Abstract
Cholangiocarcinoma is a malignancy arising from the epithelial lining of the intrahepatic or extrahepatic biliary tract. Timely diagnosis is challenging due to its silent clinical course. As reliable laboratory markers are lacking, diagnostic imaging plays a pivotal role. While cross-sectional imaging studies are usually conclusive for intrahepatic lesions, endoscopy plays an essential role in cases of extrahepatic tumors. Rational utilization of different diagnostic methods based on available evidence is needed. This article focuses on the diagnostic role of advanced biliary endoscopy, including endoscopic retrograde cholangiopancreatography, cholangioscopy, endoscopic ultrasonography, and intraductal sonography.
1. Introduction
A timely diagnosis of cholangiocarcinoma remains challenging due to its silent clinical course. As reliable laboratory markers are lacking, diagnostic imaging plays a pivotal role. While cross-sectional imaging studies are usually conclusive for intrahepatic lesions, endoscopy plays an essential role in cases of extrahepatic tumors. Nevertheless, tissue specimen is usually necessary in both locations. This article addresses cholangiocarcinoma from the perspective of endoscopic imaging and tissue sampling.
2. Epidemiology
CCA is a malignancy arising from the epithelial lining of the intrahepatic or extrahepatic biliary tract, excluding the gallbladder, cystic duct, and ampulla of Vater. It accounts for 2% of all human malignancies and is the second most common primary hepatic malignancy following hepatocellular carcinoma.
The highest global incidence of CCA is in north-east Thailand where infestation by the liver fluke Opisthorchis viverrini is endemic. In this geographic area, age-standardized incidence rates of approximately 100 per 100,000 individuals among men and 50 per 100,000 individuals among women have been reported, while it ranges between 0.5 and 2.0 per 100,000 individuals in the Western world [1, 2].
As the incidence of CCA increases with age, the median age at diagnosis is 65-68 years. Sex- and race-specific disparities have also been noted. In the USA, males and specifically Asians are affected more frequently than females and non-Hispanic whites [3].
Worldwide time trends in mortality for CCA are changing. Multiple studies have reported that the incidence of ICCA has increased by up to tenfold in high-income countries, while the incidence of ECCA has decreased at a similar rate around the turn of the 20th century. Nevertheless, it has also been suggested that this shift may have been influenced by implementing different classification systems [4, 5].
3. Prognosis and Treatment
The prognosis of biliary malignancies is dismal with the overall five-year survival as low as 10%. Radical surgery remains the only option for curative therapy offering a median disease-free survival of 12-36 months. However, due to the silent clinical course, two-thirds of CCAs are diagnosed at an inoperable stage. The natural course without chemotherapy or radiotherapy leads to overall survival of 3.9 months only. If palliative chemotherapy is used, median survival can be prolonged up to 12-15 months [6, 7]. After resection, negative margin (R0 resection) is the most important variable associated with long-term survival [8]. Recently, an excellent five-year survival of 65% in patients with “very early” perihilar cholangiocarcinoma was demonstrated after neoadjuvant chemoradiation followed by liver transplantation [9].
4. Etiopathogenesis
While the etiopathogenesis remains unknown, the development of biliary cancer is linked to a wide spectrum of conditions causing biliary inflammation, cholestasis, and inflammation of the liver. Different precancerous conditions and recommendations for the follow-up of patients with increased risk are described in detail elsewhere. Despite increasing knowledge within this field, the diagnosis of CCA at an early stage remains a challenge since the majority of cases are sporadic, affecting patients without the presence of any known risk factor [10].
5. Diagnostic Approach
The clinical presentation of CCA depends on tumor location. The typical triad of ECCA consists of symptoms of biliary obstruction, right upper quadrant pain, and weight loss. However, patients with jaundice have mostly advanced disease already. Therefore, making a diagnosis of ECCA in a nonjaundiced person remains a crucial issue. It has been shown by Sugiyama et al. that abnormalities in hepatic function and tumor marker tests combined with transabdominal ultrasonography (US) can be used for early diagnosis of ECCA in patients without jaundice, with resulting resectability of 83% and survival of 50% at 5 years [11].
A reliable laboratory test for CCA is still lacking. Extrahepatic cholestasis is reflected in elevated conjugated bilirubin, alkaline phosphatase, and gamma-glutamyltransferase levels. As for “tumor markers,” it is widely accepted that testing for the carbohydrate antigen (CA) 19-9 is useful in diagnostics, as a prognostic factor and indicator of tumor resectability. However, the levels of CA 19-9 can also increase in other hepatobiliary conditions, including cholangitis. On the other hand, about 4-14% of the population with Lewis-negative phenotype are not able to secrete CA 19-9 even when malignant tumor is present [12].
Due to the unspecific clinical presentation and the absence of accurate laboratory tests, the diagnosis of CCA depends mainly on imaging with or without tissue sampling. For ICCA, cross-sectional imaging methods are mostly conclusive. On the other hand, the role of advanced pancreatobiliary endoscopy is essential in the cases of ECCA, as will be discussed further in this text.
6. Classifications
Most classifications of CCA were developed to guide surgical and oncological treatment. Nonetheless, they can also be used for tailoring palliative treatment including endoscopic or percutaneous drainage and locoregional therapy.
As with most other tumors, CCA can be staged according to the tumor, node, metastasis (TNM) system [13]. Yet, the TNM system is unable to provide the information necessary for an assessment of surgical resectability in the case of ECCA. Therefore, additional staging systems based on anatomical, pathological, and surgical characteristics are also used [14].
According to the anatomical location, CCA can be divided into ICCA and ECCA with the anatomical border at the level of second-order bile ducts. As for ECCA, it can be subdivided into PCCA and DCCA, two entities anatomically separated by the insertion of the cystic duct into the common bile duct. From all CCAs, the proportion of PCCA, DCCA, and ICCA is 50-60%, 20%, and 20%, respectively [15] (Figure 1).
Figure 1.
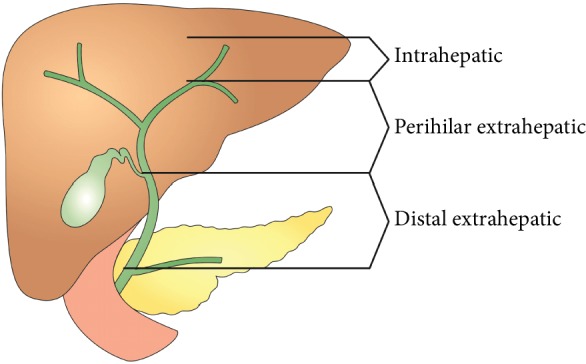
Classification of cholangiocarcinoma according to the anatomical location.
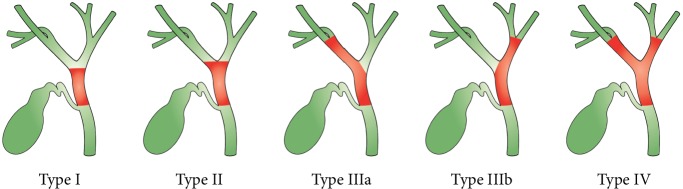
PCCA, usually referred to as a Klatskin tumor, can be further described according to the Bismuth and Corlette classification as types I-IV: type I involves the common hepatic duct below the confluence of the right and left hepatic ducts, type II involves the confluence, type IIIa involves the confluence while extending into the right hepatic duct, type IIIb involves the confluence while extending into the left hepatic duct, and type IV involves the confluence while extending into both the right and left hepatic ducts, or it is multifocal [16, 17] (Figure 2).
