Abstract
Introduction
The life expectancy of HIV-infected patients has been increased by highly effective therapies. People living with HIV (PLWH) in Romania are exposed to age-related comorbidities occurring earlier than in uninfected individuals. Multidisciplinary care is required to maintain the general health and quality of life in these patients. Currently, the communication among different specialties needs to be enhanced and formalized.
Methods
A panel consisting of 8 Romanian experts in infectious diseases, cardio-metabolic, bone, and kidney diseases and psychology met in May 2019 in Bucharest Romania to discuss the need to evaluate and monitor the most prevalent comorbidities in PLWH. The meeting resulted in practical guidance on the management of several non-infectious associated diseases. The algorithms were endorsed by the Society for Infectious Diseases and HIV/AIDS, Romania.
Results
The consensus statement offers practical guidance on how to assess and monitor associated diseases in adult PLWH. The recommendations are grouped for each cluster of comorbidities and are based on international guidelines and clinical experience, including landmarks for referral of PLWH to cardiology, endocrinology, nephrology specialist or clinical psychologist for additional investigations and adequate treatment. Specific indications for diagnosis or treatment were beyond the scope of this consensus.
Conclusions
Screening for associated diseases and adequate management are required to maintain the overall health status of PLWH. When implemented in clinical practice, the recommended algorithms should be used in addition to diagnosis and treatment guidelines and protocols. The infectious diseases specialist plays a key role in coordinating the overall treatment strategy and working within the multidisciplinary team.
Keywords: HIV, PLWH, comorbidities, consensus statement, monitoring
Introduction
The deployment of large HIV prevention campaigns and the availability of antiretroviral therapy (ART) contributed to a great extent to decreasing the impact of the global epidemic of HIV infection. Current estimates show a reduction of new cases by 40% since the peak observed in 1997.(1) Ongoing efforts are still required to achieve the UNAIDS targets, considering that only 62% of the people living with HIV (PLWH) are on treatment, worldwide, and there is a lower percentage with evidence of viral suppression.(1)
Data recently reported in Romania indicate that 77% of PLWH are receiving ART, and among them almost 70% are virally suppressed.(2) Since 2001, ART has been fully reimbursed and available in Romania to all patients diagnosed with HIV infection, immediately after diagnosis, and irrespective of the CD4 count.(3) This strategy was also included in the European AIDS Clinical Society (EACS) Guidelines in 2015.(4) Furthermore, considering the advances in therapeutic options, current guidelines recommend the use of a regimen offering durable virologic control,(5) as there is strong evidence that early initiation of ART and long-term maintenance of effective viral suppression significantly decrease the occurrence of serious AIDS-related or unrelated events.(6)
With an increasing life-expectancy, PLWH are more likely to be affected by age-related diseases common to uninfected individuals, but also by specific HIV-related or ART-related complications. A growing body of evidence indicates a premature aging effect of HIV infection on the immune system, multi-morbidity becoming the common feature of this patient population.(7-9) Risk factors for comorbidities may be associated with HIV infection per se (through microbial translocation, low grade persistent chronic inflammation, immune system activation, pro-coagulant mechanisms), co-infections (with hepatitis viruses, herpes viruses, etc.), as well as ART (toxicities, drug-drug interactions) adding to classical risk factors, such as age, dyslipidemia, diabetes, lipodystrophy, high blood pressure, obesity, smoking, and drug use. HIV- and ART-related comorbidities are implicated in causing higher mortality rates than in the general population despite advances in the therapeutic care.(10,11)
The radical change in the paradigm on treatment of HIV infection, from reduction of immediate risk of death to improvement of the quality of life and prevention of chronic diseases, is highly applicable to clinical practice in Romania. The early history of HIV infection in Romania was marked by the formation of a cohort of patients infected at an early age, in the late 1980s, who grew up living with HIV infection, initially in the absence of effective ART options, and subsequently starting monotherapy, double therapy and then progressing to highly active ART (HAART) once it became available as standard of care.(2,12) The epidemic has changed in the past years, with newly diagnosed cases having different risk factors for infection and different transmission routes, adding to the pool of patients from the initial pediatric cohort. As evidenced by the latest published report,(2) the majority of Romanian PLWH (62%) are young adults aged between 25 and 39 years. One third of the total population of PLWH make up the initial pediatric cohort who have now reached 30-34 years of age, and another third includes adults aged over 40.(2) The role of the infectious diseases (ID) specialist has transitioned from an initial all-encompassing approach, in which the ID physician was responsible for diagnosing and managing all concurrent diseases and comorbidities, to now playing a key role as part of a complex multidisciplinary team, which includes a wide array of medical specialties along with nursing and psychological health experts, with the goal of maintaining the general health and the quality of care in this patient population. This multidisciplinary approach has become an essential tool for delivering key aspects of HIV-related care, including prevention, diagnosis and monitoring of associated comorbidities.
