Abstract
A 30‐year‐old man with severe hypoalbuminemia (serum albumin: 0.9 g/dL) was admitted with severe bilateral leg edema and unilateral pleural effusion. Serum anti‐SS‐A and SS‐B antibody levels were abnormally elevated, and his symptoms fulfilled the diagnostic criteria for Sjögren's syndrome. Technetium‐99m albumin scintigraphy revealed protein leakage from a large area of the small intestine. Immunohistochemistry revealed perivascular deposition of C1q, C3d, and immunoglobulin G in the duodenal mucosa. The patient was diagnosed with protein‐losing gastroenteropathy associated with Sjögren's syndrome. Within 2 months of treatment with oral prednisolone and mycophenolate mofetil, the clinical symptoms of hypoalbuminemia and Sjögren's syndrome disappeared completely.
Keywords: human serum albumin scintigraphy, hypoalbuminemia, protein‐losing gastroenteropathy, Sjögren's syndrome
A middle‐age man with severe hypoalbuminemia and severe edema. The patient was diagnosed with Sjögren's syndrome associated with protein‐losing enteropathy by protein scintigraphy and pathological examination of colon. After treatment of immunosuppressant, the clinical symptoms disappeared completely.
1. INTRODUCTION
Sjögren's syndrome (SS) is an autoimmune disorder that manifests with various systemic clinical symptoms, along with problems in lacrimation and salivation.1 Protein‐losing gastroenteropathy (PLGE) is one such systemic complication of the disease. Although rare and treatable, PLGE associated with SS could cause severe health disturbance in patients if the diagnosis is delayed. The cause of hypoalbuminemia can be identified by routine blood and urine tests if the condition is due to liver failure or nephrotic syndrome. However, if hypoalbuminemia is caused by leakage from the gastrointestinal tract, the diagnosis becomes difficult and requires additional disease‐specific diagnostic examinations. In this report, we present a case of severe hypoalbuminemia in a young man, together with the histopathological findings and a literature review of the condition.
2. CASE
A 30‐year‐old man with no remarkable medical history was admitted initially because of progressive bilateral leg edema and hydrocele testis, accompanied by a decreased serum albumin level. He had also noticed the symptoms of dry eyes and mouth. His serum albumin level was 1.5 g/dL. He received symptomatic treatments at another hospital previously; however, his albumin level further decreased to 1.2 g/dL in the following three months. Contrast‐enhanced abdominal computed tomography (CT) revealed a thickened small intestinal wall with increased contrast enhancement in an extensive intestinal segment accompanied by multiple swollen mesenteric lymph nodes.
Four months after the clinical onset, he was referred to our hospital because of progressive hypoalbuminemia without clinical improvement. Upon admission, severe pitting edema in both legs and eye oil secretions were confirmed on physical examination. His appetite was normal with normal stool appearance. His body temperature was 36.8°C, blood pressure was 140/84 mm Hg, heart rate was 74 bpm, height was 174 cm, and body weight was 66.2 kg; the latter was significantly greater than that observed prior to the clinical onset. A blood test revealed normal liver and renal functions, total protein level of 4.0 g/dL (normal: 6.6‐8.1), albumin level of 0.9 g/dL (4.1‐5.1), prealbumin level of 11.3 mg/dL (22‐40), C‐reactive protein level of 0.21 mg/dL (0.00‐0.14), erythrocyte sedimentation rate of 83 mm/1 h (2‐10), d‐dimer level of 7.1 μg/mL (0.0‐1.0), fibrinogen level of 533 mg/dL (200‐400), free light‐chain κ/λ ratio of 2.10 (0.26‐1.65), antinuclear antibody (ANA) result of 1:1280, anti‐dsDNA level of 7.3 U/mL (0‐12), anti‐SS‐A level >1200 U/mL (0‐9.9), anti‐SS‐B level >1000 U/mL (0‐9.9), rheumatoid factor level of 11.7 U/mL (0‐15), MPO‐ANCA level <1.0 U/mL (0‐3.4), PR3‐ANCA level of 3.8 IU/mL (0‐3.4), sIL‐2R level of 802 U/mL (122‐496), C3 level of 63 mg/mL (73‐138), C4 level of 19.6 mg/mL (11‐31), and CH50 level of 23.4 U/mL (31.6‐57.6). Urinalysis revealed urine protein (±), urine occult blood (−), and urine white blood cells (−). Pleural effusion revealed a protein level of 1.5 g/dL, LDH level of 58 U/L, and cell count of 225/μL. Serum protein electrophoresis showed a relative increase in the α2 and γ fractions without a monoclonal spike. Chest X‐ray revealed unilateral pleural effusion on the right side. Upper and lower endoscopy revealed no abnormal findings except for suspected mild duodenitis. Histopathological study of the biopsied specimens from the colonic mucosa showed highly edematous stroma of the lamina propria mucosa with lymphocytic infiltration (Figure 1A left). Immunostaining was performed in the specimens biopsied from the duodenal mucosa, which revealed strong C1q, C3d, and immunoglobulin G (IgG) staining mainly around the vessels (Figure 1A middle).