Figure 2.
Classification of perihilar carcinoma according to Bismuth and Corlette.
As for macroscopic morphology, three types of CCA have been described by the Liver Cancer Study Group of Japan including (1) periductal-infiltrating, (2) intraductal-papillary, and (3) exophytic or mass-forming types. The American Joint Committee for Cancer also recognizes mixed periductal-infiltrating and intraductal-growing types. The periductal infiltrative type is the most common, representing 70% of cases [18] (Figure 3).
Figure 3.
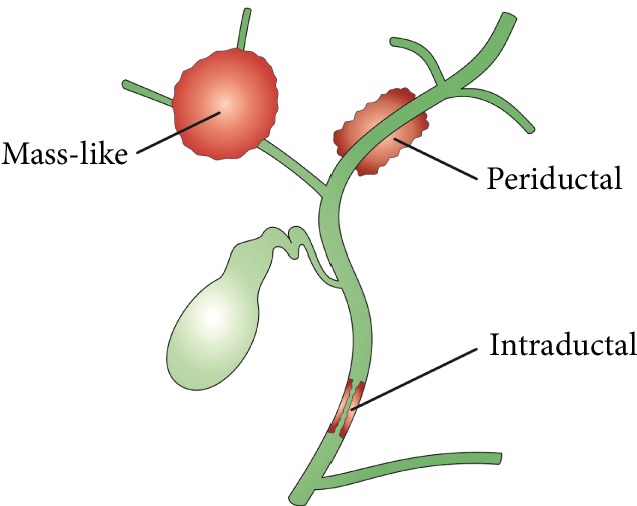
Classification of cholangiocarcinoma carcinoma according to the macroscopic morphology.
It has been shown that the type of tumor spread correlates with the morphology growth pattern. While mucosal extension is predominantly seen with intraductal-papillary and mass-forming (nodular) tumors, submucosal extension is mainly seen with periductal-infiltrating types. Generally, the extension occurs more frequently to the hepatic side, where it also tends to reach further [19, 20].
Histologically, 90% of CCAs are adenocarcinomas that are well, moderately, or poorly differentiated. They consist of either cylindrical mucin-producing glands or cuboidal non-mucin-producing cells. All types of CCAs are associated with the rapid proliferation of tumor-associated stroma cells that contribute to desmoplasia.
Recently, a new staging system integrating important anatomic, pathologic, and surgical features of PCCA has been suggested by a group of international experts. Attempting to reflect a unique complexity of this tumor, it employs eight characteristics for staging: tumor extent, tumor size, tumor growth type, vascular involvement, hepatic lobar atrophy, underlying hepatic disease, lymph node metastases, and distant metastases [21].
7. Cross-Sectional Imaging
Cross-sectional imaging involves any technique that produces an image in the form of a plane through the body with the structures cut across.
7.1. Transabdominal Ultrasonography
Transabdominal ultrasonography (US) is frequently used as a first-line imaging test in jaundiced patients. While ICCA presents as a hypoechoic mass, the direct visualization of ECCA on US is usually impossible. In one study, the sensitivities of US in demonstrating hilar tumor, middle bile duct tumor, and distal bile duct tumor were 86%, 59%, and 33%, respectively [22]. US has nevertheless proven to be useful in detecting biliary tract dilation, level of obstruction, and the presence of gallstones.
7.2. Computed Tomography/Magnetic Resonance Imaging (PET/CT)
Computed tomography (CT) is performed in the vast majority of CCAs. This is not the place to discuss the role of CT in the diagnosis of ICCA. The performance of a CT scan as a diagnostic tool for PCCA was evaluated in a meta-analysis of 16 studies demonstrating an accuracy of 86% for the ductal extent of the tumor. Sensitivity and specificity were 89% and 92% for the evaluation of portal vein involvement, 83% and 93% for hepatic artery involvement, and 61% and 88% for lymph node involvement, respectively [23]. As for the PCCA resectability assessment, another meta-analysis calculated the pooled sensitivities of CT, MRI, and PET/CT to be 95%, 94%, and 91%, respectively, with corresponding specificities of 69%, 71%, and 81%. As concluded by the authors, CT is the most frequently used modality. The diagnostic performance of MRI proved to be generally comparable with CT, while PET/CT appears to be the best method in detecting lymph node and distant metastasis [24].
8. The Role of Endoscopy
8.1. Endoscopic Retrograde Cholangiopancreatography (ERCP)
Since its introduction in 1968, ERCP has been widely used for imaging of the biliary tree and is still considered the gold standard of biliary imaging. Nevertheless, ERCP remains an invasive procedure with considerable risks. As reported by Cotton et al., unavoidable overall, severe, and lethal complication rates of 4%, 0.7%, and 0.06%, respectively, must be expected even in the hands of experts [25].
The most important ERCP-related complication is pancreatitis occurring in 2.6% of patients followed by ascending cholangitis. Although several preventive measures have proven effective in post-ERCP pancreatitis prophylaxis, including the administration of certain drugs, guidewire cannulation technique, vigorous intravenous hydration, and pancreatic duct stenting, the exclusion of patients in whom ERCP is unnecessary remains a crucial point [26].
In expert centers, ERCP has been replaced by less-invasive diagnostic modalities such as CT, MRCP, and EUS at the position of the first-line imaging method. It has been shown that MRCP in combination with MRI has a comparable diagnostic accuracy with direct (ERCP or percutaneous transhepatic) cholangiography combined with CT in the detection and staging of ECCA [27]. However, ERCP is still being widely utilized for transpapillary tissue sampling. Moreover, the availability of MRI and EUS is not universal, and the position of ERCP in the diagnostic algorithm for ECCA may, therefore, vary according to the level of local expertise [28].
In ERCP, CCA presents as a stricture or a filling defect with or without upstream dilation of the biliary tract. Malignant etiology is suggested in a long (≥10 mm), asymmetric, and irregular stricture opposed to a short, regular, and symmetric stricture, which is typical for benign disease. As shown by Park et al., using these criteria, the diagnostic sensitivity and specificity for ERCP were 74% and 70%, respectively [29].
When necessary, material for histopathology examination can be obtained by one of 3 different approaches during ERCP: (1) bile aspiration, (2) brush cytology, and (3) endobiliary biopsy. While the specificity of diagnosing malignancy approaches 100% uniformly for each method, sensitivity varies widely and generally remains unsatisfactorily low. For instance, the sensitivity of cytology examination of aspirated bile ranges between 6 and 24% [30, 31].
Endoscopic brush cytology may be considered a standard method that is safe and easy to perform. Its diagnostic performance has been evaluated in many studies with the sensitivity for CCA in the range of 23-80%. In a meta-analysis of 16 studies including 1556 patients, Burnett et al. calculated its sensitivity as 42%. Two to five brush passes through the stricture have been suggested in various studies [28, 32].
As for endoluminal forceps biopsy, the sensitivity for CCA is in the range of 52-81%. Compared to brushing, forceps biopsy is technically more demanding and may require sphincterotomy. Biopsy can also be difficult or impossible in a narrow duct. The number of biopsy bites in studies ranges between 1 and 6. As shown by Tamada et al., three biopsy bites are sufficient to obtain sensitivity of 100% in patients with the papillary type CCA, while multiple biopsies are necessary for the infiltrating type [33–37] (Figure 4).
Figure 4.