Methods
In May 2019, eight experts from several specialties (infectious diseases, cardiology, endocrinology, nephrology and clinical psychology) participated in a meeting to discuss the need for assessing and monitoring the most prevalent comorbidities in PLWH: cardio-metabolic disease, bone and kidney disease, along with depression, treatment fatigue and other psychological issues.
Before the meeting, each expert identified a number of clinically relevant questions suitable for consensus discussion and provided the available scientific evidence. During the meeting, agreement was reached on these topics and on the need to publish the results of the discussion. The findings are reported here. An advanced draft of the manuscript also circulated among the key representatives of the Society for Infectious Diseases and HIV/AIDS (Romania) and the coordinators of all 9 regional centers for the evaluation and monitoring of HIV/AIDS in Romania (Extended Consensus Group); their comments are included in the final version of the article.
This document aims to offer practical guidance on how to assess and monitor associated diseases in PLWH. No systematic literature search was performed. The algorithms presented here are based on international guidelines and clinical experience and include landmarks for referral of PLWH to other specialists for additional investigations and adequate treatment.
Consensus statement
Cardio-metabolic disease
General information
PLWH have a 1.5 to 2-fold higher risk for cardiovascular (CV) diseases than the general population.(13) Evidence shows that chronic inflammation associated with HIV infection and CV side effects of some protease inhibitors (PIs) and abacavir are the main specific risk factors, in addition to the traditional risk factors such as smoking, obesity, dyslipidemia and diabetes.(13,14)
High blood pressure
Of all the CV disorders, hypertension is the most prevalent CV condition among individuals with HIV infection.(15,16) There are multiple contributing mechanisms, from chronic inflammation and endothelial dysfunction to renin-angiotensin-aldosterone system activation and lipodystrophy. Considering the increasing number of patients with high blood pressure, clinicians – both ID specialists and cardiologists – should pay special consideration to drug-drug interactions between HIV treatments and diuretics, beta blockers and calcium channel blockers.(5)
Recommendation: Measure blood pressure at every visit.
Myocardial infarction (MI) and stroke
The risk for acute CV events is higher in PLWH than in people without HIV infection. Low CD4 cell count and HIV-induced immune disorders were associated in clinical studies with the excessive risk for myocardial infarction (MI) and stroke.(13) The risk for MI remains high even in patients receiving appropriate treatment for HIV infection and associated CV risk factors.(13) A first essential step in the prevention of cardiovascular events is evaluating and addressing modifiable risk factors.
Recommendation: Inform PLWH about the risk associated with CV acute events; discuss potential lifestyle changes for prevention.
Heart failure
The presence of HIV infection represents an important risk factor for the occurrence of heart failure (HF),(17) especially the preserved ejection fraction (EF) type.(18)
Recommendation: Active screening for HF by echocardiography for detection of subclinical CV dysfunction.
Diabetes
PLWH often present metabolic changes favoring the onset of diabetes mellitus.(19) It is believed that underlying mechanisms are linked to the effects of PIs on glucose transport,(20) with the association of the chronic inflammatory status seen in HIV infection, and of lipodystrophy. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study has shown that uncontrolled high levels of blood glucose doubles the risk for acute CV events in PLWH.(21)
Recommendation: Check fasting plasma glucose at every visit that includes blood workup; discuss potential lifestyle measures for glucose control.
Cardiovascular risk assessment
Prediction of the CV disease risk over the next 5 to 10 years helps initiate specific therapeutic measures to reduce the probability of acute events that would otherwise be either potentially fatal or leading to major disabilities. Several CV risk scores have proven their validity in clinical practice for the general population, the most established being the Framingham score and the SCORE chart.(22,23) Large trials in PLWH comparing different risk calculators led to the development of a new model, the D:A:D score, which incorporates traditional risk factors for atherosclerotic cardiovascular disease plus CD4 cell count and cumulative exposure to PIs and nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs).(24) Although most CV risk prediction tools seem to produce similar results, our multidisciplinary group voted in favor of using the D:A:D score considering the above arguments. One freely available D:A:D score calculator – English version - can be found at https://www.chip.dk/Tools-Standards/Clinical-risk-scores. In addition, clinicians should keep in mind that in PLWH presenting CVD-risk enhancers (long-term high viremia levels, low CD4 cell count, HIV treatment failure or low adherence, co-infection with hepatitis C virus), the actual risk may be greater than calculated.(13)
Recommendation: CV risk should be assessed at diagnosis and ART initiation, as well as every year at follow-up visits.
