Abstract
Objective:
In recent years, ecological momentary assessment (EMA) has been used to repeatedly assess eating disorder (ED) symptoms in naturalistic settings, which has allowed for increased understanding of temporal processes that potentiate ED behaviors. However, there remain notable limitations of self-report EMA, and with the rapid proliferation of technology there are ever-increasing possibilities to improve ambulatory assessment methods to further the understanding and treatment of EDs. Therefore, the purpose of this review was to (a) systematically review the studies in EDs that have utilized ambulatory assessment methods other than self-report, and (b) provide directions for future research and clinical applications.
Method:
A systematic literature search of electronic databases was conducted, and data regarding study characteristics and methodological quality were extracted.
Results:
The search identified 17 studies that used ambulatory assessment methods to gather objective data, and focused primarily on autonomic functioning, physical activity, and cognitive processes in ED and control groups.
Discussion:
Together the literature demonstrates the promise of using a range of ecologically valid ambulatory assessment approaches in EDs, though there remains limited research that has utilized methods other than self-report (e.g., wearable sensors), particularly in recent years. Going forward, there are several technology-enhanced momentary assessment methods that have potential to improve the understanding and treatment of EDs.
Keywords: ambulatory assessment, eating disorder, ecological momentary assessment, experience sampling, naturalistic assessment, precision medicine
Abstract
Resumen
En años recientes, la evaluación momentánea ecológica (en inglés, ecological momentary assessment (EMA)) ha sido utilizada repetidamente para evaluar los síntomas de trastorno de la conducta alimentaria (TCA) en ambientes naturalistas, lo que ha permitido un incremento en el entendimiento de los procesos temporales que potencían las conductas de trastornos alimentarios. Sin embargo, todavía hay notables limitaciones del autoreporte EMA y con la rápida proliferación de la tecnología hay todavía mayores posibilidades de mejorar los métodos de evaluación ambulatoria para avanzar el entendimiento y el tratamiento de los TCAs. Objetivo: Por lo tanto, el propósito de esta revisión fue 1) revisar sistemáticamente los estudios en TCAs que han utilizado métodos de evaluación ambulatoria además del auto-reporte, y 2) proveer las pautas para futuras investigaciones y aplicaciones clínicas. Método: Se realizó una búsqueda sistemática de la literatura en las bases de datos electrónicas, y los datos con las características del estudio y calidad metodológica fueron extraídos. Resultados: La búsqueda identificó 17 estudios que utilizaron métodos de evaluación ambulatoria para recopilar datos objetivos y se centraron principalmente en el funcionamiento autónomo, la actividad física y los procesos cognitivos en los grupos de control y de TCA. Discusión: En conjunto, la literatura demuestra la promesa de utilizar una variedad de abordajes de evaluación ambulatoria ecológicamente válidos en los TCA, aunque sigue habiendo investigaciones limitadas que han utilizado métodos distintos al autoinforme (porejemplo, sensores portátiles), particularmente en los últimos años. En el futuro, hay varios métodos de evaluación momentánea mejorados con tecnología que tienen potencial para mejorar la comprensión y el tratamiento de los TCA.
1 |. INTRODUCTI ON
Rapid advances in technology have brought about increasing possibilities to harness the potential of mobile devices to better understand and treat a range of behavioral health issues (e.g., Kay, Santos, & Takane, 2011). Similar to other fields of clinical and health psychology, over the past decades there has been tremendous growth in the integration of technology in the assessment and treatment of eating disorders (EDs). A particularly well-utilized methodology in ED research over recent years is ecological momentary assessment (EMA), which allows for repeated assessment of symptoms in naturalistic settings using mobile devices (e.g., Engel et al., 2016; Shiffman, Stone, & Hufford, 2008). While EMA research has significantly advanced ED research, it is important to acknowledge the limitations of this literature in EDs in light of current technological capabilities and consider ways in which the use of mobile technology in EDs can be advanced. In particular, one way forward is through the use of objective or passive ambulatory data collection methods (i.e., information obtained from assessments other than self-report questions, such as wearable sensors) to complement data obtained through more traditional self-report EMA approaches. As such, the goals of this review are to (a) provide a brief historical perspective on EMA in EDs, (b) systematically review existing literature using objective (i.e., other than self-report) ambulatory assessment approaches in EDs, and (c) provide directions for future integration of ambulatory assessment in ED research and treatment.
1.1 |. History and applications of ambulatory assessment in EDs
Ambulatory assessment refers to the measurement of individuals’ behavior, physiology, experience, and environment in naturalistic settings. This may include passive monitoring of physiological functioning or environmental parameters (e.g., location), as well as more traditional self-report based EMA or experiencing sampling methods (Society of Ambulatory Assessment, 2019). EMA measures constructs at periodic intervals, which can include both event-contingent (i.e., participant-initiated) and interval-contingent (i.e., device-prompted) responses on mobile devices (Stone & Shiffman, 1994). EMA emerged from earlier work that primarily involved the use of written diaries to assist memory in health care research (Verbugge, 1980) and was developed to address limitations of more traditional assessment approaches and earlier methods of naturalistic data collection. For instance, a related earlier approach was the experience sampling method (e.g., Csikszentmihalyi & Hunter, 2003), which used a signaling device to inform participants to complete an assessment via paper and pencil. While this methodology was effective, participant responses were not electronically time-stamped, which meant that completion times could be inaccurate and surveys could be backlogged (Stone, Shiffman, Schwartz, Broderick, & Hufford, 2002). In contrast, the use of electronic EMA methods, which automatically record the date and time of participant responses, greatly enhances researchers’ ability to locate events in time and examine the temporality of participant experiences.
Because EMA gathers data from current or recent events, it also minimizes the recall biases typically associated with retrospective self-report measurement (Schwarz & Sudman, 1994). Furthermore, EMA maximizes ecological validity and generalizability by avoiding the artificiality of a laboratory environment and gathering data in real-world settings (e.g., Shiffman et al., 2008; Stone & Shiffman, 1994). Finally, EMA provides an opportunity to precisely examine temporal patterns and momentary processes by intensively measuring constructs of interest many times per day for several days, weeks, or months. For instance, EMA research has been used to show that negative affect increases prior to and decreases following binge-eating episodes (e.g., Engel et al., 2013; Smyth et al., 2007). In sum, the use of mobile technologies to capture data in real-time data with EMA has provided numerous important advantages over other assessment methods.
Although there have been a large number of EMA studies published on EDs in the past two decades, the findings can be reasonably categorized into four groups: (a) model testing; (b) antecedents and consequents of ED behavior; (c) description, frequency, and patterning of ED behavior; and (d) psychometric properties of EMA-based assessments (Engel et al., 2016). Regarding model testing, a number of momentary ED models have been tested using EMA. Some examples include a state-based dual pathway model of bulimia nervosa (Holmes, Fuller-Tyszkiewicz, Skouteris, & Broadbent, 2014), cognitive behavioral theory (Lavender et al., 2013), and the interpersonal model of loss of control eating (Ranzenhofer et al., 2014).
Relatedly, EMA has also been used to study the antecedents and consequents of ED behavior. Specifically, research has studied how variables change prior to and subsequent to ED behaviors, with most research focusing on self-reported affective state (e.g., Engel et al., 2013; Hilbert & Tuschen-Caffier, 2007; Stein et al., 2007). This literature reliably indicates that negative affect increases and positive affect decreases prior to binge-eating episodes as well as other ED behaviors (e.g., Haedt-Matt & Keel, 2011). While some evidence is inclusive regarding the nature of affective change following binge-eating episodes, differences in measurement time scales and analytic approaches may account for such discrepancies (Berg et al., 2017). Together these findings have largely supported affect regulation theories of EDs, which posit that ED behaviors function to reduce negative affect, thereby maintaining the pathology via negative reinforcement processes.
In addition to affect assessments, a related line of EMA work has investigated cognitive processes relevant to ED symptoms in naturalistic settings (e.g., appearance-related social comparisons; Leahey, Crowther, & Ciesla, 2011; repetitive negative thinking; Sala, Brosof, & Levinson, 2019). The overall pattern of findings across this EMA literature has demonstrated the importance of temporal fluctuations in cognitive and affective factors that precipitate or follow ED behaviors, which has also informed new ED interventions such as Integrative Cognitive Affective Therapy (Wonderlich et al., 2008; Wonderlich, Peterson, & Smith, 2015). Importantly, the theoretical model underlying Integrative Cognitive Affective Therapy is based on EMA data regarding momentary cognitive and affective processes that serve to maintain bulimic behaviors, with a particular emphasis on affect and emotion regulation. Accordingly, intervention strategies target processes that elicit momentary negative affective states (e.g., negative self-oriented cognitions or self-discrepancy) and support adaptive coping responses to negative affect (Wonderlich, Peterson, & Smith, 2015).
EMA has also been employed to carefully document and describe the frequency and temporal patterning of ED behaviors. For example, EMA has been used to study the times of the day and week at which ED patients are most likely to engage in ED behaviors (Lavender et al., 2013; Smyth et al., 2009). Similarly, EMA has been applied to elucidate the duration of binge-eating episodes in participants with binge-eating disorder (Schreiber-Gregory et al., 2013).
Finally, some ED research has also examined the psychometric properties of EMA. Wonderlich et al. (2015) found moderate-to-strong convergence between momentary assessments of ED behaviors and retrospectively recalled assessments of emotion, binge eating, and exercise in samples of individuals with eating disorders and/or obesity. Other EMA studies have also evaluated the ecological validity of traitlevel measures of affect and ED-related constructs in anorexia nervosa (Mason et al., 2018) and among individuals with obesity with and without binge-eating disorder (Mason, Pacanowski, et al., 2017; Mason, Smith, et al., 2017).
Despite the substantial body of research that has investigated EDs with EMA, there are some notable limitations and gaps in this literature. For example, traditional EMA approaches that rely on participants’ survey responses are nevertheless subject to reporting biases associated with self-report measures. In addition, EMA involves intensive longitudinal data collection, typically multiple times per day for several days, and therefore introduces challenges related to participant burden and noncompliance with assessment protocols. As the number of bio-behavioral assessments that are available in ambulatory form continues to grow, there is increasing opportunity to utilize passive data collection approaches in naturalistic settings to gather objective information, which could be valuable independently or in conjunction with self-report EMA data. Thus, in order to further advance the ED field, it will be important to consider both the limitations of prior EMA work and the growing potential of technologyfacilitated ambulatory assessment.
