Abstract
AIM
To describe using spectral-domain optical coherence tomography the regeneration of the foveal morphology after pars plana (re)vitrectomy surgery and gas tamponade combined with injection of autologous platelet concentrate to treat full-thickness macular holes, and to describe different anatomical outcome.
METHODS
A retrospective case series of 8 eyes of 8 patients was described.
RESULTS
In all cases investigated, the platelet-assisted closure of macular holes was associated with a rapid resolution of cystic cavities in the foveal walls. In two patients, there was a regular regeneration of the foveal morphology after hole closure; the regenerated central fovea had a regular structure with a foveola and photoreceptors. In three other patients, there was an irregular regeneration of the fovea; a foveola was not formed, photoreceptor cells were absent from the foveal center, and the center was composed of Müller and retinal pigment epithelial (RPE) cells. The foveal regeneration after hole closure may proceed with or without a temporary detachment of the foveal center from the RPE, and with or without a direct contact between the central outer nuclear layer (ONL) and the RPE. Contacts between the ONL and RPE were observed only in patients with an irregular foveal regeneration after hole closure.
CONCLUSION
The data show that there are different modes of foveal regeneration after closure of macular holes with (re)vitrectomy and platelet concentrate. It is suggested that the regular regeneration of the foveal morphology proceeds by Müller cell-mediated tissue movements without cell proliferation, whereas the irregular foveal regeneration proceeds in part by proliferation of Müller and RPE cells.
Keywords: macular hole, platelet concentrate, fovea, Müller glia, retinal pigment epithelium
INTRODUCTION
The fovea as the site of the sharpest vision is a pit in the inner retina over an area of elongated thin photoreceptors. The fovea consists of the foveola which is surrounded by foveal walls. The inner retinal layers are absent from the foveola and radially shifted to the parafovea; this configuration needs special structural support. Mechanical stabilization of the retina is normally provided by macroglia, especially Müller cells[1]. The structure of the fovea is stabilized by Müller glia, the only type of macroglia in the fovea[2]–[3]. The central fovea does not contain blood vessels; a vascular ring in the foveal slope delimitates the foveal avascular zone[4].
The fovea contains two populations of Müller cells: foveolar Müller cells which constitute the so-called Müller cell cone, and Müller cells of the foveal walls; because the outer processes of the Müller cells of the foveal walls traverse horizontally or obliquely the Henle fiber layer (HFL), they have a Z-shape[2],[5]–[6]. The horizontal layer of the Müller cell cone constitutes the innermost layer of the foveola; the vertical stalk of the Müller cell cone is localized in the center of the foveola[3],[7]. The Müller cell cone increases the resistance against mechanical stress resulting from anteroposterior and tangential tractions[2]–[3],[7]. Disruption of the Müller cell cone produces a macular hole[2]. On the other hand, tractions exerted by foveal Müller cells are also suggested to be involved in the regeneration of the foveal morphology after spontaneous or surgical closure of macular holes[8]–[9].
The mechanisms which mediate the closure of macular holes are largely unclear. Because astrocytes are absent from the fovea[3], the closure is likely mediated by Müller cells. Small macular holes may close spontaneously with a low rate, between 0 and 6% in various studies[10]–[11]. It was suggested that the closure of small holes proceeds by proliferation of glial cells which bridge the gap in the central fovea[10]. On the other hand, the finding that the volume of the foveal tissue remains unaltered after surgical closure of macular holes[12] suggests that glial proliferation is less relevant. It was also suggested that the hole closure proceeds without glial proliferation[13] by Müller cell-mediated tissue movements[8]–[9]. The finding that only small holes with diameters of less than 400 µm may close spontaneously[10]–[11] was explained with the assumption that the range of the Müller cell-mediated tissue displacement is defined by the diameter of the foveola[9].
The current surgical management of macular holes includes pars plana vitrectomy, excision of the posterior vitreous cortex with or without internal limiting membrane (ILM) peeling, intraocular gas tamponade, and postoperative facedown position[14]. Various adjuvant therapies to increase the surgical success rate were described. One of the most effective adjuvant is administration of autologous platelet concentrate onto the macular hole[15]–[18]. The mechanisms of the positive effect of platelets on the closure of macular holes are unclear. There is evidence that this effect is mediated by growth factors released from the cells, but independent on the cellular adhesion capability, i.e., the sealing action of aggregated platelets[19]. Platelets release numerous growth factors[20] which accelerate tissue repair[21] and stimulate the proliferation and migration of retinal glial and retinal pigment epithelial (RPE) cells[22]–[26]. In the present study, we describe using spectral-domain optical coherence tomography (SD-OCT) the anatomical regeneration of the fovea in 8 eyes of 8 patients which underwent (re)vitrectomy with autologous platelet concentrate to close persistent macular holes.
SUBJECTS AND METHODS
Ethical Approval
This retrospective study followed the ethical standards of the Declaration of Helsinki. Eight eyes of 8 patients with idiopathic macular hole, referred to the Department of Ophthalmology, Medical Faculty of the University of Leipzig, Germany, between February 2009 and March 2019, were included in the study. Informed consent was obtained from all patients.
The patients underwent a 20-, 23-, or 25-gauge three-port pars plana (re)vitrectomy. If applicable, the ILM was peeled after staining with Brilliant Blue G (0.125 mg; 0.25 g/L; ILM-Blue, DORC, The Netherlands). In one patient, an epiretinal membrane was removed. After fluid-air exchange, two drops of autologous platelet concentrate were instilled onto the macular hole using a vitrectomy back-flush needle mounted on a 2-mL syringe; this was followed by an internal tamponade with sulfur hexafluoride (SF6) or octafluoropropane (C3F8). In one patient, vitrectomy was combined with cataract surgery. After surgery, the patients maintained a supine position for 2 or 24h and thereafter a facedown position for 3-5d. Autologous platelet concentrates were prepared as described[27]; the concentrates contained mean numbers of platelets between 1 and 5×1010/mL. Cross-sectional images of the macula were recorded with SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany). The best-corrected visual acuity (BCVA) was established with a Snellen chart and is given in decimal units.
Seven patients were of Caucasian descent and one of African descent. All patients had central visual loss and metamorphopsia in one or both eyes. With the exception of one case, the foveas in the fellow eyes had a normal morphology with or without vitreomacular adhesions. Patient 1 was a 63 year-old man who presented a full-thickness macular hole in the right eye (Figure 1A). Standard vitrectomy with ILM peeling was performed 1.1mo after the first visit. Because this treatment was unsuccessful (Figure 1A), a revitrectomy with ILM peeling and instillation of autologous platelets was performed 2.6mo after the first visit. Cataract surgery was performed 14.4mo after the first visit. Patient 2 was a 59-year-old woman with a full-thickness hole in the left eye (Figure 2B). Vitrectomy with ILM peeling and instillation of an autologous platelet concentrate was performed 0.5mo after the first visit. Patient 3 was a 67-year-old woman with central visual loss in both eyes. Five years before the first visit to treat the full-thickness hole of the right eye (Figure 3B), the left eye underwent unsuccessful pars plana vitrectomy without platelet administration because of the presence of a full-thickness hole (Figure 3A). Thereafter, this eye remained untreated. The macular hole in the right eye was treated one day and 9.7mo after the first visit with vitrectomy and instillation of autologous platelet concentrate, without ILM peeling.