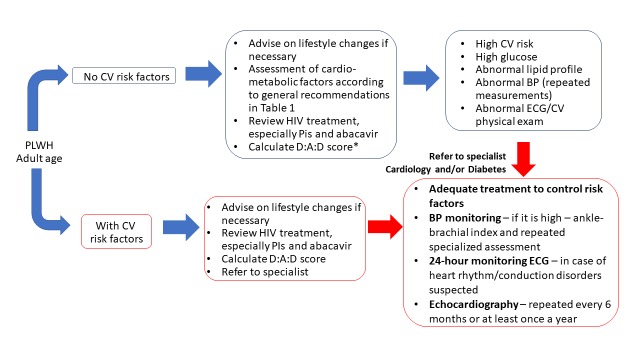
Table 1 summarizes the recommendations expressed by our expert group regarding the screening and monitoring of cardio-metabolic comorbidities in adult patients with HIV infection. Particular attention should be paid to modifiable risk factors. Specifically, general lifestyle advice is given, to quit smoking, reduce sedentary behavior, and improve dietary habits, and referral to specialized lifestyle interventions is considered whenever appropriate. HIV-related treatment should be reviewed at every visit, considering the specific CV issues associated with particular ARV drugs. If a change in ART is warranted, a thorough assessment of the risk-benefit ratio should pe performed first, to ensure that the new treatment regimen will not only be associated with a decreased CV risk, but also with good tolerability, adherence and HIV viral load suppression. Referral to CV or diabetes specialists should not be delayed in cases with uncontrolled CV risk factors or worsening symptomatology, as well as in cases with high CV risk calculated with the available tools (Figure 1).
Table 1. General recommendations for screening and follow-up of cardio-metabolic comorbidities in patients with HIV infection.
| Assessment | At HIV infection diagnosis and ART initiation | Follow-up |
|---|---|---|
| History and physical exam | + | Every visit*, as needed** |
| Lifestyle optimization | + | Every visit |
| HIV treatment review and DDI check | + | Every visit |
| Full blood count | + | Every visit |
| Blood pressure | + | Every visit |
| Lipid profile | + | Annually |
| Fasting plasma glucose | + | Every 6-12 months |
| BMI | + | Every visit |
| ECG | + | Every 6 months, or more often if needed*** |
| Echocardiography**** | + | Every 6-12 months |
| CV risk assessment (D:A:D score) | + | Annually |
BMI – body mass index; CV – cardiovascular; DDI – drug-drug interactions; ECG – electrocardiogram.
A visit is defined as a regular consult with an ID specialist, which may occur every 6 months, or sooner if medically indicated.
According to symptoms. With respect to comorbidities, history will focus on medication adherence, CV symptoms evolution/onset, and patients’ concerns related to CV disease and treatment.
Follow-up ECGs should be indicated at a frequency dependent on the results of the initial evaluation, i.e., if abnormal findings are present on the initial ECG more frequent follow-up would be warranted. One example of particular interest is QT prolongation, especially in connection with ART regimens that may also associate further QT prolongation.
If echocardiography is not available, assessment of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) could be considered.
Figure 1. Algorithm for monitoring and managing cardio-metabolic disorders in patients with HIV infection.
ART - antiretroviral therapy; BMI - body mass index; BP - blood pressure; CV - cardiovascular; ECG - electrocardiogram; PLWH - people living with HIV.
*If there are any HIV-related CVD-risk enhancers, the risk may be greater than calculated.
Blue arrows represent the steps that can be managed by the ID specialist; red arrows represent enhanced collaboration with the cardiologist and/or diabetologist in higher risk patients for adequate monitoring and treatment.
Summary
All adult PLWH should be adequately informed about the CV risk associated with HIV infection and the importance of controlling modifiable risk factors – smoking, obesity, blood pressure and plasma glucose levels.
Cardiovascular risk should be assessed yearly in all patients with HIV infection, regardless of age.
HIV-related treatment should be reviewed at every visit, considering the specific CV issues associated with particular ARV drugs. If a change in ART is warranted, a thorough assessment of the risk-benefit ratio should be performed first.
Clinicians should continue regular monitoring of CV risk factors in patients with adequate ART.
Bone disease
General information
Bone disease in patients with HIV infection was assessed in clinical trials and extensive meta-analyses,(25,26) which showed a significant increase (almost 2-fold) in the risk of fractures in patients with HIV infection compared with matched controls, and the risk increases with age.(27) As with CV risk factors, in addition to the risk factors that apply to the general population, including age, smoking, exposure to glucocorticoids, vitamin D insufficiency, hypogonadism and low bone mineral density (BMD), PLWH may have additional risk factors such as exposure to particular ARVs.(28)
Risk assessment for bone disease
The FRAX score is generally recommended for assessing and monitoring the risk of fracture, however it does not take into account the impact of HIV infection or ART and has been validated for people over 40 years of age.
