-test, there was a significant difference between the two groups in terms of overall hospital costs (based on the cost of one night in the ICU: Costs calculated on the ICU bed) (P<0.001). In other words, the ICU cost in the control group was nearly two times the cost of that in the intervention group, which was due to a lower average duration of hospitalization in the intervention group.
Discussion
The aim of this study was to determine the effect of applying a protocol on some clinical outcomes of patients under MV in the ICU (duration of MV, length of stay in the unit, and final outcome of the patients including transfer to general ward and death). The results showed that the use of sedation protocol can provide better sedation for patients under MV in the ICU and the number of hours the patients keep in the ideal range of RASS Scale increases substantially. This finding is consistent with the results of some studies in this regard.12,25,29-31 In contrast to the findings of this study and most similar studies in this area, Bucknall et al., concluded that the use of nursing sedation protocol does not contribute much to the sedation of patients in ICUs. Bucknal et al., have suggested that their different research findings compared to the other studies indicate that "Australian nurses care a lot about the patients’ level of pain and sedation even when they do not use the sedation protocol". This is the reason why patients are often as comfortable as they can be.32 it seems that this difference is due to the investigation of the sedation level by Australian ICU nurses; so that applying the protocol or otherwise, does not make any difference in the sedation of the patients.
The application of sedation protocol in this study reduced the duration of MV in ICU patients, which is consistent with the results of many studies24,30 and contradicts the results of some other studies.31-33 It seems that the observed difference can be due to the frequency of using sedation,31-33 lack of nurses to assess the level of sedation, difference in drugs Midazolam and Fentanyl33 and the design of the research in the form of before and after the intervention, using a different sedation scale (Ramsay), and difference in the research samples.34
However, the present study was designed as a single-blind clinical trial in which the patients were evaluated hourly by the researcher assistants using RASS scale. Meanwhile, the drugs used for all patients in both groups were identical and according to the orders of the patients, including fentanyl, midazolam, thiopental, haloperidol, and morphine.
The results of this study showed that the application of sedation protocol caused a significant decrease in the duration of hospitalization in patients in the intervention group. This result is consistent with the results of some studies in this regard,21,24,35 and contradicts the results of some others.,32 Different results can be attributed to the nurses’ sense of responsibility in examining the sedation level as a professional and cultural factor,32 using different scales for evaluating sedation (Ricker scale), and patients under study (patients under coronary artery bypass graft surgery).
No statistically significant difference was found between the two groups regarding the final outcome of the patients, including transfer to general ward and death, according to the number of deaths in the control group (1 death in the experimental group and 5 deaths in the control group). This case is in line with the results of some studies,10,32,36 however, the results of some studies in this regard contradict ours.30,35,37 This difference in results can be due to the large number of samples used in the study,30,35,37 design of the study in form of before and after the intervention,35 and the type of intervention used for simultaneous application of sedation and delirium protocols.24
A survey of self-extubation rate in patients showed that, despite the fact that the prevalence of spontaneous self-extubation in the control group was higher than that of the intervention group, Fisher's exact test did not show any significant difference between the two groups. This is in line with the results of some studies in this area.,32 Of course, Girard et al., reported a significant difference in this regard, indicating that patients in the intervention group experienced less self-extubation;21 this difference could be due to the type of intervention in which the sedation and ventilator weaning protocols were used simultaneously.
In this study, the sedation protocol reduced the rate of reintubation in patients. This result is different from the results of Marshall et al., and Gigard et al., studies. This difference can be attributed to the use of continuous infusion for sedation18 and the type of patients under study,18,21 since all ICU patients in these studies were examined, while in the present study, only traumatic patients under MV were included in the study.
According to the results of this study, the average cost of the whole intervention group was significantly lower than that in the control group. The average cost of admission to the ICU for the control group was nearly twice as high as the cost of the intervention group. These results are consistent with the findings of Awissi et al., of course, some studies also reported that applying a sedation protocol can reduce the drug costs between 22% to 94% in the protocol group compared to the non-protocol group.9
By using sedation protocols (such as Ramsey, Ricker, and Richmond), nurses evaluate the causes of insecurity prior to administering sedation to patients, such as presence of possible noise in the unit, inappropriate position of the patient, and prevent the unnecessary injection of drugs (very deep sedation), low infusion of sedative medications than needed (agitation and pain); and giving appropriate dose of drugs.25 Many common causes of pain and restlessness in patients in the ICU such as inappropriate bone traction, inappropriate pressure banding, pressure in the trachea, and the nasogastric tubes cannot be eliminated even by high doses of sedation and accommodation, and only eliminating the causes of these problems can relieve pain and sedation in patients. By investigating the level of patient agitation based on protocols, nurses can identify these problems and provide patients with comfort in an effective way with lower complications and costs .12
Regarding the duration of implementing the sedation protocol, there have been studies carried out within only 24 or 48 hours with every 2, 3, or 4-hour intervals,12,25,31 while there are other studies in which the sedation protocol has been used during the ICU stay,23 due to the selection of the acute type of illness under study and the shorter hospitalization time. In the present study, the protocol was applied during the total duration of MV by every one-hour interval to examine the effects of longer usage of the sedation protocol.