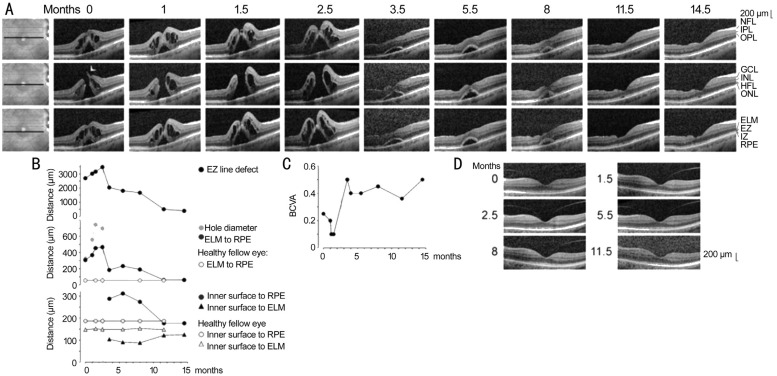
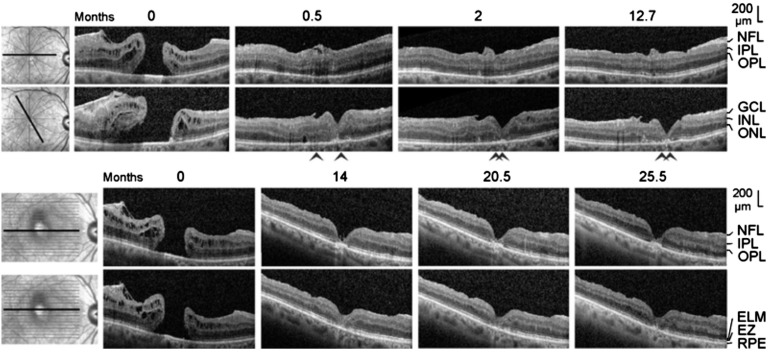
Figure 1. Closure of a macular hole after revitrectomy with autologous platelet concentrate in the right eye of patient 1.
A: Linear horizontal scans through the fovea and parafovea of the right eye. The orientations of the scans are shown at the left side. The months after the examination (0) are indicated above the images. Standard vitrectomy with ILM peeling and revitrectomy with ILM peeling and instillation of autologous platelets were performed 1.1 and 2.6mo after the first visit, respectively. B: Time-dependent alterations of the following parameters measured in linear horizontal scans through the fovea of the right eye: the horizontal diameter of the EZ line defect, the minimum diameter of the macular hole, the vertical distance between the central ELM and the RPE, the vertical distance between the inner surface of the central fovea and the RPE, and the vertical distance between the inner surface of the central fovea and the ELM. In addition, the distances in the fovea of the healthy left eye are shown. The broken vertical lines indicate the time of vitrectomy with ILM peeling. The unbroken vertical lines indicate the time of revitrectomy with ILM peeling and instillation of the platelet concentrate. C: Time dependence of the BCVA. D: Linear horizontal scans through the fovea of the left eye.
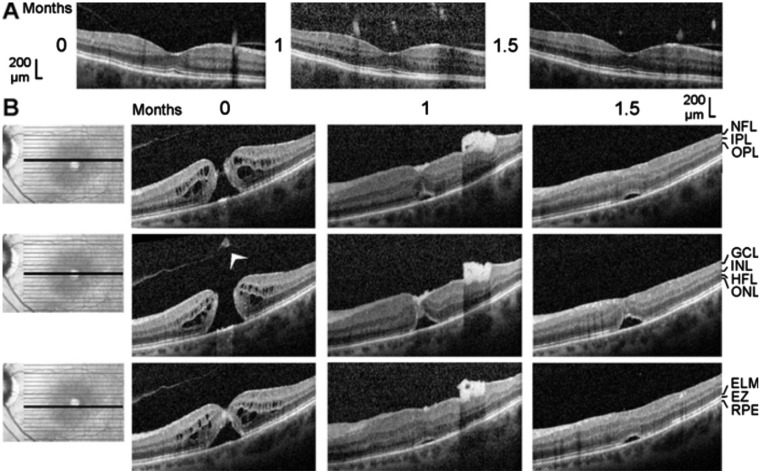
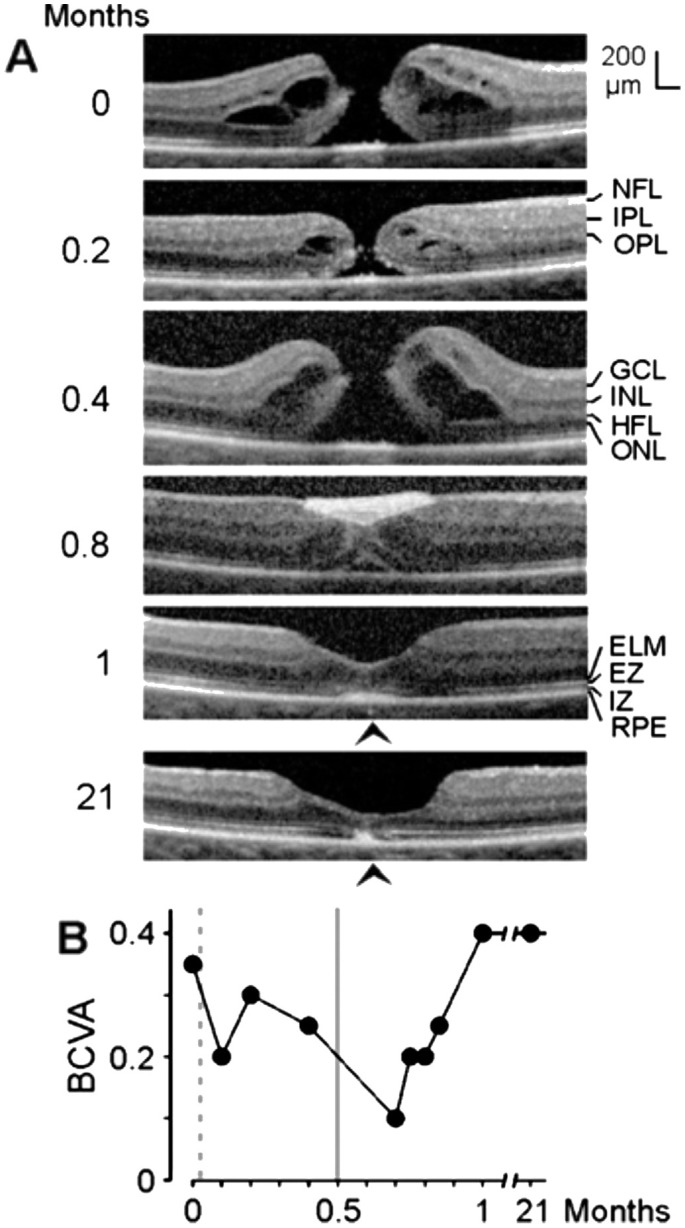
Figure 2. Closure of a macular hole after vitrectomy with autologous platelet concentrate in the left eye of patient 2.
The optical coherence tomographical images were recorded during the first visit (0mo) and 1 and 1.5mo later. A: Linear horizontal scans through the fovea of the right eye. The fovea showed no structural abnormalities with the exceptions of the vitreomacular adhesions at the parafoveas and the thickened hyperreflectivity in the inner layer of the foveola. B: Linear horizontal scans through the fovea and parafovea of the left eye. The orientation of the scans are shown at the left side. Surgery was performed 0.5mo after the first visit. Arrowhead: Operculum.
