Abstract
Introduction
Helicobacter pylori infection is a well-established etiological factor for a variety of diseases such as peptic ulcer and gastric cancer. On the other hand, there is ongoing research suggesting that H. pylori might have a beneficial effect through a pivotal influence in the immunological response especially in asthma. The aim of the current case-control study was to evaluate the prevalence of H. pylori infection in asthmatic children.
Methods
Twenty-seven children with exacerbation of persistent asthma, aged 8.6±4.5 years (18 males, 9 females) and 54 age-sex-matched non-asthmatic controls were enrolled. Clinical examination and laboratory investigations were performed. Detection of H. pylori antigen (HpSA) in stool samples was performed by a commercial kit (bioNexia® kit, BioMérieux). Serum specific IgG antibodies were detected by a rapid chromatographic immunoassay (DIAsourceImmunoAssays). Serum IgE concentration was determined by electrochemiluminescence (ECL) (Roche Elecsys) and IgE levels ≥ 90 IU/mL were considered significantly elevated.
Results
In 3 (11.1%) of the 27 asthmatic children H. pylori infection (based on both detection of HpSA and specific IgG-Abs) was established, whereas as many as 16 of the 54 (29.6%) non-asthmatic ones were found infected (odds ratio 0.1; 95%CI, 0.039-0.305, p=0.026).
Conclusions
Our findings reveal an inverse relationship between H. pylori infection and children's persistent asthma in Greece.
Keywords: Asthma, persistent, Helicobacter pylori, children
Introduction
Helicobacter pylori is a Gram-negative, spiral-shaped, microaerophilic bacterium, first isolated about 30 years ago with a number of potential virulence factors for disease generation, mainly gastric infection.1 The most important of them are represented by mucinase activity and adherence factors, which enable the organism to penetrate the mucus layer promptly and to be secured among intracellular junctions of enteric cells. Therefore, H. pylori colonization of gastric mucosa brings about infection with infiltration of inflammatory cells and release of hydrolytic enzymes causing gastritis.2 This chronic active gastritis induces peptic ulcer disease, gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. It is the first officially recognized bacterial carcinogen and has been classified as a group I gastric cancer carcinogen by the International Agency of Research on Cancer.3
Additionally, there is ongoing research regarding the extragastric diseases related to H. pylori infection. These manifestations include neurological disorders namely multiple sclerosis and dementia, metabolic syndrome and a huge variety of miscellaneous diseases such as immunologic impairment, allergy, asthma and respiratory diseases. Thus, due to great efforts that have been made to create new, prompt diagnostic methods and treatment strategies to eradicate H. pylori, a decrease in its prevalence has been seen in many countries of the world, especially the developed ones, although the overall infection in adults still remains at least 50% worldwide.4,5 Presumably, improvements in sanitation and widespread use of antibiotics are considered to be the main parameters for this decrease.6
On the other hand, this reduction coincided with the estimated increase in allergies such as asthma, atopy and allergic rhinitis.7,8 Due to the fact that H. pylori infection is mainly acquired in childhood, there are many studies that depict a possible relationship between the two conditions firstly in children and secondly in adults by different mechanisms.5,9 Some epidemiological studies revealed an inverse association between H. pylori infection and asthma or asthma-related diseases especially in children.10 Unfortunately, the results are in great dispute, notably concerning also the adults and the studies’ design limitation parameters.11
H. pylori might have a beneficial effect through a pivotal influence in the immunological response especially in asthma. The aim of the current case-control study was to evaluate the prevalence of H. pylori infection in Greek children with persistent asthma, a condition linked with chronic inflammation.
Methods
Study population
This study enrolled 27 children with exacerbation of persistent asthma, aged 8.6±4.5 years (18 males, 9 females) and 54 age-sex-matched non-asthmatic controls.
All consecutive subjects with exacerbation of persistent asthma presenting to the Accidents and Emergency Department (A&E) of the General University Hospital of Alexandroupolis from January 1st 2017 to January 1st 2018 were included. For each study group subject, 2 age-sex-matched non-asthmatic children who presented at the A&E in the same period were included in the control group. An informed consent was obtained from the parents and an assent was obtained from the children in the study and the control groups.
