Abstract
AIM
To evaluate the accuracy of three commonly used biometric formulae across different axial lengths (ALs) at one United States Veterans Affairs teaching hospital.
METHODS
A retrospective chart review was conducted from November 2013 to May 2018. One eye of each patient who underwent cataract surgery with a monofocal intraocular lens (IOL) was included. The range of postoperative follow-up period was from 3wk to 4mo. The Holladay 2, Barrett Universal II, and Hill-Radial Basis Function (Hill-RBF) formulae were used to predict the postoperative refraction for all cataract surgeries. For each formula, we calculated the prediction errors [including mean absolute prediction error (MAE)] and the percentage of eyes within ±0.25 diopter (D) and ±0.5 D of predicted refraction. We performed subgroup analyses for short (AL<22.0 mm), medium (AL 22.0-25.0 mm), and long eyes (AL>25.0 mm).
RESULTS
A total of 1131 patients were screened, and 909 met the inclusion criteria. Resident ophthalmologists were the primary surgeons in 710 (78.1%) cases. We found no statistically significant difference in predictive accuracy among the three formulae over the entire AL range or in the short, medium, and long eye subgroups. Across the entire AL range, the Hill-RBF formula resulted in the lowest MAE (0.384 D) and the highest percentage of eyes with postoperative refraction within ±0.25 D (42.7%) and ±0.5 D (75.5%) of predicted. All three formulae had the highest MAEs (>0.5 D) and lowest percentage within ±0.5 D of predicted refraction (<55%) in short eyes.
CONCLUSION
In cataract surgery patients at our teaching hospital, three commonly used biometric formulae demonstrate similar refractive accuracy across all ALs. Short eyes pose the greatest challenge to predicting postoperative refractive error.
Keywords: cataract surgery, biometry, intraocular lens, power calculation
INTRODUCTION
Cataract surgery is one of the most frequently performed procedures in the Veterans Health Administration, the largest integrated health care system and the largest provider of health care training in the United States (US)[1]. Advances in optical biometry and intraocular lens (IOL) power formulae have led to continued improvements in postoperative refractive outcomes[2]–[3]: in 2017, 97.3% of cataract surgeries were within ±1 diopter (D) of predicted postoperative refraction[4]. Determining the postoperative effective lens position (ELP) and better accounting for the role of axial length (AL) remain challenges to further improvements in the accuracy of preoperative biometry[2],[5].
While studies done in the past five years have generally found the Barrett Universal II formula to be most accurate[4],[6]–[9], the relative accuracy of different formulae is dependent on a multitude of factors, including AL[4],[7],[9]–[10], the type of biometry used [optical low-coherence reflectometry (OLCR) versus partial coherence interferometry (PCI)][11], preoperative anterior chamber depth (ACD) values[12], and interocular AL and corneal power differences[13].
Evaluating biometric accuracy in a teaching hospital setting is important as this is where residents are learning their approach to patient care. In teaching hospitals, refractive outcomes may be impacted not only by resident surgeons with variable experience[14]–[15], but also different personnel who may perform biometry and refractions[16]. However, the few studies published within the last five years in teaching hospitals are limited by size (<300 patients) or focus (eyes with AL>25.0 mm)[2],[12]. The primary objective of this study was to identify the most accurate biometric formula at a single US Veterans Affairs teaching hospital. The secondary objective was to evaluate which biometric formula had the lowest prediction error in patients with short, medium, and long ALs.
SUBJECTS AND METHODS
Ethical Approval
All study conduct adhered to the tenets of the Declaration of Helsinki. Because of its retrospective nature, the requirement of informed consent was waived. The Providence Veterans Affairs Medical Center (PVAMC) Institutional Review Board approved this retrospective study. The Holladay 2, Barrett Universal II, and Hill-Radial Basis Function (Hill-RBF, first version) formulae were used to predict the postoperative refraction for all cataract surgeries. We did not include older formulae such as the SRK-T and Hoffer Q in our analysis as prior studies have demonstrated the superiority of current generation formulae[2],[4],[7],[11]. Optical biometry was performed using the Lenstar optical biometer (Haag-Streit USA, Mason, OH, USA). We included patients who received cataract surgery using monofocal spherical SN60WF IOLs at the PVAMC teaching hospital between November 2013 and May 2018. Only one eye was included from each patient to prevent compounding of data with the use of bilateral eyes; correlation between outcomes between a patient's two eyes would decrease the power of the study[17]. Furthermore, as not all cataract patients at the PVAMC received bilateral surgery, including both eyes from eligible patients would have disproportionately weighted outcomes from these patients. If a patient had cataract surgery in both eyes, we included the eye with the better postoperative best corrected visual acuity (BCVA) in the study as refraction accuracy decreases with worsening BCVA[17]. If both eyes had the same postoperative BCVA, we included the earlier cataract surgery[4]. These inclusion criteria are based on recommendations by Hoffer et al[17] for optimized study protocol in examining IOL formula accuracy. Patients were excluded if they had no postoperative refraction within 3wk to 4mo[4],[11], AL or lens thickness (LT) not measurable by optical biometry, history of corneal disease, history of refractive surgery, posterior capsular rupture, sulcus IOL, or BCVA worse than 20/40.
