Abstract
Our purpose is to provide a framework for diagnosing the inherited causes of marked high-density lipoprotein (HDL) deficiency (HDL cholesterol levels <10 mg/dL in the absence of severe hypertriglyceridemia or liver disease) and to provide information about coronary heart disease (CHD) risk for such cases. Published articles in the literature on severe HDL deficiencies were used as sources. If apolipoprotein (Apo) A-I is not present in plasma, then three forms of ApoA-I deficiency, all with premature CHD, and normal low-density lipoprotein (LDL) cholesterol levels have been described: ApoA-I/C-III/A-IV deficiency with fat malabsorption, ApoA-I/C-III deficiency with planar xanthomas, and ApoA-I deficiency with planar and tubero-eruptive xanthomas (pictured in this review for the first time). If ApoA-I is present in plasma at a concentration <10 mg/dL, with LDL cholesterol that is about 50% of normal and mild hypertriglyceridemia, a possible diagnosis is Tangier disease due to mutations at the adenosine triphosphate binding cassette protein A1 (ABCA1) gene locus. These patients may develop premature CHD and peripheral neuropathy, and have evidence of cholesteryl ester–laden macrophages in their liver, spleen, tonsils, and Schwann cells, as well as other tissues. The third form of severe HDL deficiency is characterized by plasma ApoA-I levels <40 mg/dL, moderate hypertriglyceridemia, and decreased LDL cholesterol, and the finding that most of the cholesterol in plasma is in the free rather than the esterified form, due to a deficiency in lecithin:cholesterol acyltransferase activity. These patients have marked corneal opacification and splenomegaly, and are at increased risk of developing renal failure, but have no clear evidence of premature CHD. Marked HDL deficiency has different etiologies and is generally associated with early CHD risk.
Keywords: Apolipoprotein A-I deficiency, ATP binding cassette protein 1 dysfunction, Coronary heart disease, High-density lipoprotein deficiency, Lecithin:cholesterol acyltransferase deficiency, Tangier disease
The purpose of this review is to provide the clinical lipidologist with clues that should help distinguish the inherited causes of marked plasma high-density lipoprotein (HDL) cholesterol deficiency with values <10 mg/dL in the absence of severe hypertriglyceridemia (values >1000 mg/dL after an overnight fast) or significant liver disease with evidence of cirrhosis. With severe hypertriglyceridemia, HDL cholesterol levels are rarely <10 mg/dL, and will usually increase to >20 mg/dL when the triglycerides are reduced to <500 mg/dL.1 In patients with chronic liver disease, an HDL cholesterol <10 mg/dL is a sign of significant hepatocellular damage, indicating that the liver can no longer make adequate amounts of the enzyme lecithin: cholesterol acyl transferase (LCAT).2 Severe HDL deficiency can also occur in subjects using anabolic steroids or the combination of a fibrate and a thiazolidinedione, as well as patients with monoclonal gammopathies.3 HDL-cholesterol concentrations <10 mg/dL are uncommon in patients affected with apolipoprotein (Apo) A-IMilano or other ApoA-I variants.4
In this review, we not only provide a conceptual framework of the etiology of severe HDL-deficiency states, but also an explanation for why some of these disorders are linked to premature coronary heart disease (CHD) and others are not. In the general population, low levels of HDL cholesterol (<40 mg/dL) have been defined as an independent risk factor for CHD by the third Adult Treatment Panel of the National Cholesterol Education Program.5
In family studies of patients with premature CHD, about 15% have familial dyslipidemia (elevated triglycerides and decreased HDL cholesterol), about 15% have familial combined hyperlipidemia (elevated triglycerides and low-density lipoprotein [LDL] cholesterol, and decreased HDL cholesterol), and about 5% have isolated low HDL also known as hypoalphalipoproteinemia.5–8 If one selects for patients with CHD and low HDL cholesterol, as occurred in the Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT), one often finds patients who are overweight or obese with elevated insulin levels.9–11 However, patients with HDL cholesterol levels <10 mg/dL are rare. Three disorders with definable genetic causes usually fall into one of three categories: ApoA-I deficiency, Tangier disease (defective cellular cholesterol efflux due to defects in the ATP binding cassette transporter A1 or ABCA1), or LCAT deficiency.12–49
Clinical diagnosis
The astute and experienced lipidologist can often make the diagnosis of the cause of severe HDL deficiency after obtaining a complete history, doing a complete physical examination, and, most importantly, reviewing all the laboratory data, including a comprehensive metabolic profile, a lipid profile, and obtaining a plasma ApoA-I level (see Tables 1–3). Very low levels of HDL cholesterol can be observed in patients with marked hypertriglyceridemia, monoclonal gammopathy or severe liver disease, or in subjects taking anabolic steroids or the combination of a fibrate and a thiazolidinedione.3 These conditions can be distinguished by a metabolic profile including bilirubin, alkaline phosphatase, and liver transaminase measurements, protein electrophoresis, a fasting lipid profile, and a good medical history. If it is due to medications, baseline HDL concentration should be restored within a few weeks after discontinuation.
Table 1.
Characteristics of homozygotes in kindreds with severe high-density lipoprotein deficiency
| Approximate laboratory values | ApoA-I deficiency | Tangier disease | LCAT deficiency* |
|---|---|---|---|
| Total cholesterol (mg/dL) | 150 | 90 | 110 |
| Triglyceride (mg/dL) | 80 | 180 | 200 |
| HDL-C (mg/dL) | 2 | 3 | 9 |
| LDL-C (mg/dL) | 130 | 60 | 65 |
| ApoA-I (mg/dL) | 0 | 4 | 35 |
| Clinical findings | |||
| Xanthomas | Can have planar or tubero-eruptive | Negative | Negative |
| Corneal opacification | Mild–moderate | Very mild | Striking |
| Coronary heart disease risk | +++ | + | ? |
Table 3.
Characteristics of homozygotes with marked high-density lipoprotein deficiency and detectable plasma apolipoprotein A-I levels
| Disorder | Tangier disease* | Partial LCAT deficiency (fish-eye disease)* | LCAT deficiency* |
|---|---|---|---|
| Triglyceride | Increased | 50% increased | 50% increased |
| LDL-C | Decreased | 50% of normal | Decreased |
| HDL-C | <5 mg/dL | 10% of normal | <5 mg/dL |
| ApoA-I | <10 mg/dL | 20% of normal | <40 mg/dL |
| ApoC-III | Present | Present | Present |
| ApoA-IV | Present | Present | Present |
| Xanthomas | Negative | Negative | Negative |
| Corneal opacification | Mild | Positive | Positive |
| Premature CHD | Positive | Not striking | Not striking |
Apo, apolipoprotein; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol; LCAT, lecithin:cholesterol acyl transferase; LDL-C, low-density lipoprotein cholesterol.