1.2 |. Advantages of objective ambulatory assessment data
In particular, there are important reasons to consider integration of objective data-collection methods in ambulatory assessment research going forward. First, reliance on subjective self-report measures of some constructs relevant to EDs may introduce significant biases and limitations given that prior literature suggests that self-reported and objectively measured data do not always converge. For instance, objective and subjective measures of cognitive functioning among individuals with depression and attention-deficit/hyperactivity disorder were only moderately correlated (Potvin, Charbonneau, Juster, Purdon, & Tourjman, 2016), significant discrepancies emerge between subjective and objective measurement of sleep quality (e.g., O’Brien, Hart, & Wing, 2016; Rezaie, Fobian, McCall, & Khazaie, 2018), and correlations between direct and self-report measures of physical activity are low-to-moderate (Prince et al., 2008). Given the limited correspondence between objective and self-reported data, there is a need for ambulatory assessment research to integrate methods that may more accurately characterize participants’ affective, cognitive, behavioral, and biological functioning.
Second, objective measures may complement and deepen our understanding of constructs and symptom relationships that are assessed via self-report (e.g., affect, binge eating, cognitive rumination). That is, while self-report EMA data can yield valuable insight into participants’ internal experiences (e.g., the sense of loss of control over eating), it is possible that self-report EMA methods are not able to capture other relevant physiological and cognitive processes that occur outside individuals’ conscious awareness, fluctuate over time, and predict or follow subjective experiences and ED behaviors. Repeated measurements of potentially dynamic biomarkers of psychological constructs could therefore allow for a more integrative understanding of bio-behavioral mechanisms underlying ED symptomatology and add meaningful units of analysis by which we can understand psychological phenomena.
Third, objective parameters of physiological and neurocognitive constructs in ED research are often assessed once during a laboratory session, which introduces additional limitations given that single assessments may not capture an individual’s average functioning or typical performance over time, and variability in the assessments over time is not measured. With EMA data, multilevel analyses partition variance into between-person and within-person components. This may provide a more reliable depiction of individual differences in average levels of variables assessed across multiple assessments (i.e., between-person effects), as well as intraindividual variability in constructs (i.e., within-person effects).
Fourth, utilizing objective, passively measured data could reduce participant burden in gathering data in some domains, as well as provide fine-grained assessment of temporal patterns. For instance, data collection via wearable sensors (i.e., electronic devices worn on the body that monitor physiological and environmental parameters) is less burdensome than asking participants multiple times per day to report on physical activity levels or to report each morning on sleep time the previous night. As a result, integration of passively collected objective data may improve participant compliance and engagement with ambulatory assessment protocols, which has been noted as a challenge in the EMA literature (Engel et al., 2016). Furthermore, there has been increasing focus on determining the time scale by which different effects may unfold, and how to best determine the appropriate sampling frequency in EMA research (e.g., Kockler, Santangelo, & Ebner-Priemer, 2018). Utilizing continuous, passive data collection therefore could mitigate the concern that researchers will fail to detect potentially meaningful microtemporal fluctuations in constructs.
1.3 |. The present review
Taken together, EMA research has significantly advanced the study of EDs, most notably in the examination and development of theoretical models and by enhancing our understanding of micro-temporal processes that potentiate ED symptoms. Consequently, as a field we have learned the importance of both individual differences and momentary cognitive and affective states that have proximal influences on ED behaviors. However, there are several limitations to consider with respect to self-report EMA data collection, including issues related to participant burden, subjectivity, and an incomplete understanding of how momentary fluctuations in other relevant processes (e.g., biology, neurocognition, location) influence ED symptoms.
Therefore, the overarching goal of this review is to further advance the ED field by first synthesizing research that has utilized ambulatory assessment methods other than self-report. Second, based on the extant literature in EDs, we provide directions for future research and interventions.
2 |. METHODS
2.1 |. Study selection
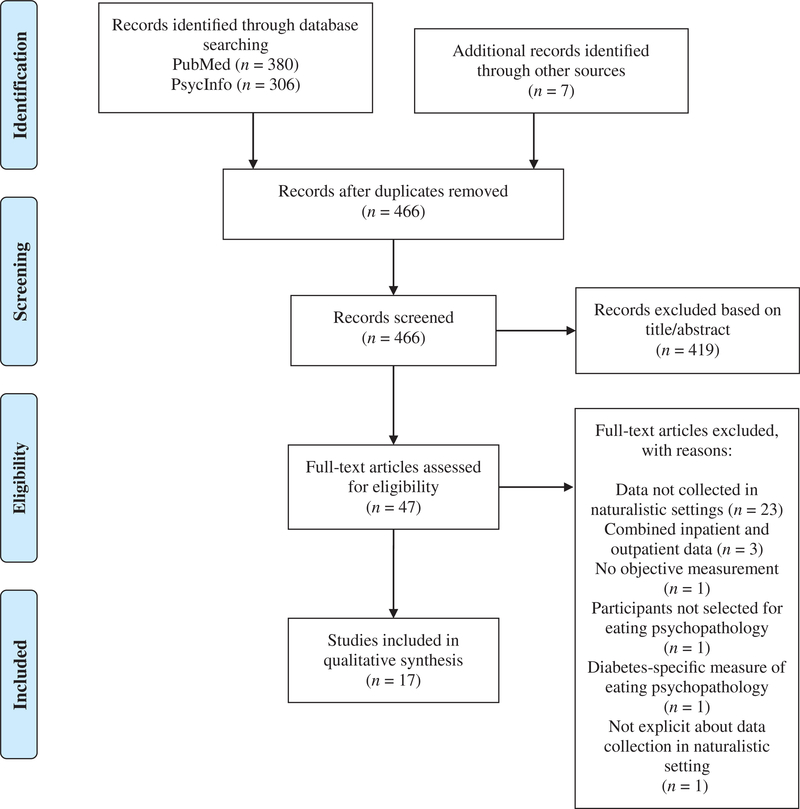
A systematic literature search in PsycInfo and PubMed electronic databases was conducted in December 2018 to identify relevant studies. Each database was queried using the following search terms, which were agreed upon by all authors: (a) “eating disorder” or “binge” or “bulimia” or “anorexia” or “disordered eating” or “eating pathology” or “eating psycho-pathology” in the article title in conjunction with (b) “ecological momentary assessment” or “experience sampling” or “ambulatory assessment” or “mobile assessment” or “real time” or “real world” or “naturalistic” or “ecological momentary intervention” or “just in time adaptive intervention” or “mobile intervention” or “smart phone” or “sensor” or “micro-randomized trial” or “mHealth” or “mobile health” or “actigraph” or “accelerometry” or “objective monitoring” or “wearable” or “heart rate variability” or “physical activity” in the title and/or abstract. Articles were included only if the methodology reflected use of ambulatory assessment to gather data in naturalistic settings from methods other than self-report (e.g., data from wearable sensors). Other eligibility criteria were the inclusion of participants with EDs or participants who were selected based on the presence of current or past eating psychopathology, availability in English, and articles that described empirical studies. The search included all available studies up until December 2018 and did not exclude unpublished dissertations or theses. Articles were excluded for the following reasons: (a) if it was not clear that data were collected in naturalistic environments, (b) if data collection was limited to a controlled setting (e.g., laboratory or inpatient/day treatment settings), (c) or if inpatient and outpatient data were combined; however, studies that included separate analyses or comparisons of inpatient and outpatient data were included (e.g., changes during and after intensive treatment). The study selection process is summarized in Figure 1; authors K.S. and L.S. independently screened articles for inclusion and resolved any discrepancies via discussion.
FIGURE 1.
PRISMA diagram summarizing study selection
2.2 |. Data collection and synthesis
2.2.1. |. Data extraction
A coding scheme was developed to systematically extract data regarding study characteristics and results, including sample demographics, ambulatory assessment or intervention methodology, and key findings. Authors K.S. and T.M. reviewed and summarized information for all studies and resolved any discrepancies via discussion.
2.2.2. |. Quality assessment
After articles were identified for inclusion, authors K.S. and T.M. independently evaluated the quality of each article. Given that there is no established tool for evaluating the quality of mobile assessment or intervention studies, as well as the substantial variability in methodology observed across the included studies, a checklist was developed based on the Newcastle-Ottawa Scale adapted for cross-sectional studies (Modesti et al., 2016), the National Heart Lung, and Blood Institute Study Quality Assessment Tools (National Institutes of Health, 2014), and the adapted STROBE Checklist for Reporting EMA Studies (Liao, Skelton, Dunton, & Bruening, 2016). Checklist items were agreed upon by all authors and are available in Data S1. One point was assigned for each of the eight items and summed to calculate a total quality score for each article, with higher scores reflecting higher methodological quality. Inter-rater reliability was adequate (kappa = .71). All discrepancies were resolved between raters before calculating the total score for each article.
3 |. RESULTS
3.1 |. Study characteristics and quality
The systematic search yielded a total of 17 articles, which ranged in year of publication from 1996 to 2017. Study characteristics, key findings, and quality scores for each article are summarized in Table 1. Notably, the most common reason for excluding studies at the level of full-text screening was inclusion of participants in inpatient or day hospital settings. Among included studies, total sample size ranged from 9 to 109 (M = 46.941, SD = 28.35), and the sample size of included ED groups ranged from 6 to 61 (M = 24.35, SD = 15.12). The mean quality score was 6.12 (SD = 1.41; Range: 4–8) out of 8. Upon review of quality ratings, information was consistently lacking regarding participant training on ambulatory assessment protocols as well as reporting of missing data, compliance, and/or attrition during study protocols. Across all studies, the most frequently studied diagnostic category was anorexia nervosa, which was examined in 12 of the 17 studies. The majority of research was conducted with adult women, though five studies included adolescents. Limited information was available regarding race or ethnicity across samples. Across studies, six examined autonomic functioning (i.e., specifically heart rate variability), six examined physical activity, four examined sleep–wake patterns, and one examined cognitive processes.
TABlE 1.
