Abstract
Scleritis and other autoimmune diseases are characterized by an imbalance in the levels of pro-inflammatory and anti-inflammatory molecules with the balance tilted more towards the former due to the failure of recognition of self. The triggering of inflammatory process could be ascribed to the presence of cytoplasmic DNA/chromatin that leads to activation of cytosolic DNA-sensing cGAS-STING (cyclic GMP-AMP synthase linked to stimulator of interferon genes) pathway and enhanced expression of NF-κB that results in an increase in the production of pro-inflammatory bioactive lipids. Bioactive lipids gamma-linolenic acid (GLA), dihomo-GLA (DGLA), prostaglandin E1 (PGE1), prostacyclin (PGI2) and lipoxin A4, resolvins, protectins and maresins have anti-inflammatory actions, bind to DNA to render it non-antigenic and are decreased in autoimmune diseases. These results suggest that efforts designed to enhance the production of anti-inflammatory bioactive lipids may form a new approach to autoimmune diseases. Local injection or infusion of lipoxins, resolvins, protectins and maresins or their precursors such as arachidonic acid may be exploited in the prevention and management of autoimmune diseases including scleritis, uveitis and lupus/rheumatoid arthritis.
Keywords: scleritis, autoimmune diseases, bioactive lipids, inflammation, micronucleus, cytokines, resolution of inflammation
INTRODUCTION
Scleritis characterized by inflammation of the sclera, the exterior part of the eye, is usually associated with auto-immune diseases such as rheumatoid arthritis (RA), lupus, Crohn's disease, and other vasculitis. Scleritis is idiopathic and autoimmune inflammation and infection are the two main causes, though trauma can be an inciting factor. Despite the fact that scleritis may occur in patients with autoimmune diseases such as RA, it could be a separate manifestation of the autoimmune disease. It is well documented that scleritis can sometimes be a presenting manifestation of a potentially serious systemic disease. At times, scleritis may precede the systemic disease by many months or even a few years, one reason as to why it is critical for patients to have regular visits to the physician/ophthalmologist. Clinical and laboratory evaluation need to be performed to search for possible autoimmune or infectious causes. Scleral biopsy and microscopic evaluation can give important information on specific patterns of inflammation seen and the presence or absence of certain infectious organisms.
It is not uncommon to have an extension of scleral inflammation to the anterior uveal tract in severe disease with ocular complications that may lead to progressive visual loss. In general, occurrence of anterior uveitis in the course of scleritis indicates poor prognosis. Hence, the anterior uveal tract should be evaluated at every follow-up visit of a patient with scleritis, and if, anterior uveitis is noted it is imperative to start systemic immunotherapy[1].
There are two main types of scleritis: anterior and posterior scleritis (PS). Anterior scleritis, the most common type, affects the front part of the sclera and is of three types: diffuse scleritis, the most common type that causes widespread redness and inflammation throughout the whole or front portion of the sclera; nodular scleritis, is known for nodules, often tender to the touch, on the surface of the eye; necrotizing scleritis, the most severe form of anterior scleritis that can destroy scleral tissues and may lead to loss of the eye(s) and causes extreme pain and tenderness (although a rare form can occur without pain). PS, the rarer form, affects the back part of the eye and often not related to an autoimmune disease. It can develop on its own or with the anterior form of scleritis. It is characterized by pain and tenderness and often associated with complications resulting in retinal detachment and angle-closure glaucoma.
In view of the uncommon nature of PS, which is often misdiagnosed or under-diagnosed, Dong et al[2], performed a retrospective systemic evaluation of the clinical features, associated systemic diseases, and risk factors in those with PS with retinal detachment and evaluated choroidal thickness (CT) noninvasively employing enhanced depth imaging optical coherence tomography (EDI-OCT) in PS with serous retinal detachment. Their results revealed that PS with retinal detachment can present with a variety of symptoms and concluded that typical T-sign detected by B-scan ultrasound is a useful confirmatory sign for diagnosing PS. It was observed that pathological increases in CT may be useful as a potential predictor of inflammation in PS. Despite their thorough clinical and imaging evaluation of the patients using best corrected visual acuity (BCVA), intraocular pressure (IOP), fundus examination, posterior coats thickness (PCT) determination by B-scan ultrasound, and CT measurement by EDI-OCT, no efforts have been made to evaluate inflammatory markers such as cytokines, and did not report the results of the treatments offered to these subjects. It would have been helpful had the authors documented and reported the response of the study subjects to various treatments offered (such local and systemic steroids, immunosuppressive therapy, biologics used, etc.) and the corresponding prognosis. In these days of investigative medicine, it is important that clinicians collaborate with scientists who have knowledge of molecular biological approaches to various diseases and identify whether such molecular approaches will give better clues to the underlying pathobiology of a disease that may lead to a better understanding of the specific condition and development of novel approaches to diagnosis and management.
Self and Non-self-discrimination by Immune System
There are two critical issues that need close scrutiny in scleritis and other eye-related autoimmune diseases including uveitis: 1) what makes these tissues antigenic; 2) what inflammatory markers/events occur that can be exploited in their therapy, to predict prognosis and evaluate response to treatment offered. It will be interesting if the molecular and biochemical events of the disease could be correlated to the clinical picture.
One of the cruxes in autoimmune diseases, in general, and, in specific, eye-related autoimmune diseases such as scleritis and uveitis is why and how self is recognized as non-self to mount an immune attack. This suggests that under some very specific circumstances, DNA (since anti-DNA and DNA-related antibodies are present in majority of the autoimmune diseases) becomes antigenic.
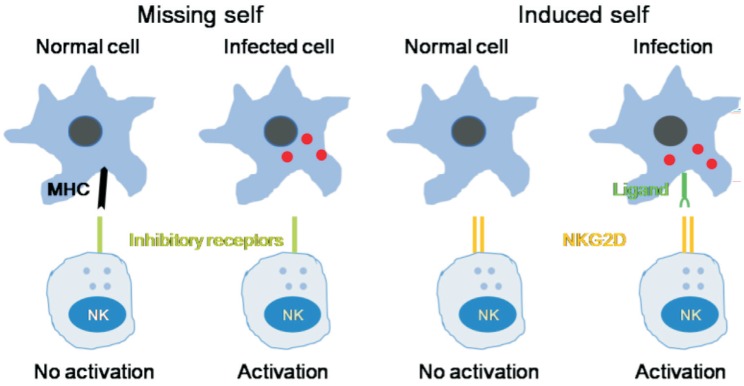
Our immune system has evolved a complex mechanism to discriminate between self and non-self. This innate recognition system is mainly based on receptors that are meant to recognize non-self-molecules present in pathogens or invading organisms or even tumor cells but are not present in the host. Thus, innate receptors are meant to recognize self-molecules that are not present in a healthy state but present in diseases. This suggests that innate immune system is capable of recognizing changes that occur in a normal cell when it is infected. The “missing self” hypothesis proposes that target cells expressing major histocompatibility complex (MHC) class I molecules are (more) resistant to natural killer (NK) cell mediated killing compared to virally infected cells which have lost the expression of MHC class I molecules implying that NK cells are able to differentiate “self” from “missing self”. In humans, these inhibitory receptors are represented by immunoglobulin (Ig)-like receptors (KIRs) and lectin-like CD94/NKG2A/B heterodimer. It is likely that interaction of MHC class I with these inhibitory receptors prevents/inhibits the activation of NK cells and the lysis of the target cells (Figure 1). In addition, microbes are recognized by the innate immune system based on a system of receptors that recognize pathogen-associated molecular patterns (PAMPs) that are unique to microbes. The best example of this system is the toll-like receptors (TLRs). TLR4 detects lipopolysaccharide (LPS), which is the major membrane component of all Gram-negative bacteria that is essential for the structural integrity of bacteria. LPS consists of a lipid that is covalently bound to a polysaccharide. It is noteworthy that polysaccharide part varies in different Gram-negative bacteria that is recognized by the adaptive immunity whereas the lipid part is highly conserved and recognized by the innate immunity-a classic example of the co-operation between innate and adaptive immunity arms of the immune system. Since most structurally variable molecules in nature (in this instance bacteria) are proteins and they (proteins) form the structural basis of distinction from one species from another and are recognized by the adaptive immunity though, innate immunity also recognizes some proteins such as flagellin that is conserved across many microbial organisms and is a ligand for TLR5[3]–[7]. In addition, innate immunity also recognizes microbial enzymes such as coagulase of Staphylococci, fungal proteases and microbial toxins, such as the adenosine diphosphate (ADP)-ribosyl transferase of diphtheria toxin. Some “danger” ligands such as uric acid, heat shock protein 70 (HSP70) and high-mobility group box-1 (HMGB-1) that are proinflammatory in nature are also recognized by the innate immune system[7]–[11], suggesting that all receptors of the innate immune system do not fit the self and non-self-paradigm.