In the absence of these conditions, the next step is to measure plasma ApoA-I levels by immunoassay. Patients who lack plasma ApoA-I can be distinguished from Tangier patients and those with LCAT deficiency because they have undetectable plasma ApoA-I levels, and normal or decreased triglyceride levels, as well as normal LDL cholesterol values (see Table 1).12–49 If ApoA-I is undetectable, then ApoC-III and ApoA-IV should be measured because these proteins are all coded for by the adjacent A-I, C-III, and A-IV genes. With regard to those without plasma ApoA-I, patients who lack plasma ApoA-I, ApoC-III, and ApoA-IV have no xanthomas, but do have evidence of fat malabsorption, as well as decreased triglyceride levels and an increased risk of premature CHD.12,13,18 Patients who lack ApoA-I and ApoC-III have planar xanthomas, have not been reported to have any evidence of fat malabsorption, and have decreased triglycerides, as well as an increased risk of premature CHD.19–25 Patients who have isolated ApoA-I deficiency usually have either planar xanthomas, tubero-eruptive xanthomas, or both, and normal triglyceride and LDL cholesterol levels.26–35 Moreover these latter cases are also at markedly increased risk of developing premature CHD.
Patients with Tangier disease have ApoA-I present in their plasma, and have evidence of orange mucosa in their oral pharynx and on colonoscopy, as well as somewhat elevated triglycerides and LDL cholesterol levels that are about 50% of normal.13,36–46 Patients with various forms of LCAT deficiency have marked corneal opacification, elevated triglycerides, and LDL cholesterol levels that are about 50% of normal.13,47–50 Laboratory values and clinical features of such cases are shown in Tables 1–3.
Standard diagnostic testing
Plasma or serum cholesterol, triglycerides, and HDL cholesterol can be measured after an overnight fast, and LDL cholesterol is calculated using the Friedewald formula (LDL cholesterol = total cholesterol − HDL cholesterol − triglyceride/5). In our experience, this formula is quite accurate for estimating LDL cholesterol, provided the patient is fasting and triglyceride values are <250 mg/dL, and also provided that the laboratory participates in a recognized standardization program. In addition to our experience, values obtained for online direct HDL cholesterol are very similar to those obtained following precipitation, and the same is true for direct online LDL cholesterol levels compared to estimated or calculated LDL cholesterol, or values obtained following ultracentrifugation (1.006 g/mL infranatant − HDL cholesterol). Serum or plasma ApoA-I, ApoC-III, and ApoA-IV levels can easily be assessed by immunoassay using assays obtained from Wako Chemicals, Inc. (Richmond, VA) or other vendors as described previously.31,40,44–46 If patients lack both ApoA-I and ApoC-III, then a deletion or a DNA rearrangement within the ApoA-I/C-III/A-IV gene complex is likely, whereas if only ApoA-I is undetectable, then a mutation in the ApoA-I gene is likely.12,17–35 If ApoA-I is present, then the amount of free cholesterol relative to cholesteryl ester in plasma and the HDL fraction, as well as LCAT mass and activity, should be determined as previously described.47–49 If approximately 70% of the total cholesterol in plasma is in the esterified form, ie, as cholesteryl ester, then the diagnosis of LCAT deficiency is ruled out. Tangier disease then becomes the most likely diagnosis.36–46 In this disorder, homozygotes have defective cellular cholesterol efflux due to mutations in ABCA1, resulting in only pre–β-1 HDL being present in plasma, whereas in LCAT deficiency states both pre–β-1 and α-4 HDL are present in plasma, based on two-dimensional gel electrophoresis.40,45 Therefore, two-dimensional gel electrophoresis of HDL particles is also very helpful in distinguishing these various causes of low HDL.
Use of two-dimensional gel electrophoresis of ApoA-I–containing HDL particles is very useful in distinguishing between the various forms of these disorders. Normal plasma ApoA-I values are about 140 mg/dL, and of this amount about 14 mg/dL or 10% is found in two small, discoidal pre–β-1 HDL particles, and about 2 mg/dL or 1.5% is found in small discoidal α-4 and pre–α-4 HDL. The remainder of about 126 mg/dL or 90% is found mainly in larger spherical α-2 and α-3 HDL containing both ApoA-I and A-II or even larger spherical α-1 HDL or in the adjacent large, spherical pre–α-1 HDL, containing ApoA-I, but not ApoA-II. Lumped together with these particles are their adjacent pre–-αHDL particles containing ApoA-I, but no ApoA-II. In the past we have lumped α-3 and α-4 particles together because we could not separate them well.
Description of individual disorders
ApoA-I/C-III/A-IV deficiency
The index case of A-I/C-III/A-IV deficiency was described in 1982.12 This patient had marked HDL deficiency, low triglycerides, normal LDL cholesterol levels, no xanthomas, and severe premature coronary artery disease. She had no history of diabetes, smoking, or hypertension, and was premenopausal. At the age 43 years, she died during coronary artery bypass surgery.12,13 At her autopsy, severe diffuse coronary atherosclerosis was documented.12 The defect in this kindred was subsequently found to be a large deletion of the entire APOA1/C3/A4 gene cluster.18 The index case proved to be a homozygote in a kindred of English origin, residing in northern Alabama. This patient also had decreased plasma levels of the fat-soluble vitamins A, D, and E (<50% of normal) and a moderately prolonged prothrombin time, consistent with malabsorption of fat and fat-soluble vitamins.7,18 Heterozygotes were found to have plasma HDL cholesterol, ApoA-I, ApoC-III, and ApoA-IV levels that were about 50% of normal.18 ApoA-I gene transfection studies indicated that ApoA-I was essential for HDL formation.18
ApoA-I/C-III deficiency
Familial ApoA/C-III deficiency was reported in two sisters with marked HDL deficiency, planar xanthomas, and CHD at ages 29 and 30 years.19 They had no history of smoking, hypertension, or diabetes, and no fat malabsorption. Their triglyceride levels were reduced, and their LDL cholesterol levels were normal. The genetic defect was subsequently found to be a DNA rearrangement affecting the adjacent APO-A1 and APO-C3 genes, resulting in total lack of production of these two apolipoproteins, and their absence from plasma.19,20 It was also subsequently reported that these homozygotes had small amounts of ApoA-II–containing HDL, and enhanced clearance of very low–density lipoprotein triglyceride and ApoB, presumably because there was no ApoC-III present in plasma to inhibit lipolysis.22–25 This kindred was described as having familial APO-AI/CIII deficiency. A second kindred with premature CHD, marked HDL deficiency, and absence of ApoA-I and C-III in plasma has also been described.27
Familial ApoA-I deficiency
A deficiency of only ApoA-I was initially described in a 56-year-old Japanese female with premature CHD, yellow-orange planar xanthomata, normal triglycerides, and LDL-cholesterol levels, marked HDL deficiency, and undetectable plasma ApoA-I levels.28 Heterozygotes were noted in the kindred. A nonsense mutation was reported in codon 84.
