Abstract
Melanogenesis is the biological process which the skin pigment melanin is synthesized to protect the skin against ultraviolet irradiation and other external stresses. Abnormal biology of melanocytes is closely associated with depigmented skin disorders such as vitiligo. In this study, we examined the effects of maclurin on melanogenesis and cytoprotection. Maclurin enhanced cellular tyrosinase activity as well as cellular melanin levels. We found that maclurin treatment increased the expression of microphthalmia-associated transcription factor (MITF), tyrosinase-related protein- (TRP-) 1, TRP-2, and tyrosinase. Mechanistically, maclurin promoted melanogenesis through cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein-dependent upregulation of MITF. CREB activation was found to be mediated by p38 mitogen-activated protein kinase (MAPK) or cAMP-protein kinase A (PKA) signaling. In addition, maclurin-induced CREB phosphorylation was mediated through the activation of both the cAMP/PKA and the p38 MAPK signaling pathways. Maclurin-induced suppression of p44/42 MAPK activation also contributed to its melanogenic activity. Furthermore, maclurin showed protective effects against H2O2 treatment and UVB irradiation in human melanocytes. These findings indicate that the melanogenic effects of maclurin depend on increased MITF gene expression, which is mediated by the activation of both p38 MAPK/CREB and cAMP/PKA/CREB signaling. Our results thus suggest that maclurin could be useful as a protective agent against hypopigmented skin disorders.
1. Introduction
In recent years, an increase in fine dust caused by industrialization, abnormal climate change, and ozone layer destruction has created conditions that can cause damage to the human body, especially the skin. To reduce the damage caused by external stress factors, the skin biosynthesizes melanin, a skin pigment. This process of skin pigment synthesis is called melanogenesis. However, various stresses can cause defects in melanogenesis, leading to depigmentation skin disorders such as vitiligo [1]. Depigmented skin disorders have been widely studied, but their mechanisms remain largely unknown.
Melanogenesis plays a critical protective role against photocarcinogenesis in the skin [2]. Skin pigmentation depends on several factors, including the type, production, and distribution of melanin, the melanocyte number, the enzymatic activity of melanogenic proteins [3], melanocyte dendricity [4], and melanosome transfer [5]. Tyrosinase-related protein- (TRP-) 1, TRP-2, and tyrosinase are melanocyte-specific enzymes involved in melanin biosynthesis. The expression of those melanogenic genes is regulated by microphthalmia-associated transcription factor (MITF), which has a basic helix-loop-helix leucine zipper [6]. Specifically, MITF increases the expression of TRP-1, TRP-2, and tyrosinase by binding to the M-box that the three genes share in their promoter regions.
Various stimuli are involved in the induction of pigmentation. They include ultraviolet irradiation, abnormal release of α-melanocyte-stimulating hormone, inflammation, and rubbing of the skin [7]. In addition, skin melanogenesis is mediated via several melanogenic signaling pathways, including p38 mitogen-activated protein kinase (MAPK) signaling, the cyclic adenosine monophosphate- (cAMP-) mediated pathway, the protein kinase C- (PKC-) mediated pathway, the phosphatidylinositol 3 kinase (PI3K)/AKT signaling, and the p44/42 MAPK pathway. That is, p38 MAPK phosphorylation increases the expression of MITF and tyrosinase, leading to the induction of melanin synthesis [8]. Elevated levels of intracellular cAMP also result in the activation of protein kinase A (PKA), which phosphorylates the cAMP-responsive element binding (CREB) binding protein and CREB protein, increasing the expression of MITF [9]. The contribution of the protein kinase C- (PKC-) mediated pathway to melanogenesis is unclear; the phosphatidylinositol 3 kinase (PI3K)/AKT signaling pathway suppresses melanogenesis by reducing the expression of tyrosinase, MITF, and TRPs [10, 11]. p44/42 MAPK reduces melanin synthesis by degrading the tyrosinase protein [12].
The structural name of maclurin is (3,4-dihydroxyphenyl)-(2,4,6-trihydroxyphenyl) methanone, and it is a member of the benzophenone family (Figure 1(a)). It exists in Morus alba (white mulberry) and Garcinia mangostana (purple mangosteen). Although previous reports demonstrated that maclurin has antioxidant and antimetastatic effects, inhibiting cancer cell migration and invasion in non-small-cell lung cancer cells [13–15], the involvement of maclurin in skin cell biology has not been elucidated. Specifically, its effects on the signal transduction pathways of melanogenesis in human epidermal melanocytes have not been previously reported.
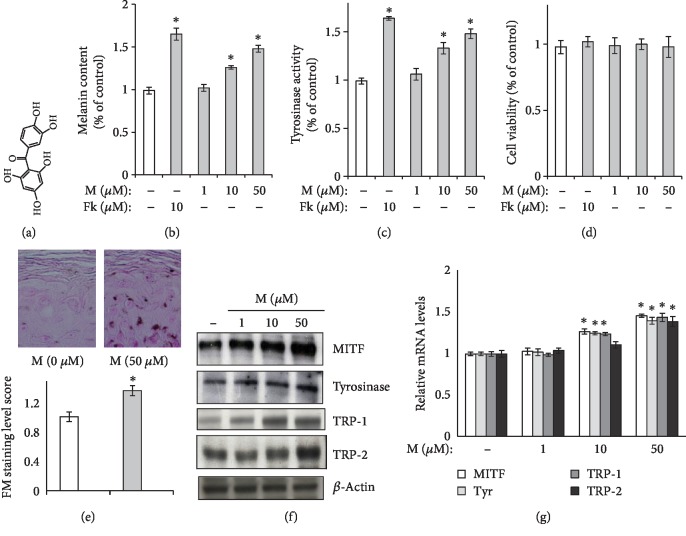
Figure 1.