Figure 1. Innate immunity recognizes changes in cells induced by infection.
NK cells have inhibitory receptors on their surface to differentiate “self” from “missing-self”. The lack of expression of MHC class I molecules (missing self) promotes the activation of NK cells and subsequent lysis of the target cells. NK cells also express activating receptors such as NKG2D that directly recognize ligands induced in response to infection (induced self). NK cell-mediated cytotoxicity against malignant target cells or infected cells is regulated by both activating and inhibitory cell surface immunoreceptors. In humans, such receptors are of three types: 1) the killer immunoglobulin receptors (KIRs), 2) natural cytotoxicity receptors (NCRs), and 3) the c-type lectin receptors. NKG2D is one member of the c-type lectin-activating receptor family that is evolutionarily conserved and is located within the NK gene complex on human chromosome 12p12-p13. NKG2D is expressed on all NK cells and is a promiscuous receptor that recognizes at least 6 counter ligands that include: the MHC class I-like molecules, MICA and MICB, and members of the ULBP family (ULPB1-4), named for the ability of some members to bind to the UL-16 protein of cytomegalovirus (CMV).
The adaptive immune response is different from innate that has the ability to mount a specific immune response against any microbe or stimuli that our body encounters. The T and B cells that constitute the adaptative immunity are able to somatically generates large repertories of specific receptors [(T cell receptor (TCR) and B cell receptor (BCR)] that have the ability to virtually recognize any non-self-antigen. As a consequence of this specific and efficient adaptive immune system, it is essential to discriminate self from non-self in order to avoid anti-self-reaction (in the form of autoimmune reactions). Thus, lymphocytes that bear these high avidity autoreactive receptors need to be eliminated or suppressed or regulated by an efficient negative feed-back control system. Since such a process is likely to be imperfect, it explains the high frequency of autoimmune diseases seen in the clinic. At the same time, the adaptive immune system needs to develop effect or mechanisms that are capable of eliminating pathogens that calls for it to be coupled with the innate immune system and use the same system to eliminate the attacking pathogens.
Cytoplasmic DNA and Inflammation
It is evident from the preceding discussion that autoimmunity is due to failure in self and non-self discrimination. Several pathogenic mechanisms proposed for the development of autoimmune diseases include molecular mimicry, exposure of hidden antigens, loss of suppressor cell function, T and B cell dysfunction, epitope spreading and epitope drift and polyclonal B cell activation by superantigens. In this context, it is noteworthy that recognition of microbial nucleic acids by the host is an important strategy that is needed to respond to various infectious agents. Several microbial DNAs are introduced into the host cells during infections that need to be recognised appropriately and eliminated without triggering abnormal immune responses. The intracellular DNA that is introduced into cells during infections are known to trigger inflammatory responses by triggering induction of anti-viral type I interferons (IFNs), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-18. If nucleases such as DNase II or DNase III (Trex1) fail to clear cytosolic DNA, then accumulated DNA drives inflammatory responses that lead to autoimmune diseases. There appears to exist various ways to recognize and respond to cytosolic DNA. Thus, it is imperative that there are specific sensors that can couple cytosolic DNA recognition to immune signaling. One such pathway leads to the proteolytic activation of the cysteine protease caspase-1 that is associated with maturation and secretion of the IL-1β and IL-18. The second pathway could involve the transcriptional induction of type 1 IFN and pro-inflammatory genes (Figure 2). IL-1β activates neutrophils, macrophages, dendritic cells, and T cells whereas IL-18 incites IFN-γ production by NK and T cells. All these events ultimately lead to the formation of inflammasome that occurs as a result of immune responses to intracellular DNA of bacterial or viral origin[12]–[16]. These results suggest that while DNA-induced immune responses are critical to immunity, failure to recognize self-DNA can lead to inappropriate consequences namely autoimmune diseases such as lupus in which type I IFN and autoantibodies directed against dsDNA, RNA and nucleosomes can be found. Thus, failure of the multiple fail-safe mechanisms employed by the host are subverted leading to DNA-induced immune responses and inflammation (Figure 2). One such regulation provided by the body include cellular endonucleases such as DNase I, DNase II and DNase III (also known as Trex1) that are normally involved in the clearance of extracellular, lysosomal and cytosolic DNA respectively. This implies that functional defects in these enzymes are present in lupus and other autoimmune diseases.
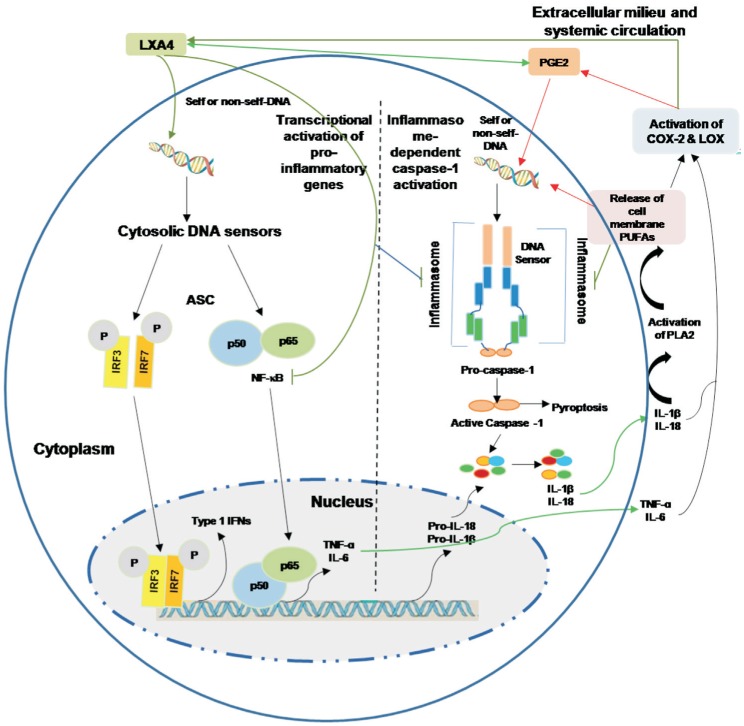
Figure 2. Cytoplasmic DNA triggers transcription of inflammatory genes and inflammasome-dependent proteolytic activation of caspase-1.