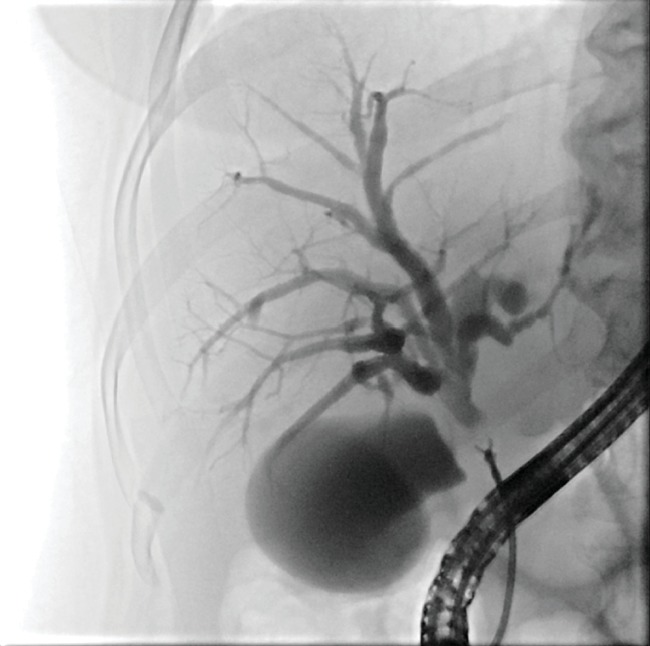
Endoscopic retrograde biopsy of biliary stricture.
It makes sense that different sampling methods could be combined. In a meta-analysis of 9 studies, Navaneethan et al. found the pooled sensitivity of endoscopic brush cytology, intraductal biopsy, and combination of both to be 45%, 48%, and 59%, respectively, with corresponding specificity of 99%, 99%, and 100%. The authors concluded that both brushing and biopsy are comparable with limited sensitivity, which can only modestly be increased by the combination of both [38].
Several factors contribute to frequent false-negative results of CCA tissue analysis. Among them, general difficulties in tissue sampling, the desmoplastic paucicellular character of CCA, and background inflammatory changes are the most prevalent. In a study devoted to endoscopic brush cytology, Logrono et al. proved that tissue sampling error was the cause of false-negative findings in 67%, followed by technical error in 17% and interpretive error in the remaining 17% [39].
Different technical solutions have been investigated in order to increase successful tissue sampling. Among them, using a larger cytology brush (3 mm × 5 cm) increased cellularity but did not improve the cancer detection rate when compared to the standard brush. The combination of biliary dilation, endoscopic needle aspiration, and subsequent brushing cytology showed higher sensitivity (85%) than brushing alone (57%). A cytological evaluation of postbrushing biliary lavage fluid increased sensitivity by 24%. A dedicated basket for tissue grasp provided better sensitivity than brushing (80 vs. 40%). Moreover, combining brush cytology with CA 19-9 assessment brought better diagnostic accuracy in patients with primary sclerosing cholangitis [40–44].
To improve the sensitivity of routine cytology, advanced cytological methods can be used. Among them, fluorescence in situ hybridization (FISH) is a test employing fluorescently labelled DNA probes to detect gains or losses of chromosomal regions. As shown by Fritcher et al., a combination of FISH probes 1q21, 7p12, 8q24, and 9p21 identified pancreatobiliary malignancies with sensitivity of 93% and specificity of 100%. The typical finding was polysomy, indicating a presence of five or more cells with gains detected for two or more probes. In other studies, FISH proved to increase sensitivity from 20% to 43% as compared to routine cytology. In cases with negative brush cytology and forceps biopsy, FISH could predict malignancy in 62% of patients with an indeterminate biliary stricture [45, 46]. Next-generation DNA sequencing (NGS), another ancillary cytological method, has also turned out to be promising. In a study combining NGS with cytology, NGS increased sensitivity to 85% as compared to 67% for cytology alone [47]. Triple modality testing combining brush cytology, FISH, and biopsy showed overall sensitivity of 82% and specificity 100% [48].
To summarize the above-mentioned, while positive ERCP-guided tissue sampling can be regarded as diagnostically conclusive, a negative finding should be interpreted with caution. The term “indeterminate stricture” is used to indicate biliary strictures remaining of a likely malignant etiology despite negative pathology results. In such cases, surgical exploration must be considered in order not to miss the opportunity of curative surgery for a potential malignancy. With that being said, 5-30% of surgically treated lesions were reported benign on final histopathology according to different studies [49]. Currently, novel endoscopic modalities, including cholangioscopy, can be used to avoid unnecessary surgery.
8.2. Cholangioscopy
The history of cholangioscopy dates back to the 1970s when two different endoscopic approaches to the biliary tract were described: (1) the “mother-baby” technique using a small endoscope that was passed through the working channel of a duodenoscope and (2) peroral direct cholangioscopy using a pediatric or dedicated forward viewing endoscope with an additional bending function that were advanced through the mouth [50–52].
As shown mainly by Japanese endoscopists, cholangioscopy increases sensitivity to 96-100% for the diagnosis of malignancy when combined with ERCP [53–56]. Nonetheless, both these techniques had important limitations that prevented their widespread usage. The former “mother-baby” endoscopy required two operators and the “babyscope” was fragile. The peroral cholangioscope was difficult to manipulate, especially within nondilated ducts, plus the dedicated video cholangioscope did not become commercially available in the West [57–61].
The current technological standard for the “mother-baby” approach is represented by a digital single-operator cholangioscope (DSOC, SpyGlass DS; Boston Scientific, Inc., Natick, Massachusetts, USA) commercially available since 2015. SpyGlass comprises a disposable 10.5 Fr scope with an integrated digital sensor and portable processor. The scope allows four-way tip deflection and enables suction, irrigation, and passage of miniaturized biopsy forceps (SpyBite; Boston Scientific, Inc.) or any of several other accessories [62].
DSOC enables direct visualization of the biliary tract and intraluminal biopsy. Although no formal consensus on visual diagnostic criteria has been established, the presence of either nodular or papillary masses, irregular surface, tortuous dilated vessels, and fragile mucosa are considered features typical of neoplasia. For benign lesions, a flat surface, fine network of vessels, regular granular appearance, nonfragile mucosa, convergence of folds, and presence of pseudodiverticula are deemed diagnostic. The sensitivity and specificity of visual diagnosis were reported in the range of 90-100% and 76-96%, respectively [63–67] (Figures 5 and 6).
Figure 5.
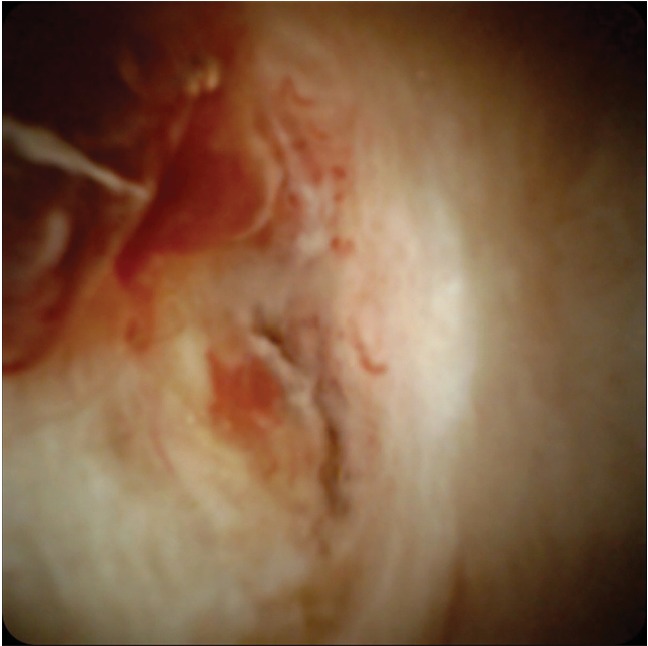
Cholangioscopy (SpyGlass) view of the intraductal-type cholangiocarcinoma.