Melanogenesis was upregulated in human epidermal melanocytes. (a) Chemical structure of maclurin. (b, c) Maclurin increased both the (b) melanin level and (c) activity of cellular tyrosinase. ∗p < 0.05 vs. control group. (d) Maclurin did not show cytotoxicity at the concentrations tested. (e) Maclurin increased melanin levels in the reconstructed epidermis. (f) Maclurin increased the protein levels of melanogenesis-related genes: MITF, TRP-1, tyrosinase, and TRP-2. (g) Maclurin increased the mRNA levels of melanogenesis-related genes: TRP-1, TRP-2, MITF, and tyrosinase. ∗p < 0.05 vs. control group. M: maclurin; Fk: forskolin.
In the present study, we investigated the effects of maclurin on melanogenesis and its action mechanism in human epidermal melanocytes.
2. Materials and Methods
2.1. Materials and Cell Viability Assay
Moloney murine leukemia virus reverse transcriptase, random primers, and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA). TaqMan reverse transcription polymerase chain reaction (RT-PCR) reagents, primers, and probes were obtained from Applied Biosystems. Phorbol myristate acetate, anti-β-actin, forskolin, SB202190, KT5720, and antityrosinase were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-TRP-1, anti-MITF, and anti-TRP-2 were obtained from NeoMarkers (Fremont, CA, USA). Maclurin (purity: 99%) was purchased from Chirochem (Daejeon, Korea). Human epidermal melanocytes were purchased from Cascade Biologics (Portland, OR, USA) and cultured in Medium 254 (Cascade Biologics) supplemented with human melanocyte growth supplement at 37°C and 5% CO2. For the measurement of cell viability, we used the 5-bromo-2′-deoxyuridine (BrdU) incorporation assay [16]. BrdU incorporation was detected by an enzyme-linked immunosorbent assay (ELISA) using a BrdU Cell Proliferation Assay Kit (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's instructions.
2.2. Assay for Melanin Content
Melanin content was determined as previously described by Lee et al. [17]. Briefly, human epidermal melanocytes were treated with maclurin and then washed with PBS, harvested, and subjected to a melanin content assay. The harvested cell pellets were dissolved in 1 N NaOH (60°C) for 1 h, and the colorimetric analysis was conducted at 475 nm using a spectrophotometer. Melanin content is presented as the ratio of the maclurin-treated group to the control group (% of control).
2.3. Cellular Tyrosinase Activity Assay
The enzymatic activity of cellular tyrosinase was measured as described previously [18], with slight modifications. Briefly, human melanocytes were treated with maclurin and then washed with PBS and homogenized with buffer solution (50 mM sodium phosphate (pH 6.8), 1 mM phenylmethanesulfonyl fluoride, and 1% Triton X-100) at 4°C in a Dounce homogenizer. To collect the supernatant as the source of crude cellular tyrosinase, the lysates were centrifuged at 15,000 rpm for 15 min. A Bradford assay was used to measure the protein content in the supernatant, and bovine serum albumin was used as the protein standard. Cellular tyrosinase activity was then determined by measuring the absorbance at 470 nm. In this assay, 3,4-dihydroxyphenylalanine was used as a tyrosinase substrate.
2.4. Histochemistry of Reconstructed Epidermis
A reconstructed epidermis (MatTek) was incubated in a medium containing 50 μM maclurin for 2 weeks, with the medium replaced every two days. The epidermis was fixed with 4% formalin in PBS and subjected to Fontana-Masson staining to determine the levels of melanin pigments. For staining of the reconstructed epidermis, a Fontana-Masson Staining Kit (American MasterTech, Lodi, CA) was used, and quantification of the Fontana-Masson staining was conducted using ImageJ software (NIH, Bethesda, MD, USA) [19].
2.5. Analysis of mRNA Levels Using Real-Time RT-PCR
The real-time RT-PCR analysis was performed using an ABI7900HT Instrument (Applied Biosystems, Waltham, MA, USA). For the TaqMan analysis, predesigned or optimized assays on demand (Applied Biosystems) were used, including MITF (ID: Hs01117294_m1), tyrosinase (ID: Hs00165976_m1), TRP-1 (ID: Hs00167051_m1), TRP-2 (ID: Hs01098278_m1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ID: Hs00266705_g1), hypoxanthine-guanine phosphoribosyltransferase (HPRT) (Hs02800695_m1), and 18S (Hs03003631_g1). The data were analyzed using ABI Sequence Detector Software version 2.0 (Applied Biosystems). Total RNA was extracted from cells using TRI reagent® according to the manufacturer's instructions and stored at -70°C until use. cDNA was synthesized from total RNA (1 μg) using MuLV reverse transcriptase according to the manufacturer's instructions. The real-time RT-PCR analysis was conducted as previously described [20]. The results were normalized to the expression level of endogenous GAPDH and were also tested against two additional housekeeping genes (18S and HPRT). We found that the results were not significantly different from those obtained using GAPDH. Expression levels of the target genes were normalized to the levels observed in the controls. The results were verified by repeating each experiment four times in triplicate.