Presence of DNA in the cytoplasm leads to the activation of two distinct signaling pathways 1) activation of IRF3, IRF7 and NF-κB that results in the transcriptional induction of type 1 IFN genes or pro-inflammatory genes IL-6 and TNF-α; 2) cytosolic DNA leads to the assembly of inflammasome leading to caspase-1 activation and subsequent cleavage of pro-IL-β and pro-IL-18 that results in the formation of biologically active and mature forms of IL-1β and IL-18. Caspase activation mediates the cell death under some very specific conditions (such as tumor cell death). Enhnced synthesis and secretion of pro-inflammatory cytokines leads to activation of COX-2 and LOX enzmes resulting in the produciton of pro-inflammatory bioactive lipids such as PGE2, LTs and TXs and a simultaenous decrease of anti-inflammatory LXa, resolvins, protectins and maresins from their respetive precursor PUFAs (AA, EPA and DHA). These PUFAs, LXA4, resolvins, protectins and maresins can suppress NF-κB and IRF3 and IRF7 activation and inhibit the production of pro-inflammatory cytokines. Under some very speific situations PGE2 may function as an anti-inflammatry molecule. PGE2 and IL-1β have been shown to trigger release of AA/EPA/DHA from the cell membrane pool by activating PLA2 that can lead to the formation of anti-inflammatory LXA4/resolvins/protectins/maresins that, in turn, suppress production of IL-6 and TNF-αto restore homeostasis. Thus, there is a very close and a positive and negative feed-back regulation between pro- and anti-inflammatory cytokines, bioactive lipids and respective signaling pathways.
The fact that cytoplasmic DNA could incite inflammatory process is supported by the recent studies that showed that cytoplasmic chromatin (chromatin is a complex of DNA, RNA, and protein found in eukaryotic cells) activates the innate immunity cytosolic DNA-sensing cGAS-STING (cyclic GMP-AMP synthase linked to stimulator of interferon genes) pathway, that leads to two downstream events: type 1 IFN through IRF3, and pro-inflammatory response through NF-κB (Figure 2). Further studies showed that cGAS-STING pathway is connected to the NF-κB-mediated senescent associated secretory phenotype (SASP) that results in the secretion of pro-inflammatory cytokines (into the surrounding milieu and systemic circulation), recruitment of immune cells, modulate their activity and consequently alters tissue microenvironment[17]–[20]. It is noteworthy that when wild-type and Sting-null mice are exposed to sub-lethal ionizing radiation to induce DNA damage, senescence and SASP program, the production of IL-1α was significantly reduced in the null mice indicating that STING mediates DNA damage-induced SASP and tissue inflammation[17]–[18]. Subsequently, it was noted that STING is essential for Ras-induced SASP and immune surveillance since expression of STING restored cytokine expression, inflammation and immune mediated clearance of malignant cells. It is interesting that short-term inflammation and senescence serve as barriers to tumorigenesis, persistent inflammation produces tissue damage and enhances tumor growth that explains increased incidence of cancer (especially lymphomas) in those with lupus, Behcet's disease and rheumatoid arthritis (RA) (conditions in which micronuclei cells are common and are frequently associated with uveitis and scleritis) and cancer cells frequently contain extra-nuclear chromatin[21]–[24]. But, paradoxically, cytoplasmic chromatin (DNA) incidence was never studied in those with scleritis and uveitis despite the fact that these are autoimmune diseases.
Cytokines and Essential Fatty Acid Metabolism
The cytoplasmic DNA/chromatin (micronuclei) that is frequent in lupus and RA and other autoimmune diseases (possibly in uveitis and scleritis)-induced increase in the production of proinflammatory cytokines IL-1β, TNF-α, IL-18, IL-6 and IFN-γ that spill over into the intercellular surrounding milieu and systemic circulation can be detected in the form of their enhanced plasma levels. In view of the putative role of TNF-α and IL-6 in lupus and RA, monoclonal antibodies against these cytokines are now being employed in their management[25]–[30]. Despite the belief that autoimmune process is involved in the pathobiology of scleritis and uveitis, anti-TNF-α and anti-IL-6 antibodies and other biologics are not routinely employed in their management except in rare instances[31]–[33]. This is understandable since, in majority of the patient's systemic manifestations of the suspected autoimmune disease is not evident. This also suggests that perhaps, understanding local inflammatory events are more crucial to manage scleritis/uveitis. In this context, the relationship between inflammatory cytokines and the bioactive lipids is important.
Essential Fatty Acids Metabolism
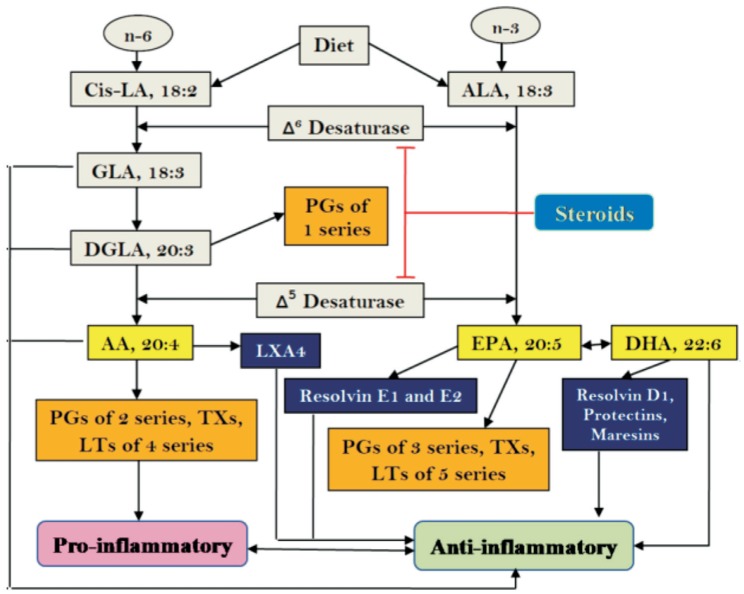
Our diet is rich in essential fatty acids (EFAs) linoleic acid (LA, 18:2 n-6) and alpha-linolenic acid (ALA, 18:3 n-3) and are acted upon by delta-6-desaturase and delta-5-desaturase and respective elongases to form their long-chain metabolites gamma-linolenic acid (GLA, 18:3 n-6), dihomo-gamma-linolenic acid (DGLA, 20:3 n-6) and arachidonic acid (AA, 20:4 n-6) and eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3) respectively. LA, GLA, DGLA, AA, ALA, EPA and DHA are called as polyunsaturated fatty acids (PUFAs) but only LA and ALA are EFAs since they cannot be formed in the body. DGLA forms the precursor of prostaglandins (PGs) of 1 series; AA forms the precursor of 2 series PGs, thromboxanes (TXs) and 4 series leukotrienes (LTs), whereas EPA is the precursor of 3 series PGs, TXs and 5 series LTs. Most of the PGs, TXs and LTs are pro-inflammatory in nature (2 series PGs and TXs>3 series PGs and TXs and 4 series LTs>5 series LTs). The conversion of DGLA, AA and EPA to their respective PGs, TXs and LTs is due to the action of cyclo-oxygenases (COX-1 and COX-2) and 5-, 12- and 15-lipoxygenases (5-LOX, 12-LOX and 15-LOX). It is noteworthy that AA is also the precursor of a potent anti-inflammatory product lipoxin A4 (LXA4) while EPA is the precursor of similar anti-inflammatory metabolites called as resolvins whereas DHA gives rise to resolvins, protectins and maresins[34] (Figure 3).
Figure 3. Scheme showing metabolism of essential fatty acids and their pro- and anti-inflammatory products.
Steroids block the activities of desaturases that leads to a decrease in the concentrations of GLA, DGLA, AA, EPA and DHA. This results in decreased formation of not only pro-inflammatory PGs, LTs and TXs but also lipoxins, resolvins, protectins and maresins (due to precursor deficiency). Hence, resolution of inflammation will be incomplete. This ultimately causes continuous of inflammation to chronic phase and failure of healing of wound due to deficiency of lipoxins, resolvins, protectins and maresins.