As mentioned above, patients from the Romanian HIV cohort were infected during childhood and may have a different profile compared to patients who acquire HIV infection during adulthood. Furthermore, several observational studies report insufficient levels of vitamin D and a relatively high incidence of osteoporosis and osteopenia in PLWH, at younger ages than expected for the general population.(12,29,30)
In the cases where infection was acquired during childhood, the optimal hormonal impregnation of the bone matrix may not have occurred, thus affecting the initial phase of bone mass formation. Together with low levels of calcium and vitamin D, this change influences the maximum bone mass reached around the age of 20. It is believed that PLWH start to lose bone mass from the age of 30, almost 10 years earlier than the general population, with an increased risk of fracture associated with a low BMD and bone structural changes. Therefore, special monitoring is warranted (Table 2 and Figure 2).
Table 2. General recommendations for screening and follow-up of bone disease in patients with HIV infection.
| Assessment | At HIV infection diagnosis and ART initiation | Follow-up |
|---|---|---|
| History and physical exam | + | Every visit*, as needed** |
| Lifestyle optimization | + | Every visit |
| Total calcium, 25-OH vitamin D | + | Annually |
| Serum phosphorus | + | Annually |
| DXA scan | + | Every 3 years in premenopausal women with normal BMD at first assessment and in men <50 years |
| Every 2 years in postmenopausal women and men ≥50 years | ||
| Annually in patients with a) reduced BMD according to their age (e.g., premenopausal women and men <50 years) or b) diagnosed osteopenia/osteoporosis, or c) secondary hypogonadism | ||
| CTX, P1NP | + | Annually |
BMD – bone mineral density; DXA - bone densitometry by dual-energy X-ray absorptiometry; CTX – C-telopeptide; P1NP – procollagen type 1 N-terminal propeptide.
A visit is defined as a regular consult with an ID specialist, which may occur every 6 months, or sooner if medically indicated.
According to symptoms. With respect to comorbidities, history will focus on medication adherence, bone fractures/clinical symptoms of vertebral fractures, and patients’ concerns related to bone disease and treatment.
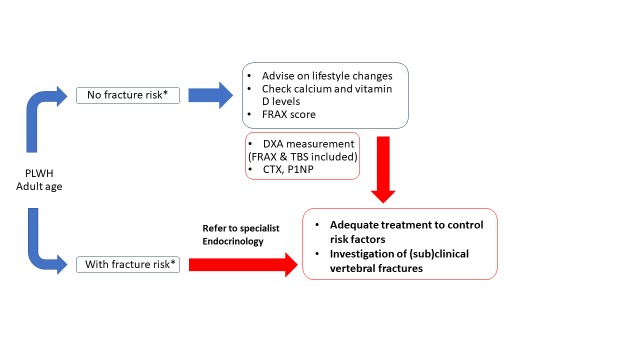
Figure 2. Algorithm for monitoring and managing bone disease in patients with HIV infection.
DXA - bone densitometry by dual-energy X-ray absorptiometry; CTX - C-telopeptide; P1NP - procollagen type 1 N-terminal propeptide; TBS - trabecular bone score.
*As indicated in EACS Guidelines.
Blue arrows represent the steps that can be managed by the infectious disease specialist; red arrows represent enhanced collaboration with the endocrinologist or other bone disease specialist in higher risk patients for adequate monitoring and treatment.
Recommendation: Evaluate fracture risk with BMD measurement by dual-energy X-ray absorptiometry (DXA) in all adults at diagnosis of HIV infection and treatment initiation and then every 2-3 years, as clinically indicated.
Summary
Smoking cessation, increase in physical activity and reduction of alcohol consumption should be recommended to all adult PLWH, irrespective of ART treatment regimen.
The FRAX score could be used for assessing and monitoring the risk of fracture.
Periodic monitoring with DXA measurement, especially in high-risk patients could be beneficial to identify the candidates for medical intervention.
Adequate supplementation with calcium and vitamin D, as well as hormonal substitution should only be prescribed based on laboratory results and in collaboration with an endocrinologist for treatment adjustment and monitoring.
Kidney disease
General information
The incidence of chronic kidney disease (CKD) has increased in PLWH.(16) A recent observational study performed in Romania showed that approximately 30% of patients were reported to have stage II CKD based on estimated glomerular filtration rate (eGFR).(12) Progression of CKD and cases requiring renal replacement therapy are increasing in patients with HIV infection despite the use of HAART.(31)
Lifestyle changes include smoking cessation, weight control, low sodium diet. Further interventions for modifiable risk factors include good blood pressure control.
Risk assessment for kidney disease
The monitoring approach for kidney disease in patients with HIV infection is similar to that in the general population (Table 3). Screening for renal disease should include routine urine and plasma biochemistry assessments. Special attention should be paid to identifying proteinuria, calculating eGFR and assessing plasma creatinine levels. Urinary protein/creatinine ratio or urinary albumin/creatinine ratio should be recommended if dipstick test is positive for protein, and can also aid in identifying glomerular and tubular, or glomerular impairment, respectively.