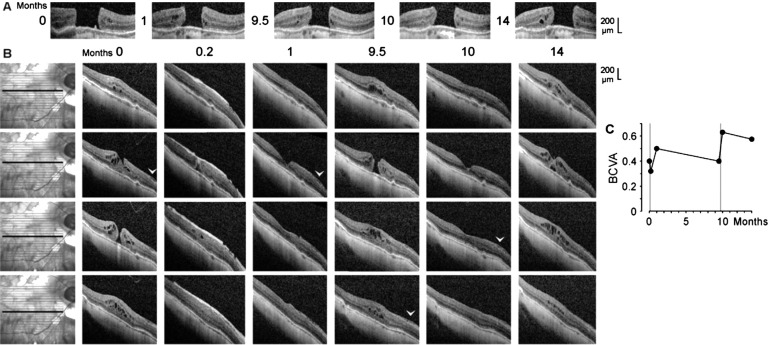
Figure 3. Closure of a macular hole after vitrectomy with autologous platelet concentrate and reformation of the hole in the right eye of patient 3.
A: Linear horizontal scans through the fovea of the left eye. The months after the examination (0) are indicated left of the images. B: Linear horizontal scans through the fovea and parafovea of the right eye. The orientations of the scans are shown at the left side. The months after the first examination (0) are indicated above the images. Vitrectomies with instillation of platelet concentrates were performed one day and 9.7mo after the first visit. The arrowheads indicate an epiretinal membrane. C: Time dependence of the BCVA. The vertical lines indicate the times of vitrectomy with instillation of platelet concentrate.
Patient 4 was a 63 year-old man who presented, in the right eye, a fovea with a detached foveola which developed to a lamellar macular hole and then to a full-thickness hole (Figure 4B). Vitrectomy with ILM peeling and instillation of platelet concentrate was performed 8.6mo after the first visit. Patient 5 was a 68 year-old woman with a traction-induced full-thickness hole in the right eye (Figure 5). The surgery included vitrectomy, removal of the epiretinal membrane, and instillation of platelet concentrate. Patient 6 was a 56 year-old man with a full-thickness hole in the left eye (Figure 6A). Patient 7 was a 73 year-old woman with a full-thickness hole in the right eye. Six months before the first visit, this eye underwent a standard vitrectomy with ILM peeling to treat the hole; however, the hole reopened thereafter (Figure 6B). Revitrectomy with platelet concentrate combined with cataract surgery was performed 1.8mo after the first visit. Patient 8 was a 70 year-old woman with a full-thickness macular hole in the right eye (Figure 7A). Standard vitrectomy with ILM peeling and a revitrectomy with instillation of autologous platelets were performed one day and 0.5mo after the first visit, respectively.
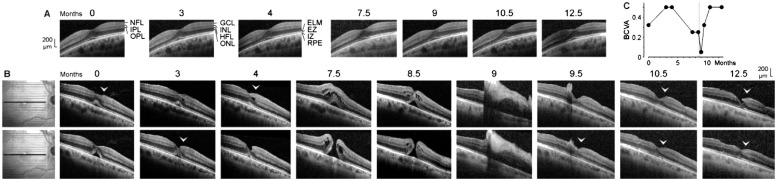
Figure 4. Development of a full-thickness macular hole and restoration of the foveal structure followed by a formation of a degenerative lamellar hole after vitrectomy with autologous platelet concentrate in the right eye of patient 4.
The months after the first examination (0) are indicated above the images. A: Linear horizontal scans through the fovea of the left eye. The fovea showed no structural abnormalities. B: Linear horizontal scans through the fovea and parafovea of the right eye. The orientations of the scans are shown at the left side. Surgery was performed 8.6mo after the first visit. The arrowheads indicate lamellar hole-associated epiretinal proliferation. C: Time dependence of the BCVA. The vertical line indicates the time of vitrectomy with instillation of autologous platelets.
Figure 5. Closure of a macular hole after vitrectomy with peeling of an epiretinal membrane and instillation of an autologous platelet concentrate in the right eye of patient 5.
The images show linear scans through the fovea and parafovea; the orientations of the scans are shown at the left side. The months after the first examination (0) are indicated above the images. Surgery was performed one day after the first visit. The arrowheads indicate sites of a direct contact between the central ONL and the RPE.
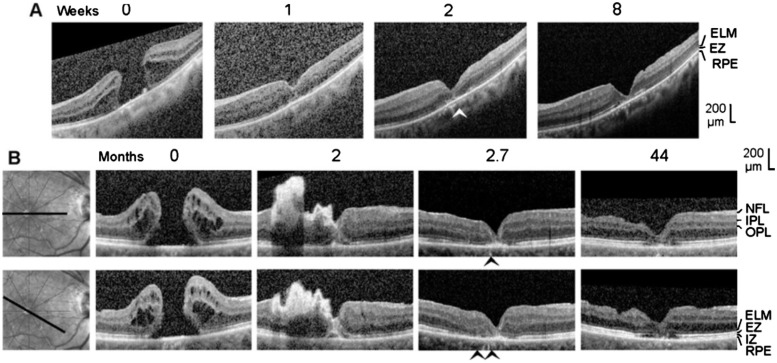
Figure 6. Closure of macular holes after vitrectomy with autologous platelet concentrate in the left eye of patient 6 (A) and the right eye of patient 7 (B).
The images show linear scans through the fovea and parafovea; the orientations of the scans are shown at the left side (B). The weeks (A) and months (B), respectively, after the first clinical examination (0) are indicated above the images. A: Surgery was performed one day after the first visit. B: Revitrectomy with autologous platelet concentrate combined with cataract surgery was performed 1.8mo after the first visit. The arrowheads indicate sites of a direct contact between the central ONL and the RPE.
Figure 7. Closure of a macular hole after revitrectomy with autologous platelet concentrate in the right eye of patient 8.
A: The images show linear scans through the fovea and parafovea. The months after the first examination (0) are indicated at the left side. Standard vitrectomy with ILM peeling was performed one day after the first visit, and revitrectomy with instillation of autologous platelets was performed 0.5mo after the first visit. The arrowheads indicate sites of a direct contact between the central ONL and the RPE. B: Time dependence of the BCVA. The broken vertical line indicates the time of vitrectomy with ILM peeling. The unbroken vertical line indicates the time of revitrectomy with instillation of autologous platelets.
We also reevaluated data of 34 eyes of 33 patients (m/w, 2/31; mean±SD age, 70.9±7.4y) which were included in the study of Degenhardt et al[27].
RESULTS
Patient 1
The full-thickness macular hole in the right eye of patient 1 was formed by a disruption of the junction between the inner layer of the foveola and the temporal foveal wall (arrowhead in Figure 1A). Large cystic cavities between the outer plexiform layer (OPL) and HFL, and smaller cysts in the inner nuclear layer (INL), produced a high elevation of the foveal walls around the hole (Figure 1A). The elevation of the foveal walls increased time-dependently until the closure of the hole; this resulted from the increase in the size and number of the cystic cavities in the foveal walls (Figure 1A). The elevation of the foveal walls was accompanied by an increase in the diameter of the hole, a detachment of the central outer nuclear layer (ONL) [resulting in an increased distance between the central external limiting membrane (ELM) and the RPE], and increases in the central defects of the ellipsoid zone (EZ) and interdigitation zone (IZ) lines (Figure 1A, 1B). The EZ line defect indicates a loss of the integrity of the photoreceptor segments, and the defect of the IZ line reflects a rupture of the outer photoreceptor segment-RPE junction[28].
The hole did not close after standard vitrectomy with ILM peeling which was performed 1.1mo after the first visit (Figure 1A). Therefore, a revitrectomy with ILM peeling and instillation of autologous platelets was carried out 2.6mo after the first visit; hole closure occurred at the level of the ONL (Figure 1A). This was associated with a full disappearance of the cystic cavities in the foveal tissue (Figure 1A). The central foveal tissue remained detached from the RPE until 5.5mo after surgery (8mo after the first clinical examination; Figure 1A). Within the next 3.5mo, the central foveal tissue reattached at the RPE (Figure 1A). This was associated with a decrease of the distances between the inner surface of the central fovea and the RPE and between the central ELM and the RPE to values similar to the healthy fellow eye, the reformation of a foveal pit, and an increased thickness of the ONL in the central fovea (as indicated by the increased distance between the inner tissue surface and the ELM; Figure 1B). The decrease of the distance between the central ELM and the RPE was a precondition for the regeneration of the photoreceptor segments, as indicated by the regeneration of the EZ and IZ lines (Figure 1A, 1B). At 14.5mo after the first examination, both lines were regenerated, with the exception of a small patch under the lower temporal foveal walls (Figure 1A).