Interaction(s) among cytokines, phospholipases, PUFAs, COX-2, LOX enzymes, corticosteroids and their relevance to inflammation and resolution of inflammation
It is likely that under normal physiological conditions, a delicate balance is maintained between pro- and anti-inflammatory eicosanoids (similar to the balance struck between pro- and anti-inflammatory cytokines). It is interesting to note that when the inflammatory process reaches its peak, the anti-inflammatory pathway is triggered that results in the formation of adequate amounts of LXA4, resolvins, protectins and maresins and anti-inflammatory cytokines accompanied by suppression of reactive oxygen species (ROS) generation and enhancement of anti-oxidant defences that results in the resolution of inflammation and restoration of homeostasis. Lipoxins, resolvins, protectins and maresins are essential for resolution of inflammation and wound healing since they inhibit polymorphonuclear leukocytes (PMNLs) trans-endothelial migration, reduce leucocyte infiltration, and suppress dendritic cells (DC) migration and IL-12 production. Lipoxins, resolvins, protectins and maresins enhance the expression of anti-inflammatory genes and attenuate LTB4-stimulated proinflammatory signals[34]. In general, lipoxins, resolvins, protectins and maresins seem to have similar and overlapping anti-inflammatory and pro-resolving actions.
In this context, the interactions between pro- and anti-inflammatory cytokines and PUFAs metabolism is interesting. Proinflammatory cytokines IL-1, IL-6, TNF-α and IFN-γ activate phospholipases, enhance ROS generation[35]–[39], increase activity of COX-2 and LOX enzymes to augment the production of pro-inflammatory PGE2, TXA2 and LTs. The precursors for the formation of these proinflammatory eicosanoids are derived from the cell membrane lipid pool by the activation of phospholipase A2 (PLA2) by pro-inflammatory cytokines (Figures 2 and 3). It is important to note that the release of PUFAs from the cell membrane lipid pool occurs in two waves by their respective phospholipases. There are three classes of phospholipases that regulate the release of PUFAs: calcium-independent PLA2 (iPLA2), secretory PLA2 (sPLA2), and cytosolic PLA2 (cPLA2). Each class of PLA2 is further divided into isoenzymes for which there are 10 for mammalian sPLA2, at least 3 for cPLA2, and 2 for iPLA2. The first wave of release of PUFAs from the cell membrane occurs due to the action of iPL2 that leads to the formation of pro-inflammatory PGE2, TXA2 and LTB4. The second wave of release of PUFAs occurs by the action of sPLA2 and cPLA2 at the time of resolution of inflammation leading to the formation of lipoxins, resolvins, protectins and maresins that are essential for the suppression of inflammation. It is paradoxical to know that formation of adequate amounts of PGE2 is necessary to both induce optimal inflammation and at the same time to trigger the initiation of resolution of inflammation. Thus, PUFAs released at the instance of inflammatory stimuli by the activation of iPLA2 are directed to form pro-inflammatory PGs, TXs and LTs whereas PUFAs released from the cell membrane at the time of resolution of inflammation triggered by sPLA2 and cPLA2 are directed to form lipoxins, resolvins, protectins and maresins. This delicate balance and switch over from pro-inflammatory to anti-inflammatory molecules are determined by the type of PLAs that are activated in response to pro- and anti-inflammatory cytokines and the activities of COX-2 and 5-, 12- and 15-LOX enzymes. This close co-operation and interaction(s) among PLAs, COX-2, LOX enzymes and various cytokines is essential for the appropriate inflammation to occur for gradual, smooth and orderly onset of anti-inflammatory events and resolution of inflammation and restoration of homeostasis[34]. Any defects in this process (dysfunction of cytokines, PLAs, COX, LOX enzymes, cell membrane stores of PUFAs, etc.) could result in persistance of inflammation and damage to the target tissues as is seen in autoimmune diseases including scleritis and uveitis.
In this context, it is important to note that IL-6, TNF-α and corticosteroids suppress the activities of desaturases resulting in deficiency of AA, EPA and DHA that results in decreased formation of LXA4, resolvins, protectins and maresins (due to precursor deficiency) but ironically excess formation of PGs, LTs and TXs persists[34]. In contrast, IL-6 and TNF-α activate PLA2, COX-2 and LOX enzymes whereas corticosteroids suppress them. This is supported by the observation that supplementation of AA during active inflammatory process when PGs, LTs and TXs are being synthesized in excess actually results in an increase in the formation of LXA4 (and possibly, resolvins, protectins and maresins) with or without any change in the concentrations of PGE2 (and thus, tilting the balance more towards anti-inflammatory molecules) and suppresses the inflammation process[34],[40]–[41]. AA, EPA, DHA, LXA4, resolvins, protectins and maresins inhibit the production of IL-6, TNF-α and ROS. Thus, corticosteroids, IL-6 and TNF-α can induce an EFA (PUFAs)-deficiency state by their ability to block the activities of desaturases as a result formation of lipoxins, resolvins, protectins and maresins is decreased leading to failure of resolution of inflammation. In the initial stages of inflammation, corticosteroids suppress inflammation by blocking the activities of PLA2, desaturases, COX-2 and LOX enzymes. In contrast, IL-6 and TNF-α induce inflammation by activating PLA2, COX-2 and LOX enzymes and enhancing the formations of PGs and LTs. This may explain why steroids are potent suppressors of acute inflammation but in the long run fail to induce wound healing since they block the formation of LXA4, resolvins, protectins and maresins that are needed for resolution of inflammation[34],[42]–[43]. IL-1β that is markedly increased during the inflammatory process induces PG biosynthesis and also up regulates the formation of LXA4 and maresins that are needed for resolution of inflammation. Both LXA4 and maresins (resolvins and protectins) are potent down-regulators of PGE2 production. Increased 15-prostaglandin dehydrogenase (15-PGDH) expression seems to augment the formation of LXA4, resolvins, protectins and maresins and regeneration of tissues to aid reestablish tissue homeostasis[34],[42]–[48]. Thus, IL-1β and PGE2 seem to have both pro- and anti-inflammatory actions depending on the context (Figure 4). These results imply that in order to suppress acute and chronic inflammation and inhibit the production of pro-inflammatory IL-6 and TNF-α, one need to employ LXA4, resolvins, protectins and maresins in combination with corticosteroids with/without AA/EPA/DHA in inflammatory conditions such as scleritis and uveitis.
Figure 4. Scheme showing the relationship among pro- and anti-inflammatory cytokines, PGs, LTs, lipoxins, resolvins, protectins and maresins and steroids. Metabolism and actions of arachidonic acid is shown as a representative of various PUFAs (DGLA, EPA and DHA).
(+) Indicates increase in the synthesis/action or positive effect. (-) Indicates decrease in the synthesis/action or negative effect.