Table 3. General recommendations for screening and follow-up of kidney disease in patients with HIV infection.
| Assessment | At HIV infection diagnosis and initiation of ART | Follow-up |
|---|---|---|
| Risk assessment | + | Every visit*, as needed** |
| Lifestyle optimization | + | Every visit |
| Urine analysis | + | Annually |
| Proteinuria (dipstick) | +*** | Annually |
| Serum creatinine | + | Annually or more frequently, if indicated |
| Estimated GFR (CKD-EPI formula) | + | 3-12 months |
ART – antiretroviral therapy; CKD – chronic kidney disease; GRF – glomerular filtration rate.
A visit is defined as a regular consult with an ID specialist, which may occur every 6 months, or sooner if medically-indicated.
CKD risk factors and treatment with ARV agent with potential nephrotoxicity; the D:A:D score recommended for renal risk assessment (medium risk 0-4, high risk >5).
If dipstick test for proteinuria is positive, recommend urinary protein/creatinine ratio.
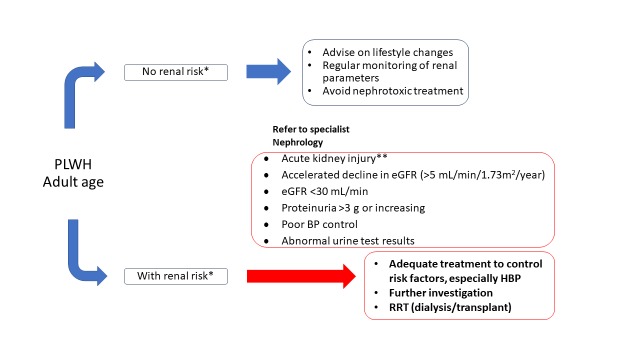
Tests should be repeated depending on the initial evaluation (low risk – repeat at 12 months, moderate risk – at 6 months, high risk – at 3 months). If proteinuria is higher than 1+ or eGFR is lower than 60 mL/min/1.73m2 repeat in a 3 months interval, and if persistently abnormal, diagnose CKD. A significant or progressive increase in serum creatinine, accelerated decline in eGFR, increasing proteinuria, and persistent abnormal urine test results are indicators for referral to nephrologist (Figure 3).
Figure 3. Algorithm for managing and monitoring kidney disease in patients with HIV infection.
eGFR - estimated glomerular filtration rate; HBP - high blood pressure; RRT - renal replacement therapy.
*D:A:D score for risk assessment.
**Serum creatinine increased 1.5-fold from baseline, known or suspected to have occurred in the last 7 days.
Blue arrows represent the steps that can be managed by the infectious disease specialist; red arrows represent referral to nephrologist in higher risk patients for adequate monitoring and treatment.
Recommendation: Check for proteinuria, assess creatinine levels and calculate eGFR annually or more frequently, as indicated.
Summary
The risk of CKD is higher in PLWH than in uninfected individuals. It should be assessed at HIV infection diagnosis and ART initiation and then at every visit.
In clinical practice, we recommend risk assessment with the D:A:D score, checking for proteinuria and assessing serum creatinine, and repeating tests depending on the initial response (low risk – at 12 months, moderate risk – at 6 months, high risk – at 3 months).
If proteinuria is higher than 1+ or eGFR is lower than 60 mL/min/1.73m2 for at least 3 months, diagnose CKD.
Increase in creatinine, accelerated decline of eGFR, increasing proteinuria, and persistent abnormal urine test results are indicators for referral to nephrology department.
In addition to lifestyle changes (smoking cessation, weight control, low sodium diet), clinicians should pay attention to potentially nephrotoxic ARV agents or drugs administered for the management of comorbidities, as well as to drug-drug interactions.
Depression
General information
Young patients with long-lasting HIV infection (the Romanian pediatric cohort) have a different perception of time compared to the general population, because of limitation, compression and lack of future projection, correlated to individual experiences related to HIV diagnosis.
The PLWH’s journey is characterized by many emotional ups and downs, which can vary from person to person. PLWH may have to deal with several issues, including self-marginalization and hanging between two worlds, “with” and “without” HIV, which induces psycho-emotional discomfort. It may take a long time to accept and cope with “living with HIV”, which may be linked with occurrence of depression.
If the onset of depression is linked to an event that triggers it, the brain – which already passed several times through depression – keeps reactivating depression again and again. Therefore, depression is cumulative over the years, usually throughout the life of PLWH, predisposing them to more intense and more frequent depressive episodes.