Study characteristics, key findings, and quality scores
| Study | ED group(s) and sample size(s) |
Mean age (years) | Mean BMI (kg/m2) | Gender (% female) |
Ethnicity | Ambulatory assessment/ intervention |
Key findings | Total quality score |
|---|---|---|---|---|---|---|---|---|
| Bomba et al.(2014) | 21 adolescents with AN; 21 adolescents with FHA (10 Hy-FYA; 11 N-FHA); 21 controls | AN: 15.9 (SD = 1.1); Hy-FHA: 16.5 (SD = 0.8); N-FHA: 15.9 (SD = 0.9); control: 16.2 (SD = 1.0) | AN: 15.1 (SD = 2.6); Hy-FHA: 18.1 (SD = 1.1); N-FHA: 18.7 (SD = 1.1); control: 19.7 (SD = 1.8) | 100 | N.S. | HRV was measured by a 24-hour ECG recording using the Accuplus 363 holter (DelMarAvionics, Irvine, CA). | The AN group evidenced dysregulation in HRV parameters (i.e., higher SDNN and rMSSD) compared to control and N-HFA groups. | 7 |
| Bouten and Westerterp (1996) | 11 women with AN; 13 NW women | AN: Range = 21–48; NW control: Range = 20–35 | AN: Range = 12.5–18.3; NW control: Range = 19.2–26.7 | 100 | N.S. | Physical activity over 7 days was determined using output from a portable motion sensor (Tracmor accelerometer). Physical activity was measured by average daily activity in terms of energy expenditure (PAL). | AN and NW groups did not differ in physical activity levels; in AN only, BMI was positively correlated with physical activity levels. | 7 |
| Dellava, Hamer, Kanodia, Rodríguez, and Bulik (2011) | 15 participants with AN-rec; 22 controls | AN-rec: 32.5 (SD = 14.3); control: 28.9 (SD = 10.7) | AN-rec: 21.4 (SD = 2.0); control: 23.6 (SD = 4.4) | 100 | N.S. | Physical activity was assessed for 3 weekdays and 1 weekend day by an Actigraph monitor. | Total daily activity (mean counts) did not differ between groups. | 7 |
| Galetta et al. (2003) | 25 participants with AN; 25 age-matched thin controls; 25 age matched NW controls | AN: 17.5 (SD = 4.2); thin controls: 17.7 (SD = 3.9); NW controls: 18.1 (SD = 4.5) | AN: 15.3 (SD = 1.4); thin controls: 18.7 (SD = 1.7); NW controls: 21.9 (SD = 2.8) | 100 | N.S. | HRV was measured via 24-hour ECG monitoring using a 2-channel amplitude modulated tape recorder (Diagnostic Monitoring System, Santa Ana, CA). | The AN group was higher on all HRV parameters compared to thin and NW controls, reflecting increased vagal tone. | 4 |
| Gianini et al. (2016) | 61 participants with AN; 24 age-matched NW controls | AN: 24.4 (SD = 6.5); control: 26.0 (SD = 3.9) | AN low weight: 16.1 (SD = 1.0); AN weight restored: 20.2 (SD = 0.7); AN follow-up: 19.5 (SD = 2.0) | 100 | N.S. | Physical activity was assessed for 3 days at 3 time points (during inpatient at a low weight, during inpatient when weight-restored, and outpatient follow-up 4–6 weeks after discharge); controls were assessed at 1 time. Physical activity was assessed by the intelligent device for energy expenditure and activity accelerometer (IDEEA; MiniSun, Fresno, CA). | Post-treatment AN patients were more physically active than controls during the day but less active at night. Fidgeting did not differ between post-treatment AN and control groups. Total activity count at follow-up did not predict BMI change in the year following inpatient discharge. | 8 |
| Keyes et al. (2015) | 37 outpatients with AN; 18 inpatients with AN; 24 participants with moderate anxiety (GAD-7 score ≥ 10); 30 controls | AN groups: 29 (range: 18–67); anxiety group: 27 (range: 18–54); control: 29 (range: 20–52) | AN outpatient: 16.04 (SD = 1.44); AN inpatient: 14.1 (SD = 2.11); anxiety group: 22.24 (SD = 3.88); control:21.2 (SD = 1.53) | 100 | N.S. | Physical activity over 1 week was assessed by actigraphy using the Actiwatch AW4 (Cambridge Neurotechnology, Cambridge, UK); objective activity was measured as average and peak actimetry data (counts per minute). | No group differences were found in objective measures of physical activity. | 6 |
| Karr et al. (2017) | 9 participants with AN | 26.38 (SD = 4.81) | 17.46 (SD = .88) | 100 | N.S. | Physical activity was measured for 7 days using Actigraph GT3X; objective activity was measured as total minutes of light and moderate-vigorous activity. EMA simultaneously measured positive and negative affect. | Within the same day, greater physical activity time was related to less instability in positive affect and stress but not negative affect. Higher physical activity predicted greater positive affect within the same hour and at the next hour, and vice versa. | 8 |
| Kostrzewa et al. (2013) | 37 adolescents with AN | 15.15 (SD = 1.21) | 15.66 (SD = 1.38) | 100 | N.S. | Physical activity was measured for 3 days by Actiwatch AW4 (Cambridge Neurotechnology, Cambridge, UK) at admission, end of treatment (12 months), and 1-year follow-up. Participants were divided into low and high physical activity groups based on moderate or vigorous physical activity measured by the Actiwatch | The high activity group had longer illness duration. Physical activity stabilized and was maintained at follow-up, in that patients initially high in activity decreased in activity, whereas patients low in activity increased activity. Patients who recovered who had higher initial activity had higher fat mass at the follow-up assessment. | 7 |
| Latzer, Tzischinsky, Epstein, Klein, and Peretz (1999) | 25 participants with BN; 21 controls | BN: 22.3 (SD = 8.3); control: 24.0 (SD = 8.11) | BN: 21.7 (SD = 2.9); control: 20.3 SD = 1.4) | 100 | N.S. | Sleep-wake patterns were assessed using mini Actigraphs (Min-Act; AMA-32, AMI, Ardsley, NY) for 1 week. | The BN group evidenced sleep onset and offset 1 hr later than controls. | 5 |
| Latzer, Tzischinsky, and Epstein (2001) | 21 participants with AN; 16 controls | AN: 18.7 (SD = 3.5); control: 19.4 (SD = 3.9) | AN: 16.8 (SD = 1.3); control: 20.0 (SD = 1.3) | 100 | N.S. | Sleep-wake patterns were assessed using mini Actigraphs (Min-Act; AMA-32, AMI, Ardsley, NY) for 1 week. | AN and control groups did not differ on any actigraph sleep parameters | 5 |
| Melanson, Donahoo, Krantz, Poirier, and Mehler (2004) | 6 participants with AN; 10 controls | AN: 29 (SD = 3); control: 24 (SD = 3) | N.S. | 100 | N.S. | HRV was assessed using a holter monitor for 2 days. | AN group evidenced reduced ambulatory HRV (rMSSD, pNN50, SDNN, and SDANN parameters) compared to controls | 4 |
| Mont et al. (2003) | 31 adolescents with AN | 16.3 (SD = 1.4) [second exam] | 19.2 (SD = 1.0) [second exam] | 80.6 | N.S. | HRV was measured by 24-hour holter monitoring (Marquette 8500 ambulatory tape recorder) at admission and 3- to 18-months later (after weight stabilization). | HRV normalized after weight restoration. | 7 |
| Myers, Ridolfi, and Crowther (2015) | 30 undergraduates who met criteria for EDs based on the EDDS (17 BN, 3, BED, 5 sub-threshold AN, 5 sub-threshold BN); 63 undergraduate controls | 19.53 (SD = 3.37) | 24.15 (SD = 5.41) | 100 | 78.5% Caucasian | RTs to a modified word-stem task were used an implicit measure of appearance schema activation. Participants completed a 5-day EMA protocol using palm handheld personal data assistant (PDA) model Z22. RTs (ms) for selection of appearance and nonappearance related words were assessed at EMA recordings. | Women with ED psychopathology evidenced longer RTs when selecting appearance-related words on the word-stem task compared to women without ED psychopathology. | 7 |
| Petretta et al. (1997) | 13 participants with AN; 13 thin controls; 10 NW controls | AN: 20.0 (SD = 2.0); thin control: 22.0 SD = 3.0); NW control: 21.0 (SD = 3.0) | AN: <19; thin control: <20; NW control: 20–27 | 100 | 100 | HRV was measured by 24-hour holter monitoring. | The AN group evidenced higher HRV (i.e., high-frequency power and all time domain measures) compared to control groups. | 6 |
| Ranzenhofer et al. (2016) | 17 adolescents with LOC eating | 14.77 (SD = 1.55) | 2.17 (SD = .48) [BMI-Z] | 100 | N.S | HR and HRV were measured for 2 days using a Mortara H12 holter monitor; EMA was conducted for 2 weeks that assessed momentary LOC eating. | Higher HR and lower HRV (i.e., rMSSD) prior to eating predicted higher LOC eating ratings at the within-subjects level | 6 |
| Tzischinsky and Latzer (2004) | 22 participants with nocturnal binge eating (10 BN, 12 BED) | 38.1 (SD = 12.3) | 30.1 (SD = 10.0) | 100 | N.S. | Sleep–wake patterns were monitored using Actigraph (Mini-Act, Ardsley, NY) for 1 week. | Objective sleep parameters did not differ between BN and BED groups. Compared to previously published data on non-NES BN and BED groups, the BN + NES but not BED +NES group evidenced lower sleep efficiency. | 6 |
| Tzischinsky and Latzer (2006) | 44 adolescents (15 OB + BE, 17 OB-BE, 12 NW controls) | OB groups: 15.0 (SD = 1.7); NW control: 15.6 (SD = 1.4) | OB + BED: 36.5 (SD = 6.0); OB-BED: 32.1 (SD = 3.1); NW control: 15.6 (SD = 1.4) | OB groups: 53.1 | N.S. | Sleep–wake patterns were monitored using Actigraph (Mini-Act, Ardsley, NY) for 1 week. | Objective sleep parameters did not differ between the 3 groups. | 5 |
Abbreviations: AN, anorexia nervosa; AN-BP, AN binge/purge type; AN-R, An restricting type; AN-rec, recovered from AN; BE, binge eating; BMI, body mass index; BN, bulimia nervosa; BP, blood pressure; ED, eating disorder; EDDS, Eating Disorder Diagnostic Scale; EDE-Q, Eating Disorder Examination Questionnaire; EDNOS, eating disorder not otherwise specified; EMA, ecological momentary assessment; FHA, functional hypothalamic amenorrhea; GAD-7, General Anxiety Disorder-7 questionnaire; HF, high frequency; HR, heart rate; HRV, heart rate variability; HyFHA, hypogonadotropic FHA; LF, low frequency; LOC, loss of control; MESOR, Midline Estimating Statistic of Rhythm; ms, millisecond; N.S., not specified; NES, nocturnal eating syndrome; N-FHA, normogonadotropic FHA; NW, normal weight; OB + BED, obesity with binge eating disorder; OB-BED, obesity without binge eating disorder; pNN50, percentage of intervals that differ by >50 ms; RAR, rest-activity circadian rhythm; rMSSD, square root of mean successive difference of successive NN intervals; RT, reaction time; SDANN, standard deviation of average NN intervals over 5-minute periods; SDNN, standard deviation of normal beat (NN) intervals.
3.2 |. Assessment methodology
With respect to the most commonly utilized methods of assessment, ambulatory heart rate variability was typically assessed by holter monitoring. Holter monitors are small devices that record continuous electrocardiography signals through wires attached to the chest, which provide indices of heart rate and heart rate variability (American Heart Association, 2019). Physical activity and sleep–wake cycles were assessed via actigraphy, which uses a small portable device (i.e., Actigraph) resembling a watch face (often worn on the wrist, such as the Actiwatch, Mini-Mitter Co., Inc., Bend, OR) to monitor periods of activity and inactivity. The occurrence and degree of motion is assessed multiple times per second using an accelerometer, and then movement data are grouped into 1- to 60-second epochs for analyses (Sadeh, 2011). Algorithms are then applied to these data to estimate various actigraphy parameters of sleep–wake cycles (e.g., sleep latency, total sleep time, awakenings, sleep efficiency) and physical activity (e.g., total amount, intensity).
