Dear Editor,
In the past, enucleation has been considered the only available option and the standard of care for the management of malignant intraocular tumors. Thanks to the advances in the field of radiotherapy, new therapeutic approaches have been developed in the last decades, such as plaque brachytherapy and proton beam therapy (PBT)[1]. These techniques allow to preserve the eyeball and obtain local control of the tumor, maintaining a certain visual function in an increasingly higher proportion of cases, without any difference in terms of survival and metastasis-free outcomes compared to enucleation[1].
However, sight- and eyeball-threatening complications including neovascular glaucoma (NVG), radiation retinopathy and optic neuropathy can complicate the postoperative course of ocular irradiation, with detrimental effects on patients' vision and quality of life[1]–[4]. In particular, NVG occurs in up to 27% of these cases[1], and represents one of the most common causes of secondary enucleation[5]. In fact, despite maximum anti-glaucoma medications, intraocular pressure (IOP) is often not well controlled in this setting. In addition, European Glaucoma Society (EGS) guidelines discourage the use of prostaglandin analogues and pilocarpine due to the theoretical risk of promoting metastasis dissemination[6]. A further issue is related to patients' intolerance to active agents and preservatives of anti-glaucoma medications, which can worsen dry eye disease and corneal/conjunctival epitheliopathy secondary to anterior segment irradiation. Finally, EGS guidelines as well as other authors suggest also that incisional glaucoma surgery is indicated only after successful irradiation therapy with local tumor control to prevent extraocular tumor extension due to the possible risks of inoculation metastasis and local recurrence[7]–[9].
Taking into account all these factors, the management of glaucoma secondary to ocular irradiation for choroidal melanoma represents a real therapeutic challenge for ophthalmologist.
We report herein the feasibility and the outcomes of ultrasound cyclo plasty (UCP) performed as first-line surgical treatment in 3 eyes of 3 patients with glaucoma secondary to ocular irradiation (PBT and brachytherapy) for the treatment of choroidal melanoma.
UCP device (EyeOP1, Eye Tech Care, Rillieux-la-Pape, France) uses high-intensity focused ultrasound (HIFU) to achieve a selective and more precise coagulation of the ciliary body, while sparing the adjacent ocular structures. The procedure has been previously described in details by our group[10]. Briefly, the device is composed by a coupling cone and a ring probe containing 6 piezoelectric transducers. The 6 transducers produce and deliver HIFU beams operating at a frequency of 21 MHz with an acoustic power of 2 W, being able to determine the fast increase of ciliary body temperature up to 90°C (avoiding tissue boiling) and treating up to 45% of the entire ciliary body circumference. The procedure is performed in operating room under loco-regional anesthesia. After the procedure, the treated eye was medicated with antibiotic plus steroid ointment and patched for 24h.
Written informed consent was obtained from all the subjects included in this case series before any procedure. This case series was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board.
CASE 1
A 44 year-old woman was referred to the Retina Service of S. Orsola-Malpighi University Hospital (Bologna, Italy) for a mushroom-shaped choroidal melanoma of 6 mm thickness and 5 mm diameter, located in the infero-temporal mid-peripheral region of her left eye (Figure 1A-1C). Total body Positron emission tomography/computed tomography examination excluded systemic dissemination of the neoplastic disease. The ocular lesion was treated with PBT at an International Referral Center (Jules Gonin University Eye Clinic, Lausanne, Switzerland) according to their standard protocol[11]. Progressive regression of the size of the melanoma accompanied with scarring and atrophy of the surrounding retina was detected 3mo after PBT (Figure 1D-1F). Full best corrected visual acuity (20/20 Snellen) was maintained after the treatment due to macular sparing.
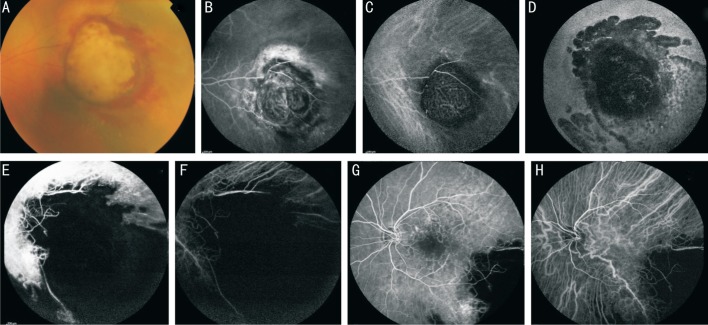
Figure 1. Case 1.