2.6. Western Blot Analysis, PKA Kinase Activity Assay, and Assay for Phosphorylated CREB Protein Levels
Levels of melanogenic proteins were measured by Western blot analysis after treatment with maclurin for 5 days. The Western blot analysis was conducted as previously described [9]. In brief, proteins separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis were transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA), which were then probed with appropriate primary and secondary antibodies (phospho CREB, phospho p44/42 MAPK, phospho nuclear factor-kappa B (NF-κB) p65 (Ser536), phospho c-Jun N-terminal kinase (JNK), phospho p38 MAPK, NF-κB p65, tyrosinase, CREB, TRP-2, p38 MAPK, MITF, p44/42 MAPK, TRP-1, and JNK). Finally, protein levels were measured using an enhanced chemiluminescence kit (Amersham, Piscataway, NJ, USA). A PKA kinase activity assay kit (Stressgen, Ann Arbor, MI, USA) was used according to the manufacturer's protocols to measure PKA kinase activity [21]. A PathScan® Phospho-CREB (Ser133) sandwich ELISA kit was used according to the manufacturer's instructions to determine phosphorylated CREB protein levels [22].
2.7. UV Irradiation and Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
Before UV irradiation, cultured cells were incubated with 1% serum medium for 12 h. Then, the cells were exposed to UVB radiation at an intensity of 20 mJ/cm2 (Luzchem Research Inc., Ottawa, Canada) [23]. After UVB irradiation, the cells were treated with maclurin. After incubation for the indicated time, cells were harvested and subjected to the ROS assay. To exclude the possibility that UVB irradiation was cytotoxic, cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were treated with MTT (0.1 mg/mL) for 3 h at 37°C in a 5% CO2 atmosphere. The medium was then removed, and the cells were solubilized with dimethyl sulfoxide (1 mL). After complete solubilization, the presence of blue formazan was evaluated spectrophotometrically by measuring the absorbance at 570 nm. For ROS measurement, cells were preloaded with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate for 30 min and then irradiated with UVB and treated with maclurin. The cells were lysed using 0.1% Triton X-100 solution, and the fluorescence intensity of the lysate was determined using a fluorometer (INFINITE M200, Tecan, Männedorf, Switzerland). In addition, ROS levels were observed directly using an Evos fluorescent microscope [24].
2.8. Statistical Analysis
For statistical analysis of the collected data, one-way analysis of variance was used, and statistical significance was accorded to a p value less than 0.05.
3. Results
3.1. Maclurin Promotes Melanogenesis in Human Epidermal Melanocytes
Maclurin concentration dependently increased both melanin content (Figure 1(b)) and cellular tyrosinase activity (Figure 1(c)) without any cytotoxicity at the concentrations tested (Figure 1(d)). In these experiments, forskolin was introduced as a positive control [17] because it increases both melanin content and cellular tyrosinase activity. In the Fontana-Masson staining and photography analysis, we found that maclurin treatment increased the level of melanin in the reconstructed epidermis (Figure 1(e)). The protein levels of MITF, tyrosinase, TRP-1, and TRP-2 increased with maclurin treatment (Figure 1(f)), and so did their mRNA levels (Figure 1(g)).
3.2. Maclurin Activates cAMP/PKA/CREB Signaling
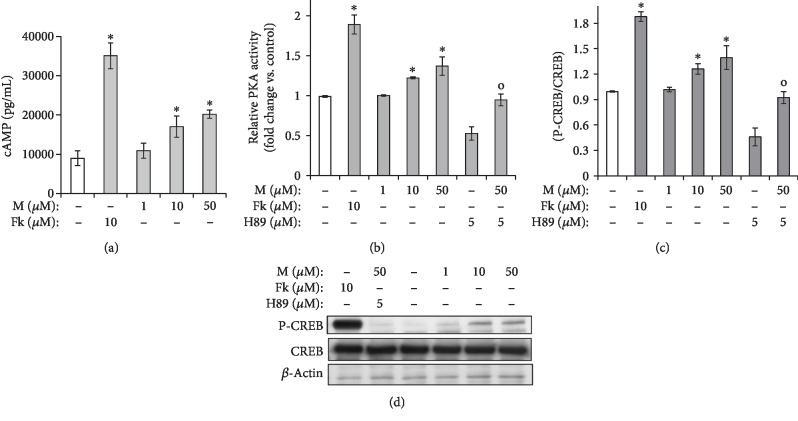
The cAMP/PKA/CREB signaling pathway is well characterized in melanogenic signaling [9]. Therefore, we investigated the effect of maclurin on signaling molecules in that pathway using ELISA for cAMP, PKA activity, and phosphorylated CREB and a Western blot analysis to confirm the phosphorylated CREB ELISA results. As shown in Figures 2(a)–2(c), maclurin treatment increased cAMP production, PKA activity, and CREB phosphorylation. The effect of maclurin on CREB phosphorylation was also confirmed in the Western blot analysis (Figure 2(d)). Those molecular effects were attenuated by treatment with H89 (a PKA inhibitor) (Figures 2(b)–2(d)). In these experiments, forskolin and H89 were introduced as a positive control and a negative control, respectively [17]. Forskolin increased cAMP production, PKA activity, and CREB phosphorylation, and H89 reduced PKA activity and CREB phosphorylation.
Figure 2.