Figure 6.
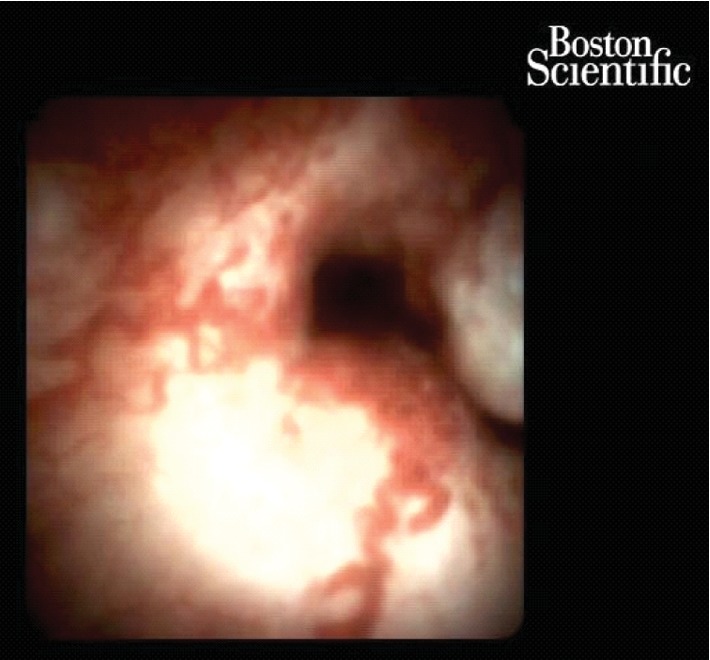
Cholangioscopy (SpyGlass) view of the periductal-type cholangiocarcinoma.
As for SpyBite biopsies, a sensitivity of 85-86% and specificity of 100% can be expected. Moreover, as shown by Varadarajulu et al., biopsy sensitivity can be increased up to 94% by using rapid on-site examinations of touch imprint cytology [68].
It has been suggested by Sugiyama et al. that the evaluation of ECCA extension may influence surgical strategy. Yet, as shown by Itoi et al., the proximal tumor margin can be visualized only in a limited number of patients and submucosal tumor extension is not possible to estimate at all. In one study comparing DSOC and ERCP, DSOC provided no additional information regarding the local spread of the disease in three surgically treated cases. Among these cases, an intraoperative frozen section revealed a spread of malignant cells beyond the predicted tumor margin in one of them, while the remaining two corresponded in preoperative and intraoperative findings. Thus, the results of local staging with DSOC should be interpreted cautiously [67, 69, 70].
As for the complications of the method, a retrospective study comparing ERCP with and without cholangioscopy showed increased morbidity when cholangioscopy was performed. Of note, the risk of cholangitis increased five times [71]. It was suggested that the higher risk of cholangitis is caused mainly by an increase in intraductal pressure due to intermittent water irrigation during the procedure. In a recent meta-analysis of 49 studies including 2193 patients who underwent either diagnostic or therapeutic peroral cholangioscopy, Korrapati et al. reported an overall and a serious adverse event rate of 7% and 1%, respectively. To prevent cholangitis, antibiotic prophylaxis and adequate biliary drainage are necessary [72].
Despite excellent operating characteristics, the position of DSOC in the diagnostic algorithm of uncertain biliary strictures must be further evaluated in regard to other modalities. Because of its costs, complexity, and procedure-related morbidity, DSOC should be considered mainly in the case of indeterminate biliary strictures in which previous ERCP tissue sampling was not conclusive. Even so, DSOC is becoming widely available and may become an option at hand during index ERCP in the future, particularly in cases with proximal strictures. Although such an approach could theoretically shorten the diagnostic process, its impact on safety remains to be investigated.
8.3. Endoscopic Ultrasound
Endoscopic ultrasound (EUS) was introduced in clinical practice in the 1980s. Since then, the position of EUS in diagnosing and staging pancreatobiliary diseases, including CCA, has been firmly established. During the EUS procedure, CCA can be readily visualized in both cross-sectional and longitudinal views. If a mass is present, it usually appears hypoechoic or, less frequently, heterogenous (Figure 7). The upstream dilatation of the biliary tract can also be estimated. In addition to tumor depiction, EUS allows for the identification of the hilum, celiac axis, and para-aortic lymph nodes facilitating the staging process.
Figure 7.
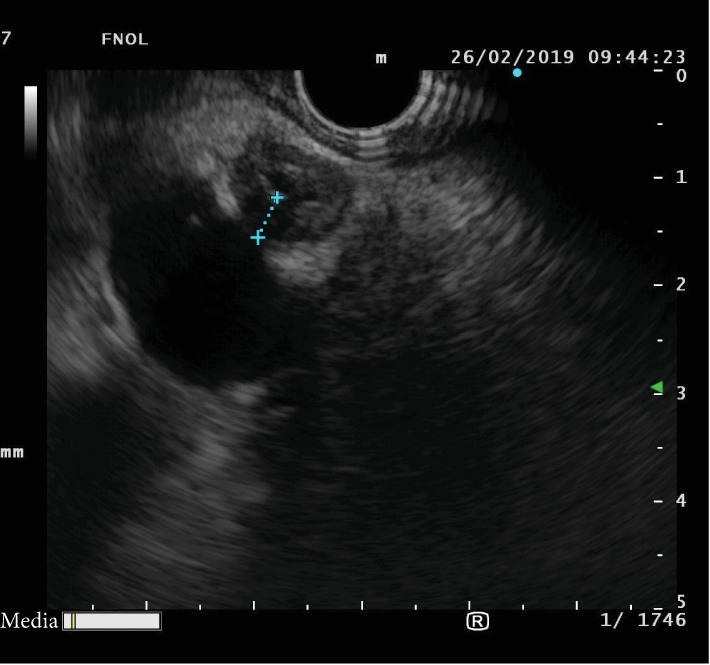
Linear endosonography view of a distal cholangiocarcinoma.
As for malignant biliary stricture detection, EUS without fine needle aspiration (FNA) was found to provide sensitivity of 78% and specificity of 84% [73]. Another study proved EUS to be superior in biliary cancer detection when compared to CT and MRI/MRCP (94%, 30%, and 42%, respectively), with a statistical significant difference. In the same study, EUS detected 100% of distal and 83% of proximal CCAs while demonstrating sensitivity of 53% and specificity of 97% in the assessment of unresectability [74].
The development of linear echoendoscopes enabled EUS-FNA which further improved the diagnostic capability of EUS. Across several studies, EUS-FNA sensitivity and specificity for differentiating ECCA from benign biliary lesions range between 43-89% and 79-100%, respectively. Moreover, sensitivity of 45% could be achieved even in the case of ECCA with no definite mass on cross-sectional imaging. Similar to EUS alone, the operating characteristics of EUS-FNA proved better for distal as compared to proximal lesions. One study has demonstrated sensitivity of 81% for distal and 59% for proximal lesions with the overall sensitivity being 73%.
Based on these results, EUS-FNA can be readily recommended for tissue sampling in cases with inconclusive results from ERCP brushing. A meta-analysis of patients with negative brush cytology has revealed the sensitivity and specificity of EUS-FNA to be 59% and 100%, respectively, for the diagnosis of ECCA [75–78].