A second kindred with ApoA-I deficiency was found in Canada in a kindred of mixed European ancestry.29,30 The proband was a 34-year-old female who presented with premature CHD and plasma HDL cholesterol of 2 mg/dL, but elevated triglycerides and LDL cholesterol levels. She had mildly thickened Achilles tendons, xanthelasma, mild mid-line cerebellar ataxia, and asymmetric bilateral neurosensory hearing loss. She also had bilateral cataracts, and bilateral subretinal lipid deposition with exudative proliferative retinopathy, with resultant bilateral retinal detachments requiring surgical repair. Her ApoA-I levels were undetectable and the investigators noted a nonsense mutation in the ApoA-I gene was at codon −2. Four other homozygotes were described from this pedigree with marked HDL deficiency (mean 4 mg/dL), normal triglycerides (mean 123 mg/dL), and elevated LDL cholesterol (mean 175 mg/dL). One homozygous sister at age 38 years had xanthelasma, Achilles tendon xanthomas, planar xanthomas in the interdigital folds of the hands and the anticubital and popliteal fossae. She had a myocardial infarction at age 34 years, and had coronary artery bypass grafting surgery at age 37 years. A second sister homozygous for ApoA-I deficiency had angina and documented reversible myocardial ischemia on stress testing, as well as cerebellar ataxia. The two other homozygotes at ages 26 and 28 years, as well as four heterozygotes (ages 14–39 years) were asymptomatic, and had no evidence of CHD, neuropathy, or visual impairment. The authors concluded that the combined hyperlipidemia in this kindred was probably not related to the APOA1 gene mutation.
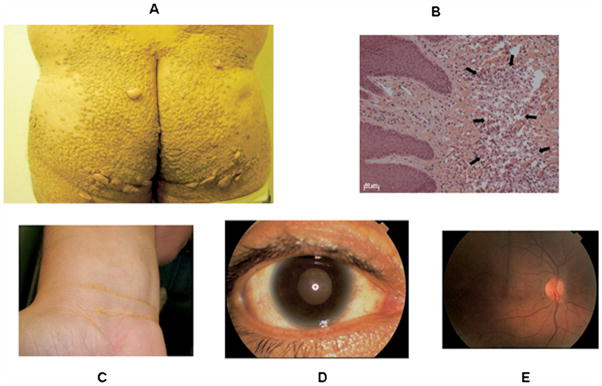
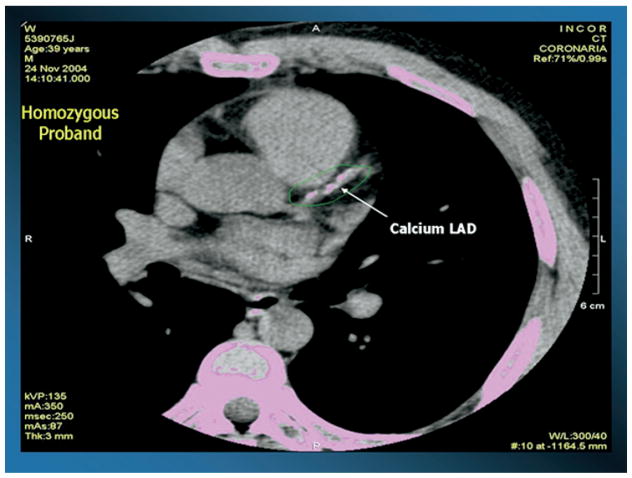
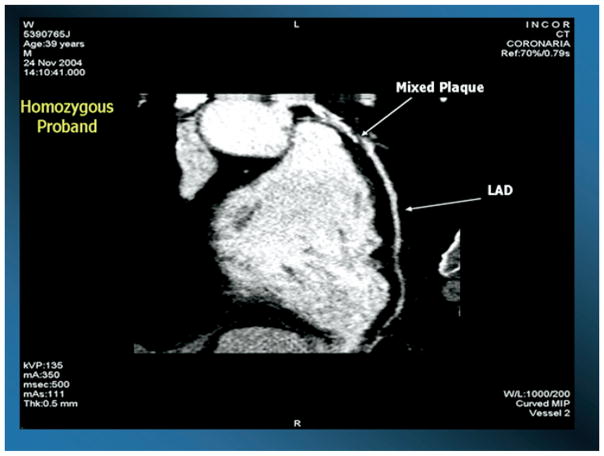
Another kindred with the same mutation was recently reported by our group,31 and is highlighted in this review. The index case for this kindred was a 39-year-old male who presented with striking tubero-eruptive xanthomas on his buttocks and lower back. Biopsy of these lesions confirmed lipid-laden macrophages (see Fig. 1). He also had palmar and planar xanthomas as evidenced by yellow creases on his palms and wrist, as well as bilateral corneal arcus and corneal opacification detected on slit-lamp examination. Examination of his retina was normal, as was his neurological examination (see Fig. 1). On physical examination, height was 1.80 m, weight was 94.0 kg, body mass index (calculated as kilograms divided by meters squared) was 29.0, and waist circumference was 105 cm. Blood pressure of 120/80 mmHg, and there was no evidence of hepatosplenomegaly or enlarged orange tonsils. He had normal liver, renal, and thyroid function, and a normal complete blood count. His fasting glucose was 92 mg/dL. His most striking laboratory finding was an HDL cholesterol level of 4 mg/dL. He had no history of chest pain, heart disease, hypertension, diabetes, or cigarette smoking. He was asymptomatic, and on coronary stress testing had no significant evidence of ischemia. However, on computed tomographic examination of his heart, he had increased coronary calcification (see Fig. 2), as well as a complete obstruction of his right coronary artery and a 90% narrowing of his left anterior descending coronary artery as documented by 16-slice multidetector computed tomographic angiography, and underwent successful coronary artery bypass surgery (see Fig. 3). His initial total cholesterol, LDL cholesterol, and triglycerides were 174, 146, and 94 mg/dL, respectively. Statin therapy was effective in this patient in lowering LDL cholesterol.
Figure 1.