Maclurin activated the cAMP-PKA-CREB pathway. (a) cAMP production assay and (b) ELISA were performed to analyze PKA activity. (c) The CREB phosphorylation levels were measured using an ELISA kit and confirmed by (d) a Western blot analysis. ∗p < 0.05 vs. control group, op < 0.05 vs. maclurin (50 μM)-treated control. M: maclurin; Fk: forskolin.
3.3. p38 MAPK and p44/42 MAPK Are Affected by Maclurin
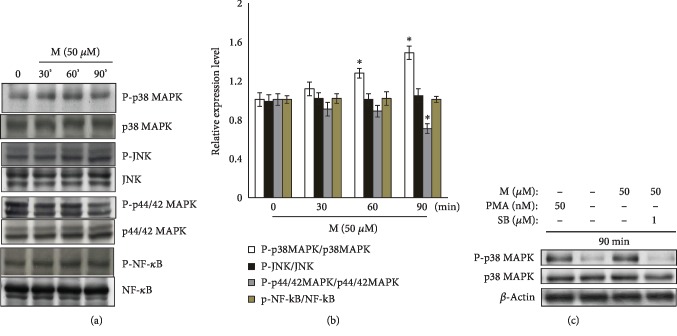
The MAPKs and NF-κB are involved in melanin synthesis, so we examined their involvement in maclurin-induced melanogenesis. Among the MAPKs tested, maclurin had no effect on JNK, but it did affect p38 MAPK and p44/42 MAPK. Specifically, maclurin induced the phosphorylation of p38 MAPK and suppressed the phosphorylation of p44/42 MAPK (Figures 3(a) and 3(b)). The effects of maclurin on p38 MAPK were attenuated by treatment with SB203580 (a p38 MAPK inhibitor) (Figure 3(c)). Maclurin had no effect on NF-κB activation (Figures 3(a) and 3(b)).
Figure 3.
Maclurin activated p38 MAPK and inhibited p44/42 MAPK. (a) Maclurin activated p38 mitogen-activated protein kinase (MAPK) and inhibited p44/42 MAPK but not JNK or NF-κB. ∗p < 0.05 vs. control group. (b) A Western blot densitometric analysis was performed. (c) SB203580 attenuated the effects of maclurin on p38 MAPK phosphorylation. M: maclurin; PMA: phorbol 12-myristate 13-acetate; SB: SB203580; P-NF-κB: phospho-NF-κB p65 (Ser536); NF-κB: NF-κB p65.
3.4. The Effects of Maclurin on Pigmentation Are Mediated through the Activation of p38 MAPK and cAMP/PKA Signaling
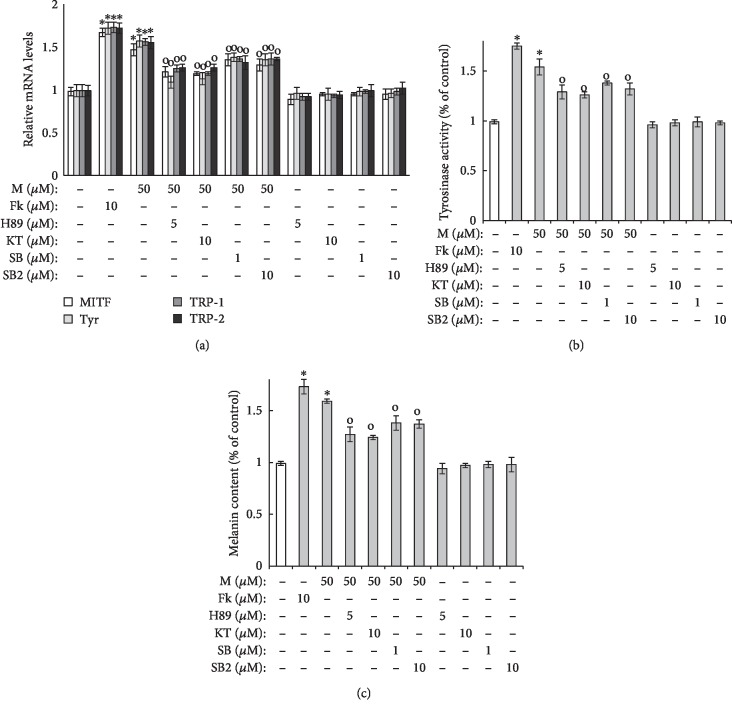
As shown in Figure 4(a), maclurin treatment enhanced the mRNA levels of TRP-1, TRP-2, MITF, and tyrosinase, and that effect was reduced by the p38 MAPK inhibitors SB203580 and SB202190 and the PKA inhibitors H89 and KT5720. Similar results were found in the cellular tyrosinase activity and melanin content assays using SB203580/SB202190 and H89/KT5720. As shown in Figures 4(b) and 4(c), tyrosinase activity and melanin content were increased by maclurin treatment, and those effects were attenuated by SB203580/SB202190 and H89/KT5720.
Figure 4.
p38 MAPK and cAMP-PKA pathways mediated maclurin-induced melanogenesis. (a) Real-time PCR analysis, (b) cellular tyrosinase activity assay, and (c) melanin content assay results. ∗p < 0.05 vs. control group, op < 0.05 vs. maclurin (50 μM)-treated control. SB: SB203580; SB2: SB202190; KT: KT5720; M: maclurin; Fk: forskolin.