Figure 1.
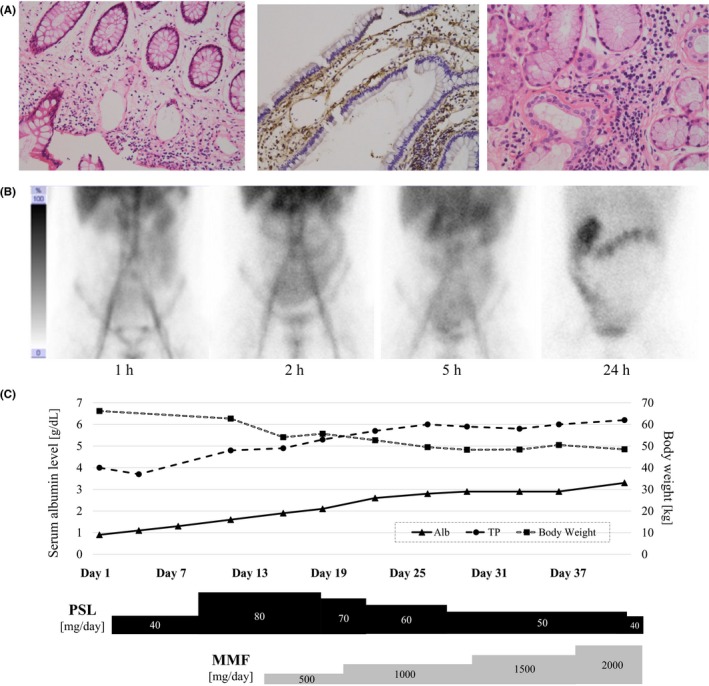
Histopathology, 99mTc‐labeled human serum albumin scintigraphy, and the treatment course of the patient. (A; left) Specimen from the ascending colon showed edematous stroma of the lamina propria mucosa with lymphocytic infiltration. (A; middle) Immunostaining for the complement C1q showed positive staining predominantly around the vessels. (A; right) Specimen from the lower lip showed findings compatible with the diagnosis of Sjögren's syndrome. (B) Technetium‐99m (99mTc)‐labeled human serum albumin (HSA) scintigraphy revealed protein leakage from an extensive area of the small intestine. The injected radioisotope accumulated mainly in the ileum 2 h after the injection, which later moved to the colon 24 h after the injection. (C) The treatment course and chronological change in serum albumin level
Based on the findings of abnormally high levels of serum anti‐SS‐A and anti‐SS‐B antibodies, the presence of oral and ocular symptoms, and a decreased unstimulated whole‐saliva flow rate of 0.046 mL/min, the patient was diagnosed with SS.2 Biopsy of the lower lip was performed, which revealed lymphocytic infiltration around the ducts, accompanied by atrophic acini and fibrillation of the ducts (Figure 1A right). The patient did not meet the diagnostic criteria for systemic lupus erythematosus developed by the Systemic Lupus Collaborating Clinics.3, 4 At this stage, PLGE associated with SS was suspected, and technetium‐99m (99mTc)‐labeled human serum albumin (HSA) scintigraphy was performed, which revealed protein leakage from an extensive area of the small intestine (Figure 1B).
Immune suppression therapy with oral prednisolone (40 mg/d from day 3) and mycophenolate mofetil (500 mg/d from day 14) was initiated, instead of cyclophosphamide pulse therapy, which the patient refused because of its possible carcinogenicity. The treatments resulted in drastic improvements both in the hypoalbuminemia and in the systemic edema (Figure 1C). At present, after treatment for 12 months with prednisolone (8 mg/d) and mycophenolate mofetil (2000 mg/d), the serum albumin level has stabilized over 4.0 g/dL without any clinical symptoms.
3. DISCUSSION
The cause of hypoalbuminemia can be roughly categorized into the following two patterns: (a) decreased amount of oral protein intake or protein production in the liver and (b) increased amount of protein consumption or protein discharge from the body.5, 6 Hypoalbuminemia based on liver failure is included in the former and that based on nephrotic syndrome is included in the latter. In addition to the discharge via the urinary tract, proteins can also be lost through the gastrointestinal tract, known as PLGE, as presented in this case report. As many factors can cause PLGE, comprehensive diagnostic examinations, such as contrast‐enhanced systemic CT, serum autoantibodies, upper and lower endoscopy, or echocardiography, are required for accurate diagnosis.