PUFAs and their metabolites regulate cytoskeleton system
Since PUFAs (especially GLA, DGLA, AA, EPA and DHA) and their pro- and anti-inflammatory metabolites are potent regulators of the expression and concentrations of pro- and anti-inflammatory cytokines, ROS, and pro- and anti-inflammatory events, it is important to know mechanism(s) of their action. In addition to their ability to enhance or suppress the activation of various immunocytes (PMNLs, T cells, macrophages, dendritic cells, etc.), it is noteworthy that these bioactive lipids can alter the cell membrane (not only cell membrane, but also that of mitochondrial membrane, nuclear membrane, etc.) fluidity by virtue of their incorporation into it. Thus, when the membrane content of PUFAs is high it will become more fluid and when their PUFAs content is low (this is likely to happen when saturated fatty acids and cholesterol content of the membrane is increased at the cost of PUFAs) the membrane will be more rigid. It was reported that increasing the PUFAs content of the cell membranes not only increases membrane fluidity but also increases the number of insulin receptors and their affinity to its receptors, whereas higher content of saturated fatty acids decreases membrane fluidity and decreases the number of insulin receptors and their affinity to the receptors[49]–[54]. These studies imply that PUFAs can alter cell membrane fluidity and thus, alter the expression of receptors and their binding to their respective receptors. Cell membrane configuration/properties are controlled by elements of the cytoskeleton (that include microfilaments, microtubules, and intermediate filaments) that play a critical role in maintaining the cell shape, cell movement, intracellular transport/trafficking, cytokinesis and cytoplasmic streaming. Since PUFAs and their metabolites regulate phagocytosis, cell mobility, cell receptor number and secretion of cytokines and other molecules, it is reasonable to propose that these bioactive lipids can modulate cytoskeleton system. It was reported that treatment of mouse lymphocytes with LA produced alterations in their cytoskeleton and contractile proteins indicating that PUFAs have the ability to alter the interaction of surface receptors with the cytoskeleton and thus, could affect cytoplasmic distribution of the proteins[55]–[56]. It is interesting to note that 15 deoxyΔ[12]–[14] PGJ2 that is essential for resolution of inflammation and has potent anti-inflammatory actions with proteins that are involved in cytoskeletal organization, such as actin, tubulin, vimentin, and tropomyosin and induced early reorganization of vimentin and tubulin in cultured mesangial cells of the kidney[57]. 12(S)-hydroxyeicosatetraenoic acid (12-HETE), derived from AA, that has chemotactic actions on neutrophils and macrophages is known to influence glucose transporter 4 (GLUT4) translocation and thus, regulate glucose transport by contributing to rearrangement of actin cytoskeletal elements[58]. HETE is important for corneal epithelial cell migration during wound healing[59] suggesting that AA metabolites participate in the restoration of functional integrity of cornea and sclera once the inflammation process subsides. It is noteworthy that LXA4, a potent anti-inflammatory metabolite of AA that is known to modulate leukocyte trafficking and stimulate nonphlogistic macrophage phagocytosis of apoptotic neutrophils and thus, promotes the resolution of inflammation and wound healing has been shown to facilitate actin cytoskeleton rearrangement and cell polarization[60]. These evidences[55]–[60] highlight the critical role of PUFAs and their metabolites not only in inflammation and its resolution, stimulation of nonphlogistic macrophage phagocytosis of apoptotic neutrophils and debris removal at the site of inflammation, but also their regulatory role incorneal and scleral cell proliferation, migration and restoring their (corneal and scleral) functional integrity.
PUFAs and their metabolites and micronuclei cells
Cytoplasmic DNA/chromatin triggers inflammationby enhancing the expression of NF-κB and such micronuclei cells are frequent in autoimmune diseases[16]–[24] (Figure 2). In this context, it is noteworthy that studies showed that GLA, DGLA, PGE1 and PGI2, which have anti-inflammatory actions[61]–[70] can prevent/decrease radiation, benzo(a)pyrene, and other chemicals-induced incidence of micronucleus containing human lymphocytes and mouse bone marrow cells[71]–[78]. These results suggest that PUFAs and their anti-inflammatory metabolites including LXA4 can prevent the generation of cytoplasmic DNA/chromatin in cells and thus, prevent inflammation and consequent autoimmune process. In addition, PUFAs and their metabolites seem to be capable of binding to DNA and regulate the expression of several genes to bring about their critical actions[79]–[82]. Our recent studies revealed that PUFAs and their metabolites especially LXA4 can suppress the expression of NF-κB and alter the expressions of p53, Ras, Myc, Ros, Ras, Bax, Bcl-2, caspases, lipocalin-2, PDX1, Nrf2, GLUT-2 and other genes in vitro and in vivo[83]–[87]. It was noted that peroxidized products of PUFAs but not PUFAs bind to DNA and thus, regulate gene(s) expression[88]–[90]. These results suggest that PUFAs and their metabolites such as PGs and LXA4 bind to DNA not only to regulate gene expression but also to render DNA non-antigenic to prevent cytoplasmic DNA/chromatin-induced inflammatory process. Thus, PUFAs and their metabolites may aid T cells and other immunocytes in self and non-self-discrimination. This may explain the apparently paradoxical results obtained by us wherein GLA supplementation to patients who are on long-term treatment with DPH (diphenylhydantoin for epilepsy) showed decreased number of micronuclei containing peripheral lymphocytes but DNA ladder pattern (an indication of DNA damage) was increased suggesting apoptosis of cells harboring DNA damage. This implies that GLA prevents cells from accumulating genetic damage[77].
CONCLUSIONS AND THERAPEUTIC IMPLICATIONS
It is evident from the preceding discussion that cytoplasmic DNA/chromatin triggers pro-inflammatory process by enhancing the expression of NF-κB that, in turn, leads to enhanced production and secretion of IL-1β, TNF-α, IFNs, IL-10 and IL-6 (Figure 2), which ultimately results in the onset of autoimmune diseases lupus, RA, scleritis and uveitis[16]–[24]. It is known that in auto-immune diseases the number of buccal mucosal cells and circulating lymphocytes containing micronuclei is increased[21]–[24]. The enhanced secretion of pro-inflammatory cytokines upregulates the activities of PLA2, COX-2 and LOX enzymes that leads to an increase in the production of pro-inflammatory PGs, LTs and TXs and decrease in anti-inflammatory bioactive lipids lipoxins, resolvins, protectins and maresins. Thus, there is a cross-talk between cytokines and bioactive lipids in the pathobiology of auto-immune diseases including scleritis and uveitis. Hitherto, it has been customary to measure plasma, synovial and tissue fluid(s) content of cytokines, PGs, LTs and TXs to assess and quantify the inflammatory process. Based on the preceding discussion, I propose that the number of micronucleus containing cells, plasma and tissue fluid(s) content of PGs, LTs, TXs, lipoxins, resolvins, protectins and maresins in addition to the concentrations of cytokines could be employed to assess the degree of inflammation, response to therapy and prognosis of the underlying auto-immune disease(s). Thus, it is recommended that in those with scleritis (and other auto-immune diseases): 1) Measure the number of micronucleus containing cells in the scleral scrapings and other appropriate tissues. 2) Estimate the concentrations of various cytokines and bioactive lipids (PUFAs, PGs, LTs, TXs, lipoxins, resolvins, protectins and maresins) in the tears, plasma and other body fluids (such as vitreal fluid in the case of uveitis). 3) These measurements could be used to assess the degree of inflammation, and changes in their concentrations may aid in assessing the progress of the disease, response to therapy and prognosis. It is likely that if the number of micronucleus containing cells and pro-inflammatory cytokines and PGs, LTs, TXs are decreasing with therapy the patient is responding to the treatment offered and prognosis is good. Furthermore, the number of micronucleus containing cells could be correlated with the cytokine profile and concentrations of bioactive lipids to assess the balance between pro- and anti-inflammatory events to assess response to therapy, progress and prognosis of the disease.
Measuring the concentrations of various cytokines and bioactive lipids in the tears of patients with scleritis and other inflammatory conditions of eye (such as uveitis wherein vitreal fluid can be used for such measurements) will form a simple, elegant and objective method of both clinical and laboratory assessment of progress of the disease that could be correlated with the clinical picture. Wherever facilities and expertise permit, perhaps, cellular scrapings (or cell/tissue samples) could be used for measuring the expressions of NF-κB, various cytokines, desaturases, COX and LOX enzymes, PG synthetases and other gene expression studies and correlated with the concentrations of their products and correlated to the clinical picture. Such in depth studies employing molecular, biochemical and genetic studies would enhance our understanding of the pathogenesis of the diseases.