3.5. p38 MAPK and cAMP/PKA Signaling Reciprocally Communicate through CREB Phosphorylation in Maclurin-Induced Pigmentation
To examine the relationship between cAMP/PKA signaling and p38 MAPK signaling in maclurin-induced melanogenesis, we examined the effects of H89 and SB203580 on CREB phosphorylation levels. As shown in Figure 5, H89 and SB203580 both reduced the CREB phosphorylation induced by maclurin. In addition, the combined treatment of SB203580 and H89 synergistically inhibited the effects of maclurin on CREB phosphorylation.
Figure 5.
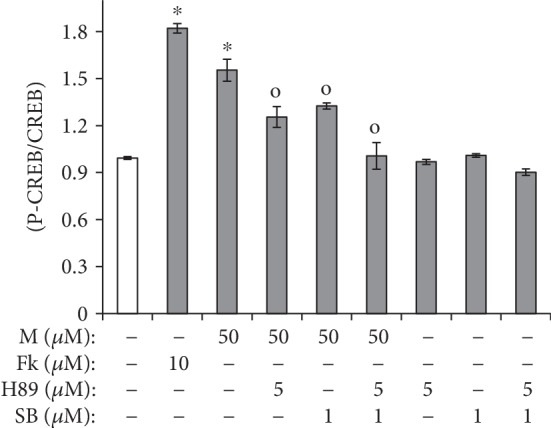
Maclurin activated CREB phosphorylation through both PKA and p38 MAPK activity. Enzyme-linked immunosorbent assays (ELISAs) were performed to analyze protein kinase A (PKA). ∗p < 0.05 vs. control group, op < 0.05 vs. maclurin (50 μM)-treated control. SB: SB203580; M: maclurin; Fk: forskolin.
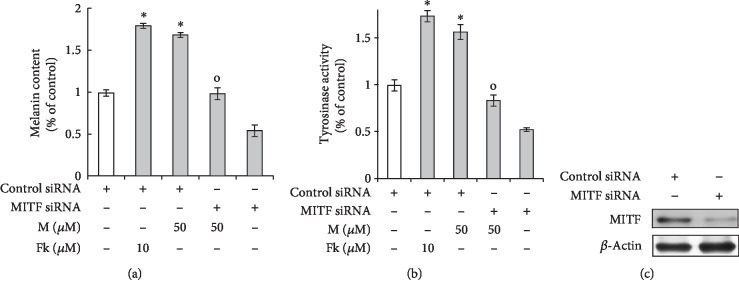
3.6. The Melanogenic Effects of Maclurin Depend on Increased MITF Gene Expression
We confirmed that the melanogenic effects of maclurin are mediated by upregulation of the MITF gene. As shown in Figures 6(a) and 6(b), we found that knockdown of MITF using siRNA attenuated the effects of maclurin, such as increased melanin levels and tyrosinase activity. MITF siRNA successfully knocked down MITF protein in human melanocytes compared with the level in cells transfected with control siRNA (Figure 6(c)). However, we did not perform related experiments under overexpression of MITF gene. These data indicate that maclurin increases pigmentation by inducing MITF-dependent signaling.
Figure 6.
Maclurin-induced pigmentation was attenuated by MITF knockdown. (a, b) Knockdown of MITF attenuated the effects of maclurin on (a) melanin levels and (b) cellular tyrosinase activity. (c) Western blot analysis of MITF. ∗p < 0.05 vs. control group, op < 0.05 vs. maclurin (50 μM)-treated control. M: maclurin; Fk: forskolin.
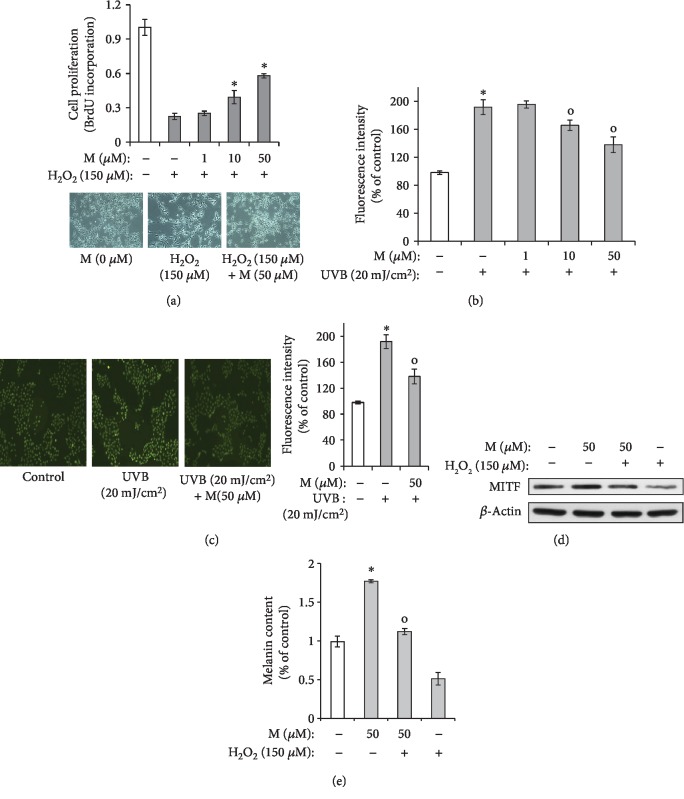
3.7. Maclurin Protects Melanocytes against H2O2 and UVB
As shown in Figure 7(a), we found that maclurin significantly inhibited hydrogen peroxide-induced reductions in cell survival. In addition, UVB-induced production of ROS was attenuated by maclurin treatment (Figures 7(b) and 7(c)). As shown in Figures 7(d) and 7(e), hydrogen peroxide reduced MITF expression levels and melanin content. However, maclurin treatment attenuated the effects of H2O2. These results indicate that maclurin protects melanocytes from H2O2-induced damage.