Fundus photography (A), fluorescein angiography (B) and indocyanine green angiography (C) showing the mushroom-shaped choroidal melanoma. Note the characteristic “double circulation” pattern of the choroidal melanoma consisting of normal retinal vessels overlying the internal circulation within the lesion. Fundus autofluorescence (D), fluorescein angiography (E) and indocyanine green angiography (F) showing the lesion 1y after PBT with atrophy of the surrounding retina and choroid. Fluorescein angiography (G) and indocyanine green angiography (H) showing radiation retinopathy involving the macular region, capillary leakage and retinal capillary dropout with foveal avascular zone enlargement.
One year after irradiation treatment, patient came to our attention complaining of decreased visual acuity (20/50) in the treated eye. Slit lamp examination showed iris neovascularization in about 3 clock hours and IOP was found to be 35 mm Hg. Gonioscopic examination revealed an open but disrupted angle with diffuse neovessels, peripheral anterior synechiae in the inferior and temporal sectors, and inferior dense pigmentation of the trabecular meshwork. Ophthalmoscopy showed the presence of radiation retinopathy, with cystoid macular edema and foveal avascular zone enlargement (Figure 1G, 1H), that was treated with multiple (n=6) anti-vascular endothelial growth factor (aflibercept) intravitreal injections.
Topical anti-glaucoma therapy was initiated with fixed combination dorzolamide-timolol eye drop twice daily and brimonidine eye drop twice daily. In the following months, the further addition of oral carbonic anhydrase inhibitor (CAI) tablets (250 mg, 1 tablet twice daily) was necessary to better control IOP values. Two years after PBT, best corrected visual acuity was stable at 20/50, while IOP was found again high (32 mm Hg) despite maximum topical hypotensive eye drops (prostaglandin analogues were avoided as suggested by EGS guidelines) and oral CAI tablets twice daily. In order to preserve visual potential avoiding the risk of metastasis inoculation, we decided to treat the patient with UCP rather than with incisional surgery or shunt implantation.
One day postoperatively, IOP was reduced to 10 mm Hg. Then, the same preoperative anti-glaucoma eye drops were continued except for oral CAI tablets that were poorly tolerated by the patient. In addition, dexamethasone eye drops 4 times daily were prescribed for 2wk and then tapered to twice daily for other 2wk according to the standard postoperative protocol.
During the following scheduled visits, IOP was approximately stable (values of 15, 18 and 19 mm Hg were recorded 1, 3 and 6mo after UCP, respectively). IOP values along with hypotensive medication number are showed in Figure 2. No major intra- or post-operative complications occurred and best corrected visual acuity remained stable at 20/50. Conjunctival hyperemia and mild anterior chamber uveal reaction (tyndall 1+) were recorded in the first postoperative days, and resolved within the first postoperative month. Neither systemic metastatic disease nor local recurrence of the tumor was detected after UCP procedure.
Figure 2. IOP changes of the treated patients during the 6-months follow-up period.
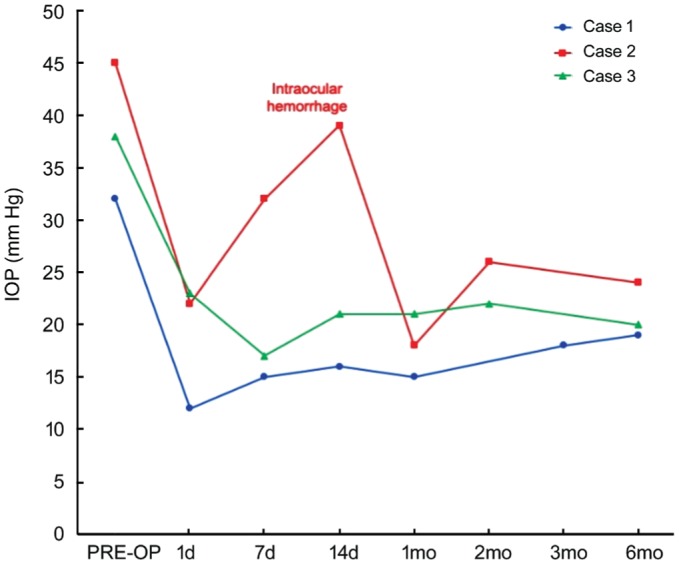
The IOP spike in Case 2 was found when intraocular hemorrhage occurred, with subsequent spontaneous resolution and IOP reduction.