The clinical examination, classification of asthma and inclusion in the study was performed by a pediatric physician (DC). The exclusion of children with intermittent asthma was decided because the triggering factor in these cases is more often a viral infection and chronic inflammation does not exist.
IgE determination
To enhance the data of asthma condition, serum IgE concentration was determined to evaluate the allergy status of the children by electrochemiluminescence (ECL) (Roche Elecsys, Basel, Switzerland) and IgE levels ≥ 90 IU/mL were considered significantly elevated.
H. pylori infection status
The H. pylori infection status was assessed by two methods. First, the detection of H. pylori antigen (HpSA) in stool samples was performed by a commercial kit (bioNexia® kit, BioMérieux, Lyon, France). It is a non-invasive test with a good performance versus ELISA microplate assay (overall percent agreement: 94.9% [90.9%; 97.2%]). Serum specific anti-H. pylori IgG were measured using a chromatographic immunoassay (DIAsourceImmunoAssays S.A., Louvain-La-Neuve, Belgium). The DIA source Helicobacter pylori test is a simple test that utilizes a combination of H. pylori antigen coated particles and anti-human IgG to detect H. pylori antibodies in plasma, serum or whole blood. This method has a sensitivity of 96.8% and a specificity of 93.0% in comparison to Biopsy/Histology/R Rapid Urease Test.
Statistical analysis
Continuous variables were analyzed using Student's t-test, and p values <0.05 were considered statistically significant. All measures of associations are presented as OR with their 95% confidence intervals (CI). Statistical analyses were performed using SPSS version 23.0 for Windows (IBM Corp, Armonk, NY, USA).12
The study was approved by the Bioethics Committee of the University General Hospital of Alexandroupolis, Greece (Ethics Committee identification code: 384).
Results
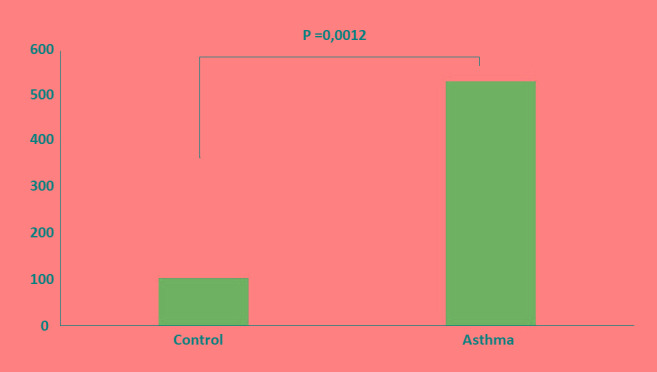
The study group was comprised of children with exacerbation of persistent asthma and more specifically 2 with severe, 9 with moderate and 16 with mild asthma. The treatment during the exacerbation (asthma attack) included inhaled short acting bronchodilators in 20 (74.1%) patients, inhaled long acting bronchodilators in combination with inhaled corticosteroids in 7 and oral corticosteroids in 2 patients. None of the patients in the study group required hospital admission. All the patients were followed up in the outpatient clinic of the Pediatric Department for at least 2 years. Inhaled long acting bronchodilators in combination with inhaled corticosteroids were used in 11 patients (40.7%) with moderate or severe asthma. Inhaled corticosteroids with occasional use of short acting bronchodilators only, were used in 16 patients (59.3%) with mild persistent asthma. Antileukotrienes were also used in 10 (37%) patients, 3 with mild, 5 with moderate and in 2 with severe asthma. Clinical and demographic characteristics of study groups are presented in Table 1. Asthmatic patients had elevated level of total IgE in comparison to controls (534.1 vs 103.13 IU/mL, p=0.001, Figure 1). Our study revealed an inverse relationship between H. pylori infection and children with asthma in Greece. In 3 (11.1%) of the 27 asthmatic children H. pylori infection (based on both detection of HpSA and specific IgG-Abs) was established, whereas as many as 16 of the 54 (29.6%) non-asthmatic ones were found infected (odds ratio 0.1; 95%CI, 0.039-0.305, p=0.026).