Depression may be associated with a negative impact on patient outcomes, including quality of life, life plans, social and vocational functioning and treatment adherence.
Left untreated, psychological disorders may lead to poor adherence and, eventually, interrupting ART, which, in turn, increases the overall morbidity and mortality rates.(32)
As a particularity of the Romanian pediatric cohort, young adult patients infected at an early age show a decrease in self-efficacy beliefs and invest less energy in the learning (educational) process, leading to a risk of professional failure, twice as much when they compare themselves to their healthy peers, the result being a distorted perception of what a job means, which may result in low job offers.
For this reason, they should be offered support in building up successful life-experiences. This may be achieved by modifying the ineffective attributional style, increasing the level of persistence in the event of resilience failure and making them understand that success comes from commitment in attitude towards HIV diagnosis, ART and necessary experiences to their age.
Patients’ attitude and behavior are influenced by their beliefs and experiences, as well as by the interventions of the medical team. Survival of PLWH depends on adherence to the correct treatment. During the sessions dedicated to ART adherence, patients should receive support in order to learn to value and capitalize the information on durable antiviral efficacy and long-term safety, so that they trust their treatment and live an active life. These sessions are indicated at ART initiation, at any subsequent change in ART regimen, or on a case by case basis. Through counseling, patients with HIV infection learn to live with their real-life problems which they know they will continue facing, while learning strategies to anticipate events and how to use their abilities to control them.
Psychological intervention allows modification of behavioral patterns by “unlearning” the negative experiences and “re-learning” experiences with positive value. Consequently, the patients will be able to identify alternative behaviors necessary to function in every-day life.
Risk assessment for depression
The first two weeks after diagnosing HIV infection may represent a critical timeframe for the patient, due to multiple reasons. These include the diagnosis per se and the associated impact of a permanent change in the patient’s routine generated by starting therapy and being engaged in medical care. Other potential triggers for depression may include any major life changes on personal, social or professional level. Therefore, the ID physician should pay close attention, to ensure timely identification of any signs of depression, which would warrant referral to a psychologist.
Because patients may not recognize or report symptoms of depression, physicians should not be afraid to ask questions about psychological health and refer them to a psychologist and/or psychiatrist in case of identifying common symptoms of depression in their patients with HIV infection. The psychologist can then choose among multiple standardized depression scales, for diagnosis and identification of actionable directions for the psychological intervention. Most of the psychological evaluation instruments rely on self-reporting symptoms. Based on the severity of depression, the patient may be subsequently referred to a psychiatrist. During psychological support sessions, psychotherapy specialists try to guide patients towards rediscovering the meaning of life and regaining control over the situation, so that they can achieve good functional adjustment on a social, professional, and personal level.
Recommendation: A multidisciplinary approach is necessary for identifying depression. To avoid unrecognized depression, the ID physician should pay close attention to ensure timely identification of any signs of depression, which would warrant referral to a psychologist, particularly in the first two weeks after diagnosis of HIV infection and whenever the clinician suspects depression symptoms.
Summary
The PLWH’s journey is characterized by many emotional ups and downs, which can vary from person to person.
Depression may be associated with a negative impact on patient outcomes, including quality of life, life plans, social and vocational functioning and treatment adherence.
The first two weeks after diagnosing HIV infection may represent a critical timeframe for the patient. Other potential triggers for depression may include any major life changes on a personal, social or professional level.
The ID physician should pay close attention, to ensure timely identification of any signs of depression, which would warrant referral to a psychologist.
Discussion
Ongoing intensive efforts are still required to reach the UNAIDS targets 90-90-90 for 2020 imposing timely diagnosis and treatment initiation, as well as robust healthcare management to prioritize resources at country and European region levels.(2,3) The basis for successful living of patients with HIV infection starts with a correct diagnosis and adequate treatment, and these premises are achievable in Romania based on extensive experience and availability of highly effective treatment.
Although the characteristics of the Romanian cohort render it quite unique in Europe due to the age at acquiring the infection and the cluster of HIV subtype F,(12) the issues associated with ageing are not different. Several observational studies initiated in the adult Romanian PLWH showed a high burden of bone and renal impairment and cardio-metabolic comorbidities,(12,29,30) both in patients from the pediatric cohort and in patients who acquired HIV infection through other transmission routes. Additionally, current figures available from June 2019 show that most of the Romanian patients are young adults who would benefit from screening programs to identify comorbidities otherwise occurring at older ages. In this context, the formulation of practical guidance to build and enhance communication between infectious diseases specialists and other healthcare professionals is required. This Consensus Statement should be used in conjunction with European guidelines and established national protocols. It offers both a reason to start discussing patients’ journey with colleagues from other specialties and a practical approach of management to be adapted and implemented into routine clinical practice. The recommendations and algorithms are not focused on the diagnosis or specific use of certain ARVs, however, we support the use of highly effective ART, with a good tolerability profile and a low pill burden, to increase adherence.