CASE 2
A 76 years-old woman was treated at the Retina Service of Hospital La Paz (Madrid, Spain) with I-125 brachytherapy for a choroidal melanoma of 15 mm base and 5 mm thickness in her left eye. After ocular irradiation, a residual best corrected visual acuity of 20/100 was maintained. Four years after brachytherapy, patient underwent phacoemulsification and intraocular lens implantation for a dense cortico-nuclear cataract that hampered ocular fundus and lesion examination. After surgery, radiation retinopathy was detected (Figure 3A) and treated with focal laser photocoagulation of leaking vessels. In the following months, dense vitreitis with fibrin membrane and exudative retinal detachment developed, requiring pars plana vitrectomy, surgical capsulotomy and endolaser panretinal photocoagulation. Subsequently, NVG complicated further the clinical picture with an IOP of 29 mm Hg that was managed with 2 topical hypotensive medications (fixed combination dorzolamide-timolol eye drop twice/daily and brimonidine eye drop twice/daily). However, a second session of capsulotomy with iridotomy was needed due to pupillary blockage with fibrin membrane formation, and 2 intravitreal implants of dexamethasone were injected in the vitreous chamber in the following months to control intraocular inflammation and fibrin reaction.
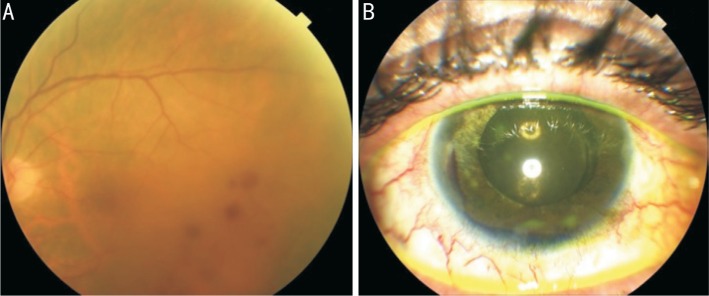
Figure 3. Case 2.
Fundus photography (A) showing radiation retinopathy with scattered dot and blot retinal hemorrhages in the temporal macular region. Anterior segment photography (B) showing the hyphema occurred 1wk after UCP procedure.
The postoperative course was stable until the 8-year post-brachytherapy visit, when best corrected visual acuity was 20/200 and IOP was 45 mm Hg despite 2 topical hypotensive medications (as above) plus 3 daily oral CAI tablets. In the attempt of reducing oral and topical hypotensive medication, the patient was treated with a session of UCP. One day postoperatively, IOP was 22 mm Hg without any hypotensive medication. However, 1wk after treatment, hyphema occurred (Figure 3B) and IOP increased to 32 mm Hg, requiring the resumption of fixed combination dorzolamide-timolol eye drop twice/daily. Progressively, the hemorrhage self-resolved and IOP decreased to 18 mm Hg at 1mo, 26 mm Hg at 2mo and 24 mm Hg at 6mo, without eye pain. Intraocular pressure values along with hypotensive medication number are showed in Figure 2.
CASE 3
A 58 years-old man was diagnosed with a large choroidal melanoma of 7.6 mm base and 7.5 mm thickness in inferotemporal region of his right eye (Figure 4A, 4B) and treated with I-125 brachytherapy at the Retina Service of Hospital La Paz (Madrid, Spain). After treatment, residual best corrected visual acuity was 20/100. One year after treatment, a dense vitreous hemorrhage with fibrin reaction and NVG developed requiring pars plana vitrectomy with endolaser panretinal photocoagulation. In addition, fixed combination dorzolamide-timolol eye drop twice/daily was necessary to control IOP. In the subsequent year, phacoemulsification and intraocular lens implantation was performed and best corrected visual acuity returned to the value of 20/100, while IOP increased again to 45 mm Hg. Additional hypotensive medications were prescribed but IOP remained poorly controlled with values of 38 mm Hg recorded 3y after brachytherapy despite 2 topical hypotensive medications (fixed combination dorzolamide-timolol eye drop twice/daily, brimonidine eye drop twice/daily) plus 3 daily oral CAI tablets.
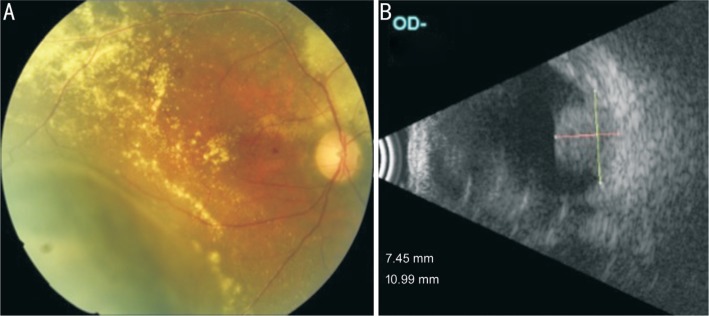
Figure 4. Case 3.