(A) Tubero-eruptive xanthomas on the buttocks of a patient with homozygous familial apolipoprotein A-I deficiency as described in Matsunaga and colleagues.28 (B) Light microscopy of a biopsy of one of these xanthomas documenting lipid-laden macrophages. (C) Planar xanthomas in the skin creases of the wrist. (D) Photograph of the eye of a patient with homozygous familial apolipoprotein A-I deficiency as described in Santos and colleagues,31 documenting moderate corneal opacification, especially in the limbic area, and (E) fundus of the same patient with no significant abnormalities noted.
Figure 2.
Computed tomographic image of the heart of a 39-year-old patient with homozygous familial apolipoprotein A-I deficiency as described in Santos and colleagues,31 indicating marked coronary calcification in the left anterior descending (LAD) coronary artery.
Figure 3.
Coronary angiography using 16-slice multidetector computed coronary angiography and injection of contrast material documenting complete obstruction of the left anterior descending (LAD) coronary artery in a 39-year-old patient with homozygous familial apolipoprotein A-I deficiency.
The proband had three children who were healthy at ages 3, 6, and 8 years with HDL cholesterol levels of 14, 21, and 34 mg/dL, respectively. The proband’s 41-year-old brother had previously had a myocardial infarction followed by coronary artery bypass graft surgery at the age of 38 years. His brother was also noted to have tubero-eruptive xanthomas, as well as corneal arcus, and his HDL cholesterol was found to be 2 mg/dL. He also had a significant reduction in his LDL cholesterol with statin therapy. His daughter was in good health at age 12 years, and was presumably a heterozygote, but did not consent for phlebotomy. Two other siblings of the index case were examined, and were found not to have xanthomas, with HDL cholesterol levels of 15 and 22 mg/dL, consistent with heterozygosity. They were in good health at ages 33 and 35 years. Their children were in good health at ages 3, 8, and 10 with HDL cholesterol levels of 21, 45, and 42 mg/dL, respectively. The parents of the index case were alive and well at ages 65 and 66 years, both with HDL cholesterol levels that were 24 mg/dL. The fathers of the proband parents were nonidentical twin brothers. Therefore, the index case, his homozygous brother, and his two heterozygous siblings were products of a consanguineous marriage.
DNA sequencing of the APOA1 gene was completed using genomic DNA. The proband was found to be homozygous for a mutation at APOA1 codon-2, namely Q[-2]X, identical to the mutation in the second kindred described here.29,30 This mutation results in generation of a termination codon, and the lack of any mature ApoA-I being expressed in homozygotes. Molecular analysis in the family revealed two homozygotes, eight heterozygotes, and two normal subjects, the latter of whom were the offspring of a heterozygote and had HDL cholesterol levels of 42 and 45 mg/dL, respectively. APOE genotyping in all family members was either the E3/3 or E4/3 genotype; moreover, the proband’s APOE gene was sequenced, and was found to be normal.
With two-dimensional gel electrophoresis of HDL from the two homozygotes, no ApoA-I–containing particles were detectable by immunoblotting with antibody specific for ApoA-I (Fig. 3). ApoC-III and ApoA-IV was present in both homozygotes. Heterozygotes had virtually undetectable large α-1 particles and decreases in α-2 HDL particles.31 Other kindreds with ApoA-I deficiency have also been described.32–35
Defective ABCA1 transport (Tangier disease)
Tangier disease was originally described in two siblings (a boy aged 5 and a girl aged 6) from Tangier Island, a small island located near the mouth of the Chesapeake Bay in the United States, settled by immigrants from Wales in the 1600s.36 These children both had their tonsils removed and they were noted to have an unusual orange color. The pathologist sent the tissue blocks to pathologists at the Armed Forces Institute of Pathology in Washington, DC, who noted cells consistent with lipid storage and referred them to the National Heart Institute, National Institutes of Health in Bethesda, MD. On pathologic examination at the National Institutes of Health, the tonsils were found to be full of cholesterol-laden macrophages, and the patients were also noted to have very low levels of plasma α-migrating lipoproteins or HDL, as well as hepatosplenomegaly.36 A kindred analysis revealed that the parents of the two probands had approximately half normal levels of HDL. It was noted subsequently that some patients with this disorder can develop permanent or transient peripheral neuropathy.14 The histopathology of Tangier disease was examined by light and electron microscopy and documented lipid-laden macrophages in tonsils, liver, and Schwann cells in nerves of this patients.15 More details of the blood plasma of Tangier homozygotes described moderate hypertriglyceridemia, decreased levels of triglyceride-enriched LDL, and very small amounts of very small HDL particles.13,16 Later studies of the original (index cases) Tangier homozygotes revealed markedly increased fractional catabolism of HDL proteins using radioiodinated HDL, with ApoA-I being cleared more rapidly than ApoA-II.13 Moreover ApoA-I was documented to be on a very small HDL particle in homozygotes, whereas ApoA-II was mainly found in the 1.063 g/mL infranatant fraction. In addition, these authors reported that the two obligate heterozygous parents had twofold increased fractional catabolism of HDL protein and ApoA-I. The rapid clearance of HDL particles was confirmed by studies by infusion of HDL into Tangier homozygotes.13 Therefore, it was already clear in the 1980s that these patients had rapid clearance of a very small ApoA-I–containing HDL particle.
Cholesteryl ester deposition in macrophages has been found in liver, spleen, and Schwann cells, as well as omental macrophages, and the entire gastrointestinal mucosa has been described as having a yellowish color.37,38
Tangier disease had been reported in many countries all over the world. The major clinical findings in these cases were hepatosplenomegaly, mild corneal opacification, enlarged orange tonsils or a history of a tonsillectomy, permanent or transient peripheral neuropathy in about 50% of the cases, and, in some cases, premature CHD.37–39 It has been suggested that homozygotes with Tangier disease have increased risk of developing premature CHD in their 50s and 60s, but may not develop CHD earlier because their LDL cholesterol levels are about 50% of normal.37,38 Interestingly, increased prevalence of CHD was described in heterozygotes compared to unaffected relatives in a large kindred originally from Sicily.40 In this large kindred, it was estimated that homozygotes had threefold increased CHD risk and estimated 1.5-fold increased risk in heterozygotes compared to unaffected kindred members.