Table 1. Clinical and demographic characteristics of patients and control.
| Patients n=27 | Controls n=54 | p | |
|---|---|---|---|
| Age (years), mean±SD | 8.6±4.5 | 9.1±3.3 | 0.14 |
| Systolic BP (mmHg), mean±SD | 102.3±17.1 | 109.7±9.9 | 0.23 |
| Diastolic BP (mmHg), mean±SD | 78.5±11.5 | 74.7±7.5 | 0.68 |
| Gender, male (%) | 18 (66.7%) | 38 (70.4%) | |
| Disease duration (years), mean±SD | 3±0.5 | ||
| Comorbidity | |||
| Allergic rhinitis | 14 (51.8%) | 6 (11.1%) | |
| Atopic dermatitis | 9 (33.3%) | 0 | |
| Food allergy | 11 (40.7%) | 0 | |
| Treatment | |||
| Corticosteroids | 2 (7.4%) | 0 | |
| Short acting bronchodilators | 20 (74.1%) | 0 | |
| Long acting bronchodilators in combination with corticosteroids | 7 (25.9%) | 0 | |
Figure 1. Level of total IgE in study group.
Discussion
Our results demonstrate that H. pylori infection is inversely associated with persistent asthma. A possible mechanism of action of H. pylori is presented in Figure 2. These results are comparable with those described by other investigators.10,13 Some population surveys have demonstrated this negative relationship bringing about the potential protective role of H. pylori against disease.
Figure 2. Hypothesis of possible immune mechanism related to Helicobacter pylori infection and asthma.

Gastric colonization by H. pylori, through dendritic cells (DC) mediated T cell expression, affects the Th1/Th2 response. The rise of gastric mucosal T regulatory cells (T regs) in H. pylori infection subsequently can provoke increase in the levels of interleukin (IL)-10 release, that inhibits a Th2 response and the activation of Th17. Thus H. pylori infection is favoring a Th1 response and not a Th2, that is related with allergic manifestations and asthma.
The cornerstone of this assumption is the ‘hygiene hypothesis’14 which Strachan introduced in 1989, emphasizing that good sanitary conditions reduce microbial exposures especially in infancy and childhood and the subsequent immune response is protective against atopy, asthma and autoimmune diseases.15 The linkage between the ‘hygiene hypothesis’ and the H. pylori infection-colonization is a field with a bulk of current research.16
Besides that, another hypothesis in favor of the aforementioned negative association is the ‘decreasing microbiota hypothesis’ which proposes the interplay between intestinal microbiota and the immune system.17 In this theory, the Th1/Th2 balance is influenced by the commensal microbes. H. pylori is implicated in the process of induction of naïve T cells to 2 subpopulations of Th1 and Th2. It has been well depicted that in H. pylori-colonized subjects T cells of the intestine mucus layer express more interferon-γ, interleukin-12, tumor necrosis factor-ɑ (TNFɑ) and less interleukin-4 compared to uninfected subjects.18 A shift to Th1 type and suppression of Th2 immune response may explain the downregulation of Th2-mediated clinical manifestations in H. pylori positive children and the possible protective role against the development of asthma and allergy.19 Moreover, in several studies the expansion of T regulatory cells (T regs) is attributed to the H. pylori human gastric mucosa infection with the consequent distortion to immune tolerance.20,21
Contradictory results of recent studies show that allergy based on IgE levels has a higher prevalence when it is accompanied by past H.pylori infection22 and also higher prevalence of asthma in children who are H. pylori-positive. Instead, colonization with a certain strain of H. pylori (CagA-negative) at age 6 correlated with asthma in European children.11 Nevertheless, the selected population of children, in our study, with persistent asthma, is more equivalent to chronic inflammation and Th1/Th2 dysregulation.
The multifactorial nature of asthma and allergy is well established; it includes genetic predisposition and environmental factors and the fact that the ‘hygiene hypothesis’ could not apply worldwide. In addition, the diversity and abundancy of gastric microbiota seem to be correlated to the presence or absence of H. pylori in children and might be an important co-player in the asthma development as a biomarker of poor hygiene.23
Different studies have demonstrated that wide and persistent eradication strategies of H. pylori might contribute significantly in minimizing the incidence of peptic ulcer, gastric cancer and gastric atrophy.24 Having considered these efforts, it has already been proposed by Noverr et al. in 2005 that an eradication plan of H. pylori could lead to undesirable results if the microbe is indeed protective towards asthma and atopy.25
Potential limitations of our study were that we had a small sample size of patients. Moreover, we did not measure Th subtypes in the study groups subjects. Also, it was not possible to fully evaluate the laboratory allergic profile, by measuring specific IgEs.