Fundus photography (A) showing the choroidal melanoma in the inferotemporal region with surrounding retinal exudation. B-scan ultrasonography (B) showing the solid hyperechoic choroidal mass protruding in the vitreous chamber.
In the attempt of reducing IOP and controlling eye pain, UCP treatment was performed in the right eye. One day postoperatively, IOP dropped to 23 mm Hg without any hypotensive medication. One, two, four and eight weeks postoperatively, IOP reduction was maintained stable with values of 17, 21, 21 and 22 mm Hg. Six months after UCP treatment, IOP was 20 mm Hg with the use of timolol eye drop once daily. Residual best corrected visual acuity of 20/200 was preserved. IOP values along with hypotensive medication number are showed in Figure 2.
Intraocular tumors can cause secondary glaucoma through different ways such as solid tumor invasion and tumor seeding into the angle, angle closure, and iris neovascularization[9]. However, secondary glaucoma can often complicate the postoperative course after ocular irradiation treatments[8].
UCP uses HIFU beams to induce a precise, well-controlled and selective tissue coagulation of the ciliary body, while sparing adjacent ocular structures[12]–[13]. This non-incisional minimally-invasive procedure allows for a more predictable and controlled IOP reduction, with lower complications rates and better preservation of visual acuity compared to traditional cyclodestructive techniques. In fact, the latter are currently generally indicated for eyes with refractory or end-stage glaucoma and poor visual potential, due to their non-selectivity and unpredictable dose-effect relationship, with the risk of severe complications as chronic hypotony, vision loss and phthisis[14].
In previous studies, UCP procedure showed to be safe and effective, reducing both IOP values and hypotensive medications, without detrimental effects on visual function[10],[12]–[13],[15]–[17].
To date, UCP has been used with a good profile of safety and efficacy in different stages and types of glaucoma, such as primary open angle, chronic angle closure, pseudoexfoliative, uveitic, neovascular and others[12]–[13],[15]–[17]. To the best of our knowledge, no previous reports of glaucoma secondary to ocular irradiation for choroidal melanoma treated with UCP have been described to date.
Nowadays, the progresses in ocular radiotherapy allow preserving a certain visual function in an ever-increasing number of cases, even after highly invasive treatments. In the attempt of being less invasive and trying to preserve patients' residual visual function, we performed UCP that is a more conservative treatment with lower complications rates compared to traditional cyclodestructive techniques. The other additional advantage over conventional incisional surgical techniques (e.g., trabeculectomy, shunt implantation) is the presumed lower risk of inoculation metastasis and local recurrence, as stated by EGS guidelines and other authors[6],[8]–[9]. In all cases, UCP showed a good hypotensive effect, with an IOP reduction of about 40% 6mo after the procedure, allowing at the same time the reduction of the daily number of topical and oral hypotensive medications used. Since the UCP procedure is “conjunctival-sparing”, it is indicated also in eyes with chronic conjunctival inflammation and fibrotic or necrotic tissues alterations affecting wound healing, as can occur after ocular proton beam irradiation or in multi-operated eyes.
The main limitation of the study is the limited follow-up of 6mo. In fact, like for other cyclodestructive procedures, the efficacy of UCP may be partial or time-limited, mainly due to the possible re-epithelialization process of the ciliary processes with progressive recovery of their function[18]. However, repeated treatments are possible in case of qualified success in order to enhance efficacy and further reduce IOP without any detrimental effect, as previously described[17].
In conclusion, UCP might add a new additional step between medical and surgical therapeutic options for challenging cases of glaucoma, like those presented in this case series, thus providing a new minimally invasive option to be added in the current armamentarium of glaucoma treatments.
Acknowledgments
Conflicts of Interest: Sebastiani S, None; Asencio-Durán M, None; Lavín-Dapena C, None; Manzano-Muñoz B, None; D'Anna-Mardero O, None; Cordero-Ros R, None; Pellegrini M, None; Bernabei F, None; Mercanti A, None; Scorcia V, None; Giannaccare G, None.