Given that the primary focus of this project was to provide an overview of technology-enhanced methodologies, a comprehensive review of specific content areas is beyond the scope of the present review. The following summary of the identified literature is organized by content area, with a brief introduction of the relevance of each construct to EDs followed by a review of the included studies.
3.3 |. Autonomic functioning
3.3.1 |. Relevance of heart rate variability in EDs
Altered autonomic nervous system functioning has been the focus of a substantial body of research in the ED field, particularly heart rate variability (for reviews see Mazurak, Enck, Muth, Teufel, & Zipfel, 2011; Peschel et al., 2016). Heart rate variability involves a complex interplay between the sympathetic and parasympathetic branches of the autonomic nervous system. Researchers propose that parasympathetic activation, mediated by the vagus nerve, contributes to high levels of heart rate variability, which has been thought to be broadly connected to behavioral constructs, such as self-regulation, cognitive control, and increasingly, various forms of psychopathology (Beauchaine & Thayer, 2015; Geisler, Kubiak, Siewert, & Hannelore Weber, 2013; Thayer & Lane, 2000). One prominent model, the neurovisceral integration model (Thayer, Hansen, Saus-Rose, & Johnsen, 2009) proposes that the autonomic nervous system adjusts physiological arousal via inhibitory processes controlled by the parasympathetic nervous system in response to situational demands. High levels of heart rate variability are generally associated with high levels of social engagement and adaptive self-regulation (Geisler et al., 2013) whereas low levels of heart rate variability have been shown to be associated with a wide range of psychopathology and deficits in cognitive control (Hovland et al., 2012; Kemp, Quintana, Quinn, Hopkinson, & Harris, 2014; Santerre & Allen, 2007). Recent reviews in EDs suggest that bulimia nervosa is characterized by heightened levels of resting state heart rate variability and impaired stress response to stress-related cues (Peschel et al., 2016), and anorexia nervosa is associated with a range of disturbances in autonomic nervous system functioning, including imbalances between the parasympathetic nervous system and sympathetic nervous system (Mazurak et al., 2011). However, there has been a great deal of heterogeneity in methods and the pattern of results across studies.
3.3.2 |. Review of naturalistic studies of heart rate variability
Among the studies reviewed that utilized naturalistic heart rate variability assessment methods, all studies used holter monitoring for a period of one to two days of ambulatory assessment in samples that ranged in size from 16 to 34. A range of heart rate variability parameters in the time (e.g., standard deviation of the normal-to-normal interval, root mean square of successive interval differences) and frequency (e.g., low frequency, high frequency, power) domains were reported (Task Force, 1996). Similar to the broader heart rate variability literature in anorexia nervosa and bulimia nervosa (Mazurak et al., 2011; Peschel et al., 2016), ambulatory heart rate variability assessments in naturalistic settings yielded inconsistent findings; two studies documented higher heart rate variability parameters in anorexia nervosa (Bomba et al., 2014; Galetta et al., 2003), though one study indicated reduced sympathetic activity in anorexia nervosa and bulimia nervosa samples (Melanson et al., 2004). One study demonstrated that heart rate variability normalized after weight restoration in anorexia nervosa (Mont et al., 2003). It is important to note that variations in heart rate variability within individuals may be associated with the occurrence of ED behaviors, as demonstrated by one study that integrated ambulatory heart rate variability assessment with self-report EMA of symptoms (Ranzenhofer et al., 2016). Results indicated that higher heart rate and lower heart rate variability prior to eating predicted higher loss of control eating ratings at the within-subjects level. In sum, heart rate variability appears to be a transdiagnostic biomarker of psychopathology that reflects variations of self-regulation, cognitive control, and affect regulation. While results of group comparisons in EDs have been inconsistent, some EMA evidence suggests that it may serve as a viable and significant indicator of momentary risk for ED behaviors.
3.4 |. Sleep
3.4.1 |. Relevance of sleep in EDs
The relationship between sleep disturbances and psychopathology is well-established, as sleep problems (i.e., inadequate continuity, duration, or quality) are considered both a risk factor and symptom of several psychiatric illnesses (American Psychiatric Association [APA], 2013; Breslau, Roth, Rosenthal, & Andreski, 1996). Poor sleep is also thought to impede adaptive emotion regulation, which is a transdiagnostic factor broadly related to various forms of psychopathology, including EDs (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Palmer & Alfano, 2017). In addition to the potential relationship between sleep and impaired self-regulatory processes that are often seen in EDs, some presentations of disordered eating are specifically characterized by aberrant sleep processes. For instance, in the current Diagnostic and Statistical Manual of Mental Disorders (DSM-5), night eating syndrome (a subtype of Other Specified Feeding or Eating Disorder) is associated with episodes of night eating with awareness of eating, while sleep disorders may involve episodes of night eating with varying degrees of awareness (APA, 2013). Given this evidence, characterizing sleep–wake patterns among individuals with EDs has emerged as a relevant focus of naturalistic assessment studies.
3.4.2 |. Review of naturalistic studies of sleep
Three of the four articles identified in the present review used objective sleep measurement to assess binge-type EDs (bulimia nervosa: Latzer et al., 1999; bulimia nervosa, binge-eating disorder: Tzischinsky & Latzer, 2004; binge-eating disorder in obesity: Tzischinsky & Latzer, 2006), and one study compared an anorexia nervosa sample to controls (Latzer et al., 2001). All studies used actigraphy to measure sleep–wake patterns for a one-week monitoring period, which was assessed by a variety of indices, including latency, onset, duration, and efficiency, with sample sizes ranging from 22 to 46. One study found no differences in objective sleep parameters between anorexia nervosa and control groups (Latzer et al., 2001). Latzer et al. (1999) observed sleep onset and offset 1 hr later in a bulimia nervosa group compared to controls, and a later study found no differences between bulimia nervosa and binge-eating disorder in objective sleep measures (Tzischinsky & Latzer, 2006). Regarding binge eating in the context of obesity, one study found no differences in any sleep parameters between individuals with obesity and binge eating and those with obesity without binge eating (Tzischinsky & Latzer, 2006). Furthermore, some studies found no group differences in objective sleep measures despite differences in subjective sleep quality (e.g., Latzer et al., 2001; Tzischinsky & Latzer, 2006). Taken together, the number of studies and sample sizes in this area remain limited, with only one study finding differences in objective sleep parameters between ED and control groups.
3.5 |. Physical activity
3.5.1 |. Relevance of physical activity in EDs
Like heart rate variability and sleep, physical activity is a domain that is salient to psychopathology in a variety of ways, and particularly to EDs. Broadly, heightened levels of physical activity have been shown to be related to better psychological functioning and health outcomes (e.g., Chekroud et al., 2018; Penedo & Dahn, 2005). However, this relationship is certainly more complex and nuanced in the context of EDs. Excessive exercise is a prominent symptom for many individuals with EDs, especially those with anorexia nervosa, and is thought to be an important etiological and maintenance factor (e.g., Davis et al., 1997). While it is well-documented that exercise is often linked to overvaluation of shape and weight (i.e., core features of anorexia nervosa and bulimia nervosa) for many with EDs, it is also possible that physical activity that is not compulsive or excessive could have psychological and/or physical health benefits for other individuals with EDs. That is, physical activity is a potentially modifiable domain that is thought to facilitate regulation of eating behavior (Joseph, Alonso-Alonso, Bond, Pascual-Leone, & Blackburn, 2011), and a recent review of randomized control trials of physical activity interventions for binge-eating disorder found that physical activity interventions were associated with reductions in binge eating and depression, and in some cases weight loss (Blanchet et al., 2018). Thus, for a variety of reasons physical activity has been a domain of particular interest in the study of EDs.
3.5.2 |. Review of naturalistic studies of physical activity
Of the five articles included in the present review, all examined individuals with current or past anorexia nervosa, with total sample sizes ranging from 9 to 109. Each monitoring period ranged from 3 to 7 days, during which physical activity was measured via Actiwatch or other accelerometer devices. Physical activity was indexed in a variety of ways, including average daily activity in terms of energy expenditure, mean counts per minute or day, total minutes of light, moderate, and vigorous activity, as well as counts per minute in peak activity. Three studies found no differences in objective physical activity between individuals with current or past anorexia nervosa and control groups (Bouten & Westerterp, 1996; Hechler et al., 2008; Keyes et al., 2015), whereas one study found that after treatment, individuals with anorexia nervosa were more active during daytime hours compared to controls (Gianini et al., 2016). One study of adolescents with anorexia nervosa found that physical activity levels stabilized over the course of treatment and at follow-up assessment (i.e., those with high initial levels decreased activity, and those with low initial levels increased activity; Kostrzewa et al., 2013). Findings were somewhat discrepant with respect to symptom severity or course. While some data indicated that BMI was positively correlated with activity in anorexia nervosa (Bouten & Westerterp, 1996), and that individuals with AN who had higher activity levels at the start of treatment evidenced higher fat mass at a follow-up assessment (Kostrzewa et al., 2013), it was also reported that higher activity levels at the beginning of treatment were associated with longer duration of illness (Kostrzewa et al., 2013). Lastly, one pilot study of 9 women with anorexia nervosa examined within-person relationships between minutes of physical activity and affect by integrating actigraphy with EMA (Karr et al., 2017). Within the same day, greater physical activity time was related to less instability in positive affect and stress, but not negative affect. Bidirectional relationships were also found between physical activity and positive affect at the momentary level, in that greater physical activity predicted greater positive affect within the same hour and at the next hour, and vice versa. Collectively, similar to the sleep and heart rate variability literature, inconsistent findings emerged from group comparisons using modest sample sizes, and notably, there were no available studies of bulimia nervosa or binge-eating disorder. Additionally, some evidence suggested that higher activity levels were related to improved outcome in anorexia nervosa samples, and findings of Karr et al. (2017) demonstrated the potential affect regulatory function of physical activity in anorexia nervosa, which is broadly consistent with literature outside of EDs (Liao, Shonkoff, & Dunton, 2015).
3.6 |. Cognitive processes
3.6.1 |. Relevance of cognitive processes in EDs
Prominent models of EDs highlight the importance of various cognitive processes in the etiology and maintenance of symptoms (e.g., Fairburn, Cooper, & Shafran, 2003; Vitousek & Hollon, 1990; Wonderlich et al., 2008). For instance, it has been suggested that cognitive schemas of individuals with EDs facilitate processing of appearance-related information (Vitousek & Hollon, 1990), that cognitive rumination exacerbates and maintains negative affect in EDs (Smith, Mason, & Lavender, 2018), and that attentional biases may maintain ED symptoms by directing cognitive resources toward disorder-salient stimuli, thereby interfering with tasks and distorting interpretations about the environment (Aspen, Darcy, & Lock, 2013; Stojek et al., 2018). In recent years, several cognitive processes that are thought to be relevant to EDs have been investigated by measuring individuals’ performances on behavioral tasks, though this work has largely been limited to laboratory settings (Smith, Mason, Johnson, Lavender, & Wonderlich, 2018).