Conclusions
In conclusion, our results reveal H. pylori is inversely associated with asthma, and this infection seems to be protective in the development of asthma in children. H. pylori infection might be beneficial but future studies need to include data for variations in prevalence, limitations in methodology, treatment options – odds and considerations about eradication-vaccination programs. Controversies in different studies demand new prospective research to elucidate the possible protective role of H. pylori infection with respect to allergy, asthma and atopy.
Footnotes
Authors’ contributions statement: CT designed the research plan of this study and contributed to writing the manuscript. TGK coordinated the data analysis, and contributed to writing the manuscript. DC supervised all clinical data of patients. AK and AG carried out all laboratory tests. MP and AgT participated in the main role of editing the manuscript. AtT participated in the main role of revising the manuscript. All authors reviewed and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–5. [PubMed] [Google Scholar]
- 2.Murray PR. Medical microbiology. St Louis, MO, USA: Mosby; 1994. [Google Scholar]
- 3.Møller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer. 1995;60:587–9. doi: 10.1002/ijc.2910600502. [DOI] [PubMed] [Google Scholar]
- 4.Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016;21(Suppl 1):3–7. doi: 10.1111/hel.12332. [DOI] [PubMed] [Google Scholar]
- 5.Lim JH, Kim N, Lim SH, et al. Inverse relationship between Helicobacter pylori infection and asthma among adults younger than 40 years: a cross-sectional study. Medicine (Baltimore) 2016;95:e2609. doi: 10.1097/MD.0000000000002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HR. Prevalence of asthma. BMJ. 2005;330:1037–8. doi: 10.1136/bmj.330.7499.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 9.Miftahussurur M, Nusi IA, Graham DY, Yamaoka Y. Helicobacter, hygiene, atopy, and asthma. Front Microbiol. 2017;8:1034. doi: 10.3389/fmicb.2017.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amberbir A, Medhin G, Erku W, et al. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. 2011;41:1422–30. doi: 10.1111/j.1365-2222.2011.03831.x. [DOI] [PubMed] [Google Scholar]
- 11.den Hollander WJ, Sonnenschein-van der Voort AM, Holster IL, et al. Helicobacter pylori in children with asthmatic conditions at school age, and their mothers. Aliment Pharmacol Ther. 2016;43:933–43. doi: 10.1111/apt.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SPSS v. 23 . IBM Corp. Armonk, NY, USA: [Google Scholar]
- 13.Zevit N, Balicer RD, Cohen HA, Karsh D, Niv Y, Shamir R. Inverse association between Helicobacter pylori and pediatric asthma in a high-prevalence population. Helicobacter. 2012;17:30–5. doi: 10.1111/j.1523-5378.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 14.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011;127:1097–107. doi: 10.1016/j.jaci.2011.02.012. quiz 1108-9. [DOI] [PubMed] [Google Scholar]
- 18.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 20.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–93. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codolo G, Mazzi P, Amedei A, et al. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10:2355–63. doi: 10.1111/j.1462-5822.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SP, Lee SY, Kim JH, et al. Correlation between Helicobacter pylori infection, IgE hypersensitivity, and allergic disease in Korean adults. Helicobacter. 2015;20:49–55. doi: 10.1111/hel.12173. [DOI] [PubMed] [Google Scholar]
- 23.Kalach N, Bontems P, Raymond J. Helicobacter pylori infection in children. Helicobacter. 2017;22(Suppl 1) doi: 10.1111/hel.12414. [DOI] [PubMed] [Google Scholar]
- 24.Graham DY, Uemura N. Natural history of gastric cancer after Helicobacter pylori eradication in Japan: after endoscopic resection, after treatment of the general population, and naturally. Helicobacter. 2006;11:139–43. doi: 10.1111/j.1523-5378.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 25.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun. 2005;73:30–8. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