In 1995, two research groups documented that there was a cellular defect in Tangier fibroblasts resulting in defective removal of cellular cholesterol and phospholipids by HDL or ApoA-I.41,42 Later a chromosomal locus (9q31) for Tangier disease was described based on positional cloning.43 In early 1999, the function of the ABCA1 transporter in the efflux of cellular cholesterol as phospholipid to HDL and ApoA-I was described.44 Later that year, three different research groups reported various mutations in ABCA1 as causes of homozygous Tangier disease in a single issue of Nature Genetics.45–47 In the following year (2000), three different research groups confirmed the finding that a genetic defect in the gene ABCA1 could cause Tangier disease.44,48,49 Tangier heterozygotes had 50% of normal cellular cholesterol efflux, and a loss of large α-1 and β-2 HDL particles on two-dimensional gel electrophoresis.50 In this type of analysis, homozygotes had only pre–β-1 HDL present in their plasma. The intestinal cholesterol absorption was found to be normal in Tangier homozygote. The reduced LDL ApoB values are about 50% of normal, due to enhanced fractional catabolism of small triglyceride-rich, cholesteryl ester–poor LDL.51 The rapid clearance of very small HDL particles by the kidney appears to explain the very low levels of ApoA-I–containing particles in blood plasma. It has been proposed that the relative enrichment of the core of LDL by β-carotene, resulting in enhanced uptake by the reticuloendothelial cells resulting in the orange coloration of these tissues.46
Familial LCAT deficiency
The first case of LCAT deficiency was a 33-year-old woman admitted to the National Hospital of Norway in Oslo because of diffuse, clearly visible, grayish corneal opacities, anemia, proteinuria, and hyperlipidemia. Kidney function was otherwise normal. A kidney biopsy revealed foam cells in the glomerular tufts.52 Plasma cholesterol and triglyceride levels were moderately increased, but most of the cholesterol was unesterified, and no pre–β-1 or α-lipoproteins could be detected on electrophoresis. Two of her sisters were similarly affected and the blood plasmas of all three siblings were found to lack the ability to transfer a fatty acid from phosphatidylcholine (lecithin) to cholesterol to form cholesteryl ester and lysolecithin. This process is known to be due to a protein known as lecithin:cholesterol acyl transferase (LCAT). In 1969, a Swedish kindred with LCAT deficiency was reported and, since that time, kindreds have been documented from many countries, including from Germany, the United Kingdom, France, Canada, Japan, the United States, Italy, and Bulgaria.13
Not only were these patients found to have very low levels of HDL, but also elevations of free cholesterol in all lipoproteins. Unusual lipoprotein forms with VLDL flotation characteristics is the ultracentrifuge, but β instead of pre–β-mobility on electrophoresis. Moreover their LDL was found to be large and heterogeneous, and enriched in free cholesterol, phospholipids, and triglycerides, with a very low cholesteryl ester content. These particles have also been found to be low in ApoB, and enriched in the C apolipoproteins.13
Corneal opacification observed in familial LCAT deficiency presents early in life and consists of numerous, minute, grayish dots in the entire corneal stroma, and is much more striking than that reported for Tangier disease or the ApoA-I deficiency states.13,39,53 The opacification is more striking near the limbal area, forming a circular band resembling arcus senilis. Surprisingly, vision is usually not impaired. The anemia in these patients is moderate, with hemoglobin levels of around 10 g/dL, associated with enhanced fractional clearance of red cells. Renal disease presents as proteinuria early in life, and increases in the fourth or fifth decades of life as renal function deteriorates. Early atherosclerosis has been reported in some patients with familial LCAT deficiency with aortic, carotid, and femoral atherosclerosis, but premature CHD usually does not occur.13
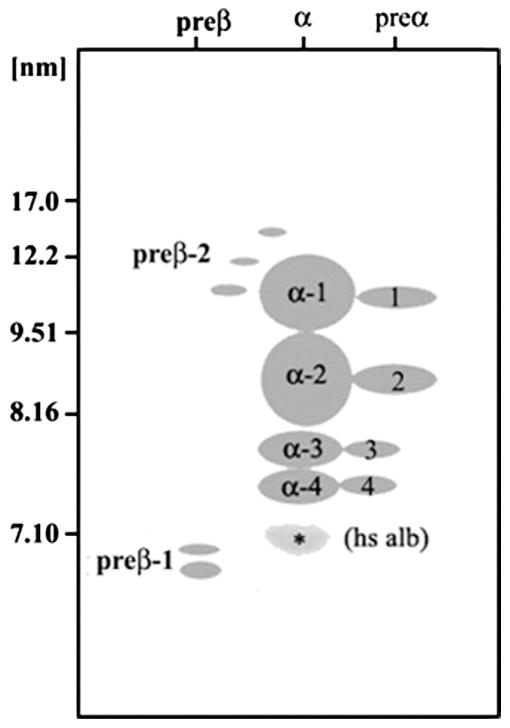
Multiple mutations have been reported in the gene for LCAT.48 Hypercatabolism of HDL proteins, especially ApoA-II in LCAT has been reported in LCAT-deficient homozygotes.48,49 Patients homozygous for LCAT deficiency have ApoA-I in plasma present mainly in pre–β-1 and α-4 discoidal HDL particles, with a lack of large α-1 particles.54 Therefore, the technology of two-dimensional gel electrophoresis of whole plasma followed by immunoblotting with specific antibody for ApoA-I can readily be used to distinguished homozygous ApoA-I deficiency, ABCA1 deficiency, or LCAT deficiency (see Figs. 4 and 5).31,40,54
Figure 4.
Schematic representation of apolipoprotein A-I–containing high-density lipoprotein (HDL) subpopulations in human plasma separated by nondenaturing agarose-polyacrylamide gel electrophoresis. Nomenclature is based on HDL particle separation by electrophoretic charge relative to albumin (pre-β, α, and pre-α) in the horizontal dimension, and by size (17.0 to ≤ 7.1 nm) relative to the molecular weight standards in the vertical dimension by two-dimensional, nondenaturing agarose polyacrylamide gel electrophoresis as described in references 31,40,54–57. *Position of albumin on the gel. hs alb, human serum albumin.
Figure 5.