3.6.2 |. Review of naturalistic studies of cognitive processes
One EMA study examined a behavioral index of cognitive processing in undergraduate women with and without ED psychopathology (N = 93; Myers et al., 2015). Specifically, a word-stem completion task was embedded within an EMA protocol to assess reaction times for the selection of appearance and non-appearance-related words, which was used as an implicit measure of appearance schema activation. Results indicated that women with ED psychopathology, on average, had longer reaction times to appearance-related words than non-ED controls, which the authors suggest could reflect increased processing and rumination about disorder-relevant stimuli among those with ED symptoms. While the authors specifically interpreted this task as a measure of schema activation, these findings are also supported by the broader attention bias literature in EDs demonstrating selective attention toward weight and shape stimuli on modified Emotional Stroop paradigms, as indexed by longer reaction times to such stimuli (Aspen et al., 2013; Stojek et al., 2018).
4 |. DISCUSSIO N
Collectively, the 17 studies reviewed illustrate the various ways in which technology has been harnessed to gather objective physiological and behavioral data in naturalistic settings, which primarily focused on assessment of autonomic functioning, sleep, and physical activity. Overall, there remains limited research that has utilized ambulatory monitoring methods to gather objective data in naturalistic settings, and significant discrepancies and limitations emerged across studies. Notably, despite the rapid technology advances in recent years, there have been no published studies since 2017 utilizing passive data collection in naturalistic settings among individuals with EDs. Together these findings highlight key areas for future research and clinical applications that could integrate novel ambulatory assessment and intervention methods.
With respect to overall findings across studies, the heart rate variability literature was largely characterized by inconsistencies when comparing ED and control groups. Most studies found no differences in objective physical activity between individuals with current or past anorexia nervosa compared to controls, though the relationship between activity and illness severity or prognosis remains unclear. Regarding sleep, there was some evidence for sleep impairment in bulimia nervosa compared to controls, though impairments did not consistently emerge in other ED groups. Only one study was identified that examined a behavioral index of cognitive processing, with evidence suggesting increased processing of appearance-related words among individuals with ED psychopathology. While these studies had particular strengths in the use of naturalistic assessment methods and collection of objective data, several limitations were also observed. In the following sections, key limitations and directions for future research and clinical applications are reviewed.
4.1 |. Generalizability and sample size
Key limitations that emerged across all areas reviewed were modest sample sizes and insufficient level of methodological detail. The small samples may in part account for the inconsistent findings across this literature. Furthermore, quality assessments revealed that information was often lacking regarding important methodological details (i.e., attrition and compliance). Both of these issues question the reproducibility and generalizability of findings, which highlights the need for larger EMA studies in the future that consistently report all aspects of study design and methodology to confirm or disconfirm previous findings.
4.2 |. Within-person variability in naturalistic settings
Most assessment studies focused on categorical comparisons of aggregated momentary variables (e.g., average reaction time; Myers et al., 2015) between ED and control groups, which precludes examination of dynamic, momentary relationships between ED symptoms and relevant physiological, behavioral, and cognitive processes. It was also notable that while there have been several studies of EDs using self-report measures of physical activity (e.g., see Blanchet et al., 2018), which were excluded from the review, there is a dearth of research examining objective physical activity levels in daily life and especially in binge-type EDs. In addition, many studies were excluded that applied objective monitoring techniques in controlled settings, particularly inpatient and day hospital programs. Although there are certainly advantages of this research (e.g., controlling confounds and characterizing acute states of illness), examination of research questions in naturalistic settings offers potential to enhance ecological validity and evaluate the influence of other relevant time-varying contextual factors (e.g., affect, location, ED symptoms), as demonstrated by Karr et al. (2017) and Ranzenhofer et al. (2016).
4.3 |. Integration across units of analysis
There was also a lack of integration across methodologies within studies. That is, only two studies to date integrated analysis of objective ambulatory measures with self-report data reported via EMA (Karr et al., 2017; Ranzenhofer et al., 2016). These studies demonstrated dynamic, within-person relationships between autonomic functioning and ED behavior (Ranzenhofer et al., 2016), and within-person relationships between physical activity and affective functioning (Karr et al., 2017). Such research highlights the relevance of assessing bio-behavioral mechanisms underlying ED psychology using multiple modalities and units of analysis.
4.4 |. Measurement considerations
It is also important to consider potential limitations and methodological concerns pertaining to the ambulatory assessments reviewed earlier, as well as those currently emerging in the literature, given the rapid growth in the availability of wearable sensors. Regarding ambulatory assessment of cardiac functioning, there are a range of wrist-worn devices that allow for more convenient monitoring compared to the holter monitoring approach, which is generally considered a “gold standard” method (e.g., Akintola, van de Pol, Bimmel, Maan, & van Heemst, 2016). However, studies assessing the validity and reliability of newer wrist-worn heart monitoring devices are limited and incon-clusive (e.g., Benedetto et al., 2018; Shcherbina et al., 2017; Thiebaud et al., 2018), which may be due in part to methodological variability (Sartor, Papini, Cox, & Cleland, 2018). This evidence therefore highlights the need for future research in this area.
There also remains a wide range of available accelerometer devices that vary in scoring algorithms, output parameters, as well as reliability and validity (Evenson, Goto, & Furberg, 2015; Migueles et al., 2017; Plasqui, Bonomi, & Westerterp, 2013; Sadeh, 2011). Importantly, different device specifications and the methods by which researchers collect and analyze data may have a significant impact on interpretation of results (Migueles et al., 2017). For instance, physical activity intensity, step counts, energy expenditure estimation, and sedentary behavior estimates may vary on accelerometer placement (Migueles et al., 2017). The majority of studies have indicated that hip placement results in more accurate classifications of intensity, energy expenditure, and sedentary behavior, and other studies have found substantial discrepancies in step counts depending on device placement (Migueles et al., 2017). Furthermore, while Actigraph devices have been most commonly used device in research, there is increasing utilization of a range of commercially available devices (e.g., Fitbit) in research settings (Evenson et al., 2015; Migueles et al., 2017), which may or may not converge with measurements from research-grade devices such as the Actigraph (e.g., Montgomery-Downs, Insana, & Bond, 2012; Redenius, Kim, & Byun, 2019). This underscores the need for investigators to carefully consider their decisions regarding device selection, data collection methods, and data processing criteria (e.g., device placement, epoch length, intensity classification, scoring algorithms) based on their study questions and sample (see Migueles et al., 2017 for a review of relevant issues and recommendations for Actigraph devices).
With respect to sleep assessment, evidence across studies suggests that actigraphy assessment of sleep has reasonable validity and reliability when compared with polysomnography (considered the “gold standard” of sleep assessment). However, evidence for the validity and reliability of actigraphy is strongest in estimating total sleep time among healthy individuals. Thus, actigraphy may be a somewhat less accurate and reliable tool among individuals with poor sleep quality (Sadeh, 2011). In particular, actigraphy may underestimate sleep latency and overestimate total sleep time during periods when individuals may be awake but lying down (Sadeh, 2011). Given such evidence, it is recommended to use complementary subjective assessment (e.g., self-report sleep logs and questionnaires assessing sleep times and quality) along with actigraphy data (Berry & Wagner, 2014; Sadeh, 2011). This recommendation is a particularly important in light of the observed discrepancies between objective and subjective sleep measures in the ED literature. That is, it may be that subjective sleep disturbances provide clinically useful information about sleep quality in EDs (e.g., not feeling rested) that may not always be detected via objective parameters, particularly with small samples.
4.5 |. Future directions in ambulatory assessment
4.5.1 |. Physiological assessment
While heart rate variability, sleep, and physical activity have been the primary focus of ambulatory physiological assessment in EDs, it will be particularly useful to study other aspects of physiological functioning as time-varying factors and to examine how these variables relate to the occurrence of ED behaviors and cognitions. Notably, research outside of EDs has seen tremendous growth in the use of ambulatory biosensors to assess a range of other physiological indices including cortisol, electrodermal activity, and glucose (e.g., Kleckner et al., 2018; Rodbard, 2016; Smyth, Zawadzki, Juth, & Sciamanna, 2017). Combining ambulatory assessment of such physiological indices and self-report data in EDs may be particularly useful to gain insight into both the subjective and physiological components of experiences relevant to EDs (e.g., emotion valence and arousal; Wilhelm & Grossman, 2010).
4.5.2 |. Nutrition and diet assessment
Mobile assessment of nutrition and diet is still emerging but may provide more valid and reliable data beyond traditional assessments (e.g., 24-hour recalls, food frequency questionnaires; Schembre et al., 2018). EMA has successfully been used to study diet (Schembre et al., 2018), although data on portion size and specific nutrient intake has proved difficult to capture. In order to collect more detailed information about food intake that is difficult to collect via self-report EMA, researchers have had participants take photos of their food consumed throughout the day (e.g., Costello, Deighton, Dyson, Mckenna, & Jones, 2017). In addition, new mobile and ambulatory developments have been studied to automatically detect eating, including wrist-worn bite counters (Desendorf, Bassett Jr, Raynor, & Coe, 2014; Dong, Hoover, Scisco, & Muth, 2012), ambulatory audio-based detection (Kalantarian & Sarrafzadeh, 2015), and necklaces with embedded sensors (Kalantarian, Alshurafa, Le, & Sarrafzadeh, 2015). In future ED research, these devices could be utilized in combination with EMA to detect eating and initiate participants’ self-report of food intake and ED psychopathology.
4.5.3 |. Geospatial assessment
No studies were identified that used geospatial assessment in ED research. Geospatial assessment allows for monitoring of individuals’ environment in real-time through mobile phone or smart watch via global positioning system (GPS) location information (Mennis, Mason, Ambrus, Way, & Henry, 2017). This monitoring provides information about where someone is located at a given time (e.g., at home, at a fast food restaurant) and what is around them (e.g., food outlets, parks) across the day, which can be linked to behavioral data. Integrating geospatial and EMA data will allow for elucidating where ED behaviors and cognitions typically occur for individuals and how someone’s environment interacts with risk factors to promote ED behaviors and cognitions. For example, elevated stress in food-rich environments may be more highly associated with binge eating; if such relationships are identified, interventions could be designed to assess symptoms and potentially intervene when individuals are in high-risk environments.