The RPE line, which is a light reflection at the mitochondria-containing basal part of the RPE[28], showed no disruption during the development and subsequent closure of the full-thickness hole (Figure 1A). The RPE line displayed a central hyperreflectivity surrounded by a hyporeflectivity; the latter roughly coincided with the IZ line defect (Figure 1A). The central hyperreflectivity of the RPE was likely caused by the light transmission through the hole while the hyporeflectivity of the RPE might be caused by the deteriorated light transmission through the thickened tissue of the foveal walls or by a loss of mitochondria in the RPE resulting from the disruption of the contact to the photoreceptor segments[9]. After regeneration of the majority of photoreceptor segments at 11.5mo and later (indicated by the nearly full restoration of the EZ and IZ lines), the RPE showed regular reflectivity (Figure 1A).
Figure 1C shows the time dependence of the BCVA. After the first vitrectomy with ILM peeling, the BCVA decreased from 0.2 to 0.1 (Figure 1C). Revitrectomy with instillation of autologous platelets was followed by a sharp increase of the BCVA from 0.1 to 0.5 (Figure 1C). Thereafter, the BCVA remained relatively stable between 0.35 and 0.45 (Figure 1C). The final increase of the BCVA to 0.5 resulted from cataract surgery.
Patient 2
The full-thickness macular hole (minimum diameter, 520 µm) in the left eye of patient 2 was formed by the removal of a part of the inner Müller cell layer of the foveola (Figure 2B). Cystic cavities between the OPL and HFL, and within the INL, ONL, and ganglion cell layer (GCL), produced an elevation of the foveal walls associated with a detachment of the central ONL from the RPE and EZ and IZ defects (Figure 2B). SD-OCT images recorded 0.5mo after vitrectomy with ILM peeling and administration of autologous platelet concentrate (1mo after the first examination) showed that the hole was closed by platelets which were visible as a hyperreflective clot that filled the center of the fovea (Figure 2B). The closure of the hole was accompanied by a full resolution of the cystic cavities in the foveal tissue (Figure 2B). This resulted in a drop of the elevated foveal walls and narrowing of the walls around the platelet-filled foveal center which remained detached from the RPE (Figure 2B). A further aggregate of hyperreflective platelets, which caused shadows in the underlying retinal tissue, was attached at the inner surface of the temporal parafovea (Figure 2B). This aggregate intruded into the underlying retina and altered the layered structure of the tissue (Figure 2B). One month after surgery (1.5mo after the first examinaton), the hyperreflective platelets disappeared (Figure 2B). At this time, the central foveal walls fused and the horizontal gaps in the central ONL and ELM were closed; however, the central ONL was still detached from the RPE and there was no foveal pit (Figure 2B). The preoperative BCVA was 0.25; the BCVA decreased to 0.16 at one month, and increased to 0.25 at 1.5mo.
Patient 3
SD-OCT scans of the fovea of the untreated left eye of patient 3 showed a stage-4 macular hole (minimum diameter, 610 µm; Figure 3A). The size of the hole varied little during the examination period (Figure 3A). SD-OCT scans of the fovea of the right eye recorded at the first visit revealed the presence of a small stage-3 hole (minimum diameter, 110 µm; Figure 3B). Cystic cavities between the OPL and HFL, and in the INL, produced an elevation of the foveal walls, a horizontal gap in the central ONL, and central defects of the ELM, EZ, and IZ lines (Figure 3B). There was an epiretinal membrane in the nasal parafovea of the right eye (Figure 3B); tangential traction exerted by the membrane likely contributed to the development of the hole.
Shortly after vitrectomy with instillation of autologous platelets performed one day after the first visit, the platelets were visible as a hyperreflective tissue which filled the hole and which lay above the inner surface of the parafovea (Figure 3B). The platelet-assisted closure of the hole was accompanied by a resolution of most cystic cavities in the foveal tissue (Figure 3B). One month after the first examination, the platelets fully disappeared, and the central fovea reattached at the RPE; this was associated with the reformation of the foveal pit (Figure 3B). Within 9.5mo, the macular hole developed again (minimum diameter, 220 µm), likely by traction exerted by the epiretinal membrane (Figure 3B). After a second administration of platelet concentrate 9.7mo after the first examination, the hole closed again; this was associated with the regeneration of the central defect of the ELM line, but not of the EZ line (Figure 3B). Four months later, the reformation of cystic cavities in the foveal tissue resulted in a renewed elevation of the foveal walls and detachment of the central ONL (Figure 3B). As shown in Figure 3C, both vitrectomies with instillation of autologous platelets were followed by increases of the BCVA.
Patient 4
SD-OCT images of the fovea of the right eye of patient 4 recorded at the first examination showed a detachment of the foveola from the RPE; this was associated with a shallow foveal pit and a loss of the integrity of the central photoreceptors, as indicated by the central defect of the EZ line (Figure 4B). In addition, there was a lamellar macular hole-associated epiretinal proliferation (LHEP) at the vitreal surface of the nasal foveal wall (Figure 4B) which may suggest that a degenerative lamellar hole[29] was present before the first examination.
During the following 3mo, the EZ line partly recovered (Figure 4B). This was associated with an increase of BCVA (Figure 4C). One month later, the foveola was largely reattached at the RPE; this was accompanied by a deepening of the foveal pit and a thickening of the central ONL in the nasal part of the fovea (Figure 4B). However, the oblique shape of the foveal pit with a cavitation in the lower nasal foveal wall which continued to a tissue disruption in the superior parafovea, and the presence of an oblique band of medium reflectivity in the foveal center (Figure 4B), may indicate that dysregulated tissue movements caused these alterations, in part mediated by contraction of an epiretinal membrane at the inner surface of the temporal parafovea. Between 4 and 7.5mo, a full-thickness hole developed (minimum diameter, 225 µm; Figure 4B); this was associated with a decrease of the BCVA (Figure 4C). Cystic cavities between the OPL and HFL, and in the INL, produced an elevation of the foveal walls including a detachment of the central ONL (Figure 4B). One month later (8.5mo), the size and number of the cystic cavities decreased; this was accompanied by a narrowing of the hole (minimum diameter, 130 µm; Figure 4B).
Vitrectomy with administration of platelet concentrate was performed 8.6mo after the first visit. Shortly after surgery, a large aggregate of hyperreflective platelets adhered at the inner surface of the fovea and the nasal parafovea which caused shadows in the underlying retinal tissue (Figure 4B) and a strong decrease of the BCVA (Figure 4C). The shape of the shadow below the platelet aggregate suggests that the hole was closed and the foveal center was attached at the RPE (Figure 4B). The hyperreflective platelets fully disappeared after 10.5mo (Figure 4B). After 9.5mo, the foveal structure and the central ELM were largely regenerated. After 10.5mo, the EZ line was regenerated at many sites, suggesting an ongoing regeneration of the photoreceptor segments (Figure 4B). However, a degenerative lamellar hole redeveloped finally. The morphology of the lamellar hole was similar at 4 and 12.5mo after the first visit (Figure 4B). After vitrectomy with autologous platelets, the BCVA increased to a plateau at 0.5 (Figure 4C).