We identified a total of 13 articles describing 14 cases of PLGE associated with SS in a PubMed search, as listed in Table 1.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Most cases were reported from Asian countries. Compared with the previous cases, the present case involved one of the youngest patients and most severe cases with the lowest serum albumin level. Based on the literature review, the most characteristic location of albumin leakage in patients with PLGE associated with SS is the small intestine, but the leakage portion can range from the stomach to the colon. Based on the literature review, characteristic findings in biopsied specimens from the involved alimentary tract wall include inflammatory cell infiltration, edematous interstitial tissue, atrophied villi, and lymphangiectasis. Immunohistochemical staining was performed in two of the aforementioned previous case studies, one of which showed perivascular deposition of IgG and C3, as that shown in the present case11, but another did not.12
Table 1.
List of cases with protein‐losing gastroenteropathy associated with Sjögren syndrome
| Author (ref) | Ethnicity | Age, Sex | Alb [g/dL] | ANA | C3/C4 [mg/dL] | Initial symptoms (duration from onset to consultation) | Pleural effusion (side) | Diagnostic test | Site of protein loss | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Hsieh7 | Asian | 37,F | 1.4 | 320 | ‐/‐ | Edema in face and legs (2 mo) | Pos. (unknown) | 99mTc‐HSA | Stomach and small intestine | PSL (p.o.), mPSL pulse, hydroxychloroquine |
| Hsieh7 | Asian | 50,F | 1.1 | 640 | ‐/‐ | Anasarca (4 mo) | No data | 99mTc‐HSA | Stomach | PSL (p.o.), mPSL pulse, hydroxychloroquine |
| Ushiyama8 | Asian | 61,F | 1.8 | 320 | 22/<6.2 | Abdominal distention, edema in lower legs (4 mo) | Pos. (unknown) | 99mTc‐HSA, α1‐AT (216 mL/d) | Stomach | PSL (iv) |
| Nagashima9 | Asian | 41,M | 1.3 | 1280 | 54/18 | Facial edema (2 mo) | Pos. (bilateral) | 99mTc‐HSA, α1‐AT (281 mL/d) | Stomach and small intestine | PSL (iv), mPSL pulse |
| Nasu10 | Asian | 59,F | 2.8 | ‐ | 35.6/20 | Facial edema (1 mo) | Pos. (right) | 99mTc‐HSA, α1‐AT (205 mL/d) | Stomach | PSL (p.o.), mPSL pulse, CPA pulse, mizoribine |
| Uraoka11 | Asian | 42,F | 1.5 | ‐ | ‐/‐ | Facial edema (no data) | Pos. (right) | 99mTc‐HSA, α1‐AT (53 mL/d) | Stomach and small intestine | PSL (p.o.), mPSL pulse, CPA pulse, rituximab |
| Kakigao12 | Asian | 58,F | 1.5 | ‐ | 55/8 | Abdominal distention (4 mo) | No data | 99mTc‐HSA, α1‐AT (233 mL/d) | Stomach, small intestine, and colon | PSL (p.o.) |
| Yamashita13 | Asian | 51,F | 1.5 | 2560 | ‐/‐ | Left chest discomfort on expiration (6 mo) | Neg. | 99mTc‐HSA | Small intestine | PSL (p.o.) |
| Liao14 | Asian | 30,F | 1.8 | 5120 | 55/9 | Edema in face and legs, dizziness (no data) | Pos. (right) | None | Unknown | PSL (p.o.), hydroxychloroquine |
| Gupta15 | White | 58,F | 2.6 | 1280 | ‐/‐ | Weight loss (60 mo), diarrhea (6 mo) | Neg. | α1‐AT (13 mL/18 h) | Unknown | PSL (p.o.), CPA (iv) |
| Izumi16 | Asian | 64,F | 3.0 | <40 | 66/15 | Diarrhea, appetite loss (1 mo) | Pos. (unknown) | 99mTc‐HSA | Small intestine | PSL (p.o.), mPSL pulse, mizoribine |
| Watanabe17 | Asian | 88,M | 2.8 | 40 | ‐/‐ | Respiratory distress and pedal edema (1 mo) | Pos. (unknown) | 99mTc‐HSA | Small intestine | PSL (p.o.), mPSL pulse, IVIG |
| This case | Asian | 30,M | 0.9 | 1280 | 63/19.6 | Edema in legs (4 mo) | Pos. (right) | 99mTc‐HSA | Small intestine | PSL (p.o.), MMF (p.o.) |
Abbreviations: Alb, albumin; ANA, antinuclear antibody; CPA, cyclophosphamide; F, female; iv, intravenous; IVIG, intravenous immunoglobulin; M, male; MMF, mycophenolate mofetil; mPSL, methyl‐prednisolone; Neg, negative; p.o., per os; Pos, positive; PSL, prednisolone; SS‐A, anti‐SS‐A antibody; SS‐B, anti‐SS‐B antibody; α1‐AT, alpha‐1‐antitrypsin clearance; 99mTc‐HSA, 99m Tc‐labeled human serum albumin.