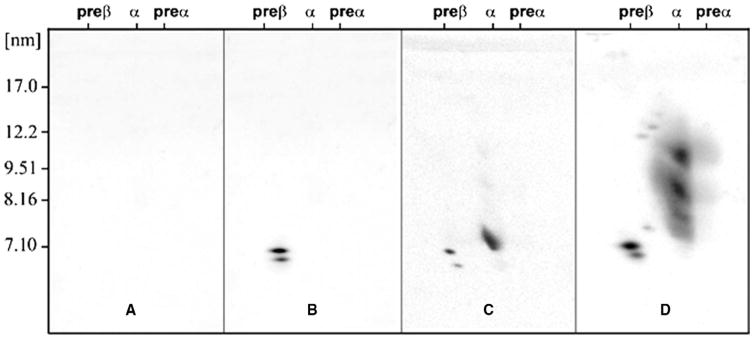
Distribution of apolipoprotein (Apo) A-I–containing high-density lipoprotein (HDL) subpopulations of homozygotes with (A) familial ApoA-I deficiency indicating no detectable ApoA-I in plasma, (B) Tangier disease (defective adenosine triphosphate binding cassette transporter A1 [ABCA1]) indicating only the presence of pre–β-1 HDL in plasma (C) familial lecithin:cholesterol acyltransferase deficiency, indicating the presence of pre–β-1 and α-4 HDL, and (D) a control subject indicating the presence of all the 13 major and minor plasma ApoA-I–containing HDL subspecies(D) separated by two-dimensional, nondenaturing agarose polyacrylamide gel electrophoresis as described in references 31,40,54–57.
Familial partial LCAT deficiency (fish-eye disease)
A variant form of LCAT deficiency was described in 1975.13 The first case was a 61-year-old Swedish female who presented with hypertriglyceridemia and was noted to have marked corneal opacification. The same abnormalities had been noted in her father and two sisters, and people had told the patient that her eyes looked like those of “boiled fish.” Hence this disorder was named fish-eye disease.13 These patients were noted to have HDL levels that were about 10% of normal, with small spherical particles.13 Subsequently, it was learned that there is both β-LCAT activity, which acts on ApoB–containing lipoproteins, and α-LCAT activity, which acts on HDL. Patients with fish-eye disease only have a deficiency of the latter activity, whereas patients with familial LCAT deficiency have a deficiency of both activities.13 No evidence of kidney disease or premature CHD has been reported in fish-eye disease kindreds. HDL abnormalities in standard LCAT deficiency and fish-eye disease appear to be virtually identical with the loss of large α HDL particles.13,52
Synthesis of the lessons from genetic disorders of HDL metabolism
Studies of inborn errors of HDL metabolism and the use of two-dimensional gel electrophoresis combined with cellular studies (in collaboration with Dr. George Rothblat of the University of Pennsylvania) have allowed the development of a theoretical framework for HDL particle metabolism.50 We propose the following:
The first step in HDL metabolism is the production of ApoA-I and its combining with phospholipids to form discoidal pre–β-1 HDL. This step is defective in patients with the homozygous forms of ApoA-I deficiency, resulting in no detectable ApoA-I in plasma (see Fig. 5).12,17–35
The second step in HDL metabolism is the effluxing of free cholesterol and phospholipids onto pre–β-1 HDL via the cellular ABCA1 transporter to form discoidal α-4 HDL. This step is defective in patients with homozygous Tangier disease (ABCA1 dysfunction), resulting in only the presence of pre–β-1 HDL in their plasma (see Fig. 5).37–40 Without picking up lipid, this form of HDL is very rapidly catabolized via the kidney.
The third step in HDL metabolism is the esterification of free cholesterol on the surface of HDL to cholesteryl ester, which goes into the core of the HDL particle along with transferred triglyceride. The donor for the fatty acid is lecithin, which is converted to lysolecithin. This process is facilitated by LCAT, which is synthesized in the liver and binds to discoidal α-4 HDL to facilitate its conversion to spherical α-3 HDL and α-2 HDL, which contain both ApoA-I and ApoA-II. When this process does not occur, only discoidal pre–β-1 and discoidal α-4 HDL are found in plasma in patients with homozygous LCAT deficiency or homozygous fish-eye disease (see Fig. 5).53 Under this circumstance, HDL is also rapidly removed presumably by the kidney, and there is lipid deposition in the kidney, which may contribute to the renal failure observed in this disease. Therefore, these three inborn errors of metabolism have taught us a great deal about the first three steps in HDL metabolism. There is additional data in this information that may teach us about the causation of atherosclerosis because patients with CHD frequently have moderate increases in pre–β-1 B1 HDL of about 10% to 15% and reductions in large α-1 and α-2 HDL of about 30% to 40%.54–57
Summary
Severe deficiency of plasma HDL (HDL cholesterol <10 mg/dL) can be due to marked hypertriglyceridemia, liver failure, monoclonal gammopathy, or use of anabolic steroids or the fibrate—thiazolidinedione combination.3 There are other more specific conditions with well-defined genetic causation that markedly reduce HDL concentrations. These include absence of ApoA-I, ABCA1 dysfunction, or deficiency of LCAT activity.12–50 Lack of ApoA-I involves mutations affecting the ApoA-I gene, both the adjacent ApoA-I and ApoC-III genes, or in one kindred, the entire AI/CIII/AIV gene complex. These patients may have planar and or tubero-eruptive xanthomas as well as very premature CHD.12,13,17–35 Patients who lack both ApoA-I and ApoC-III also have low VLDL because ApoC-III is an inhibitor of lipolysis.12–14,17–35 Patients who lack ApoA-I, ApoC-III, and ApoA-IV not only have low VLDL, but also fat malabsorption, which is evidence for involvement of ApoA-IV in intestinal lipid absorption.18 Heterozygotes with ApoA-I deficiency, in contrast to heterozygotes with Tangier disease or LCAT deficiency, have about 50% of normal pre–β-1 HDL, and about twofold increases in pre–β-2.31 In homozygous Tangier disease, due to mutations at the ABCA1 gene locus, ApoA-I is present in plasma at a concentration of <10 mg/dL, with LDL cholesterol that is about 50% of normal and mild hypertriglyceridemia (see Tables 1–3). The diagnosis is established by the presence of only pre–β-1 HDL in plasma as determined by two-dimensional gel electrophoresis, and lack of cellular cholesterol efflux (see Fig. 5). Familial LCAT deficiency is characterized by plasma ApoA-I levels of <40 mg/dL, moderate elevations of triglycerides and decreased LDL cholesterol, and the finding that most of the cholesterol in plasma is in the free rather than the esterified form, due a deficiency in LCAT activity and mass or an alteration in the sequence of ApoA-I, the activator of LCAT, resulting in decreased LCAT activity, but normal LCAT mass. These patients have severe corneal opacification, and are at increased risk of developing renal failure, but have no evidence of premature CHD. The diagnosis is established by the finding of almost all the cholesterol in plasma in the free form, decreased plasma LCAT activity, and only discoidal pre–β-1 and small discoidal α HDL particles in plasma.23 As can be seen from Tables 1 to 3, and Figure 5, the diagnosis of ApoA-I deficiency (undetectable ApoA-I), Tangier disease (only pre–β-1 HDL), and LCAT deficiency (both pre–β-1 and α-4 HDL) can easily be made with the two-dimensional gel technology.31,40,54 In our view, correctly diagnosing these different states is important because they have markedly different CHD risks.