Patient 5
SD-OCT scans of the fovea of the right eye of patient 5 recorded at the first visit showed a large full-thickness hole (minimum diameter, 650 µm; Figure 5). There was an epiretinal membrane in the dorsotemporal parafovea (Figure 5) which likely caused the development of the hole. The hole closed within 0.5mo after vitrectomy with removal of the epiretinal membrane and instillation of platelet concentrate; the closure was associated with a resolution of the cystic cavities in the foveal walls (Figure 5). There were sites of a direct contact between the central ONL and RPE (as indicated by the absence of the ELM and EZ lines; arrowheads in Figure 5). In the further course, a regular foveola was not formed. Between 0.5 and 14mo after the first examination, there was a time-dependent increase in the horizontal gap of the central ONL associated with a deepening of the foveal pit and a thinning of the foveal center (Figure 5). There remained a gap in the central ONL until the end of the examination period (Figure 5). In addition, the defects of the central ELM, EZ, and IZ lines remained, and there was a thickening of the RPE line in the center of the fovea (Figure 5). These signs indicate that there were no photoreceptors in the foveal center after closure of the hole. The absence of an ONL in the foveal center was associated with the presence of a very deep foveal pit; the base of the pit was at the level of the ELM (Figure 5). The foveal center was filled by a tissue of medium reflectivity, likely Müller cells, and hyperreflective RPE cells (Figure 5). The preoperative BCVA was 0.1. Within 2mo after surgery, the BCVA increased to 0.16 and remained at this value until the end of the examination period.
Patient 6
The first clinical examination revealed the presence of a large macular hole (minimum diameter, 690 µm) in the left eye of patient 6 (Figure 6A). The hole closed within 6d after vitrectomy with instillation of platelet concentrate (Figure 6A). There was an area of a direct contact between the central ONL and RPE with an absence of ELM and EZ lines (arrowhead in Figure 6A). During the closure of the hole, the gap in the central ONL largely closed (Figure 6A). However, the gap increased again between 2 and 8wk after the first examination (Figure 6A). Until the end of the examination period, the central fovea remained free of photoreceptors, as indicated by the central defects of the ELM and EZ lines (Figure 6A). A regular foveola was not formed; the absence of an ONL in the center was associated with a very deep foveal pit with the base at the level of the ELM (Figure 6A). The foveal center was filled by a tissue formed by medium-reflective Müller cells and hyperreflective RPE cells (Figure 6A). The BCVA at the first visit was 0.32. It developed to 0.3 at 1wk, 0.35 at 2wk, and 0.45 at 8wk after the first examination.
Patient 7
Six months before the first visit, an unsuccessful standard vitrectomy with ILM peeling was performed to close a full-thickness macular hole in the the right eye of patient 7. SD-OCT scans of the fovea recorded at the first visit showed a large full-thickness hole (minimum diameter, 590 µm) which was surrounded by foveal walls that were highly elevated and contained large cystic spaces (Figure 6B). After vitrectomy with platelet concentrate performed 1.8mo after the first visit, the hole had a smaller diameter because the foveal walls dropped down as a result of the resolution of the cystic cavities. Hyperreflective platelets filled the hole and were attached at the inner surface of the temporal foveal wall and parafovea (Figure 6B). The platelets disappeared between 2 and 2.7mo (Figure 6B). The foveal tissue was reattached at the RPE; however, there were areas with a direct contact between the central ONL and RPE as indicated by the absence of ELM and EZ lines (arrowheads in Figure 6B). In addition, the RPE line displayed a central thickening (Figure 6B). After 44mo, the foveal structure was similar as at 2.7mo: there was no regular foveola, the foveal center was filled by a tissue composed of medium-reflective Müller cells and hyperreflective RPE cells, the central RPE line was thickened (although the width of the thickened central RPE line decreased between 2.7 and 44mo), and there were no photoreceptors in the foveal center as indicated by the defects of the ELM, EZ, and IZ lines (Figure 6B). In addition, the inner layers of the parafovea displayed degenerative alterations (Figure 6B). The BCVA increased from 0.08 before vitrectomy with platelet concentrate to 0.2 at the end of the examination period.
Patient 8
SD-OCT scans recorded at the first clinical examination showed the presence of a macular hole (minimum diameter, 120 µm) in the right eye of patient 8 (Figure 7A). Standard vitrectomy with ILM peeling performed one day after the first visit was followed by a drop of the foveal walls associated with a decrease in the size of the cystic cavities; however, the hole did not close (Figure 7A). Thereafter, the cystic cavities increased and the foveal walls elevated again which was associated with an increase in the diameter of the hole (Figure 7A). After revitrectomy with administration of autologous platelets 0.5mo after the first visit, the hole closed and the foveal walls reattached at the RPE because of a full resolution of the cystic cavities (Figure 7A). The foveal pit was filled by a clot of hyperreflective platelets (Figure 7A). The foveal center, however, was still detached from the RPE and was largely filled by a tissue of medium reflectivity (Figure 7A). Between 0.8 and 1mo after the first examination, the hyperreflective platelets disappeared and the foveola contained the ONL (Figure 7A). The inner layer of the foveola was thickened, likely reflecting gliosis. In addition, the central RPE line was thickened, and there were areas in the central foveola which were devoid of ELM and EZ lines, likely reflecting an absence of photoreceptors and a direct contact between the central ONL and the thickened RPE (Figure 7A). During the next 20mo, the foveola became thinner, and the foveal pit became broader and deeper, because of a centrifugal displacement of the foveal walls (Figure 7A). The center of the foveola was composed of a tissue of medium reflectivity, likely Müller cells, and the thickened RPE; the width of the thickened central RPE line decreased between 1 and 21mo (Figure 7A). The absence of ELM and EZ lines in the center of the foveola (Figure 7A) suggests that it was devoid of photoreceptors. As shown in Figure 7B, revitrectomy with platelet concentrate was followed by a decrease and thereafter an increase of BCVA to a stable value of 0.4.
Data of Further Patients
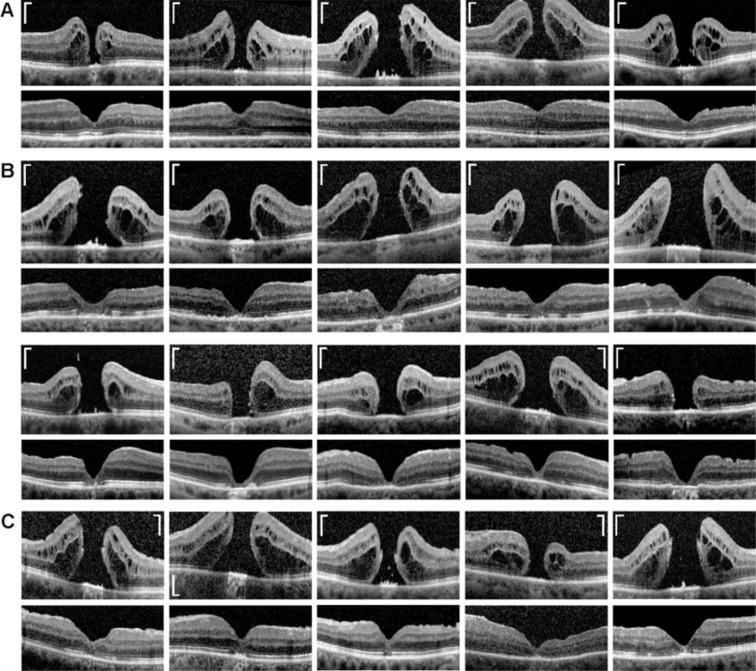
We recently described the anatomical and functional outcome after revitrectomy with application of autologous platelet concentrate in eyes with idiopathic macular hole[27]. A reevaluation of the data of 34 eyes of 33 patients, which were included in this study and which presented a successful surgery with a closure of the hole, showed in 16 eyes a regular regeneration of the central fovea and in 18 eyes an irregular regeneration. Figure 8A and B shows examples of regular and irregular regeneration of the central fovea after hole closure in 5 (Figure 8A) and 10 (Figure 8B) eyes of 15 of these patients. A regular regeneration of the central fovea was defined as a reconstruction of a foveola which contained an ONL and photoreceptors while an irregular regeneration was defined as an absence of an ONL and photoreceptors in the foveal center. Figure 8C shows examples of further 5 eyes with a “mixed type” regeneration of the fovea; this type of regeneration produced a foveola which contained an ONL, but the center of the foveola displayed a gap in the ONL and was photoreceptor-free, as also observed in patient 8 (Figure 7A).