In addition, the preceding discussion implies that newer therapeutic approaches in the management of scleritis and uveitis and other ophthalmic inflammatory conditions is a distinct possibility. LXA4/resolvins/protectins/maresins containing ophthalmic preparations could be developed and further studies could investigate the potential role of their local instillation (on the surface of the eye or intravitreally, especially for those with uveitis and possibly, diabetic retinopathy as it is also considered as an inflammatory condition[91]–[93]) in reduction of inflammation by themselves alone or in combination with local and systemic steroids and/or with immunosuppressive drugs to induce or enhance the anti-inflammatory process and accelerate healing. Such novel therapeutic approaches could be attempted in future.
Acknowledgments
Conflicts of Interest: Das UN, None.
REFERENCES
- 1.Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis-associated uveitis. Ophthalmology. 1997;104(1):58–63. doi: 10.1016/s0161-6420(97)30361-3. [DOI] [PubMed] [Google Scholar]
- 2.Dong ZZ, Gan YF, Zhang YN, Zhang Y, Li J, Zheng HH. The clinical features of posterior scleritis with serous retinal detachment: a retrospective clinical analysis. Int J Ophthalmol. 2019;12(7):1151–1157. doi: 10.18240/ijo.2019.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn M. The common sense of the self-nonself discrimination. Springer Semin Immunopathol. 2005;27(1):3–17. doi: 10.1007/s00281-005-0199-1. [DOI] [PubMed] [Google Scholar]
- 4.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102(27):9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001;181:170–184. doi: 10.1034/j.1600-065x.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 6.Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev. 2001;181:158–169. doi: 10.1034/j.1600-065x.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez S, González-Rodríguez AP, Suárez-Álvarez B, López-Soto A, Huergo-Zapico L, Lopez-Larrea C. Conceptual aspects of self and nonself discrimination. Self Nonself. 2011;2(1):19–25. doi: 10.4161/self.2.1.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 10.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227(1):248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 13.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 14.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 15.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 16.Atianand MK, Fitzgerald KA. Molecular basis of DNA recognition in the immune system. J Immunol. 2013;190(5):1911–1918. doi: 10.4049/jimmunol.1203162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dou ZX, Ghosh K, Vizioli MG, Zhu JJ, Sen P, Wangensteen KJ, Simithy J, Lan YM, Lin YP, Zhou Z, Capell BC, Xu CY, Xu MG, Kieckhaefer JE, Jiang TY, Shoshkes-Carmel M, Tanim KMAA, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umbreit NT, Pellman D. Cancer biology: Genome jail-break triggers lockdown. Nature. 2017;550(7676):340–341. doi: 10.1038/nature24146. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, Olova N, Sutcliffe H, Rainger JK, Leitch A, Osborn RT, Wheeler AP, Nowotny M, Gilbert N, Chandra T, Reijns MAM, Jackson AP. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Rawi ZS, Gorial FI, Tawfiq RF, Mohammed AK, Al-Naaimi AS, Al'aadhmi MA, Hayyawi AA. Brief report: a novel application of buccal micronucleus cytome assay in systemic lupus erythematosus: a case-control study. Arthritis & Rheumatology (Hoboken, N.J) 2014;66(10):2837–2841. doi: 10.1002/art.38764. [DOI] [PubMed] [Google Scholar]
- 22.Karaman A, Kadi M, Kara F. Sister chromatid exchange and micronucleus studies in patients with Behçet's disease. J Cutan Pathol. 2009;36(8):831–837. doi: 10.1111/j.1600-0560.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamurcu Z, Dönmez-Altuntas H, Borlu M, Demirtas H, Asçioslu O. Micronucleus frequency in the oral Mucosa and lymphocytes of patients with Behçet's disease. Clin Exp Dermatol. 2005;30(5):565–569. doi: 10.1111/j.1365-2230.2005.01876.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Remus C, Dorazco-Barragan G, Aceves-Avila FJ, Alcaraz-Lopez F, Fuentes-Ramirez F, Michel-Diaz J, Torres-Bugarin O, Ventura-Aguilar A, Zuñiga-González G. Genotoxicity assessment using micronuclei assay in rheumatoid arthritis patients. Clin Exp Rheumatol. 2002;20(2):208–212. [PubMed] [Google Scholar]
- 25.Cavalcanti A, Santos R, Mesquita Z, Duarte AL, Lucena-Silva N. Cytokine profile in childhood-onset systemic lupus erythematosus: a cross-sectional and longitudinal study. Revista Brasileira De Pesquisas Med E Biol. 2017;50(4):e5738. doi: 10.1590/1414-431X20175738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimarães PM, Scavuzzi BM, Stadtlober NP, Franchi Santos LFDR, Lozovoy MAB, Iriyoda TMV, Costa NT, Reiche EMV, Maes M, Dichi I, Simão ANC. Cytokines in systemic lupus erythematosus: far beyond Th1/Th2 dualism lupus: cytokine profiles. Immunol Cell Biol. 2017;95(9):824–831. doi: 10.1038/icb.2017.53. [DOI] [PubMed] [Google Scholar]
- 27.Foster W, Carruthers D, Lip GY, Blann AD. Inflammatory cytokines, endothelial markers and adhesion molecules in rheumatoid arthritis: effect of intensive anti-inflammatory treatment. J Thromb Thrombolysis. 2010;29(4):437–442. doi: 10.1007/s11239-009-0370-y. [DOI] [PubMed] [Google Scholar]
- 28.Bray VJ, Broadwell A, Baraf HSB, Black S, Brady BL, Tkacz J, Yarngo L, DeHoratius RJ. The effectiveness of intravenous golimumab administered directly after infliximab in rheumatoid arthritis patients. Drugs R D. 2018;18(3):211–219. doi: 10.1007/s40268-018-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leandro MJ. Anti-tumour necrosis factor therapy and B cells in rheumatoid arthritis. Arthritis Res Ther. 2009;11(5):128. doi: 10.1186/ar2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang BL, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–796.e3. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 32.Wakefield D, Di Girolamo N, Thurau S, Wildner G, McCluskey P. Scleritis: challenges in immunopathogenesis and treatment. Discov Med. 2013;16(88):153–157. [PubMed] [Google Scholar]
- 33.El-Shabrawi Y, Hermann J. Anti-TNF alpha therapy in chronic necrotizing scleritis resistant to standard immunomodulatory therapy in a patient with Wegener's granulomatosis. Eye (Lond) 2005;19(9):1017–1018. doi: 10.1038/sj.eye.6701712. [DOI] [PubMed] [Google Scholar]
- 34.Poorani R, Bhatt AN, Dwarakanath BS, Das UN. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol. 2016;785:116–132. doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 35.Das UN, Ells G, Begin ME, Horrobin DF. Free radicals as possible mediators of the actions of interferon. J Free Radic Biol Med. 1986;2(3):183–188. doi: 10.1016/s0748-5514(86)80068-x. [DOI] [PubMed] [Google Scholar]