REFERENCES
- 1.Bensoussan E, Thariat J, Maschi C, Delas J, Schouver ED, Hérault J, Baillif S, Caujolle JP. Outcomes after proton beam therapy for large choroidal melanomas in 492 patients. Am J Ophthalmol. 2016;165:78–87. doi: 10.1016/j.ajo.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Floriani I, Quaranta L, Rulli E, Katsanos A, Varano L, Frezzotti P, Rossi GC, Carmassi L, Rolle T, Ratiglia R, Gandolfi S, Fossarello M, Uva M, Hollander L, Poli D, Grignolo F, Italian Study Group on QoL in glaucoma Health-related quality of life in patients with primary open-angle glaucoma. An Italian multicentre observational study. Acta Ophthalmol. 2016;94(5):e278–e286. doi: 10.1111/aos.12890. [DOI] [PubMed] [Google Scholar]
- 3.Rulli E, Quaranta L, Riva I, Poli D, Hollander L, Galli F, Katsanos A, Oddone F, Torri V, Weinreb RN, Italian Study Group on QoL in Glaucoma Visual field loss and vision-related quality of life in the Italian primary open angle glaucoma study. Sci Rep. 2018;8(1):619. doi: 10.1038/s41598-017-19113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riva I, Legramandi L, Katsanos A, Oddone F, Rulli E, Roberti G, Quaranta L, Italian Study Group on QoL in Glaucoma Influence of sociodemographic factors on disease characteristics and vision-related quality of life in primary open-angle glaucoma patients: the Italian primary open angle glaucoma study (IPOAGS) J Glaucoma. 2018;27(9):776–784. doi: 10.1097/IJG.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 5.Fabian ID, Tomkins-Netzer O, Stoker I, Arora AK, Sagoo MS, Cohen VM. Secondary enucleations for uveal melanoma: a 7-year retrospective analysis. Am J Ophthalmol. 2015;160(6):1104–1110.e1. doi: 10.1016/j.ajo.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 6.European glaucoma society terminology and guidelines for glaucoma, 4th edition-Chapter 2: classification and terminologysupported by the EGS foundation. Br J Ophthalmol. 2017;101(5):73–127. doi: 10.1136/bjophthalmol-2016-EGSguideline.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway RM, Poothullil AM, Daftari IK, Weinberg V, Chung JE, O'Brien JM. Estimates of ocular and visual retention following treatment of extra-large uveal melanomas by proton beam radiotherapy. Arch Ophthalmol. 2006;124(6):838–843. doi: 10.1001/archopht.124.6.838. [DOI] [PubMed] [Google Scholar]
- 8.Riechardt AI, Cordini D, Rehak M, Hager A, Seibel I, Böker A, Gundlach E, Heufelder J, Joussen AM. Trabeculectomy in patients with uveal melanoma after proton beam therapy. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1379–1385. doi: 10.1007/s00417-016-3310-5. [DOI] [PubMed] [Google Scholar]
- 9.Camp DA, Yadav P, Dalvin LA, Shields CL. Glaucoma secondary to intraocular tumors: mechanisms and management. Curr Opin Ophthalmol. 2019;30(2):71–81. doi: 10.1097/ICU.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 10.Giannaccare G, Sebastiani S, Campos EC. Ultrasound cyclo plasty in eyes with glaucoma. J Vis Exp. 2018(131) doi: 10.3791/56192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger E, Schalenbourg A, Zografos L, Bercher L, Boehringer T, Chamot L, Goitein G. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys. 2001;51(1):138–147. doi: 10.1016/s0360-3016(01)01560-7. [DOI] [PubMed] [Google Scholar]
- 12.Denis P, Aptel F, Rouland JF, Nordmann JP, Lachkar Y, Renard JP, Sellem E, Baudouin C, Bron A. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci. 2015;56(2):1089–1096. doi: 10.1167/iovs.14-14973. [DOI] [PubMed] [Google Scholar]
- 13.Aptel F, Denis P, Rouland JF, Renard JP, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol. 2016;94(5):e268–e277. doi: 10.1111/aos.12913. [DOI] [PubMed] [Google Scholar]
- 14.Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007;91(12):1631–1635. doi: 10.1136/bjo.2007.116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannaccare G, Vagge A, Gizzi C, Bagnis A, Sebastiani S, Del Noce C, Fresina M, Traverso CE, Campos EC. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):599–605. doi: 10.1007/s00417-016-3563-z. [DOI] [PubMed] [Google Scholar]
- 16.Giannaccare G, Vagge A, Sebastiani S, Urbini LE, Corazza P, Pellegrini M, Carmassi L, Bergamini F, Traverso CE, Campos EC. Ultrasound cyclo-plasty in patients with glaucoma: 1-year results from a multicentre prospective study. Ophthalmic Res. 2019;61(3):137–142. doi: 10.1159/000487953. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini M, Sebastiani S, Giannaccare G, Campos EC. Intraocular inflammation after ultrasound cyclo plasty for the treatment of glaucoma. Int J Ophthalmol. 2019;12(2):338–341. doi: 10.18240/ijo.2019.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKelvie PA, Walland MJ. Pathology of cyclodiode laser: a series of nine enucleated eyes. Br J Ophthalmol. 2002;86(4):381–386. doi: 10.1136/bjo.86.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]