This interventional study examined the effect of applying a sedation protocol with the frequency of every one hour on improving the clinical outcomes of patients undergoing MV, which to our knowledge, was conducted for the first time in Iran. These results can provide an appropriate background needed to improve the sedation and prevent the agitation of patients requiring MV and, to obtain better clinical outcomes. One of the strengths of this study was implementing sedation during the total duration of MV. In most studies, this intervention lasted for only 24 to 48 hours with every 2, 3, or 4-hour intervals.12,31 On the other hand, in most of the studies, there was no comparison of imposed costs on the patients under study.
One of the limitations of this research is insufficient power of generalizability and its external validity, since it was conducted only in one of the ICUs of Ayatollah Mousavi Hospital in Zanjan and on trauma patients and may not be generalized to other ICUs and other patients. The impossibility of following up the recovery of patients who were admitted to the ICU longer than the intervention period due to the time limit for the study is another limitation of the present study. The confounding factors of the study were moderated through randomization, matching the prescribed drugs, and using Apache II criteria to the severity of the patients in two groups. The inequality of MV period during the intervention was another limitation of this study that was solved using restructuring in the statistical analysis.
Hawthorne Effect was one of the possible biases that may affect the outcome of the study. The blindness of the research assistants who performed the intervention and the record of all the clinical outcomes by the researcher could partly control the effect of this factor.
Conclusion
Many hospitalized patients are agitated due to conditions in the ICU. The control of pain and sedation has caught the attention of nurses and other members of the treatment team. Nurses are more concerned with the sedation of patients than other members of the ICU. Due to continuous presence on the patient's bedside, during the work shift, nurses can play a very important role in the application of the sedation protocol. The results of this study showed that applying sedation protocol by nurses using RASS based protocol can lead to better sedation in patients and consequently significantly reduce the duration of MV, the length of stay, and consequently reduce maintenance costs. Implementing this method in the ICU and conducting further research is recommended.
Acknowledgments
The researchers would like to express their gratitude and appreciation to the director of the Nursing Office of Ayatollah Mousavi Hospital in Zanjan, the head nurse and the nurses working in the ICU, who cooperated in this research, as well as the participants and their families who have devotedly helped us to implement this study.
Ethical issues
None to be declared.
Conflict of interest
The authors declare no conflict of interest in this study.
Citation: Taran Z, Namadian M, Faghihzadeh S, Naghibi T. The effect of sedation protocol using Richmond agitation-sedation scale (RASS) on some clinical outcomes of mechanically ventilated patients in intensive care units. J Caring Sci 2019; 8 (4): 199-206. doi:10.15171/jcs.2019.028.
References
- 1.Yeganeh MR, Gholami S, Tabari R, Atrkar Roshan Z, Rimaz S. The effect of controlled sedation based on the Richmond scale on the duration of mechanical ventilation and the changes of blood pressure in patients following coronary artery bypass graft surgery: A randomized clinical trial. Journal of Faculty of Nursing & Midwifery, Tehran University of Medical Sciences. 2018;23(4):372–86. (Persian) [Google Scholar]
- 2.McLean SE, Jensen LA, Schroeder DG, Gibney NR, Skjodt NM. Improving adherence to a mechanical ventilation weaning protocol for critically ill adults: outcomes after an implementation program. Am J Crit Car. 2006;15(3):299–309. [PubMed] [Google Scholar]
- 3.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–53. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 4.Blackwood B, Alderdice F, Burns K, Cardwell C, Lavery G, O’Halloran P. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: Cochrane systematic review and meta-analysis. BMJ. 2011;342:c7237. doi: 10.1136/bmj.c7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdannik AR, Salmani F, Irajpour AR, Abasi S. Effect of the Nurse-directed weaning readiness assessment on the duration of mechanical ventilation: a randomized clinical trial. Qom University of Medical Sciences Journal. 2013;7(4):89–94. (Persian) [Google Scholar]
- 6.Cortés I, Esteban A. Weaning from mechanical ventilation. Anaesthesia, pharmacology, intensive care and emergency medicine A.P.I.C.E. 1st ed. Italy: Springer, Milano; 2011. [Google Scholar]
- 7.Marcin JP. Unplanned extubations—they’re not accidental. Pediatr Crit Care Med. 2007;8(4):406–7. doi: 10.1097/01.PCC.0000269385.18380.D3. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET. et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Jackson DL, Proudfoot CW, Cann KF, Walsh T. A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care. 2010;14(2):R59. doi: 10.1186/cc8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale CR, Kannas DA, Fan VS, Daniel SL, Deem S, Yanez III ND. et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367–74. doi: 10.1513/AnnalsATS.201306-210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Dossow V, Moshirzadeh M, Kastrup M, Wernecke K-D, Konertz W, Spies C. Performance of the A-line Autoregressive Index (AAI) and of the Bispectral Index (BIS) at assessing depth of short-term sedation following cardiac surgery. Journal of International Medical Research. 2009;37(3):611–20. doi: 10.1177/147323000903700303. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi TE, keykha AA, Abbaszadeh A, Rafiei H, Enayati H, khodadadi HBM. et al. The effect of the sedation protocol on the level of consciousness in ventilator-dependent trauma patients hospitalized in intensive care unit (ICU) Medical - Surgical Nursing Journal. 2015;4(1):23–30. (Persian) [Google Scholar]