A limitation of the current paper stems from the fact that the first meeting of the Expert Group focused on four main comorbidities: cardio-metabolic, renal, osteo-skeletal disorders, and depression. We are fully aware that the list should also include other potentially associated diseases, such as malignancies, co-infection with hepatitis viruses, liver diseases, and neurocognitive impairment, to name only a few. Psychological changes associated with HIV infection diagnosis and stigma should also be addressed.
As scientific evidence gathers, efficient treatment increases life expectancy, showing a long duration of the disease but also multiple associated diseases. Future research will describe in more detail the HIV- and ART-related factors influencing the risk for comorbidities and will help develop specific interventions for a more individualized approach. In parallel, the multidisciplinary approach is essential for maintaining the quality of life and for improving outcomes of PLWH.
Conclusions
Increasing life expectancy of patients with HIV infection imposes a shift in the management, with a focus on the presence of comorbidities, with screening and regular monitoring, and appropriate referral to other specialists involved in their care. The multidisciplinary approach has become the norm rather than the exception. Screening for comorbidities is essential if we target a healthy life for both older patients and younger patients who have a long treatment journey ahead.
Supplementary information
Extended Consensus Group authors are listed below (in alphabetical order):
Victoria Aramă, MD, PhD, Society for Infectious Diseases and HIV/AIDS; Carol Davila University of Medicine and Pharmacy Bucharest; National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, 1 Dr. Calistrat Grozovici Street, Bucharest 021105, Romania
Emanoil Ceauşu, MD, PhD, Clinical Hospital of Infectious and Tropical Diseases “Dr. Victor Babeş”, Bucharest; Carol Davila University of Medicine and Pharmacy Bucharest, 8 Eroilor Sanitari Boulevard, Bucharest, Romania
Lucia Carmen Chiriac, MD, PhD, Mureş Clinical County Hospital; University of Medicine and Pharmacy of Tîrgu Mureş, 38 Gheorghe Marinescu Street, Tîrgu Mureş, Romania
Felicia Constandiş, MD, Clinical Infectious Diseases Hospital Braşov, 9 Mihai Viteazu Street, Braşov, Romania
Augustin Cupşa, MD, PhD, “Victor Babeş” Infectious Diseases Clinical Hospital Craiova; University of Medicine and Pharmacy of Craiova, 2 Petru Rareş street, Craiova, Romania
Corina Itu Mureşan, MD, PhD, Clinical Hospital of Infectious Diseases Cluj-Napoca; Iuliu Hațieganu University of Medicine and Pharmacy Cluj-Napoca, 8 Victor Babeş street, Cluj-Napoca, Romania
Mariana Mărdărescu, MD, PhD, National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, 1 Dr. Calistrat Grozovici Street, Bucharest 021105, Romania
Sorin Petrea, MD, PhD, Society for Infectious Diseases and HIV/AIDS; National Institute for Infectious Diseases “Prof. Dr. Matei Balş”, 1 Dr. Calistrat Grozovici Street, Bucharest 021105, Romania
Liviu Jany Prisăcariu, MD, PhD, Infectious Diseases Hospital “Sf Parascheva”, 2 Octav Botez Street, Iaşi, 700116, Romania
Sorin Rugină, MD, PhD, Clinical Hospital of Infectious Diseases Constanța; Department of Infectious Diseases, Faculty of Medicine, Ovidius University of Constanța, 124 Mamaia Boulevard, Constanța, Romania
Footnotes
Authors’ contributions statement: All authors had equal contributions. All authors reviewed and approved the final version of the manuscript.
Conflicts of interest: AnSC reports being sub-investigator in clinical trials by Merck and Gilead, principal investigator in an investigator sponsored research study funded by a Gilead grant, speaker for Gilead and participating in advisory boards for Gilead and ViiV, outside the submitted work. OS reports being sub-investigator in clinical trials by Merck and Gilead, and speaker for Gilead, outside the submitted work. AdSC reports being principal investigator in clinical trials by Merck and Gilead, speaker for Gilead and participating in advisory boards for Gilead and ViiV, outside the submitted work. AnSC, CP, MD, GM, VEL, IMD, OC and AdSC participated in the Gilead expert meeting/advisory board on the topic of comorbidities in PLWH. All other authors – none to declare.
Funding: The expert meeting was organized with financial support from Neolapharma Romania. Medical writing support was provided by Raluca Voicu, MD of MedInteractiv Plus and was funded by Gilead Science Europe Ltd. Gilead Science Europe Ltd and Neolapharma Romania had no involvement in the concept behind the consensus statement, content of the scientific material produced at the consensus statement, drafting of the manuscript, analysis and interpretation of the data, and the decision to submit for publication. They had only administrative roles in organization of the initial meeting and providing financial support for the medical writing activities.