4.5.4 |. Neurocognition
Outside of EDs, EMA has been increasingly used to administer ambulatory neurocognitive assessments on smartphones and tablets. Ambulatory neurocognitive assessment is a novel methodology that offers unique advantages over traditional lab-based measures, as extant evidence suggests there is substantial within-person variability in neurocognitive performance (Sliwinski et al., 2018). Moreover, momentary neurocognitive assessment allows for evaluation of relationships between performance and other time-varying phenomena (Sliwinski et al., 2018), particularly affective states and physiological indices. Thus far, several studies in non-ED populations have been successful in employing mobile neurocognitive assessments (Moore, Swendsen, & Depp, 2017; Sliwinski et al., 2018). For instance, using an ambulatory inhibitory control task, Powell, McMinn, and Allan (2017) demonstrated a within-person, but not between-person, effect of inhibitory control on snacking behavior; that is, at moments when individuals exhibited poorer inhibitory control, snack consumption was higher within the subsequent hour. While a wealth of literature has indicated aberrant neurocognitive functioning in EDs (Smith et al., 2018), it is yet unclear how momentary fluctuations in neurocognitive functioning may relate to time-varying affect and ED symptoms measured in real-time. Going forward, this is a particularly important research question to address in order to better understand momentary cognitive mechanisms that may underlie and maintain ED symptoms.
4.5.5 |. Digital phenotyping
Given the recent trends toward precision medicine approaches to mental health, which aim to individualize prevention and treatment strategies, there is increasing need to develop precise, efficient measures of biomarkers that can improve the accuracy of diagnosis and predict clinical course (Insel, 2017). Digital phenotyping refers to the measurement of behavior in naturalistic settings using data from personal digital devices such as smartphones (e.g., sensors, keyboard interaction, voice/speech features; Insel, 2017; Torous, Onnela, & Keshavan, 2017). Digital phenotyping may offer a viable way to precisely capture and quantify time-varying markers of psychiatric illness that relate to observable clinical features (Insel, 2017; Torous et al., 2017). If digital phenotyping approaches are shown to improve diagnostic accuracy and prediction of clinical outcomes, it has been suggested that such technology could then enhance treatment and prevention approaches by identifying signs of risk and relapse (Insel, 2017). Thus, digital phenotyping is an example of how methods of objective data collection in naturalistic settings could be meaningfully integrated to inform classification and treatment in EDs.
Although digital phenotyping research is in its early stages, and there remain significant concerns to address regarding privacy, scope, and reproducibility, its potential is supported by extant research that has linked passively collected data to relevant clinical constructs (Torous et al., 2017). For instance, studies have examined relationships between GPS indices and depressive symptoms (Saeb, Lattie, Schueller, Kording, & Mohr, 2016), utilized automated speech analysis to predict psychosis onset (Bedi et al., 2015), and identified digital biomarkers of cognitive functioning on smartphones that correlate with standard neuropsychological assessments (Dagum, 2018). Using digital phenotyping, a range of information relevant to EDs could be passively gathered on smartphones, such as markers of interpersonal processes and social networks (e.g., phone/text communications, Bluetooth signals), mood (e.g., voice samples), neurocognition (e.g., human–computer interaction such as taps and swipes), and energy expenditure (e.g., accelerometer measures). For example, there has already been some research in EDs using linguistic analysis to assess cognitive styles (Wolf, Sedway, Bulik, & Kordy, 2007), and text and social network analyses of social media use to assess communication content and patterns (Moessner, Feldhege, Wolf, & Bauer, 2018). In sum, while much remains to be learned, digital phenotyping could help to understand and organize temporally dynamic social, cognitive, affective, physiological, and environmental processes in a way that minimizes patient burden and advances existing approaches to ED diagnosis, prevention, and treatment.
4.6 |. Ecological momentary intervention applications
Innovative ambulatory assessment methods may have particular utility for mobile health (mHealth) applications in the field of EDs, which refer to the use of smartphones and wireless technology to deliver interventions. Outcomes of internet-based self-help and therapist-supported programs, as well as text message-based systems appear to be promising in EDs, and importantly, such modalities may in part address the various barriers associated with ED treatment (e.g., stigma, cost, accessibility; Aardoom, Dingemans, & Van Furth, 2016). However, such approaches have not yet harnessed ambulatory assessment methods to automatically deliver real-time interventions based on momentary user data (Juarascio, Parker, Lagacey, & Godfrey, 2018).
Increasing research outside of EDs has been devoted to development and evaluation of ecological momentary interventions (EMIs), which refer to real-time treatments delivered in naturalistic settings that can be implemented as a stand-alone intervention or adjunct to treatment as usual (Heron & Smyth, 2010). In particular, just-in-time adaptive interventions (JITAIs) are a form of EMI that have the capability to deliver highly tailored interventions to individuals in naturalistic settings based on momentary assessment of individuals’ endogenous and environmental circumstances, which may serve to increase patient engagement and skill utilization, and ultimately improve treatment outcomes. Specifically, just-in-time adaptive interventions are designed to provide interventions at moments when individuals are vulnerable to engaging in symptoms and in contexts when they are receptive to making changes and tailor the nature of interventions (e.g., type, timing, dosage) according to time-varying information (e.g., mood, location; Nahum-Shani, Hekler, & Spruijt-Metz, 2015).
Given the ability of just-in-time adaptive interventions to provide highly tailored, real-time interventions at the particular moments when individuals most need support, such methodology could have potential to advance mHealth and EMI approaches in EDs (Juarascio et al., 2018). Furthermore, just-in-time adaptive interventions have the capacity to integrate both passive (i.e., sensor-based) and active (i.e., self-report) data collection methods to identify moments of risk and receptivity and intervene accordingly. For example, future research may be able to develop just-in-time adaptive interventions that can identify the specific combinations of time-varying internal states (e.g., level of negative affect and cravings) and environmental contexts (e.g., location, time since last eating episode) associated with ED behaviors for different individuals and prompt tailored interventions. However, there are also many unanswered questions about how to design feasible, acceptable, and impactful mHealth systems. These include identifying how best to make data collection as minimally burdensome as possible, when and how to best integrate these tools into existing treatment approaches, and what type of intervention content to provide to best change in-the-moment behavior (see Juarascio et al., 2018). Microrandomized trials have potential to inform just-in-time adaptive intervention optimization by examining causal effects of just-in-time adaptive intervention components and moderating influences of individual and contextual factors (for a review see Klasnja et al., 2015), though no such studies have been conducted in EDs. Despite these unanswered questions and substantial need for additional research, the increasing sophistication and widespread use of mHealth technologies clearly offers many promising avenues to explore the potential of ambulatory assessment to augment treatment.
4.7 |. Discussion and implications for the ED field
The use of mobile and ecologically valid assessments in the natural environment has significantly contributed to the ED literature. In particular, gaining a greater understanding of the role of momentary causal factors for ED behavior has been illuminated by these methods. More broadly, they help us to understand that individuals with EDs are not fixed, static phenotypes, but they are people with dynamic and changing life circumstances, which influence the experience of their disorder. This area of research in EDs could be greatly enhanced through the use of innovative methodologies, which allow us to examine the behavioral and biological processes underlying these disorders through ambulatory assessment techniques other than self-report, as well as the administration of neurocognitive behavioral tasks in real-time in the natural environment. Also, the ability to use geo-spatial methods to more deeply and significantly understand the environmental contexts in which our patients live, and how these contexts influence their disorders, is remarkably innovative. However, each of these ambulatory methods of assessment is currently limited by a relative absence of significant empirical testing. Going forward, it is imperative for researchers to carefully consider methodological issues (i.e., sample size, reliability, and validity of various ambulatory assessment devices) to ensure that resulting findings can meaningfully advance this field.
Through more advanced empirical studies of these momentary bio-behavioral constructs, we may gain a much deeper understanding of the factors that influence individuals with EDs in moments of significant risk. As these measurement systems are empirically clarified, EMI and just-in-time adaptive intervention strategies will be significantly enhanced. Improved measurement of these fundamental bio-behavioral constructs in a momentary fashion could significantly help us to identify exactly when our patients are at greatest risk and respond accordingly. Finally, the interface between these technological interventions and traditional interventions mediated by human relationships (both face-to-face interactions and technologyfacilitated communications) is a topic of great relevance for the future, as the possibility of integrating ambulatory assessment mHealth approaches in ED treatment raises numerous practical and ethical considerations. For instance, researchers, clinicians, and application developers will need to resolve issues regarding data privacy and management, how to minimize participant burden with passive and active ambulatory data collection methods, and how to make ambulatory assessment feasible and meaningful in the context of routine clinical care (Carpenter, Wycoff, & Trull, 2016). Furthermore, it is important to note there has been a rapid emergence of smartphone applications in other areas (e.g., physical activity) that lack sufficient empirical evidence and theoretical basis (Lewis, Napolitano, Buman, Williams, & Nigg, 2017). If such trends emerge in the ED field, this will bring about increasing need for critical evaluation of these modalities and how to disseminate research findings to patients and clinicians. Lastly, examination of whether mobile intervention technologies can stand alone, or are best construed as extensions of traditional treatment methodologies is an important topic for future research.