One of the reasons underlying the most severe hypoalbuminemia in the present case seems to be the relatively long time period from the onset to the diagnosis of PLGE because all the previous cases involving a short time period from onset to diagnosis (ie, ≤1 month) did not show severe hypoalbuminemia. Considering PLGE as a differential diagnosis of hypoalbuminemia may be key to avoid a delayed diagnosis. Once the cause of PLGE has been identified to be connective tissue disorders, swift initiation of immune suppressants may result in a dramatic improvement in symptoms.
CONFLICT OF INTERESTS
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGEMENTS
We appreciate Dr Lawrence M. Tierney (University of California) for his comments and advice on this case.
Akaishi T, Yasaka K, Abe M, et al. Protein‐losing gastroenteropathy with severe hypoalbuminemia associated with Sjögren’s syndrome: A case report and review of the literature. J Gen Fam Med. 2020;21:24–28. 10.1002/jgf2.281
REFERENCES
- 1. Perzynska‐Mazan J, Maslinska M, Gasik R. Neurological manifestations of primary Sjogren's syndrome. Reumatologia. 2018;56:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: A consensus and data‐driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 3. Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 4. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiedermann CJ, Wiedermann W, Joannidis M. Causal relationship between hypoalbuminemia and acute kidney injury. World J Nephrol. 2017;6:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh TY, Lan JL, Chen DY. Primary Sjogren's syndrome with protein‐losing gastroenteropathy: report of two cases. J Formos Med Assoc. 2002;101:519–22. [PubMed] [Google Scholar]
- 8. Ushiyama A, Teraoka H, Shiba T, Saito T, Nagata J, Koike J, et al. Protein‐losing gastroenteropathy associated with Sjogren syndrome‐case report and review of the Japanese literature. Nihon Shokakibyo Gakkai Zasshi. 2004;101:1314–9. [PubMed] [Google Scholar]
- 9. Nagashima T, Hoshino M, Shimoji S, Morino N, Kamimura T, Okazaki H, et al. Protein‐losing gastroenteropathy associated with primary Sjogren's syndrome: a characteristic oriental variant. Rheumatol Int. 2009;29:817–20. [DOI] [PubMed] [Google Scholar]
- 10. Nasu T, Miyata K, Uno A, Kawashima A, Kondo M, Akamizu T, et al. Successful treatment of protein‐losing gastroenteropathy with steroid pulse and immunosuppressive therapies in a patient with Sjogren syndrome. Case Rep Gastroenterol. 2011;5:372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uraoka Y, Tanigawa T, Watanabe K, Machida H, Okazaki H, Yamagami H, et al. Complete remission of protein‐losing gastroenteropathy associated with Sjogren's syndrome by B cell‐targeted therapy with rituximab. Am J Gastroenterol. 2012;107:1266–8. [DOI] [PubMed] [Google Scholar]
- 12. Kakigao K, Fukushima N, Mizutani T, Haraguchi K, Okamoto R, Sawamura N, et al. A case of protein‐losing gastroenteropathy accompanied by Sjogren syndrome and mixed connective tissue disease. Nihon Shokakibyo Gakkai Zasshi. 2012;109:1770–5. [PubMed] [Google Scholar]
- 13. Yamashita H, Muto G, Hachiya R, Sugiyama H, Takahashi Y, Kaneko H, et al. A case of Sjogren's syndrome complicated by protein‐losing gastroenteropathy with unprecedented pulmonary interstitial lesions. Mod Rheumatol. 2014;24:877–9. [DOI] [PubMed] [Google Scholar]
- 14. Liao CY, Chien ST, Wang CC, Chen IH, Chiu HW, Liu MY, et al. Sjogren's syndrome associated with protein losing gastroenteropathy manifested by intestinal lymphangiectasia successfully treated with prednisolone and hydroxychloroquine. Lupus. 2015;24:1552–6. [DOI] [PubMed] [Google Scholar]
- 15. Gupta A, Cohen NL, McCarthy S, McHugh JB, Kwon R. Protein‐losing gastroenteropathy associated with Sjogren's Syndrome: first known case reported outside of Asia. ACG Case Rep J. 2015;2:184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izumi Y, Nakaoka K, Kamata M, Iwanaga N, Imadachi S, Kurohama H, et al. Steroid‐resistant protein‐losing gastroenteropathy complicated with Sjogren's syndrome successfully treated with mizoribine. Mod Rheumatol. 2018;28:716–20. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe K, Murakami S, Misago M, Yoshikawa M, Tamai D, Nakao S, et al. Sjogren's syndrome concurrent with protein‐losing gastroenteropathy with secondary systemic capillary leak syndrome : A case report. Clin Case Rep. 2018;6:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