The possibility of using EUS-FNA as a safer alternative to ERCP was also investigated. For instance, Onda et al. performed EUS-FNA as the first-line method in patients with suspected ECCA based on CT or other imaging modalities with sensitivity of 89% and accuracy of 87% [79]. In a recent meta-analysis, De Moura et al. calculated the mean sensitivities of ERCP and EUS-FNA for the tissue diagnosis of a malignant biliary stricture to be 49% and 75%, while specificities were 96% and 100% [80].
However, not all literature is in agreement with the superiority of EUS over ERCP in the assessment of malignant biliary strictures. For example, although the study by Rosch et al. proved EUS to be more sensitive in the diagnosis of pancreatic tumors, ERCP was a better diagnostic modality for CCA [75]. In a study by Weilert et al., ERCP brushing had the same sensitivity (79%) as compared to EUS-FNA [81].
Finally, same-session EUS-FNA and ERCP-based tissue sampling was superior to EUS-FNA alone for both pancreatic and biliary lesions [82].
It is important to note that the negative predictive values of EUS-FNA for malignancy were relatively low in most of the studies, ranging from 29% to 67%. Therefore, similarly to ERCP, a negative EUS-FNA may not exclude the malignant etiology of biliary strictures.
The short-term endoscopic risks of EUS-FNA are low, including acute pancreatitis (0.3-2%), bleeding (1%), perforation (0.4%), and infection (0.3%) [83].
Amid late complications, tumor cell seeding after transperitoneal FNA must be considered in operable patients. As reported by Heimbach et al., peritoneal metastasis occurred in 83% of patients who underwent a percutaneous or transluminal FNA biopsy of the primary hilar tumor mass as opposed to 8% in cases where no biopsy was performed [84]. As a result, the Mayo Clinic transplantation protocol excludes patients who have undergone a biopsy of the primary tumor from neoadjuvant therapy and liver transplantation. So far, there have been no reports of tumor seeding in those with distal CCA. In theory, the puncture route for a distal biliary lesion is usually nontransperitoneal and the needle tract is removed during the Whipple resection. Furthermore, a retrospective study found a significantly lower incidence of peritoneal carcinomatosis in patients with pancreatic adenocarcinoma after EUS-FNA as compared to percutaneous procedures (2.2% in the EUS group vs. 16.3% in the percutaneous group) [85].
In conclusion, EUS-FNA performs well in the detection and staging process of ECCA, although the relatively low negative predictive value is a fact that must be taken into consideration. Still, its nearly 100% positive predictive value is the utmost power when it comes to indicating aggressive treatment including surgery. Due to the concern of tumor seeding, the EUS-FNA of proximal lesions should be indicated with caution [86].
8.4. Intraductal Sonography (IDUS)
IDUS uses high-frequency (12-20 MHz) catheter-based probes of 2 mm diameter introduced into the biliary tract over a guidewire through the working channel of a duodenoscope. In most cases, preceding sphincterotomy is not necessary. IDUS is safe and technically easy to learn, but image interpretation remains challenging. It is used both for CCA detection and local staging. Lymph node assessment with this high-frequency ultrasound is not possible due to a penetration depth of only 20 mm.
IDUS distinguishes three layers of the normal bile duct wall: (1) inner hyperechoic corresponding to mucosa, (2) middle hypoechoic of smooth muscle fibers with fibroelastic tissue, and (3) outer hyperechoic corresponding to connective tissue.
Several diagnostic features of malignant strictures were described such as the disruption of the normal sonographic pattern, presence of a hypoechoic infiltrating lesion with irregular margins, and a tumor invasion into surrounding tissues. Malignancy is extremely likely if IDUS detects a tumor invasion into the hepatic artery, portal vein, or pancreatic parenchyma. Secondly, findings considered typical for a benign bile duct stricture include the preservation of the normal wall pattern, a homogeneous echo pattern, smooth margins, hyperechoic lesions, and absence of a mass lesion. The accuracy of IDUS in differentiating benign from malignant strictures ranges from 76 to 98% in the literature [87–89].
Other diagnostic features such as interrupted wall structure, the presence of a sessile tumor intraductally or outside the bile duct, or tumor size greater than 10 mm were suggested by Tamada et al. The likelihood of malignancy was as high as 97% when two or three of these features were present. To the contrary, an IDUS examination with no positive feature correlated with negative findings at the final diagnosis [90].
Even though IDUS has no capability for tissue sampling, it proved to be more accurate in distinguishing benign and malignant strictures than ERCP with transpapillary biopsy. In a retrospective study, IDUS was more specific (92% vs. 42%) and similarly sensitive (89% vs. 83%) as ERCP with biopsy. Compared to EUS, IDUS had higher sensitivity (91% vs. 75%) and specificity (80 vs. 75%) [91, 92].
IDUS has demonstrated to be a useful tool in predicting positive biopsy. When an intraductal sessile tumor could be visualized, the sensitivity of the biopsy was 92%. Moreover, as previously suggested, IDUS-directed bile duct sampling was found to be more sensitive than ERCP-guided sampling (87% vs. 67%) [28, 93].
As for the assessment of longitudinal tumor spread, IDUS proved better accuracy than ERCP (84% vs. 47%). In a study investigating bile duct wall thickness using IDUS in patients who had not undergone biliary drainage, in 95% of cases, the biliary wall of the common hepatic duct was not thicker than 1.8 mm unless the patients were diagnosed with primary sclerosing cholangitis or had longitudinal cancer extension along the bile duct [94]. A different study reported the mean length of longitudinal extension beyond the estimated measure by cholangiography to be about 6-10 mm for submucosal and 10-20 mm for mucosal spread [95].
The depth of tumor invasion can also be assessed by IDUS. When the outside hyperechoic layer is interrupted, a serosa invasion is suggested with an accuracy of 86%-93%. A vascular invasion is suspected if a high-echoic (“interface”) echo in between the tumor and vessel wall disappears. While diagnostic accuracy for portal vein and right hepatic artery invasion assessment was 86-100% and 92-100%, respectively, the visualization of the left and proper hepatic artery was poor. Therefore, CT and IDUS should be regarded as complementary staging methods [96–98].
8.5. Confocal Laser Endomicroscopy
Probe-based confocal laser microscopy is a method providing real-time, 400-fold-magnified imaging of the mucosa. The probe is introduced into the biliary tract through a catheter or the working channel of a cholangioscope. The intravenous injection of 10% fluorescein sodium is mandatory. A high diagnostic performance in malignant biliary strictures with accuracy of 79-82% has been reported. Nevertheless, this technology is costly and interpretation of images challenging [99–101]. Of note, in cases with prior biliary stenting, accuracy decreased to 45% in one study. Therefore, to decrease the rate of misclassification of benign stricture as malignant, usage of Paris (instead of Miami) Classification was recommended in this clinical scenario [102, 103].
9. Patients with PSC
The diagnosis of CCA in patients with primary sclerosing cholangitis (PSC) is cumbersome since background inflammation can obscure the clinical and morphological manifestation of the disease. According to the ESGE/EASL guidelines, ERCP with tissue sampling should be considered in patients with worsening symptoms, a rapid increase of cholestatic enzyme levels, or a new dominant stricture or progression of existing dominant strictures at MRCP. The diagnostic performance of DSOC was shown to be similar to non-PSC patients [104].
10. Conclusion
A rational utilization of cross-sectional imaging and endoscopic procedures in patients with clinical and laboratory suspicions of CCA is mandatory. The availability of advanced endoscopic methods, such as cholangioscopy and EUS, is increasing and makes the diagnostic algorithm easier. The limitation of tissue sampling resides in its low negative predictive value for malignancy. Despite the considerable progress in this field, further clinical research is needed.