Table 2.
Characteristics of homozygotes with marked high-density lipoprotein deficiency and undetectable plasma apolipoprotein A-I levels
| Disorder | ApoA-I deficiency | ApoA-I/C-III deficiency | ApoA-I/C-III/A-IV deficiency* |
|---|---|---|---|
| Triglyceride | Normal | Decreased | Decreased |
| LDL-C | Normal | Normal | Normal |
| HDL-C | <5 mg/dL | <5 mg/dL | <5 mg/dL |
| ApoA-I | Undetectable | Undetectable | Undetectable |
| ApoC-III | Present | Undetectable | Undetectable |
| ApoA-IV | Present | Present | Undetectable |
| Planar xanthomas | Positive | Positive | Negative |
| Tubero-eruptive xanthomas | Positive | Negative | Negative |
| Premature CHD | Positive | Positive | Positive |
Acknowledgments
Supported by research grants to InCor (to Dr. Santos) and the Lipid Metabolism Laboratory at Tufts University from the National Institutes of Health, Bethesda, MD, (HL-60935, HL 74753, and PO50HL083813) and the US Department of Agriculture, Washington DC (contract 53-3K06-5-10) (to Drs. Asztalos, Polisecki, and Schaefer).
References
- 1.Schaefer EJ, Levy RI, Anderson DW, Danner RN, Brewer HB, Jr, Blackwelder WC. Plasma-triglycerides in regulation of HDL-cholesterol levels. Lancet. 1978;2:391–393. doi: 10.1016/s0140-6736(78)91863-9. [DOI] [PubMed] [Google Scholar]
- 2.Jahn CE, Schaefer EJ, Taam L, Hoofnagel J, Jones EA, Brewer HB., Jr Lipoprotein abnormalities in primary biliary cirrhosis: association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology. 1985;89:1266–1278. [PubMed] [Google Scholar]
- 3.Goldberg RB, Mendez AJ. Severe acquired (secondary) high-density lipoprotein deficiency. J Clin Lipidol. 2007;1:41–56. doi: 10.1016/j.jacl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Bielicki JK, Oda MN. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 2002;41:2089–2096. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Genest JJ, McNamara JR, Salem DN, Schaefer EJ. Prevalence of risk factors in men with premature coronary artery disease. Am J Cardiol. 1991;67:1185–1189. doi: 10.1016/0002-9149(91)90924-a. [DOI] [PubMed] [Google Scholar]
- 7.Genest JJ, Martin-Munley S, McNamara JR, et al. Prevalence of familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 8.Genest JJ, Jr, Bard JM, Fruchart JC, Ordovas JM, Schaefer EJ. Familial hypoalphalipoproteinemia in premature coronary artery disease. Arterioscler Thromb. 1993;13:1728–1737. doi: 10.1161/01.atv.13.12.1728. [DOI] [PubMed] [Google Scholar]
- 9.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 10.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events. VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 11.Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease. Subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT) Arch Intern Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer EJ, Heaton WH, Wetzel MG, Brewer HB., Jr Plasma apolipoprotein A-I absence associated with marked reduction of high density lipoproteins and premature coronary artery disease. Arteriosclerosis. 1982;2:16–26. doi: 10.1161/01.atv.2.1.16. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer EJ. The clinical, biochemical, and genetic features in familial disorders of high density lipoprotein deficiency. Arteriosclerosis. 1984;4:303–322. doi: 10.1161/01.atv.4.4.303. [DOI] [PubMed] [Google Scholar]
- 14.Engel WK, Dorman JD, Levy RI, Fredrickson DS. Neuropathy in Tangier disease. Alpha-lipoprotein deficiency manifesting as familial recurrent neuropathy and intestinal lipid storage. Arch Neurol. 1967;17:1–9. doi: 10.1001/archneur.1967.00470250005001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrans VJ, Fredrickson DS. The pathology of Tangier disease. A light and electron microscopic study. Am J Pathol. 1975;78:101–158. [PMC free article] [PubMed] [Google Scholar]
- 16.Assmann G, Herbert PN, Fredrickson DS, Forte T. Isolation and characterization of an abnormal high density lipoprotein in Tangier Disease. J Clin Invest. 1977;60:242–252. doi: 10.1172/JCI108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer EJ, Ordovas JM, Law S, et al. Apolipoprotein A-I and C-III deficiency, variant II. J Lipid Res. 1985;26:1089–1101. [PubMed] [Google Scholar]
- 18.Ordovas JM, Cassidy DK, Civeira F, Bisgaier CL, Schaefer EJ. Familial apolipoprotein A-I, C-III, and A-IV deficiency with marked high density lipoprotein deficiency and premature atherosclerosis due to a deletion of the apolipoprotein A-I, C-III, and A-IV gene complex. J Biol Chem. 1989;264:16339–16342. [PubMed] [Google Scholar]
- 19.Norum RA, Lakier JB, Goldstein S, et al. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary artery disease. N Engl J Med. 1982;306:1513–1519. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- 20.Karathanasis SK, Norum RA, Zannis VI, Breslow JL. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983;301:718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- 21.Karathanasis SK, Ferris E, Haddad EA. DNA inversion within the apolipoprotein AI/CIII/AIV-encoding gene cluster of certain patients with premature atherosclerosis. Proc Natl Acad Sci U S A. 1987;84:7198–7202. doi: 10.1073/pnas.84.20.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beher WT, Gabbard A, Norum RA, Stradnieks S. Effect of blood high density lipoprotein cholesterol concentration on fecal steroid excretion in humans. Life Sci. 1983;32:2933–2937. doi: 10.1016/0024-3205(83)90643-4. [DOI] [PubMed] [Google Scholar]
- 23.Forte TM, Nichols AV, Krauss RM, Norum RA. Familial apolipoprotein AI and apolipoprotein CIII deficiency. Subclass distribution, composition, and morphology of lipoproteins in a disorder associated with premature atherosclerosis. J Clin Invest. 1984;74:1601–1613. doi: 10.1172/JCI111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginsberg HN, Le NA, Goldberg IJ, et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apoCIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest. 1986;78:1287–1295. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbaiah PV, Norum RA, Bagdade JD. Effect of apolipoprotein activators on the specificity lecithin:cholesterol acyltransferase: determination of cholesteryl esters formed in A-I/C-III deficiency. J Lipid Res. 1991;32:1601–1609. [PubMed] [Google Scholar]