Figure 7.
Maclurin protected human epidermal melanocytes against the H2O2-mediated decrease in cell viability. (a) The BrdU incorporation assay was used to measure the percentage of viable cells 14 h after H2O2 treatment. ∗p < 0.05 vs. control group, op < 0.05 vs. H2O2 (150 μM)-treated control. (b) Effects of maclurin on UVB-induced ROS production in human epidermal melanocytes. ROS-induced dichlorofluorescein (DCF) formation was measured using a spectrophotometer. ∗p < 0.05 vs. control group, op < 0.05 vs. UVB-irradiated control. (c) ROS-induced DCF fluorescence was visualized by fluorescence microscopy. ∗p < 0.05 vs. control group, op < 0.05 vs. UVB-irradiated control. (d, e) Effects of maclurin on the H2O2-induced reduction of MITF protein and melanin content in human epidermal melanocytes. ∗p < 0.05 vs. control group, op < 0.05 vs. H2O2-treated control. SB: SB203580; M: maclurin.
4. Discussion
In this study, we have demonstrated the stimulatory effects of maclurin on melanogenesis in human epidermal melanocytes. Maclurin increased MITF gene expression and melanin formation. The results indicate that the melanogenic effects of maclurin occur mainly through MITF upregulation, which is mediated by two different signaling pathways, p38 MAPK and cAMP/PKA signaling. The signals from these two pathways commonly target CREB for phosphorylation, which upregulates MITF. In addition, the results indicate that maclurin has antioxidant and protective effects against H2O2 and UVB, as well as a pigmenting effect, suggesting that maclurin could be useful as a protective agent against hypopigmented skin disorders.
The MITF protein is essential for the expression of melanogenic genes. In this study, we found that maclurin increased the production of tyrosinase, TRP-1, TRP-2, and MITF. Among them, TRP-1, a 75 kDa protein synthesized in the endoplasmic reticulum, is transported through the Golgi and transferred to melanosomes. The cytoplasmic tail of TRP-1 drives its transfer to the melanosome through a retention signal sequence [25, 26]. TRP-1 is also expressed in the melanosome on the cell surface of melanocytes [27]. In melanogenesis, TRP-1 increases tyrosinase activity by stabilizing it through complex formation. In addition, TRP-1 is involved in the proliferation and morphology of melanocytes [28]. In this study, we found that maclurin upregulated the TRP-1 gene, suggesting that maclurin could contribute to the maintenance of melanocyte biology. TRP-2 (dopachrome tautomerase) converts dopachrome (5,6-dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid) to DHICA (5,6-dihydroxyindole-2-carboxylic acid), which affects eumelanin but not pheomelanin synthesis. During pheomelanin synthesis, TRP-2 is reduced [29] and disappears in the bulbar melanocytes of mice producing predominantly pheomelanin [30]. The deletion of the TRP-2 gene in mice leads to the dilution of their coat color and a reduction of the melanin content in their hair shafts [31–34]. Therefore, our results suggest that maclurin contributes to eumelanin synthesis.
The melanogenic signaling pathways include p38 MAPK signaling, the cAMP-mediated pathway, the PKC-mediated pathway, PI3K/AKT signaling, and the p44/42 MAPK pathway. We found that maclurin induced p38 MAPK phosphorylation, and SB203580 attenuated maclurin-induced melanin synthesis. Maclurin also increased the levels of intracellular cAMP, activated PKA, increased the phosphorylation of the CREB protein, and decreased the phosphorylation levels of p44/42 MAPK. However, maclurin showed no effects on the PKC-mediated pathway or the PI3K/AKT signaling pathway. These data indicate that the melanogenic effects of maclurin are mediated by the p38 MAPK, cAMP/PKA, and p44/42 MAPK signaling pathways. Furthermore, we found that maclurin induced CREB phosphorylation through two different signaling pathways, cAMP/PKA and p38 MAPK, suggesting that cAMP/PKA and p38 MAPK-CREB phosphorylation synergistically contribute to maclurin-induced melanogenesis.
Oxidative stress induces depigmentation by downregulating MITF and the MITF-dependent melanogenic enzymes that contribute to melanocyte survival [35]. Vitiligo is a depigmentation skin disorder that results from the death of melanocytes [36]. A combination of antioxidants and melanogenic inducers has shown promising therapeutic efficiency in vitiligo patients, increasing pigmentation levels and cell survival [37]. In our study, maclurin attenuated the reduction in cell survival caused by H2O2 exposure and reduced UVB-induced ROS production, in addition to its melanogenic effects. Therefore, maclurin could be useful in treating vitiligo.
Although the antimetastatic and antioxidant activities of maclurin have been reported, its activities in skin physiology have not previously been elucidated. In this study, we demonstrated that maclurin has melanogenic activity and that its effects depend on activation of the cAMP/PKA and p38 MAPK signaling pathways. Specifically, maclurin induced melanogenesis by activating CREB through p38 MAPK- and cAMP/PKA-dependent pathways and increasing the expression of the MITF gene.