- 13.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S. et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 14.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'neal PV, Keane KA. et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 15.Tadrisi S, Madani S, Farmand F, Ebadi A, AA KZ, Mirhashemi S. et al. Richmond agitation–sedation scale validity and reliability in intensive care unit adult patients Persian version. Iranian Journal of Critical Care Nursing. 2009;2(1):15–21. [Google Scholar]
- 16.Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W. et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–15. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF. et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691–9. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 18.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36(2):427–33. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 19.Burns SM, Earven S, Fisher C, Lewis R, Merrell P, Schubart JR. et al. Implementation of an institutional program to improve clinical and financial outcomes of mechanically ventilated patients: one-year outcomes and lessons learned. Crit Care Med. 2003;31(12):2752–63. doi: 10.1097/01.CCM.0000094217.07170.75. [DOI] [PubMed] [Google Scholar]
- 20.Arabi Y, Haddad S, Hawes R, Moore T, Pillay M, Naidu B. et al. Changing sedation practices in the intensive care unit. Middle East J Anaesthesiol. 2007;19:429–47. [PubMed] [Google Scholar]
- 21.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT. et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 22.Rose L, Smith O, Gélinas C, Haslam L, Dale C, Luk E. et al. Critical care nurses’ pain assessment and management practices: a survey in Canada. Am J Crit Care. 2012;21(4):251–9. doi: 10.4037/ajcc2012611. [DOI] [PubMed] [Google Scholar]
- 23.Mirzaee M, Pourmirza Kalhori R, Moradi G, Khatoni A, Rezaee M. The effect of Riker sedation-agitation scale on clinical outcome of patients under coronary artery bypass graft surgery. Journal of Critical Care Nursing. 2013;6(4):217–22. [Google Scholar]
- 24.Mansouri P, Javadpour S, Zand F, Ghodsbin F, Sabetian G, Masjedi M. et al. Implementation of a protocol for integrated management of pain, agitation, and delirium can improve clinical outcomes in the intensive care unit: a randomized clinical trial. J Crit Care. 2013;28(6):918–22. doi: 10.1016/j.jcrc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Akbar Keykha A, Abbaszadeh A, Enayati H, Borhani F, Rafiei H, Hoseini BMK. Applying the instruction of pain control and sedation of the patients hospitalized in intensive care unit. Iranian Journal of Critical Care Nursing. 2014;6(4):243–50. (Persian) [Google Scholar]
- 26.Carlson KK. AACN Advanced Critical Care Nursing. 1st ed. Amsterdam: Elsevier Health Sciences; 2008. [Google Scholar]
- 27.Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(3):S2. doi: 10.1186/cc6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramazani J, Hosseini M. The Assessment of APACHE II Scoring System in Predicting the Result of Weaning from Ventilator. Journal of Knowledge & Health in Basic Medical Sciences. 2014;8(4):187–92. (Persian) [Google Scholar]
- 29.Porhomayon J, Nader ND, El-Solh AA, Hite M, Scott J, Silinskie K. Pre-and post-intervention study to assess the impact of a sedation protocol in critically ill surgical patients. Journal of Surgical Research. 2013;184(2):966–72. doi: 10.1016/j.jss.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka LMS, Azevedo LCP, Park M, Schettino G, Nassar AP, Réa-Neto A. et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Critical Care. 2014;18(4):R156. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeGrado JR, Anger KE, Szumita PM, Pierce CD, Massaro AF. Evaluation of a local ICU sedation guideline on goal-directed administration of sedatives and analgesics. Journal of Pain Research. 2011;4:127. doi: 10.2147/JPR.S18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Critical care Medicine. 2008;36(5):1444–50. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 33.Yılmaz C, Girgin NK, Özdemir N, Kutlay O. The effect of nursing-implemented sedation on the duration of mechanical ventilation in the ICU. Turkish Journal of Trauma & Emergency Surgery. 2010;16(6):521–6. [PubMed] [Google Scholar]
- 34.Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Medicine. 2006;32(10):1506–14. doi: 10.1007/s00134-006-0309-0. [DOI] [PubMed] [Google Scholar]
- 35.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesthesia & Analgesia. 2010;111(2):451–63. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 36.Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J. et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308(19):1985–92. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 37.Sehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Ali TM. et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Medicine. 2013;39(5):910–8. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]