- 26.Bekaert ED, Alaupovic P, Knight-Gibson CS, Laux MJ, Pelachyk JM, Norum RA. Characterization of apoA-I and apoB containing lipoprotein particles in a variant of familial apolipoprotein A-I deficiency with planar xanthomas: the metabolic significance of LP-A-II particles. J Lipid Res. 1991;32:1587–1599. [PubMed] [Google Scholar]
- 27.Hiasa Y, Maeda T, Mori H. Deficiency of apolipoproteins A-I and C-III and severe coronary artery disease. Clin Cardiol. 1986;9:349–352. doi: 10.1002/clc.4960090709. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga T, Hiasa Y, Yanagi H, et al. Apolipoprotein A-I deficiency due to a codon 84 nonsense mutation of the apolipoprotein A-I gene. Proc Natl Acad Sci U S A. 1991;88:2793–2797. doi: 10.1073/pnas.88.7.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng DS, Leiter LA, Vezina C, Connelly PW, Hegele RA. Apolipoprotein A-I Q[-2]X causing isolated apolipoprotein deficiency in a family with analphalipoproteinemia. J Clin Invest. 1994;93:223–229. doi: 10.1172/JCI116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng DS, O’Connor PW, Mortimer CB, Leiter LA, Connelly PW, Hegele RA. Case report: retinopathy and neuropathy associated with complete apolipoprotein A-I deficiency. Am J Med Sci. 1996;312:30–33. doi: 10.1097/00000441-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Santos RD, Schaefer EJ, Asztalos BF, et al. Characterization of high density lipoprotein particles in familial apolipoprotein A-I deficiency. J Lipid Res. 2008;46:349–357. doi: 10.1194/jlr.M700362-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Matsunaga A, Sasaki J, Han H, et al. Compound heterozygozity for an apolipoprotein AI gene promoter mutation and a structural nonsense mutation with apolipoprotein deficiency. Arterioscler Thromb Vasc Biol. 1999;19:348–355. doi: 10.1161/01.atv.19.2.348. [DOI] [PubMed] [Google Scholar]
- 33.Ikewaki K, Matsunaga A, Han H, et al. A novel two nucleotide deletion in the apolipoprotein A-I gene, apoA-I Shinbashi, associated with high density lipoprotein deficiency, corneal opacities, planar xanthomas, and premature coronary artery disease. Atherosclerosis. 2004;172:39–45. doi: 10.1016/j.atherosclerosis.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Deeb SS, Cheung MC, Peng R, et al. A mutation in the apolipoprotein A-I gene. J Biol Chem. 1991;266:13654–13660. [PubMed] [Google Scholar]
- 35.Pisciotta L, Miccoli R, Cantafora A, et al. Recurrent mutations of the apolipoprotein A-I gene in three kindreds with severe HDL deficiency. Atherosclerosis. 2003;167:335–345. doi: 10.1016/s0021-9150(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 36.Fredrickson DS, Altrocchi PH, Avioli LV, Goodman DS, Goodman HC. Tangier disease. Ann Intern Med. 1961;55:1016–1031. [Google Scholar]
- 37.Schaefer EJ, Zech LA, Schwartz DS, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high density lipoprotein deficiency (Tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 38.Serfaty-Lacrosniere C, Lanzberg A, Civeira F, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 39.Chu FC, Kuwabara T, Cogan PG, Schaefer EJ, Brewer HB., Jr Ocular manifestations of familial high density lipoprotein deficiency (Tangier disease) Arch Opthalmol. 1979;97:1926–1928. doi: 10.1001/archopht.1979.01020020374020. [DOI] [PubMed] [Google Scholar]
- 40.Asztalos BF, Brousseau ME, McNamara JR, Horvath KV, Roheim PS, Schaefer EJ. Subpopulations of high-density lipoproteins in homozygous and heterozygous Tangier disease. Atherosclerosis. 2001;156:217–225. doi: 10.1016/s0021-9150(00)00643-2. [DOI] [PubMed] [Google Scholar]
- 41.Rogler G, Trumbach B, Klima B, Lackner KJ, Schmitz G. HDL-mediated efflux of intracellular cholesterol is impaired in fibroblasts from Tangier disease patients. Arterioscler Thromb Vasc Biol. 1995;15:683–690. doi: 10.1161/01.atv.15.5.683. [DOI] [PubMed] [Google Scholar]
- 42.Francis GA, Knopp RH, Oram JF. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J Clin Invest. 1995;96:78–87. doi: 10.1172/JCI118082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 44.Langmann T, Klucken J, Reil M, et al. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Comm. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 45.Brooks A, Marcil M, Clee SM, et al. Mutation in ABC1 in Tangier disease and familial high density lipoprotein. Nature Gen. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 46.Bodzioch M, Orso E, Klucken J, et al. The gene encoding ATP binding cassette transporter 1 is mutated in Tangier disease. Nature Gen. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 47.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP binding cassette transporter 1. Nature Gen. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 48.Brousseau ME, Schaefer EJ, Dupuis J, et al. Novel mutations in the gene encoding ATP-binding cassette 1 in four Tangier disease kindreds. J Lipid Res. 2000;41:433–441. [PubMed] [Google Scholar]
- 49.Brousseau ME, Eberhart GP, Dupuis J, et al. Cellular cholesterol efflux in heterozygotes for Tangier disease is markedly reduced and correlates with high density lipoprotein cholesterol concentration and particle size. J Lipid Res. 2000;41:1125–1135. [PubMed] [Google Scholar]
- 50.Schaefer EJ, Brousseau ME, Diffenderfer MR, et al. Cholesterol and apolipoprotein B metabolism in Tangier disease. Atherosclerosis. 2001;159:231–236. doi: 10.1016/s0021-9150(01)00688-8. [DOI] [PubMed] [Google Scholar]
- 51.Norun KR, Gjone E. Familial plasma lecithin cholesterol acyl transferase deficient. Biochemical study if a new inborn error of metabolism. Scand J Clin Lab Invest. 1967;20:231–235. [Google Scholar]
- 52.Calabresi L, Pisciotta L, Constantin A, et al. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler Thromb Vasc Biol. 2005;25:1972–1978. doi: 10.1161/01.ATV.0000175751.30616.13. [DOI] [PubMed] [Google Scholar]
- 53.Asztalos BF, Schaefer EJ, Horvath KV, et al. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Asztalos BF, De la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1 and SRB1-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Asztalos BF, Cupples LA, Demissie S, et al. High density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants in the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 56.Asztalos BF, Collins D, Cupples LA, et al. Value of high density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 57.Asztalos BF, Batista M, Horvath KV, et al. Change in alpha 1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:847–852. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]