- 36.Das UN, Padma M, Sagar PS, Ramesh G, Koratkar R. Stimulation of free radical generation in human leukocytes by various agents including tumor necrosis factor is a calmodulin dependent process. Biochem Biophys Res Commun. 1990;167(3):1030–1036. doi: 10.1016/0006-291x(90)90626-x. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto M, Yokota S, Vilcek J, Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- 38.Berton G, Zeni L, Cassatella MA, Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- 39.Das UN, Huang YS, Begin ME, Horrobin DF. Interferons, phospholipid metabolism, immune responses and cancer. IRCS Med Sci. 1986;14:1069–1074. [Google Scholar]
- 40.Tateishi N, Kakutani S, Kawashima H, Shibata H, Morita I. Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon, but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis. 2014;13:30. doi: 10.1186/1476-511X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tateishi N, Kaneda Y, Kakutani S, Kawashima H, Shibata H, Morita I. Dietary supplementation with arachidonic acid increases arachidonic acid content in paw, but does not affect arthritis severity or prostaglandin E2 content in rat adjuvant-induced arthritis model. Lipids Health Dis. 2015;14:3. doi: 10.1186/1476-511X-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das UN. Circulating microparticles in septic shock and Sepsis-related complications. Minerva Anestesiol. 2019;85(6):571–576. doi: 10.23736/S0375-9393.19.13596-1. [DOI] [PubMed] [Google Scholar]
- 43.Das UN. Polyunsaturated fatty acids and Sepsis. Nutrition. 2019;65:39–43. doi: 10.1016/j.nut.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Dakin SG, Ly L, Colas RA, Oppermann U, Wheway K, Watkins B, Dalli J, Carr AJ. Increased 15-PGDH expression leads to dysregulated resolution responses in stromal cells from patients with chronic tendinopathy. Sci Rep. 2017;7(1):11009. doi: 10.1038/s41598-017-11188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das UN. Current and emerging strategies for the treatment and management of systemic lupus erythematosus based on molecular signatures of acute and chronic inflammation. J Inflamm Res. 2010;3:143–170. doi: 10.2147/JIR.S9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YY, Desai A, Yang SY, Bae KB, Antczak MI, Fink SP, Tiwari S, Willis JE, Williams NS, Dawson DM, Wald D, Chen WD, Wang ZH, Kasturi L, Larusch GA, He L, Cominelli F, Di Martino L, Djuric Z, Milne GL, Chance M, Sanabria J, Dealwis C, Mikkola D, Naidoo J, Wei SG, Tai HH, Gerson SL, Ready JM, Posner B, Willson JK, Markowitz SD. TISSUE REGENERATION. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348(6240):aaa2340. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FitzGerald GA. BIOMEDICINE. Bringing PGE2 in from the cold. Science. 2015;348(6240):1208–1209. doi: 10.1126/science.aac5515. [DOI] [PubMed] [Google Scholar]
- 48.Duffin R, O'Connor RA, Crittenden S, Forster T, Yu CJ, Zheng XZ, Smyth D, Robb CT, Rossi F, Skouras C, Tang SH, Richards J, Pellicoro A, Weller RB, Breyer RM, Mole DJ, Iredale JP, Anderton SM, Narumiya S, Maizels RM, Ghazal P, Howie SE, Rossi AG, Yao CC. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351(6279):1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ginsberg BH, Jabour J, Spector AA. Effect of alterations in membrane lipid unsaturation on the properties of the insulin receptor of Ehrlich ascites cells. Biochim Biophys Acta. 1982;690(2):157–164. doi: 10.1016/0005-2736(82)90318-2. [DOI] [PubMed] [Google Scholar]
- 50.Ginsberg BH, Chatterjee P, Yorek MA. Insulin sensitivity is increased in Friend erythroleukemia cells enriched in polyunsaturated fatty acid. Receptor. 1991;1(3):155–166. [PubMed] [Google Scholar]
- 51.Yorek M, Leeney E, Dunlap J, Ginsberg B. Effect of fatty acid composition on insulin and IGF-I binding in retinoblastoma cells. Invest Ophthalmol Vis Sci. 1989;30(10):2087–2092. [PubMed] [Google Scholar]
- 52.Field CJ, Ryan EA, Thomson AB, Clandinin MT. Dietary fat and the diabetic state alter insulin binding and the fatty acyl composition of the adipocyte plasma membrane. Biochem J. 1988;253(2):417–424. doi: 10.1042/bj2530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grunfeld C, Baird KL, Kahn CR. Maintenance of 3T3-L1 cells in culture media containing saturated fatty acids decreases insulin binding and insulin action. Biochem Biophys Res Commun. 1981;103(1):219–226. doi: 10.1016/0006-291x(81)91682-x. [DOI] [PubMed] [Google Scholar]
- 54.Das UN. Insulin resistance and hyperinsulinemia: are they secondary to an alteration in the metabolism of essential fatty acids? Med Sci Res. 1994;22:243–245. [Google Scholar]
- 55.Hoover RL, Fujiwara K, Klausner RD, Bhalla DK, Tucker R, Karnovsky MJ. Effects of free fatty acids on the organization of cytoskeletal elements in lymphocytes. Mol Cell Biol. 1981;1(10):939–948. doi: 10.1128/mcb.1.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marra CA, de Alaniz MJ. Microtubular integrity differentially modifies the saturated and unsaturated fatty acid metabolism in cultured Hep G2 human hepatoma cells. Lipids. 2005;40(10):999–1006. doi: 10.1007/s11745-005-1462-5. [DOI] [PubMed] [Google Scholar]
- 57.Stamatakis K, Sánchez-Gómez FJ, Pérez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Delta12, 14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17(1):89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- 58.Dransfeld O, Rakatzi I, Sasson S, Gruzman A, Schmitt M, Häussinger D, Eckel J. Eicosanoids participate in the regulation of cardiac glucose transport by contribution to a rearrangement of actin cytoskeletal elements. Biochem J. 2001;359(Pt 1):47–54. doi: 10.1042/0264-6021:3590047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada M, Proia AD. 8(S)-hydroxyeicosatetraenoic acid is the lipoxygenase metabolite of arachidonic acid that regulates epithelial cell migration in the rat Cornea. Cornea. 2000;19(3 Suppl):S13–S20. doi: 10.1097/00003226-200005001-00004. [DOI] [PubMed] [Google Scholar]
- 60.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol. 2006;176(3):1878–1888. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 61.Cao DY, Luo J, Zang WJ, Chen DK, Xu HF, Shi HP, Jing XQ. Gamma-linolenic acid suppresses NF-κΒ signaling via CD36 in the lipopolysaccharide-induced inflammatory response in primary goat mammary gland epithelial cells. Inflammation. 2016;39(3):1225–1237. doi: 10.1007/s10753-016-0358-7. [DOI] [PubMed] [Google Scholar]
- 62.Kapoor R, Huang YS. Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7(6):531–534. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
- 63.Rothman D, Allen H, Herzog L, Pilapil A, Seiler CM, Zurier RB. Effects of unsaturated fatty acids on interleukin-1β production by human monocytes. Cytokine. 1997;9(12):1008–1012. doi: 10.1006/cyto.1997.0304. [DOI] [PubMed] [Google Scholar]
- 64.Santoli D, Zurier RB. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol. 1989;143(4):1303–1309. [PubMed] [Google Scholar]
- 65.Das UN. Inhibition of sensitized lymphocyte response to sperm antigen(s) by prostaglandins. IRCS Med Sci. 1981;9:1087. [Google Scholar]
- 66.Kumar GS, Das UN, Kumar KV, Madhavi N, Das NP, Tan BKH. Effect of n-6 and n-3 fatty acids on the proliferation of human lymphocytes and their secretion of TNF-α and IL-2 in vitro. Nutr Res. 1992;12(7):815–823. [Google Scholar]