Supplementary Material
Footnotes
SUPPORTING INF ORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Aardoom JJ, Dingemans AE, & Van Furth EF. (2016). E-health interventions for eating disorders: Emerging findings, issues, and opportunities. Current Psychiatry Reports, 18(4), 42. [DOI] [PubMed] [Google Scholar]
- Akintola AA, van de Pol V, Bimmel D, Maan AC, & van Heemst D. (2016). Comparative analysis of the equivital EQ02 lifemonitor with holter ambulatory ECG device for continuous measurement of ECG, heart rate, and heart rate variability: A validation study for precision and accuracy. Frontiers in Physiology, 7, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, & Schweizer S. (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. [DOI] [PubMed] [Google Scholar]
- American Heart Association.(2019). Holter Monitor. Retrieved from: https://www.heart.org/en/health-topics/heart-attack/diagnosing-a-heart-attack/holter-monitor.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Aspen V, Darcy AM, & Lock J. (2013). A review of attention biases in women with eating disorders. Cognition & Emotion, 27(5), 820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, & Thayer JF. (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. [DOI] [PubMed] [Google Scholar]
- Bedi G, Carrillo F, Cecchi GA, Slezak DF, Sigman M, Mota NB, Corcoran CM. (2015). Automated analysis of free speech predicts psychosis onset in high-risk youths. Npj Schizophrenia, 1, 15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto S, Caldato C, Bazzan E, Greenwood DC, Pensabene V, & Actis P. (2018). Assessment of the Fitbit Charge 2 for monitoring heart rate. PLoS One, 13(2), e0192691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KC, Cao L, Crosby RD, Engel SG, Peterson CB, Crow SJ, Wonderlich SA. (2017). Negative affect and binge eating: Reconciling differences between two analytic approaches in ecological momentary assessment research. International Journal of Eating Disorders, 50(10), 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry RB, & Wagner MH. (2014). Sleep medicine pearls E-book. Elsevier Health Sciences. [Google Scholar]
- Blanchet C, Mathieu MÈ, St-Laurent A, Fecteau S, St-Amour N, & Drapeau V. (2018). A systematic review of physical activity interventions in individuals with binge eating disorders. Current Obesity Reports, 7(1), 76–88. [DOI] [PubMed] [Google Scholar]
- Bomba M, Corbetta F, Gambera A, Nicosia F, Bonini L, Neri F, Nacinovich R. (2014). Heart rate variability in adolescents with functional hypothalamic amenorrhea and anorexia nervosa. Psychiatry Research, 215(2), 406–409. [DOI] [PubMed] [Google Scholar]
- Bouten CV, & Westerterp KR. (1996). Body mass index and daily physical activity in anorexia nervosa. Medicine and Science in Sports and Exercise, 28(8), 967–973. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, & Andreski P. (1996). Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biological Psychiatry, 39(6), 411–418. [DOI] [PubMed] [Google Scholar]
- Carpenter RW, Wycoff AM, & Trull TJ. (2016). Ambulatory assessment: New adventures in characterizing dynamic processes. Assessment, 23(4), 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, & Chekroud AM. (2018). Association between physical exercise and mental health in 1· 2 million individuals in the USA between 2011 and 2015: A cross-sectional study. The Lancet Psychiatry, 5(9), 739–746. [DOI] [PubMed] [Google Scholar]
- Costello N, Deighton K, Dyson J, Mckenna J, & Jones B. (2017). Snap-N-send: A valid and reliable method for assessing the energy intake of elite adolescent athletes. European Journal of Sport Science, 17(8), 1044–1055. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, & Hunter J. (2003). Happiness in everyday life: The uses of experience sampling. Journal of Happiness Studies, 4(2), 185–199. [Google Scholar]
- Dagum P. (2018). Digital biomarkers of cognitive function. Npj Digital Medicine, 1(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K,… Kaplan AS. (1997). The prevalence of high-level exercise in the eating disorders: Etiological implications. Comprehensive Psychiatry, 38(6), 321–326. [DOI] [PubMed] [Google Scholar]
- Dellava JE, Hamer RM, Kanodia A, Reyes-Rodríguez ML, & Bulik CM. (2011). Diet and physical activity in women recovered from anorexia nervosa: A pilot study. International Journal of Eating Disorders, 44(4), 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desendorf J, Bassett DR Jr., Raynor HA, & Coe DP. (2014). Validity of the bite counter device in a controlled laboratory setting. Eating Behaviors, 15(3), 502–504. [DOI] [PubMed] [Google Scholar]
- Dong Y, Hoover A, Scisco J, & Muth E. (2012). A new method for measuring meal intake in humans via automated wrist motion tracking. Applied Psychophysiology and Biofeedback, 37(3), 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SG, Crosby RD, Thomas G, Bond D, Lavender JM, Mason T, Wonderlich SA. (2016). Ecological momentary assessment in eating disorder and obesity research: A review of the recent literature. Current Psychiatry Reports, 18(4), 37. [DOI] [PubMed] [Google Scholar]
- Engel SG, Wonderlich SA, Crosby RD, Mitchell JE, Crow S, Peterson CB, … Gordon KH. (2013). The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. Journal of Abnormal Psychology, 122(3), 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Goto MM, & Furberg RD. (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers. International Journal of Behavioral Nutrition and Physical Activity, 12 (1), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, & Shafran R. (2003). Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behaviour Research and Therapy, 41(5), 509–528. [DOI] [PubMed] [Google Scholar]
- Force T. (1996). Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93, 1043–1065. [PubMed] [Google Scholar]
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, & Pentimone F. (2003). Heart rate variability and left ventricular diastolic function in anorexia nervosa. Journal of Adolescent Health, 32(6), 416–421. [DOI] [PubMed] [Google Scholar]
- Geisler FCM, Kubiak T, Siewert K, & Hannelore Weber KS (2013). Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology, 93(2), 279–286. [DOI] [PubMed] [Google Scholar]
- Gianini LM, Klein DA, Call C, Walsh BT, Wang Y, Wu P, & Attia E. (2016). Physical activity and post-treatment weight trajectory in anorexia nervosa. International Journal of Eating Disorders, 49(5), 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK. (2011). Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler T, Rieger E, Touyz S, Beumont P, Plasqui G, & Westerterp K. (2008). Physical activity and body composition in outpatients recovering from anorexia nervosa and healthy controls. Adapted Physical Activity Quarterly, 25(2), 159–173. [DOI] [PubMed] [Google Scholar]
- Heron KE, & Smyth JM. (2010). Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. British Journal of Health Psychology, 15(1), 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, & Tuschen-Caffier B. (2007). Maintenance of binge eating through negative mood: A naturalistic comparison of binge eating disorder and bulimia nervosa. International Journal of Eating Disorders, 40 (6), 521–530. [DOI] [PubMed] [Google Scholar]
- Holmes M, Fuller-Tyszkiewicz M, Skouteris H, & Broadbent J. (2014). Tests of an extension of the dual pathway model of bulimic symptoms to the state-based level. Eating Behaviors, 15(2), 280–285. [DOI] [PubMed] [Google Scholar]
- Hovland A, Pallesen S, Hammar Å, Hansen AL, Thayer JF, Tarvainen MP, & Nordhus IH. (2012). The relationships among heart rate variability, executive functions, and clinical variables in patients with panic disorder. International Journal of Psychophysiology, 86(3), 269–275. [DOI] [PubMed] [Google Scholar]
- Insel TR. (2017). Digital phenotyping: Technology for a new science of behavior. JAMA, 318(13), 1215–1216. [DOI] [PubMed] [Google Scholar]
- Joseph RJ, Alonso-Alonso M, Bond DS, Pascual-Leone A, & Blackburn GL. (2011). The neurocognitive connection between physical activity and eating behaviour. Obesity Reviews, 12(10), 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarascio AS, Parker MN, Lagacey MA, & Godfrey KM. (2018). Just-in-time adaptive interventions: A novel approach for enhancing skill utilization and acquisition in cognitive behavioral therapy for eating disorders. International Journal of Eating Disorders, 51(8), 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantarian H, Alshurafa N, Le T, & Sarrafzadeh M. (2015). Monitoring eating habits using a piezoelectric sensor-based necklace. Computers in Biology and Medicine, 58, 46–55. [DOI] [PubMed] [Google Scholar]
- Kalantarian H, & Sarrafzadeh M. (2015). Audio-based detection and evaluation of eating behavior using the smartwatch platform. Computers in Biology and Medicine, 65, 1–9. [DOI] [PubMed] [Google Scholar]
- Karr TM, Cook B, Zunker C, Cao L, Crosby RD, Wonderlich SA, & Mitchell JE. (2017). Examining physical activity and affect using objective measures: A pilot study of anorexia nervosa. The Sport Journal, 20. [Google Scholar]
- Kay M, Santos J, & Takane M. (2011). mHealth: New horizons for health through mobile technologies. World Health Organization, 64(7), 66–71. [Google Scholar]
- Kemp AH, Quintana DS, Quinn CR, Hopkinson P, & Harris AW. (2014). Major depressive disorder with melancholia displays robust alterations in resting state heart rate and its variability: Implications for future morbidity and mortality. Frontiers in Psychology, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes A, Woerwag-Mehta S, Bartholdy S, Koskina A, Middleton B, Connan F, … Campbell IC. (2015). Physical activity and the drive to exercise in anorexia nervosa. International Journal of Eating Disorders, 48(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Klasnja P, Hekler EB, Shiffman S, Boruvka A, Almirall D, Tewari A, & Murphy SA. (2015). Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychology, 34(S), 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Jones RM, Wilder-Smith O, Wormwood JB, Akcakaya M, Quigley KS, Goodwin MS. (2018). Simple, transparent, and flexible automated quality assessment procedures for ambulatory electrodermal activity data. IEEE Transactions on Biomedical Engineering, 65(7), 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockler T, Santangelo P, & Ebner-Priemer U. (2018). Investigating binge eating using ecological momentary assessment: The importance of an appropriate sampling frequency. Nutrients, 10(1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzewa E, van Elburg AA, Sanders N, Sternheim L, Adan RA, & Kas MJ. (2013). Longitudinal changes in the physical activity of adolescents with anorexia nervosa and their influence on body composition and leptin serum levels after recovery. PLoS One, 8(10), e78251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzer Y, Tzischinsky O, & Epstein R. (2001). Sleep-wake monitoring in women suffering from anorexia nervosa. Eating Disorders, 9(2), 159–166. [DOI] [PubMed] [Google Scholar]
- Latzer Y, Tzischinsky O, Epstein R, Klein E, & Peretz L. (1999). Naturalistic sleep monitoring in women suffering from bulimia nervosa. International Journal of Eating Disorders, 26(3), 315–321. [DOI] [PubMed] [Google Scholar]
- Lavender JM, De Young KP, Wonderlich SA, Crosby RD, Engel SG, Mitchell JE, Le Grange D. (2013). Daily patterns of anxiety in anorexia nervosa: Associations with eating disorder behaviors in the natural environment. Journal of Abnormal Psychology, 122 (3), 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahey TM, Crowther JH, & Ciesla JA. (2011). An ecological momentary assessment of the effects of weight and shape social comparisons on women with eating pathology, high body dissatisfaction, and low body dissatisfaction. Behavior Therapy, 42(2), 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BA, Napolitano MA, Buman MP, Williams DM, & Nigg CR. (2017). Future directions in physical activity intervention research: Expanding our focus to sedentary behaviors, technology, and dissemination. Journal of Behavioral Medicine, 40(1), 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Shonkoff ET, & Dunton GF. (2015). The acute relationships between affect, physical feeling states, and physical activity in daily life: A review of current evidence. Frontiers in Psychology, 6, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Skelton K, Dunton G, & Bruening M. (2016). A systematic review of methods and procedures used in ecological momentary assessments of diet and physical activity research in youth: An adapted STROBE checklist for reporting EMA studies (CREMAS). Journal of Medical Internet Research, 18(6), e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TB, Pacanowski CR, Lavender JM, Crosby RD, Wonderlich SA, Engel SG, … Peterson CB. (2017). Evaluating the ecological validity of the Dutch eating behavior questionnaire among obese adults using ecological momentary. Assessment, 26, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TB, Smith KE, Crosby RD, Wonderlich SA, Crow SJ, Engel SG, & Peterson CB. (2017). Does the eating disorder examination questionnaire global subscale adequately predict eating disorder psychopathology in the daily life of obese adults? Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 23, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason TB, Wonderlich SA, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Peterson CB. (2018). Associations among eating disorder behaviors and eating disorder quality of life in adult women with anorexia nervosa. Psychiatry Research, 267, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurak N, Enck P, Muth E, Teufel M, & Zipfel S. (2011). Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: A review of the literature. European Eating Disorders Review, 19(2), 87–99. [DOI] [PubMed] [Google Scholar]