Figure 8. Examples of regular (A), irregular (B), and mixed type regeneration (C) of the central fovea after closure of full-thickness holes by (re)vitrectomy and autologous platelet concentrate.
The images show linear scans through the fovea and parafovea of different patients. Preoperative scans are shown above; postoperative scans obtained between 1 and 55mo after surgery are shown below. Scale bars, 200 µm.
DISCUSSION
Autologous platelet concentrate is a safe and effective adjunct to the surgical management of macular holes[15]–[18]. We investigated with SD-OCT the regeneration of the foveal structure in 8 eyes of 8 patients which underwent (re)vitrectomy with autologous platelet concentrate to close a full-thickness macular hole. We found different modes of foveal regeneration after hole closure. A regular regeneration resulted in the formation of a fovea which contained a foveola and photoreceptors in the center (Figure 9A, 9B). Irregular regeneration resulted in the formation of a fovea which did not contain a foveola and central photoreceptors; the foveal center was filled by a tissue formed by Müller and RPE cells (Figure 9A, 9B). We found using data of 34 eyes of 33 further patients that approximately one half of these eyes showed a regular regeneration of the central fovea after hole closure (Figure 8A) and the other half an irregular regeneration (Figure 8B).
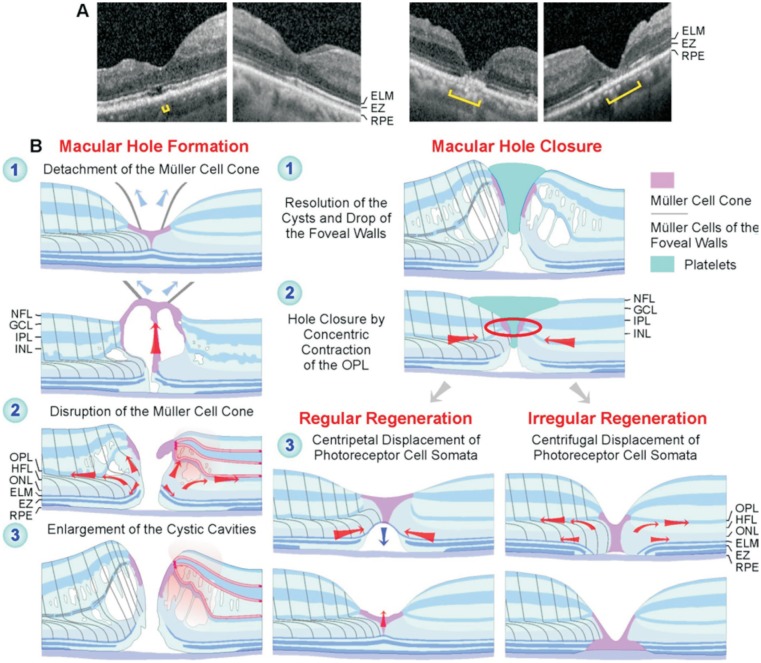
Figure 9. Regular and irregular regeneration of the fovea after vitrectomy with instillation of autologous platelet concentrate to stimulate the closure of a full-thickness macular hole.
A: Final foveal structure after hole closure in the eyes of patients 1, 4, 5, and 6 (from left to right). The foveas of patients 1 and 4 regenerated regularly while the foveas of patients 5 and 6 regenerated irregularly. Brackets: EZ defect. B: Putative mechanisms of macular hole formation and regular and irregular foveal regeneration after platelet-assisted closure of macular holes.
The mechanisms which mediate the closure of macular holes are incompletely understood. Because the fovea is free of astrocytes[3], the closure is likely mediated by Müller cells. Normally, Müller cells maintain the structural stability of the fovea[2]–[3]. Müller cell-mediated tissue movements may be involved in the regeneration of the foveal shape after surgical or spontaneous closure of macular holes[8]–[9]. The cardinal event of hole closure is the sealing of the horizontal gap in the central ONL[9]. The spontaneous closure of small holes was suggested to be mediated by two tissue movements exerted by Müller cells of the foveal walls: 1) a concentric contraction of the Müller cell side processes in the OPL and 2) a concentric contraction of the Müller cell processes which envelop the photoreceptor cells at the ELM resulting in a centripetal shift of the central photoreceptor cell somata[9]. Both movements cause a centripetal shift of the foveal walls, a narrowing and fusion of the remnants of the Müller cell cone, and the closure of the hole at the level of the OPL/inner part of the ONL[9]. Similar Müller cell-mediated tissue movements seem to contribute to the hole closure and the regeneration of a regular central fovea after platelet-assisted surgery (Figure 9B).
The spontaneous closure of small holes is followed by the regeneration of a regular central fovea, i.e., the recreation of a foveola, the centripetal shift of photoreceptor cell somata, and the regeneration of the central photoreceptor segments[9]. However, there may be a considerable time delay between the closure of the hole and the begin of the restoration of the regular foveal shape[9]. A time delay of about 5mo was also observed after platelet-assisted closure of the hole in patient 1 (Figure 1A, 1B). During this period, the central foveal tissue remained detached from the RPE (Figure 1A). The restoration of a regular foveola after spontaneous closure of small holes includes a reattachment of the tissue of the central fovea at the RPE and proceeds by a Müller cell-mediated displacement of photoreceptor cell somata towards the foveal center resulting in a thickening of the ONL in the foveola[9]. This reduces the distance between the central ELM and the RPE which allows a regeneration of the central photoreceptor segments. A similar regeneration of the foveal shape was found after vitrectomy with autologous platelet concentrate in patient 1 (Figure 1A).
We suggested that the spontaneous closure of small macular holes proceeds by Müller cell-mediated tissue movements, without cell proliferation[9]. This was likely also the case of the platelet-assisted closure of macular holes and the regular foveal regeneration. On the other hand, the irregular regeneration of the foveal structure was mediated in part by proliferation of Müller and RPE cells. In these cases, the hole closed by (at least) two mechanisms: the resolution of the cystic cavities, which resulted in a drop of the elevated foveal walls around the hole, and Müller cell-mediated centripetal tissue movements. Thereafter, when the horizontal gap in the central ONL was not closed or increased again by a centrifugal displacement of the photoreceptor cells, the foveal center was filled by a tissue formed by proliferating Müller and RPE cells (Figure 9B).
It was suggested that the stimulatory effect of platelets on the surgical closure of macular holes depends on the release of growth factors[19] which stimulate the proliferation and migration of Müller and RPE cells[22]–[26]. It could be that platelet-derived growth factors stimulate the proliferation of Müller and RPE cells in cases of irregular foveal regeneration. However, in two cases, the platelet concentrate was already removed for a relatively long time period until the onset of Müller and RPE cell proliferation (2wk in patient 6 and at least 12.7mo in the case of patient 5); thus, it seems to be rather unlikely that the cell proliferation in these cases was only stimulated by platelet-derived growth factors. The findings may suggest that platelets stimulate the closure of a hole, which is likely mediated by the resolution of the cystic cavities, which causes a drop of the elevated foveal walls, and a concentric contraction exerted by the Müller cells of the foveal walls[9], whereas the regeneration of the foveal morphology, which may proceed with a considerable time delay after hole closure, is likely not stimulated by platelets.