Abbreviations
- CCA:
Cholangiocarcinoma
- ECCA:
Extrahepatic cholangiocarcinoma
- ICCA:
Intrahepatic cholangiocarcinoma
- PCCA:
Proximal (perihilar) extrahepatic cholangiocarcinoma
- DCCA:
Distal extrahepatic cholangiocarcinoma
- ERCP:
Endoscopic retrograde cholangiopancreatography
- EUS:
Endoscopic ultrasound
- EUS-FNA:
Endoscopic ultrasound (-guided) fine needle aspiration
- IDUS:
Intraductal ultrasonography.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Sripa B., Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Current Opinion in Gastroenterology. 2008;24(3):349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergquist A., von Seth E. Epidemiology of cholangiocarcinoma. Best Practice & Research. Clinical Gastroenterology. 2015;29(2):221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Mosadeghi S., Liu B., Bhuket T., Wong R. J. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: an updated analysis of the 2000-2011 Surveillance, Epidemiology and End Results registry. Hepatology Research. 2016;46(7):669–677. doi: 10.1111/hepr.12605. [DOI] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2(1) doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi S., Khan S. A., Hallemeier C. L., Kelley R. K., Gores G. J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J., Kim M. H., Kim K. P., et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut and Liver. 2009;3(4):298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J., Wasan H., Palmer D. H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England Journal of Medicine. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.DeOliveira M. L., Cunningham S. C., Cameron J. L., et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Annals of Surgery. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapisochin G., Facciuto M., Rubbia-Brandt L., et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 10.Esnaola N. F., Meyer J. E., Karachristos A., Maranki J. L., Camp E. R., Denlinger C. S. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama M., Atomi Y., Kuroda A., Muto T. Bile duct carcinoma without jaundice: clues to early diagnosis. Hepato-Gastroenterology. 1997;44(17):1477–1483. [PubMed] [Google Scholar]
- 12.Cravo M. Is CA 19-9 of any help in the management of cholangiocarcinoma? GE Portuguese Journal of Gastroenterology. 2017;24(3):108–109. doi: 10.1159/000457910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brierley J. D., Gospodarowicz M. K., Wittekind C., editors. The TNM Classification of Malignant Tumors, 8. Oxford: Eiley Blackwell; 2017. [Google Scholar]
- 14.Edge S. B., Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Nakanuma Y., Leong A. S., Sripa B., Ponchon T., Hainaut P., Inoue H. Intrahepatic cholangiocarcinoma. In: Hamilton S. R., Aaltonen L. A., editors. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press; 2000. [Google Scholar]
- 16.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. The American Journal of Medicine. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 17.Bismuth H., Corlette M. B. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surgery, Gynecology & Obstetrics. 1975;140(2):170–178. [PubMed] [Google Scholar]
- 18.Sakamoto Y., Kokudo N., Matsuyama Y., et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122(1):61–70. doi: 10.1002/cncr.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi S., Miyazaki M., Kondo Y., Nakajima N. Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer. 1994;73(12):2922–2929. doi: 10.1002/1097-0142(19940615)73:12<2922::AID-CNCR2820731208>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Ebata T., Watanabe H., Ajioka Y., Oda K., Nimura Y. Pathological appraisal of lines of resection for bile duct carcinoma. The British Journal of Surgery. 2002;89(10):1260–1267. doi: 10.1046/j.1365-2168.2002.02211.x. [DOI] [PubMed] [Google Scholar]
- 21.DeOliveira M. L., Schulick R. D., Nimura Y., et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53(4):1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 22.Albu S., Tantau M., Sparchez Z., et al. Diagnosis and treatment of extrahepatic cholangiocarcinoma: results in a series of 124 patients. Romanian Journal of Gastroenterology. 2005;14(1):33–36. [PubMed] [Google Scholar]
- 23.Ruys A. T., van Beem B. E., Engelbrecht M. R. W., Bipat S., Stoker J., van Gulik T. M. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. The British Journal of Radiology. 2012;85(1017):1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Zhu J., Ke F., et al. Radiological Imaging for Assessing the Respectability of Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. BioMed Research International. 2015;2015:11. doi: 10.1155/2015/497942.497942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotton P. B., Garrow D. A., Gallagher J., Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointestinal Endoscopy. 2009;70(1):80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Dumonceau J. M., Andriulli A., Elmunzer B. J., et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline - updated June 2014. Endoscopy. 2014;46(9):799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 27.Park H. S., Lee J. M., Choi J. Y., et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. American Journal of Roentgenology. 2008;190(2):396–405. doi: 10.2214/AJR.07.2310. [DOI] [PubMed] [Google Scholar]
- 28.Tamada K., Ushio J., Sugano K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: advances and current limitations. World Journal of Clinical Oncology. 2011;2(5):203–216. doi: 10.5306/wjco.v2.i5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park M. S., Kim T. K., Kim K. W., et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233(1):234–240. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 30.Kurzawinski T., Deery A., Dooley J., Dick R., Hobbs K., Davidson B. A prospective controlled study comparing brush and bile exfoliative cytology for diagnosing bile duct strictures. Gut. 1992;33(12):1675–1677. doi: 10.1136/gut.33.12.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desa L. A., Akosa A. B., Lazzara S., Domizio P., Krausz T., Benjamin I. S. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut. 1991;32(10):1188–1191. doi: 10.1136/gut.32.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponchon T., Gagnon P., Berger F., et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointestinal Endoscopy. 1995;42(6):565–572. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 33.Tamada K., Tomiyama T., Wada S., et al. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 2002;50(3):326–331. doi: 10.1136/gut.50.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugiyama M., Atomi Y., Wada N., Kuroda A., Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. The American Journal of Gastroenterology. 1996;91(3):465–467. [PubMed] [Google Scholar]
- 35.Kubota Y., Takaoka M., Tani K., et al. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. The American Journal of Gastroenterology. 1993;88(10):1700–1704. [PubMed] [Google Scholar]
- 36.Pugliese V., Conio M., Nicolò G., Saccomanno S., Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointestinal Endoscopy. 1995;42(6):520–526. doi: 10.1016/s0016-5107(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 37.Howell D. A., Parsons W. G., Jones M. A., Bosco J. J., Hanson B. L. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointestinal Endoscopy. 1996;43(5):498–502. doi: 10.1016/s0016-5107(96)70294-8. [DOI] [PubMed] [Google Scholar]