- 67.Kumar GS, Das UN. Effect of prostaglandins and their precursors on the proliferation of human lymphocytes and their secretion of tumor necrosis factor and various interleukins. Prostaglandins Leukot Essent Fatty Acids. 1994;50(6):331–334. doi: 10.1016/0952-3278(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 68.Das UN. Beneficial effect of eicosapentaenoic and docosahexaenoic acids in the management of systemic lupus erythematosus and its relationship to the cytokine network. Prostaglandins Leukot Essent Fatty Acids. 1994;51(3):207–213. doi: 10.1016/0952-3278(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 69.Di Francesco L, Totani L, Dovizio M, Piccoli A, Di Francesco A, Salvatore T, Pandolfi A, Evangelista V, Dercho RA, Seta F, Patrignani P. Induction of prostacyclin by steady laminar shear stress suppresses tumor necrosis factor-alpha biosynthesis via heme oxygenase-1 in human endothelial cells. Circ Res. 2009;104(4):506–513. doi: 10.1161/CIRCRESAHA.108.191114. [DOI] [PubMed] [Google Scholar]
- 70.Zhou WS, Zhang J, Goleniewska K, Dulek DE, Toki S, Newcomb DC, Cephus JY, Collins RD, Wu PS, Boothby MR, Peebles RS., Jr Prostaglandin I2 suppresses proinflammatory chemokine expression, CD4 T cell activation, and STAT6-independent allergic lung inflammation. J Immunol. 2016;197(5):1577–1586. doi: 10.4049/jimmunol.1501063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devi GR, Das UN, Rao KP, Rao MS. Prevention of radiation-induced poly-chromatophilia by prostaglandin El and colchicine. IRCS Med Sci. 1983;11:863. [Google Scholar]
- 72.Ramadevi G, Das UN, Rao KP, Rao MS. Prostaglandins and mutagenesis: prevention and/or reversibility of genetic damage induced by benzo (a) pyrene in the bone marrow cells of mice by prostaglandin E1. Prostaglandins Leukot Med. 1984;15(3):287–292. doi: 10.1016/0262-1746(84)90128-8. [DOI] [PubMed] [Google Scholar]
- 73.Ramadevi G, Das UN, Rao KP, Rao MS. Prostaglandins and mutagenesis: modification of phenytoin induced genetic damage by prostaglandins in lymphocyte cultures. Prostaglandins Leukot Med. 1984;15(1):109–113. doi: 10.1016/0262-1746(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 74.Das UN, Ramadevi G, Rao KP, Rao MS. Prostaglandins and their precursors can modify genetic damage-induced by gamma-radiation and benzo(a)pyrene. Prostaglandins. 1985;29(6):911–920. doi: 10.1016/0090-6980(85)90216-3. [DOI] [PubMed] [Google Scholar]
- 75.Sridevi K, Rao KP, Das UN. Modification of benzo (a) pyrene induced genetic damage by prostaglandin El: a doseresponse and time course study. Med Sci Res. 1990;18:473. [Google Scholar]
- 76.Koratkar R, Das UN, Sagar PS, Ramesh G, Padma M, Kumar GS, Vijay K, Madhavi N. Prostacyclin is a potent anti-mutagen. Prostaglandins Leukot Essent Fatty Acids. 1993;48(2):175–184. doi: 10.1016/0952-3278(93)90107-8. [DOI] [PubMed] [Google Scholar]
- 77.Ponnala S, Rao KP, Chaudhury JR, Ahmed J, Rama Rao B, Kanjilal S, Hasan Q, Das UN. Effect of polyunsaturated fatty acids on diphenyl hydantoin-induced genetic damage in vitro and in vivo. Prostaglandins Leukot Essent Fatty Acids. 2009;80(1):43–50. doi: 10.1016/j.plefa.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 78.Das UN, Rao KP. Effect of gamma-linolenic acid and prostaglandins E1 on gamma-radiation and chemical-induced genetic damage to the bone marrow cells of mice. Prostaglandins Leukot Essent Fatty Acids. 2006;74(3):165–173. doi: 10.1016/j.plefa.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Das UN. Prostaglandins and gene action: possible relevance to the effect of PG system on leukocyte alkaline phosphatase enzyme activity. Med Hypotheses. 1983;11(2):185–194. doi: 10.1016/0306-9877(83)90062-2. [DOI] [PubMed] [Google Scholar]
- 80.Su RY, Chi KH, Huang DY, Tai MH, Lin WW. 15-deoxy-Delta12, 14-prostaglandin J2 up-regulates death receptor 5 gene expression in HCT116 cells: involvement of reactive oxygen species and C/EBP homologous transcription factor gene transcription. Mol Cancer Ther. 2008;7(10):3429–3440. doi: 10.1158/1535-7163.MCT-08-0498. [DOI] [PubMed] [Google Scholar]
- 81.Acarregui MJ, Snyder JM, Mitchell MD, Mendelson CR. Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology. 1990;127(3):1105–1113. doi: 10.1210/endo-127-3-1105. [DOI] [PubMed] [Google Scholar]
- 82.Benavente J, Esteban M, Jaffe BM, Santoro MG. Selective inhibition of viral gene expression as the mechanism of the antiviral action of PGA1 in vaccinia virus-infected cells. J Gen Virol. 1984;65(Pt 3):599–608. doi: 10.1099/0022-1317-65-3-599. [DOI] [PubMed] [Google Scholar]
- 83.Polavarapu S, Dwarakanath BS, Das UN. Arachidonic acid activates extrinsic apoptotic pathway to enhance tumoricidal action of bleomycin against IMR-32 cells. Prostaglandins Leukot Essent Fatty Acids. 2018;132:16–22. doi: 10.1016/j.plefa.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Hari AD, Naidu VGM, Das UN. N-6 and n-3 Fatty acids and their metabolites augment inhibitory action of doxorubicin on the proliferation of human neuroblastoma (IMR-32) cells by enhancing lipid peroxidation and suppressing Ras, Myc, and Fos. BioFactors. 2018;44(4):387–401. [Google Scholar]
- 85.Gundala NKV, Naidu VGM, Das UN. Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. Biofactors. 2017;43(2):251–271. doi: 10.1002/biof.1336. [DOI] [PubMed] [Google Scholar]
- 86.Gundala NKV, Naidu VGM, Das UN. Arachidonic acid and lipoxinA4 attenuate streptozotocin-induced cytotoxicity to RIN5 F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition. 2017;35:61–80. doi: 10.1016/j.nut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Gundala NKV, Naidu VGM, Das UN. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem Biophys Res Commun. 2018;496(1):105–113. doi: 10.1016/j.bbrc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Das UN. Essential fatty acids, lipid peroxidation and apoptosis. Prostaglandins Leukot Essent Fatty Acids. 1999;61(3):157–163. doi: 10.1054/plef.1999.0085. [DOI] [PubMed] [Google Scholar]
- 89.Karmali R, Schiller PW, Horrobin DF. Prostaglandins can prevent the binding of chloroquine to calf Thymus DNA. Prostaglandins. 1976;12(3):463–464. doi: 10.1016/0090-6980(76)90026-5. [DOI] [PubMed] [Google Scholar]
- 90.Qiu FH, Devchand PR, Wada K, Serhan CN. Aspirin-triggered lipoxin A4 and lipoxin A4 up-regulate transcriptional corepressor NAB1 in human neutrophils. FASEB J. 2001;15(14):2736–2738. doi: 10.1096/fj.01-0576fje. [DOI] [PubMed] [Google Scholar]
- 91.Shen JH, Ma Q, Shen SR, Shen SG, Xu GT, Das UN. Effect of α-linolenic acid on streptozotocin-induced diabetic retinopathy indices in vivo. Arch Med Res. 2013;44(7):514–520. doi: 10.1016/j.arcmed.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Shen JH, Bi YL, Das UN. Potential role of polyunsaturated fatty acids in diabetic retinopathy. Arch Med Sci. 2014;10(6):1167–1174. doi: 10.5114/aoms.2014.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaviarasan K, Jithu M, Arif Mulla M, Sharma T, Sivasankar S, Das UN, Angayarkanni N. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metab Clin Exp. 2015;64(9):958–966. doi: 10.1016/j.metabol.2015.04.005. [DOI] [PubMed] [Google Scholar]