5. Conclusions
Collectively, the results of this study indicate that maclurin regulates melanogenesis by increasing the expression of the MITF gene through the p38 MAPK- and cAMP/PKA-dependent activation of CREB. Therefore, although further research about its efficacy and safety in clinical studies is necessary, maclurin could be used to treat hypopigmented skin disorders such as vitiligo.
Acknowledgments
This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2017R1D1A1B03032513).
Abbreviations
- MITF:
Microphthalmia-associated transcription factor
- TRP:
Tyrosinase-related protein
- cAMP:
Cyclic adenosine monophosphate
- CREB:
cAMP-response element binding protein
- PKA:
Protein kinase A
- JNK:
c-Jun N-terminal kinase
- MAPKs:
Mitogen-activated protein kinases
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B cells
- BrdU:
5-Bromo-2′-deoxyuridine
- DCF:
Dichlorofluorescein.
Contributor Information
Jae Youl Cho, Email: jaecho@skku.edu.
Jongsung Lee, Email: bioneer@skku.edu.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
Young Sun Hwang, Sae Woong Oh, See-Hyoung Park, Jae Youl Cho, and Jongsung Lee conceived and designed the experiments; Young Sun Hwang, Sae Woong Oh, See-Hyoung Park, Jienny Lee, Ju Ah Yoo, Kitae Kwon, Se Jung Park, Jangsoon Kim, and Eunbi Yu performed the experiments; Young Sun Hwang, Sae Woong Oh, See-Hyoung Park, Jienny Lee, Ju Ah Yoo, Kitae Kwon, Se Jung Park, Jangsoon Kim, Eunbi Yu, Jae Youl Cho, and Jongsung Lee analyzed the data; and Young Sun Hwang, Sae Woong Oh, See-Hyoung Park, Jae Youl Cho, and Jongsung Lee wrote the paper. Young Sun Hwang, Sae Woong Oh, and See-Hyoung Park contributed equally to this work.
References
- 1.Bickers D. R., Athar M. Oxidative stress in the pathogenesis of skin disease. The Journal of Investigative Dermatology. 2006;126(12):2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi N., Nakagawa A., Muramatsu T., et al. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. The Journal of Investigative Dermatology. 1998;110(5):806–810. doi: 10.1046/j.1523-1747.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.del Marmol V., Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Letters. 1996;381(3):165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 4.Scott G. A., Jacobs S. E., Pentland A. P. sPLA2-X stimulates cutaneous melanocyte dendricity and pigmentation through a lysophosphatidylcholine-dependent mechanism. The Journal of Investigative Dermatology. 2006;126(4):855–861. doi: 10.1038/sj.jid.5700180. [DOI] [PubMed] [Google Scholar]
- 5.Seiberg M., Paine C., Sharlow E., et al. Inhibition of melanosome transfer results in skin lightening. The Journal of Investigative Dermatology. 2000;115(2):162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 6.Hodgkinson C. A., Moore K. J., Nakayama A., et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74(2):395–404. doi: 10.1016/0092-8674(93)90429-T. [DOI] [PubMed] [Google Scholar]
- 7.Kang W. H., Yoon K. H., Lee E. S., et al. Melasma: histopathological characteristics in 56 Korean patients. British Journal of Dermatology. 2002;146(2):228–237. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahn J. H., Jin S. H., Kang H. Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Archives of Dermatological Research. 2008;300(6):325–329. doi: 10.1007/s00403-008-0863-0. [DOI] [PubMed] [Google Scholar]
- 9.Kang M., Park S. H., Park S. J., et al. p44/42 MAPK signaling is a prime target activated by phenylethyl resorcinol in its anti-melanogenic action. Phytomedicine. 2019;58:p. 152877. doi: 10.1016/j.phymed.2019.152877. [DOI] [PubMed] [Google Scholar]
- 10.Khaled M., Larribere L., Bille K., Ortonne J. P., Ballotti R., Bertolotto C. Microphthalmia associated transcription factor is a target of the phosphatidylinositol-3-kinase pathway. The Journal of Investigative Dermatology. 2003;121(4):831–836. doi: 10.1046/j.1523-1747.2003.12420.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee J., Jung K., Kim Y. S., Park D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sciences. 2007;81(3):249–254. doi: 10.1016/j.lfs.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Chung B. Y., Kim S. Y., Jung J. M., et al. The antimycotic agent clotrimazole inhibits melanogenesis by accelerating ERK and PI3K-/Akt-mediated tyrosinase degradation. Experimental Dermatology. 2015;24(5):386–388. doi: 10.1111/exd.12669. [DOI] [PubMed] [Google Scholar]
- 13.Chang L. W., Juang L. J., Wang B. S., et al. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food and Chemical Toxicology. 2011;49(4):785–790. doi: 10.1016/j.fct.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Ku M. J., Kim J. H., Lee J., Cho J. Y., Chun T., Lee S. Y. Maclurin suppresses migration and invasion of human non-small-cell lung cancer cells via anti-oxidative activity and inhibition of the Src/FAK-ERK-β-catenin pathway. Molecular and Cellular Biochemistry. 2015;402(1-2):243–252. doi: 10.1007/s11010-015-2331-4. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Gao Y., Li F., et al. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: antioxidant evaluation and mechanistic insight. Chemico-Biological Interactions. 2014;219:221–228. doi: 10.1016/j.cbi.