- 38.Navaneethan U., Njei B., Lourdusamy V., Konjeti R., Vargo J. J., Parsi M. A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointestinal Endoscopy. 2015;81(1):168–176. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logrono R., Kurtycz D. F., Molina C. P., Trivedi V. A., Wong J. Y., Block K. P. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at 2 university hospitals. Archives of Pathology & Laboratory Medicine. 2000;124(3):387–392. doi: 10.1043/0003-9985(2000)124<0387:AOFNDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Fogel E. L., deBellis M., McHenry L., et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointestinal Endoscopy. 2006;63(1):71–77. doi: 10.1016/j.gie.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Mohandas K. M., Swaroop V. S., Gullar S. U., Dave U. R., Jagannath P., DeSouza L. J. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointestinal Endoscopy. 1994;40(2):150–154. doi: 10.1016/S0016-5107(94)70157-1. [DOI] [PubMed] [Google Scholar]
- 42.Farrell R. J., Jain A. K., Brandwein S. L., Wang H., Chuttani R., Pleskow D. K. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointestinal Endoscopy. 2001;54(5):587–594. doi: 10.1067/mge.2001.118715. [DOI] [PubMed] [Google Scholar]
- 43.Dumonceau J. M., Macias Gomez C., Casco C., et al. Grasp or brush for biliary sampling at endoscopic retrograde cholangiography? A blinded randomized controlled trial. The American Journal of Gastroenterology. 2008;103(2):333–340. doi: 10.1111/j.1572-0241.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 44.Siqueira E., Schoen R. E., Silverman W., et al. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointestinal Endoscopy. 2002;56(1):40–47. doi: 10.1067/mge.2002.125105. [DOI] [PubMed] [Google Scholar]
- 45.Barr Fritcher E. G., Kipp B. R., Halling K. C., et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136(7):2180–2186. doi: 10.1053/j.gastro.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 46.Levy M. J., Baron T. H., Clayton A. C., et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. The American Journal of Gastroenterology. 2008;103(5):1263–1273. doi: 10.1111/j.1572-0241.2007.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley J. C., Zheng Z., McDonald T., et al. Next-generation sequencing and fluorescence in situ hybridization have comparable performance characteristics in the analysis of pancreaticobiliary brushings for malignancy. The Journal of Molecular Diagnostics. 2016;18(1):124–130. doi: 10.1016/j.jmoldx.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Nanda A., Brown J. M., Berger S. H., et al. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therapeutic Advances in Gastroenterology. 2015;8(2):56–65. doi: 10.1177/1756283X14564674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerhards M. F., Vos P., van Gulik T., Rauws E. A., Bosma A., Gouma D. J. Incidence of benign lesions in patients resected for suspicious hilar obstruction. The British Journal of Surgery. 2001;88(1):48–51. doi: 10.1046/j.1365-2168.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 50.Urakami Y. Peroral cholangiopancreatoscopy (PCPS) and peroral direct cholangioscopy (PDCS) Endoscopy. 1980;12(1):30–37. doi: 10.1055/s-2007-1021706. [DOI] [PubMed] [Google Scholar]
- 51.Rösch W., Koch H., Demling L. Peroral cholangioscopy. Endoscopy. 1976;08(3):172–175. doi: 10.1055/s-0028-1098405. [DOI] [Google Scholar]
- 52.Nakajima M., Akasaka Y., Fukumoto K., Mitsuyoshi Y., Kawai K. Peroral cholangiopancreatoscopy (PCPS) under duodenoscopic guidance. The American Journal of Gastroenterology. 1976;66:241–247. [PubMed] [Google Scholar]
- 53.Fukuda Y., Tsuyuguchi T., Sakai Y., Tsuchiya S., Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointestinal Endoscopy. 2005;62(3):374–382. doi: 10.1016/j.gie.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 54.Nishikawa T., Tsuyuguchi T., Sakai Y., Sugiyama H., Miyazaki M., Yokosuka O. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointestinal Endoscopy. 2013;77(2):219–226. doi: 10.1016/j.gie.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Osanai M., Itoi T., Igarashi Y., et al. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013;45(8):635–642. doi: 10.1055/s-0032-1326631. [DOI] [PubMed] [Google Scholar]
- 56.Itoi T., Osanai M., Igarashi Y., et al. Diagnostic peroral video cholangioscopy is an accurate diagnostic tool for patients with bile duct lesions. Clinical Gastroenterology and Hepatology. 2010;8(11):934–938. doi: 10.1016/j.cgh.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 57.Meves V., Ell C., Pohl J. Efficacy and safety of direct transnasal cholangioscopy with standard ultraslim endoscopes: results of a large cohort study. Gastrointestinal Endoscopy. 2014;79(1):88–94. doi: 10.1016/j.gie.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 58.Choi H. J., Moon J. H., Ko B. M., et al. Overtube-balloon-assisted direct peroral cholangioscopy by using an ultra-slim upper endoscope (with videos) Gastrointest Endosc. 2009;69(4):935–940. doi: 10.1016/j.gie.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 59.Moon J. H., Ko B. M., Choi H. J., et al. Intraductal balloon-guided direct peroral cholangioscopy with an ultraslim upper endoscope (with videos) Gastrointestinal Endoscopy. 2009;70(2):297–302. doi: 10.1016/j.gie.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Moon J. H., Terheggen G., Choi H. J., Neuhaus H. Peroral cholangioscopy: diagnostic and therapeutic applications. Gastroenterology. 2013;144(2):276–282. doi: 10.1053/j.gastro.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 61.Beyna T., Farnik H., Sarrazin C., Gerges C., Neuhaus H., Albert J. G. Direct retrograde cholangioscopy with a new prototype double-bending cholangioscope. Endoscopy. 2016;48(10):929–933. doi: 10.1055/s-0042-110395. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y. K., Pleskow D. K. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointestinal Endoscopy. 2007;65(6):832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Sethi A., Shah R. J., Itoi T., et al. 1040 Defining Imaging Criteria for Indeterminate Biliary Strictures Utilizing Video Cholangioscopy: the Monaco Classification. Gastrointestinal Endoscopy. 2015;81(5):AB188–AB189. doi: 10.1016/j.gie.2015.03.1939. [DOI] [Google Scholar]
- 64.Sethi A., Widmer J., Shah N. L., et al. Interobserver agreement for evaluation of imaging with single operator choledochoscopy: what are we looking at? Digestive and Liver Disease. 2014;46(6):518–522. doi: 10.1016/j.dld.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Navaneethan U., Hasan M. K., Kommaraju K., et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video) Gastrointestinal Endoscopy. 2016;84(4):649–655. doi: 10.1016/j.gie.2016.03.789. [DOI] [PubMed] [Google Scholar]
- 66.Shah R. J., Raijman I., Brauer B., Gumustop B., Pleskow D. K. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy. 2017;49(07):651–658. doi: 10.1055/s-0043-106295. [DOI] [PubMed] [Google Scholar]
- 67.Urban O., Evinová E., Fojtík P., et al. Digital cholangioscopy: the diagnostic yield and impact on management of patients with biliary stricture. Scandinavian Journal of Gastroenterology. 2018;53(10-11):1364–1367. doi: 10.1080/00365521.2018.1512649. [DOI] [PubMed] [Google Scholar]
- 68.Varadarajulu S., Bang J. Y., Hasan M. K., Navaneethan U., Hawes R., Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video) Gastrointestinal Endoscopy. 2016;84(4):681–687. doi: 10.1016/j.gie.2016.03.1497. [DOI] [PubMed] [Google Scholar]
- 69.Sugiyama H., Tsuyuguchi T., Sakai Y., Ohtsuka M., Miyazaki M., Yokosuka O. Potential role of peroral cholangioscopy for preoperative diagnosis of cholangiocarcinoma. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 2012;22(6):532–536. doi: 10.1097/SLE.0b013e3182686178. [DOI] [PubMed] [Google Scholar]