It is unclear which cellular and molecular events trigger the regular and irregular regeneration of the fovea. One possibility might be the presence or absence of remnants of the disrupted Müller cell cone; the latter may prevent a regular regeneration of the fovea[9]. Another possibility is the presence or absence of a direct contact between the central ONL and RPE after the closure of the hole. The direct contact between the central ONL and RPE (Figures 5, 6A, 6B, and 7A) may induce an irregular regeneration of the fovea. A detachment of the central fovea from the RPE shortly after the closure of the hole as found in patients 1 and 2 (Figures 1A, 2B) may ensure a regular regeneration of the fovea by the prevention of a contact between the central ONL and RPE; however, a temporary detachment of the central fovea after closure of the hole seems to be unnecessary to prevent such contact if the central ELM is intact as in patient 4 (Figure 4B).
The regular foveal regeneration after hole closure involved an increase in the thickness of the central ONL mediated by a centripetal movement of photoreceptor cells (Figure 9B). In cases of irregular foveal regeneration, the gap in the central ONL was not closed or increased again after hole closure, by a centrifugal movement of photoreceptor cells. It was suggested that the centripetal movement of photoreceptor cells during the regular regeneration of the fovea is mediated by a concentric contraction of the Müller cell processes which envelop the photoreceptor cells in the outer ONL and at the ELM (Figure 9B)[9]. It could be that the direct contact between the central ONL (i.e., the outer processes of Müller cells of the foveal walls which envelop the photoreceptor cell somata in the ONL) and the RPE cells inhibits the centripetal movement of the Müller cell processes; instead, the Müller cell processes may retract from the foveal center. The retraction of the Müller cell processes from the foveal center causes the centrifugal shift of the photoreceptor cell somata which results in a widening of the gap in the central ONL.
A prominent feature of the platelet-assisted closure of macular holes observed in all investigated patients is the rapid resolution of the cystic cavities in the foveal walls. A similar rapid resolution of cystic cavities was observed after spontaneous closure of small macular holes[9]. These findings support the hypothesis of a cystic genesis of macular holes[30]. The cysts produce the elevation of the foveal walls around the hole which is associated with a widening of the hole, a detachment of the central ONL, and the formation of a gap in the ONL of the foveola (Figure 9B). Redevelopment of the cystic cavities after hole closure may result in a reopening of the hole (Figure 3B). The cystic cavities are likely result from a leakage of the foveal avascular zone-delimitating vessels in the foveal walls[31]. A dysfunction of Müller cells may contribute to the development of edematous cysts, due to a dysregulated fluid clearance[32]. Mechanical stress resulting from vitreofoveal traction or contraction of epiretinal membranes may induce a dysfunction of Müller cells; mechanical stress exterted on the tissue by the hydrostatic pressure within the increasing cysts may further induce Müller cell dysfunction.
The present data suggest that the reformation of a regular foveola after hole closure proceeds by a Müller cell-mediated displacement of photoreceptor cells towards the foveal center. A similar Müller cell-mediated movement of photoreceptor cell somata contributes to the ontogenetic development of the foveola[3]. The developmental displacement of the photoreceptor cell somata proceeds after birth and is associated with an elongation and thinning of the central receptors, and a thickening of the ONL in the foveola[33]. The latter proceeds up to 17-25y after birth[34], and the increase of the central receptor density improves the vision until 21 years of age[35]. Because the centripetal displacement of the photoreceptor cell somata continues for many years after birth, it seems to be conceivable that a similar mechanism contributes also to the reformation of a regular foveola after macular hole closure[9].
Platelets may support the closure of macular holes by various mechanisms, including 1) the factors released from platelets[19] may stimulate the resolution of the cystic cavities in the foveal walls and the concentric contraction of Müller cell processes in the OPL which both contribute to hole closure (Figure 9B); 2) platelet aggregates may provide a scaffold and may stimulate the production of extracellular matrix with junction sites for contracting Müller cell processes; and 3) platelets which fill the hole (Figures 2B, 3B, and 6B) may decrease the diameter of the hole to values smaller than 400 µm at which the hole may close spontaneously[10]–[11]. Platelet-derived growth factors are known to stimulate the contraction of Müller cells[36]–[37] and thus may induce Müller cell-mediated tissue movements implicated in hole closure[9].
The main limitations of the present study are the small sample size and the restrospective design at one institution. Further investigation of the course of the platelet-assisted hole closure and the subsequent foveal regeneration will be required for a better understanding of the factors and cellular mechanisms which drive regular and irregular regeneration of the foveola, including the question whether the contact between RPE cells and Müller cells of the foveal walls plays a role in inhibition of regular regeneration.
In summary, we describe different modes of foveal regeneration after closure of full-thickness macular holes by (re)vitrectomy with usage of autologous platelet concentrate. The foveal regeneration can proceed with or without a temporary detachment of the foveal center from the RPE, and with or without a contact between the central ONL and the RPE. Contacts between the central ONL and RPE may prevent a regular foveal regeneration, and the center of the fovea is only formed by Müller and RPE cells and does not contain photoreceptors. A regular regeneration, which results in the formation of a fovea with a foveola and photoreceptors in the center, is likely produced by Müller cell-mediated tissue movements whereas an irregular regeneration proceeds in part by proliferation of Müller and RPE cells. An irregular regeneration of the foveal structure, with the absence of photoreceptors in the foveal center, may be one reason for a rather moderate postoperative improvement of the visual acuity despite successful closure of the hole[27]. On the other hand, the decrease in the size of the photoreceptor-free area after hole closure due to the resolution of cystic cavities, which allows a reattachment of the foveal walls at the RPE, may decrease the size of the central scotoma.
Acknowledgments
Authors' contributions: Bringmann A and Wiedemann P designed the experiments. Jochmann C, Unterlauft JD, Wiedemann R, and Rehak M performed the experiments. Bringmann A performed the data analysis and wrote the paper. Wiedemann P revised the paper.
Conflicts of Interest: Bringmann A, None; Jochmann C, None; Unterlauft JD, None; Wiedemann R, None; Rehak M, None; Wiedemann P, None.