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Kortüm F., Harms F. L., Hennighausen N., Rosenberger G. αPIX is a trafficking regulator that balances recycling and degradation of the epidermal growth factor receptor. PLoS One. 2015;10(7):p. e0132737. doi: 10.1371/journal.pone.0132737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S. E., Park S. H., Oh S. W., et al. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK-mediated pathways. Scientific Reports. 2018;8(1):p. 14958. doi: 10.1038/s41598-018-33352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curto E. V., Kwong C., Hermersdörfer H., et al. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochemical Pharmacology. 1999;57(6):663–672. doi: 10.1016/S0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y., Cho J. Y., Oh S. W., et al. Globular adiponectin acts as a melanogenic signal in human epidermal melanocytes. The British Journal of Dermatology. 2018;179(3):689–701. doi: 10.1111/bjd.16488. [DOI] [PubMed] [Google Scholar]
- 20.Hwang Y. S., Park S. H., Kang M., et al. Stemness and differentiation potential-recovery effects of sinapic acid against ultraviolet-A-induced damage through the regulation of p38 MAPK and NF-κB. Scientific Reports. 2017;7(1):p. 909. doi: 10.1038/s41598-017-01089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang Y. S., Kim Y. J., Kim M. O., et al. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chemico-Biological Interactions. 2017;273:107–114. doi: 10.1016/j.cbi.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Jung E., Kim J. H., Kim M. O., et al. Afzelin positively regulates melanogenesis through the p38 MAPK pathway. Chemico-Biological Interactions. 2016;254:167–172. doi: 10.1016/j.cbi.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Shin S. W., Jung E., Kim S., et al. Antagonizing effects and mechanisms of afzelin against UVB-induced cell damage. PLoS One. 2013;8(4):p. e61971. doi: 10.1371/journal.pone.0061971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes A., Fernandes E., Lima J. L. F. C. Fluorescence probes used for detection of reactive oxygen species. Journal of Biochemical and Biophysical Methods. 2005;65(2-3):45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Vijayasaradhi S., Doskoch P. M., Houghton A. N. Biosynthesis and intracellular movement of the melanosomal membrane glycoprotein gp75, the human b (brown) locus product. Experimental Cell Research. 1991;196(2):233–240. doi: 10.1016/0014-4827(91)90256-t. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y., Vijayasaradhi S., Houghton A. N. The cytoplasmic tail of the mouse brown locus product determines intracellular stability and export from the endoplasmic reticulum. The Journal of Investigative Dermatology. 1998;110(4):324–331. doi: 10.1038/jid.1998.4. [DOI] [PubMed] [Google Scholar]
- 27.Takechi Y., Hara I., Naftzger C., Xu Y., Houghton A. N. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clinical Cancer Research. 1996;2(11):1837–1842. [PubMed] [Google Scholar]
- 28.Li C. Y., Gao T. W., Wang G., et al. The effect of antisense tyrosinase-related protein 1 on melanocytes and malignant melanoma cells. British Journal of Dermatology. 2004;150(6):1081–1090. doi: 10.1111/j.1365-2133.2004.05929.x. [DOI] [PubMed] [Google Scholar]
- 29.Furumura M., Sakai C., Potterf S. B., Vieira W. D., Barsh G. S., Hearing V. J. Characterization of genes modulated during pheomelanogenesis using differential display. Proc Natl Acad Sci U S A. 1998;95(13):7374–7378. doi: 10.1073/pnas.95.13.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T., Vieira W. D., Potterf B., Sakai C., Imokawa G., Hearing V. J. Modulation of melanogenic protein expression during the switch from eu- to pheomelanogenesis. Journal of Cell Science. 1995;108(6):2301–2309. doi: 10.1242/jcs.108.6.2301. [DOI] [PubMed] [Google Scholar]
- 31.Guyonneau L., Murisier F., Rossier A., Moulin A., Beermann F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Molecular and Cellular Biology. 2004;24(8):3396–3403. doi: 10.1128/mcb.24.8.3396-3403.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson I. J., Chambers D. M., Tsukamoto K., et al. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. The EMBO Journal. 1992;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroumpouzos G., Urabe K., Kobayashi T., Sakai C., Hearing V. J. Functional analysis of the slaty gene product (TRP2) as dopachrome tautomerase and the effect of a point mutation on its catalytic function. Biochemical and Biophysical Research Communications. 1994;202(2):1060–1068. doi: 10.1006/bbrc.1994.2036. [DOI] [PubMed] [Google Scholar]
- 34.Lamoreux M. L., Wakamatsu K., Ito S. Interaction of major coat color gene functions in mice as studied by chemical analysis of eumelanin and pheomelanin. Pigment Cell Research. 2001;14(1):23–31. doi: 10.1034/j.1600-0749.2001.140105.x. [DOI] [PubMed] [Google Scholar]
- 35.Widlund H. R., Fisher D. E. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22(20):3035–3041. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- 36.Gauthier Y., Cario-Andre M., Lepreux S., Pain C., Taieb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. British Journal of Dermatology. 2003;148(1):95–101. doi: 10.1046/j.1365-2133.2003.05024.x. [DOI] [PubMed] [Google Scholar]
- 37.Passi S., Grandinetti M., Maggio F., Stancato A., De Luca C. Epidermal oxidative stress in vitiligo. Pigment Cell Research. 1998;11(2):81–85. doi: 10.1111/j.1600-0749.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.