- 70.Itoi T., Sofuni A., Itokawa F., et al. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos) Gastrointestinal Endoscopy. 2007;66(4):730–736. doi: 10.1016/j.gie.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 71.Sethi A., Chen Y. K., Austin G. L., et al. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointestinal Endoscopy. 2011;73(2):251–256. doi: 10.1016/j.gie.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 72.Korrapati P., Ciolino J., Wani S., et al. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endoscopy International Open. 2016;4(3):E263–E275. doi: 10.1055/s-0042-100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garrow D., Miller S., Sinha D., et al. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clinical Gastroenterology and Hepatology. 2007;5(5):616–623. doi: 10.1016/j.cgh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 74.Mohamadnejad M., DeWitt J. M., Sherman S., et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single- center experience. Gastrointestinal Endoscopy. 2011;73(1):71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 75.Rösch T., Hofrichter K., Frimberger E., et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointestinal Endoscopy. 2004;60(3):390–396. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 76.Fritscher-Ravens A., Broering D. C., Sriram P. V., et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointestinal Endoscopy. 2000;52(4):534–540. doi: 10.1067/mge.2000.109589. [DOI] [PubMed] [Google Scholar]
- 77.Meara R. S., Jhala D., Eloubeidi M. A., et al. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17(1):42–49. doi: 10.1111/j.1365-2303.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 78.DeWitt J., Misra V. L., Leblanc J. K., McHenry L., Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointestinal Endoscopy. 2006;64(3):325–333. doi: 10.1016/j.gie.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 79.Onda S., Ogura T., Kurisu Y., et al. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Therapeutic Advances in Gastroenterology. 2016;9(3):302–312. doi: 10.1177/1756283X15625584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Moura D. T. H., De Moura E. G. H., Bernardo W. M., et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: systematic review and meta-analysis. Endoscopic Ultrasound. 2018;7(1):10–19. doi: 10.4103/2303-9027.193597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weilert F., Bhat Y. M., Binmoeller K. F., et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointestinal Endoscopy. 2014;80(1):97–104. doi: 10.1016/j.gie.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Jo J. H., Cho C. M., Jun J. H., et al. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. Journal of Gastroenterology and Hepatology. 2019;34(4):799–805. doi: 10.1111/jgh.14528. [DOI] [PubMed] [Google Scholar]
- 83.Murad F., Levy M. J., Topazian M. Indications, preparation, risks, and complications. In: Hawes R. V., Fockens P., Varadarjulu S., editors. Endosonography. 2nd. Philadelphia, PA, USA: Elsevier, Saunders; 2011. pp. 29–37. [DOI] [Google Scholar]
- 84.Heimbach J. K., Sanchez W., Rosen C. B., Gores G. J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB. 2011;13(5):356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Micames C., Jowell P. S., White R., et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointestinal Endoscopy. 2003;58(5):690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 86.Strongin A., Singh H., Eloubeidi M. A., Siddiqui A. A. Role of endoscopic ultrasonography in the evaluation of extrahepatic cholangiocarcinoma. Endoscopic Ultrasound. 2013;2(2):71–76. doi: 10.4103/2303-9027.117690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamada K., Ido K., Ueno N., Kimura K., Ichiyama M., Tomiyama T. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. The American Journal of Gastroenterology. 1995;90(2):239–246. [PubMed] [Google Scholar]
- 88.Tamada K., Ueno N., Ichiyama M., et al. Assessment of pancreatic parenchymal invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1996;28(6):492–496. doi: 10.1055/s-2007-1005529. [DOI] [PubMed] [Google Scholar]
- 89.Napoleon B., Alvarez-Sánchez M. V., Markoglou K., Lefort C. H. EUS in bile duct, gallbladder, and ampullary lesions. In: Hawes R., Fockens P., Varadarajulu S., editors. Endosonography. Second edition. Philadelphia, PA, USA: Elsevier Saunders; 2006. pp. 178–200. [DOI] [Google Scholar]
- 90.Tamada K., Ueno N., Tomiyama T., et al. Characterization of biliary strictures using intraductal ultrasonography: comparison with percutaneous cholangioscopic biopsy. Gastrointestinal Endoscopy. 1998;47(5):341–349. doi: 10.1016/s0016-5107(98)70216-0. [DOI] [PubMed] [Google Scholar]
- 91.Vazquez-Sequeiros E., Baron T. H., Clain J. E., et al. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointestinal Endoscopy. 2002;56(3):372–379. doi: 10.1016/S0016-5107(02)70041-2. [DOI] [PubMed] [Google Scholar]
- 92.Menzel J., Poremba C., Dietl K. H., Domschke W. Preoperative diagnosis of bile duct strictures–comparison of intraductal ultrasonography with conventional endosonography. Scandinavian Journal of Gastroenterology. 2000;35(1):77–82. doi: 10.1080/003655200750024579. [DOI] [PubMed] [Google Scholar]
- 93.Ho M. J. The usefulness of IDUS-guided transpapillary bile duct biopsy for the diagnosis of malignant biliary strictures. Endoscopy. 2011;43 doi: 10.1055/s-0031-1292124. [DOI] [Google Scholar]
- 94.Tamada K., Tomiyama T., Oohashi A., et al. Bile duct wall thickness measured by intraductal US in patients who have not undergone previous biliary drainage. Gastrointestinal Endoscopy. 1999;49(2):199–203. doi: 10.1016/s0016-5107(99)70486-4. [DOI] [PubMed] [Google Scholar]
- 95.Tamada K., Nagai H., Yasuda Y., et al. Transpapillary intraductal US prior to biliary drainage in the assessment of longitudinal spread of extrahepatic bile duct carcinoma. Gastrointestinal Endoscopy. 2001;53(3):300–307. doi: 10.1016/s0016-5107(01)70402-6. [DOI] [PubMed] [Google Scholar]
- 96.Kuroiwa M., Tsukamoto Y., Naitoh Y., Hirooka Y., Furukawa T., Katou T. New technique using intraductal ultrasonography for the diagnosis of bile duct cancer. Journal of Ultrasound in Medicine. 1994;13(3):189–195. doi: 10.7863/jum.1994.13.3.189. [DOI] [PubMed] [Google Scholar]
- 97.Tamada K., Ido K., Ueno N., et al. Assessment of portal vein invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995;27(8):573–578. doi: 10.1055/s-2007-1005760. [DOI] [PubMed] [Google Scholar]
- 98.Tamada K., Ido K., Ueno N., et al. Assessment of hepatic artery invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995;27(8):579–583. doi: 10.1055/s-2007-1005761. [DOI] [PubMed] [Google Scholar]
- 99.ASGE Technology Committee, Kantsevoy S. V., Adler D. G., et al. Confocal laser endomicroscopy. Gastrointest Endosc. 2014;70(2):197–200. doi: 10.1016/j.gie.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Löhr J.-M., Lönnebro R., Stigliano S., et al. Outcome of probe-based confocal laser endomicroscopy (pCLE) during endoscopic retrograde cholangiopancreatography: a single-center prospective study in 45 patients. United European Gastroenterology Journal. 2015;3(6):551–560. doi: 10.1177/2050640615579806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meining A., Chen Y. K., Pleskow D., et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointestinal Endoscopy. 2011;74(5):961–968. doi: 10.1016/j.gie.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Taunk P., Singh S., Lichtenstein D., Joshi V., Gold J., Sharma A. Improved classification of indeterminate biliary strictures by probe-based confocal laser endomicroscopy using the Paris criteria following biliary stenting. Journal of Gastroenterology and Hepatology. 2017;32(10):1778–1783. doi: 10.1111/jgh.13782. [DOI] [PubMed] [Google Scholar]
- 103.Caillol F., Filoche B., Gaidhane M., Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris classification. Digestive Diseases and Sciences. 2013;58(6):1784–1789. doi: 10.1007/s10620-012-2533-5. [DOI] [PubMed] [Google Scholar]
- 104.Aabakken L., Karlsen T. H., Albert J., et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) clinical guideline. Endoscopy. 2017;49(6):588–608. doi: 10.1055/s-0043-107029. [DOI] [PubMed] [Google Scholar]