Information extracted from patient charts included patient age, race, ethnicity, gender, pupil size, prior cataract surgery, preoperative refraction, preoperative BCVA, postoperative refraction, postoperative BCVA, IOL type, and IOL power. Preoperative and postoperative refractive values were recorded in spherical equivalents. The preoperative biometry and the majority of the postoperative refractions were performed by experienced technicians certified by the Joint Commission on Allied Health Personnel in Ophthalmology[18].
Information extracted from the Lenstar device included AL, ACD, preoperative flat corneal front power (K1), preoperative steep corneal front power (K2), LT, horizontal white-to-white (WTW) corneal diameter, and central corneal thickness (CCT). Predicted postoperative refractions from the Barrett Universal II and Hill-RBF formulae were extracted from the Haag-Streit EyeSuite software. Predictive measurements from the Holladay 2 formula were extracted from the Holladay IOL Consultant program.
We plotted overall refractive outcomes and calculated mean prediction error (ME), mean absolute prediction error (MAE), median absolute prediction error (MedAE), and the percentage of eyes with a prediction error of ±0.25 D and ±0.5 D for each formula. The MAE and MedAE provided a glimpse into the overall accuracy of each formula, while the ME showed whether each formula tends to produce more negative or positive refractive outcomes than predicted. These conventions follow those established by prior studies[4],[7],[11]. Statistical comparisons of MAE among the three formulas were performed using one-way repeated measures analysis of variance (Friedman test). Subgroup analyses for short (AL<22.0 mm), medium (AL 22.0-25.0 mm), and long eyes (AL>25.0 mm) were also performed. STATA 11 (StataCorp LLC, College Station, Texas, USA) was used for statistical analysis.
RESULTS
A breakdown of patient demographics can be found in Table 1. Out of 1131 total charts reviewed in the study period, we included 909 eyes from 909 patients in the final study; 170 patients were excluded due to lack of postoperative refraction within the designated follow-up period, 33 for worse than 20/40 postoperative BCVA (27 had pre-existing ocular disease), 14 for complications, and five for missing data. Resident ophthalmologists were the primary surgeons in 78.1% (710/909) of the cases.
Table 1. Demographics of patients.
| Demographics | Data |
| Left eye | 444 (49) |
| Female | 21 (2.3) |
| Race | |
| Asian | 2 (0.2) |
| Black | 32 (3.5) |
| White | 855 (94) |
| Other | 20 (2.2) |
| Ethnicity | |
| Hispanic or Latino | 4 (0.4) |
| Not Hispanic or Latino | 897 (99) |
| Unknown | 8 (0.9) |
| Axial length subgroups | |
| Short, <22.0 mm | 16 (1.8) |
| Medium, 22.0-25.0 mm | 762 (84) |
| Long, >25.0 mm | 125 (14) |
| Age, y (mean±SD) | 74.5±0.26 |
| Preoperative refraction (25%tile, median, 75%tile) | -2.125, -0.375, 1.125 |
| Postoperative refraction (25%tile, median, 75%tile) | -0.5, -0.25, 0.0 |
| IOL power (mean±SD) | 20.6±2.8 |
| Anterior chamber depth, mm (mean±SD) | 3.2±0.43 |
| Lens thickness, mm (mean±SD) | 4.61±0.50 |
| Preoperative flat corneal front power, K1 (mean±SD) | 43.1±1.52 |
| Preoperative steep corneal front power, K2 (mean±SD) | 43.8±1.75 |
| Horizontal white-to-white corneal diameter, mm (mean±SD) | 12.2±0.51 |
| Central corneal thickness, µm (mean±SD) | 547±38 |
SD: Standard deviation; IOL: Intraocular lens.