REFERENCES
- 1.MacDonald RB, Randlett O, Oswald J, Yoshimatsu T, Franze K, Harris WA. Müller glia provide essential tensile strength to the developing retina. J Cell Biol. 2015;210(7):1075–1083. doi: 10.1083/jcb.201503115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gass JD. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol. 1999;117(6):821–823. doi: 10.1001/archopht.117.6.821. [DOI] [PubMed] [Google Scholar]
- 3.Bringmann A, Syrbe S, Görner K, Kacza J, Francke M, Wiedemann P, Reichenbach A. The primate fovea: Structure, function and development. Prog Retin Eye Res. 2018;66:49–84. doi: 10.1016/j.preteyeres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000;41(10):2827–2836. [PubMed] [Google Scholar]
- 5.Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969;82(2):151–159. doi: 10.1001/archopht.1969.00990020153002. [DOI] [PubMed] [Google Scholar]
- 6.Reichenbach A, Bringmann A. Müller cells in the healthy and diseased retina. New York: Springer New York; 2010. [DOI] [PubMed] [Google Scholar]
- 7.Syrbe S, Kuhrt H, Gärtner U, Habermann G, Wiedemann P, Bringmann A, Reichenbach A. Müller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2018;167:110–117. doi: 10.1016/j.exer.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Chung H, Byeon SH. New insights into the pathoanatomy of macular holes based on features of optical coherence tomography. Surv Ophthalmol. 2017;62(4):506–521. doi: 10.1016/j.survophthal.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Bringmann A, Duncker T, Jochmann C, Barth T, Duncker GIW, Wiedemann P. Spontaneous closure of small full-thickness macular holes: presumed role of Müller cells. Acta Ophthalmol. 2019 doi: 10.1111/aos.14289. [DOI] [PubMed] [Google Scholar]
- 10.Tadayoni R, Massin P, Haouchine B, Cohen D, Erginay A, Gaudric A. Spontaneous resolution of small stage 3 and 4 full-thickness macular holes viewed by optical coherence tomography. Retina. 2001;21(2):186–189. doi: 10.1097/00006982-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Privat E, Tadayoni R, Gaucher D, Haouchine B, Massin P, Gaudric A. Residual defect in the foveal photoreceptor layer detected by optical coherence tomography in eyes with spontaneously closed macular holes. Am J Ophthalmol. 2007;143(5):814–819. doi: 10.1016/j.ajo.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Itoh Y, Inoue M, Rii T, Ando Y, Hirakata A. Asymmetrical recovery of cone outer segment tips line and foveal displacement after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2014;55(5):3003–3011. doi: 10.1167/iovs.14-13973. [DOI] [PubMed] [Google Scholar]
- 13.Funata M, Wendel RT, de la Cruz Z, Green WR. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992;12(4):289–298. doi: 10.1097/00006982-199212040-00001. [DOI] [PubMed] [Google Scholar]
- 14.García-Layana A, García-Arumí J, Ruiz-Moreno JM, Arias-Barquet L, Cabrera-López F, Figueroa MS. A review of current management of vitreomacular traction and macular hole. J Ophthalmol. 2015;2015:809640. doi: 10.1155/2015/809640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudric A, Massin P, Paques M, Santiago PY, Guez JE, Le Gargasson JF, Mundler O, Drouet L. Autologous platelet concentrate for the treatment of full-thickness macular holes. Graefes Arch Clin Exp Ophthalmol. 1995;233(9):549–554. doi: 10.1007/BF00404704. [DOI] [PubMed] [Google Scholar]
- 16.Faude F, Edel E, Dannhauer M, Petzel C, Meier P, Wiedemann P. Autologous thrombocyte administration in treatment of idiopathic macular foramen. Ophthalmologe. 1997;94(12):877–881. doi: 10.1007/s003470050215. [DOI] [PubMed] [Google Scholar]
- 17.Paques M, Chastang C, Mathis A, Sahel J, Massin P, Dosquet C, Korobelnik JF, Le Gargasson JF, Gaudric A. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology. 1999;106(5):932–938. doi: 10.1016/s0161-6420(99)00512-6. [DOI] [PubMed] [Google Scholar]
- 18.Purtskhvanidze K, Frühsorger B, Bartsch S, Hedderich J, Roider J, Treumer F. Persistent full-thickness idiopathic macular hole: anatomical and functional outcome of revitrectomy with autologous platelet concentrate or autologous whole blood. Ophthalmologica. 2018;239(1):19–26. doi: 10.1159/000481268. [DOI] [PubMed] [Google Scholar]
- 19.Engelmann K, Sievert U, Hölig K, Wittig D, Weßlau S, Domann S, Siegert G, Valtink M. Effect of autologous platelet concentrates on the anatomical and functional outcome of late stage macular hole surgery: a retrospective analysis. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(11-12):1289–1298. doi: 10.1007/s00103-015-2251-1. [DOI] [PubMed] [Google Scholar]
- 20.Su CY, Kuo YP, Nieh HL, Tseng YH, Burnouf T. Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion. 2008;48(11):2414–2420. doi: 10.1111/j.1537-2995.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 21.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 22.Velhagen KH, Druegg A, Rieck P. Proliferation and wound healing of retinal pigment epithelium cells in vitro. Effect of human thrombocyte concentrate, serum and PDGF. Ophthalmologe. 1999;96(2):77–81. doi: 10.1007/s003470050378. [DOI] [PubMed] [Google Scholar]
- 23.Castelnovo L, Dosquet C, Gaudric A, Sahel J, Hicks D. Human platelet suspension stimulates porcine retinal glial proliferation and migration in vitro. Invest Ophthalmol Vis Sci. 2000;41(2):601–609. [PubMed] [Google Scholar]
- 24.Hollborn M, Bringmann A, Faude F, Wiedemann P, Kohen L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun. 2006;344(3):912–919. doi: 10.1016/j.bbrc.2006.03.185. [DOI] [PubMed] [Google Scholar]
- 25.Burmeister SL, Hartwig D, Limb GA, Kremling C, Hoerauf H, Müller M, Geerling G. Effect of various platelet preparations on retinal Müller cells. Invest Ophthalmol Vis Sci. 2009;50(10):4881–4886. doi: 10.1167/iovs.08-3057. [DOI] [PubMed] [Google Scholar]
- 26.Bringmann A, Wiedemann P. Involvement of Müller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):865–883. doi: 10.1007/s00417-009-1082-x. [DOI] [PubMed] [Google Scholar]
- 27.Degenhardt V, Busch C, Jochmann C, Meier P, Unterlauft JD, Mößner A, Edel E, Tewari R, Wiedemann P, Rehak M. Prognostic factors in patients with persistent full-thickness idiopathic macular holes treated with re-vitrectomy with autologous platelet concentrate. Ophthalmologica. 2019;242(4):214–221. doi: 10.1159/000502386. [DOI] [PubMed] [Google Scholar]
- 28.Cuenca N, Ortuño-Lizarán I, Pinilla I. Cellular characterization of OCT and outer retinal bands using specific immunohistochemistry markers and clinical implications. Ophthalmology. 2018;125(3):407–422. doi: 10.1016/j.ophtha.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Haritoglou C, Tadayoni R, Hubschman JP. Lamellar macular hole surgery-current concepts, future prospects. Clin Ophthalmol. 2019;13:143–146. doi: 10.2147/OPTH.S188309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23(3):421–424. doi: 10.1097/00006982-200306000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Allen AW, Jr, Gass JD. Contraction of a perifoveal epiretinal membrane simulating a macular hole. Am J Ophthalmol. 1976;82(5):684–691. doi: 10.1016/0002-9394(76)90002-7. [DOI] [PubMed] [Google Scholar]
- 32.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36(5):241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 33.Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126(4):507–511. doi: 10.1001/archopht.126.4.507. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Purohit R, Patel A, Papageorgiou E, Sheth V, Maconachie G, Pilat A, McLean RJ, Proudlock FA, Gottlob I. In vivo foveal development using optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(8):4537–4545. doi: 10.1167/iovs.15-16542. [DOI] [PubMed] [Google Scholar]
- 35.Wang YZ, Morale SE, Cousins R, Birch EE. Course of development of global hyperacuity over lifespan. Optom Vis Sci. 2009;86(6):695–700. doi: 10.1097/OPX.0b013e3181a7b0ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidry C. Tractional force generation by porcine Müller cells. Development and differential stimulation by growth factors. Invest Ophthalmol Vis Sci. 1997;38(2):456–468. [PubMed] [Google Scholar]
- 37.Hardwick C, Feist R, Morris R, White M, Witherspoon D, Angus R, Guidry C. Tractional force generation by porcine Müller cells: stimulation by growth factors in human vitreous. Invest Ophthalmol Vis Sci. 1997;38(10):2053–2063. [PubMed] [Google Scholar]