- Melanson EL, Donahoo WT, Krantz MJ, Poirier P, & Mehler PS. (2004). Resting and ambulatory heart rate variability in chronic anorexia nervosa. The American Journal of Cardiology, 94(9), 1217–1220. [DOI] [PubMed] [Google Scholar]
- Mennis J, Mason M, Ambrus A, Way T, & Henry K. (2017). The spatial accuracy of geographic ecological momentary assessment (GEMA): Error and bias due to subject and environmental characteristics. Drug and Alcohol Dependence, 178, 188–193. [DOI] [PubMed] [Google Scholar]
- Migueles JH, Cadenas-Sanchez C, Ekelund U, Nyström CD, Mora-Gonzalez J, Löf M, … Ortega FB. (2017). Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sports Medicine, 47(9), 1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, … Parati G. (2016). Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PLoS One, 11, e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessner M, Feldhege J, Wolf M, & Bauer S. (2018). Analyzing big data in social media: Text and network analyses of an eating disorder forum. International Journal of Eating Disorders, 51(7), 656–667. [DOI] [PubMed] [Google Scholar]
- Mont L, Castro J, Herreros B, Paré C, Azqueta M, Magriña J, … Brugada J. (2003). Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. Journal of the American Academy of Child & Adolescent Psychiatry, 42(7), 808–813. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, & Bond JA. (2012). Movement toward a novel activity monitoring device. Sleep and Breathing, 16(3), 913–917. [DOI] [PubMed] [Google Scholar]
- Moore RC, Swendsen J, & Depp CA. (2017). Applications for self-administered mobile cognitive assessments in clinical research: A systematic review. International Journal of Methods in Psychiatric Research, 26(4), e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TA, Ridolfi DR, & Crowther JH. (2015). Reaction times to appearance-related or non-appearance-related word choice among women with and without eating psychopathology. Cognitive Therapy and Research, 39(2), 204–214. [Google Scholar]
- Nahum-Shani I, Hekler EB, & Spruijt-Metz D. (2015). Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychology, 34(S), 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. (2014). Quality assessment tool for observational cohort and cross-sectional studies. National Heart, Lung, and Blood Institute. Retrieved from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- O’Brien E, Hart C, & Wing RR. (2016). Discrepancies between self-reported usual sleep duration and objective measures of total sleep time in treatment-seeking overweight and obese individuals. Behavioral Sleep Medicine, 14(5), 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CA, & Alfano CA. (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Medicine Reviews, 31, 6–16. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, & Dahn JR. (2005). Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry, 18(2), 189–193. [DOI] [PubMed] [Google Scholar]
- Peschel SK, Feeling NR, Vögele C, Kaess M, Thayer JF, & Koenig J. (2016). A meta-analysis on resting state high-frequency heart rate variability in bulimia nervosa. European Eating Disorders Review, 24(5), 355–365. [DOI] [PubMed] [Google Scholar]
- Petretta M, Bonaduce D, Themistoclakis S, Ianniciello A, Scalfi L, De Filippo E, … Migaux ML. (1997). Heart rate variability as a measure of autonomic nervous system function in anorexia nervosa. Clinical Cardiology, 20(3), 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasqui G, Bonomi A, & Westerterp KR. (2013). Daily physical activity assessment with accelerometers: New insights and validation studies. Obesity Reviews, 14(6), 451–462. [DOI] [PubMed] [Google Scholar]
- Potvin S, Charbonneau G, Juster RP, Purdon S, & Tourjman SV. (2016). Self-evaluation and objective assessment of cognition in major depression and attention deficit disorder: Implications for clinical practice. Comprehensive Psychiatry, 70, 53–64. [DOI] [PubMed] [Google Scholar]
- Powell DJ, McMinn D, & Allan JL. (2017). Does real time variability in inhibitory control drive snacking behavior? An intensive longitudinal study. Health Psychology, 36(4), 356–364. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, & Tremblay M. (2008). A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 5 (1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer LM, Engel SG, Crosby RD, Anderson M, Vannucci A, Cohen LA, … Tanofsky-Kraff M. (2014). Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. International Journal of Eating Disorders, 47(7), 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer LM, Engel SG, Crosby RD, Haigney M, Anderson M, McCaffery JM, & Tanofsky-Kraff M. (2016). Real-time assessment of heart rate variability and loss of control eating in adolescent girls: A pilot study. International Journal of Eating Disorders, 49(2), 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redenius N, Kim Y, & Byun W. (2019). Concurrent validity of the Fitbit for assessing sedentary behavior and moderate-to-vigorous physical activity. BMC Medical Research Methodology, 19(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie L, Fobian AD, McCall WV, & Khazaie H. (2018). Paradoxical insomnia and subjective–objective sleep discrepancy: A review. Sleep Medicine Reviews, 40, 196–202. [DOI] [PubMed] [Google Scholar]
- Rodbard D. (2016). Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technology & Therapeutics, 18(S2), S2–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A. (2011). The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews, 15(4), 259–267. [DOI] [PubMed] [Google Scholar]
- Saeb S, Lattie EG, Schueller SM, Kording KP, & Mohr DC. (2016). The relationship between mobile phone location sensor data and depressive symptom severity. PeerJ, 4, e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Brosof LC, & Levinson CA. (2019). Repetitive negative thinking predicts eating disorder behaviors: A pilot ecological momentary assessment study in a treatment seeking eating disorder sample. Behaviour Research and Therapy, 112, 12–17. [DOI] [PubMed] [Google Scholar]
- Santerre C, & Allen JJ. (2007). Methods for studying the psychophysiology of emotion In Emotion and psychopathology: Bridging affective and clinical science (pp. 53–79). Washington, DC: American Psychological Association. [Google Scholar]
- Sartor F, Papini G, Cox LGE, & Cleland J. (2018). Methodological shortcomings of wrist-worn heart rate monitors validations. Journal of Medical Internet Research, 20(7), e10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembre SM, Liao Y, O’Connor SG, Hingle MD, Shen SE, Hamoy KG, … Boushey CJ. (2018). Mobile ecological momentary diet assessment methods for behavioral research: Systematic review. JMIR mHealth and uHealth, 6(11), e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber-Gregory DN, Lavender JM, Engel SG, Wonderlich SA, Crosby RD, Peterson CB, … Mitchell JE. (2013). Examining duration of binge eating episodes in binge eating disorder. International Journal of Eating Disorders, 46(8), 810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N, & Sudman S. (1994). Autobiographical memory and the validity of retrospective reports. New York: Springer Verlag. [Google Scholar]
- Shcherbina A, Mattsson C, Waggott D, Salisbury H, Christle J, Hastie T, … Ashley E. (2017). Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. Journal of Personalized Medicine, 7(2), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR. (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, & Lipton RB. (2018). Reliability and validity of ambulatory cognitive assessments. Assessment, 25(1), 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Johnson JS, Lavender JM, & Wonderlich SA. (2018). A systematic review of reviews of neurocognitive functioning in eating disorders: The state-of-the-literature and future directions. International Journal of Eating Disorders, 51(8), 798–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, & Lavender JM. (2018). Rumination and eating disorder psychopathology: A meta-analysis. Clinical Psychology Review, 61, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, & Engel SG. (2007). Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology, 75(4), 629–638. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Sliwinski MJ, Crosby RD, Engel SG, Mitchell JE, & Calogero RM. (2009). Ecological momentary assessment of affect, stress, and binge-purge behaviors: Day of week and time of day effects in the natural environment. International Journal of Eating Disorders, 42(5), 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Zawadzki MJ, Juth V, & Sciamanna CN. (2017). Global life satisfaction predicts ambulatory affect, stress, and cortisol in daily life in working adults. Journal of Behavioral Medicine, 40(2), 320–331. [DOI] [PubMed] [Google Scholar]
- Society for Ambulatory Assessment. (2019). Society for Ambulatory Assessment. Retrieved from: http://ambulatory-assessment.org.
- Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, & Wilfley DE. (2007). What’s driving the binge in binge eating disorder? A prospective examination of precursors and consequences. International Journal of Eating Disorders, 40(3), 195–203. [DOI] [PubMed] [Google Scholar]
- Stojek M, Shank LM, Vannucci A, Bongiorno DM, Nelson EE, Waters AJ, … Tanofsky-Kraff M. (2018). A systematic review of attentional biases in disorders involving binge eating. Appetite, 123, 367–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, & Shiffman S. (1994). Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine, 16, 199–202. [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, & Hufford MR. (2002). Patient non-compliance with paper diaries. BMJ, 324(7347), 1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61 (3), 201–216. [DOI] [PubMed] [Google Scholar]
- Thiebaud RS, Funk MD, Patton JC, Massey BL, Shay TE, Schmidt MG, & Giovannitti N. (2018). Validity of wrist-worn consumer products to measure heart rate and energy expenditure. Digital Health, 4, 2055207618770322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torous J, Onnela JP, & Keshavan M. (2017). New dimensions and new tools to realize the potential of RDoC: Digital phenotyping via smartphones and connected devices. Translational Psychiatry, 7(3), e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzischinsky O, & Latzer Y. (2004). Nocturnal eating: Prevalence, features and night sleep among binge eating disorder and bulimia nervosa patients in Israel. European Eating Disorders Review: The Professional Journal of the Eating Disorders Association, 12(2), 101–109. [Google Scholar]
- Tzischinsky O, & Latzer Y. (2006). Sleep-wake cycles in obese adolescents with and without binge eating episodes. European Eating Disorders Review: The Professional Journal of the Eating Disorders Association, 14(2), 111–117. [Google Scholar]
- Verbugge LM. (1980). Health diaries. Medical Care, 18, 110–116. [DOI] [PubMed] [Google Scholar]
- Vitousek KB, & Hollon SD. (1990). The investigation of schematic content and processing in eating disorders. Cognitive Therapy and Research, 14(2), 191–214. [Google Scholar]
- Wilhelm FH, & Grossman P. (2010). Emotions beyond the laboratory: Theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. Biological Psychology, 84(3), 552–569. [DOI] [PubMed] [Google Scholar]
- Wolf M, Sedway J, Bulik CM, & Kordy H. (2007). Linguistic analyses of natural written language: Unobtrusive assessment of cognitive style in eating disorders. International Journal of Eating Disorders, 40(8), 711–717. [DOI] [PubMed] [Google Scholar]
- Wonderlich JA, Lavender JM, Wonderlich SA, Peterson CB, Crow SJ, Engel SG, … Crosby RD. (2015). Examining convergence of retrospective and ecological momentary assessment measures of negative affect and eating disorder behaviors. International Journal of Eating Disorders, 48(3), 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich SA, Engel SG, Peterson CB, Robinson MD, Crosby RD, Mitchell JE, … Strauman TJ. (2008). Examining the conceptual model of integrative cognitive-affective therapy for BN: Two assessment studies. International Journal of Eating Disorders, 41(8), 748–754. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Peterson CB, & Smith TL. (2015). Integrative cognitive-affective therapy for bulimia nervosa: A treatment manual. New York: Guilford Publications. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.