Contributor Information
Extended Consensus Group:
Victoria Aramă, Emanoil Ceauşu, Lucia Carmen Chiriac, Felicia Constandiş, Augustin Cupşa, Corina Itu Mureşan, Mariana Mărdărescu, Sorin Petrea, Liviu Jany Prisăcariu, and Sorin Rugină
REFERENCES
- 1.UNAIDS Global HIV & AIDS Statistics – 2019 Fact Sheet. Accessed on: 12 September 2019. Available at https://www.unaids.org/en/resources/fact-sheet.
- 2.Romanian National Committee for Fighting Against AIDS HIV/AIDS infection in Romania - update 30 June 2019. Accessed on: 12 September 2019. Available at http://cnlas.ro/images/doc/30062019_rom.pdf.
- 3.Gokengin D, Oprea C, Begovac J, et al. HIV care in Central and Eastern Europe: How close are we to the target? Int J Infect Dis. 2018;70:121–30. doi: 10.1016/j.ijid.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 4.European AIDS Clinical Society EACS Guidelines 2015 (version 8.0). Accessed on: 12 September 2019. Available at https://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf.
- 5.European AIDS Clinical Society EACS Guidelines 2018 (version 9.1). Accessed on: 12 September 2019. Available at https://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf.
- 6.INSIGHT START Study Group Lundgren JD, Babiker AG. et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–7. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 9.Guaraldi G. The transition from co-morbidities to geriatric syndromes in HIV. Germs. 2016;6:79–81. doi: 10.11599/germs.2016.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockstroh J, Guaraldi G, Deray G. HIV and the body: a review of multidisciplinary management. HIV Med. 2010;11(Suppl2):1–8. doi: 10.1111/j.1468-1293.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 11.Hentzein M, Dramé M, Delpierre C, et al. HIV-related excess mortality and age-related comorbidities in patients with HIV aged ≥60: a relative survival analysis in the French Dat'AIDS cohort. BMJ Open. 2019;9:e024841. doi: 10.1136/bmjopen-2018-024841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streinu-Cercel A, Săndulescu O, Ceapraga G, et al. Prevalence of osteo-renal impairment in the Romanian HIV cohort. BMC Infect Dis. 2016;16(Suppl 1):93. doi: 10.1186/s12879-016-1397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American Heart Association. Circulation. 2019;140:e98–124. doi: 10.1161/CIR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorjee K, Choden T, Baxi SM, Steinmaus C, Reingold AL. Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: a systematic review and meta-analysis of results from 17 epidemiological studies. Int J Antimicrob Agents. 2018;52:541–53. doi: 10.1016/j.ijantimicag.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–97. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 16.Pelchen-Matthews A, Ryom L, Borges ÁH, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. 2018;32:2405–16. doi: 10.1097/QAD.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 17.Cerrato E, D'Ascenzo F, Biondi-Zoccai G, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34:1432–6. doi: 10.1093/eurheartj/ehs471. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: Results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–46. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guaraldi G, Orlando G, Squillace N, et al. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7:97–106. doi: 10.1310/EYWJ-8B5K-X7VQ-9CPE. [DOI] [PubMed] [Google Scholar]
- 20.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 21.Worm SV, De Wit S, Weber R, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study) Circulation. 2009;119:805–11. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 23.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Friis-Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214–23. doi: 10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 25.Stone B, Dockrell D, Bowmann C, McCloskey E. HIV and bone disease. Arch Biochem Biophys. 2010;503:66–77. doi: 10.1016/j.abb.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Ilha TASH, Comim FV, Copes RM, Compston JE, Premaor MO. HIV and vertebral fractures: a systematic review and metanalysis. Sci Rep. 2018;8:7838. doi: 10.1038/s41598-018-26312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2:e288–98. doi: 10.1016/S2352-3018(15)00077-6. [DOI] [PubMed] [Google Scholar]
- 28.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–46. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poiana C, Capatina C, Streinu-Cercel A, Sandulescu O, Streinu-Cercel A. Hypovitaminosis D in HIV-infected patients. Acta Endocrinol (Buchar) 2019;5:102–6. doi: 10.4183/aeb.2019.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horosanu GC, Streinu-Cercel A, Tudor AM, Streinu-Cercel A. A profile of specific risk factors for chronic kidney disease-mineral and bone disorder in Romanian HIV-positive patients. J Contemp Clin Pract. 2016;2:18–24. [Google Scholar]
- 31.Saracho R, Martín Escobar E, Comas Farnés J, et al. Clinical evolution of chronic renal patients with HIV infection in replacement therapy. Nefrologia. 2015;35:457–64. doi: 10.1016/j.nefro.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez J, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–7. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]