n=909, n (%)
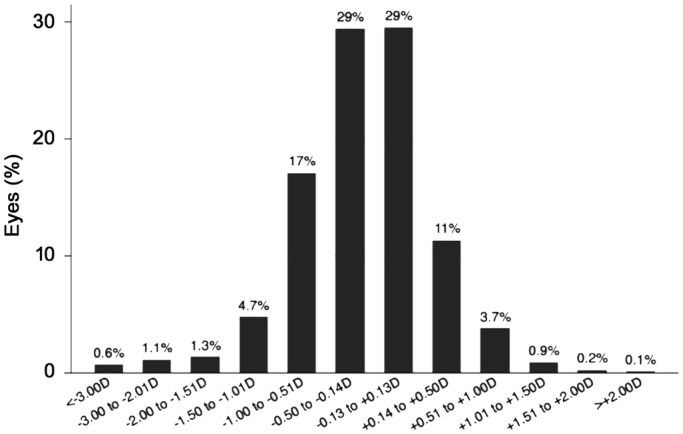
Overall refractive outcomes are displayed in Figure 1 and prediction error data for all AL subgroups are found in Table 2. While the Hill-RBF formula had the lowest MAE across the entire AL range, one-way analysis of variance showed no significant difference among the three formulae for monofocal IOL implantation (F=0.37, P=0.69). The Hill-RBF also predicted the highest percentage of eyes with postoperative refraction within ±0.25 D (42.5%) and ±0.5 D (75.5%) across the entire AL range.
Figure 1. Bar graph of the distribution of refractive outcomes for the SN60WF model intraocular lens.
Table 2. Prediction errors in different AL groups (n=909).
| Formula | MAE (D) | MedAE (D) | ME (D) | SD | Percentage of eyes within diopter range indicated |
|
| ±0.25 D (%) | ±0.5 D (%) | |||||
| Entire AL range (n=909, F=0.37, P=0.69) | ||||||
| Barrett 2 | 0.397 | 0.300 | -0.0760 | 0.564 | 41.8 | 74.1 |
| Holladay 2 | 0.399 | 0.310 | 0.0661 | 0.570 | 40.6 | 71.2 |
| Hill-RBF | 0.384 | 0.300 | -0.0023 | 0.554 | 42.7 | 75.5 |
| Short eyes (n=16, F=0.02, P=0.97) | ||||||
| Barrett 2 | 0.535 | 0.470 | 0.137 | 0.669 | 26.7 | 53.3 |
| Holladay 2 | 0.512 | 0.480 | 0.115 | 0.672 | 37.5 | 50.0 |
| Hill-RBF | 0.502 | 0.410 | 0.057 | 0.664 | 46.7 | 53.3 |
| Medium eyes (n=762, F=0.28, P=0.75) | ||||||
| Barrett 2 | 0.376 | 0.285 | -0.060 | 0.517 | 42.8 | 76.4 |
| Holladay 2 | 0.384 | 0.302 | 0.055 | 0.527 | 41.4 | 72.7 |
| Hill-RBF | 0.370 | 0.295 | 0.015 | 0.517 | 43.0 | 77.6 |
| Long eyes (n=125, F=0.08, P=0.91) | ||||||
| Barrett 2 | 0.507 | 0.355 | -0.203 | 0.772 | 37.9 | 62.9 |
| Holladay 2 | 0.483 | 0.368 | 0.130 | 0.785 | 36.4 | 65.3 |
| Hill-RBF | 0.474 | 0.335 | -0.174 | 0.763 | 39.8 | 62.5 |
AL: Axial length; MAE: Mean absolute prediction error; MedAE: Median absolute prediction error; ME: Mean prediction error; SD: Standard deviation; Hill-RBF: Hill-Radial Basis Function.
The outcomes for short, medium, and long AL subgroups were similar: no statistically significant differences were found among the three formulae for all three subgroups (P=0.97, 0.75, and 0.91 for short, medium, and long ALs, respectively). The Hill-RBF formula, however, consistently had the lowest MAE across all eye lengths. All three formulae produced their highest respective MAEs in the short AL subgroup: Holladay 2 had a MAE of 0.512 D, Hill-RBF had one of 0.502 D, and Barrett Universal II had one of 0.535 D. The Hill-RBF and Barrett also produced the lowest percentage of eyes in the short AL subgroup with postoperative refraction within ±0.25 and ±0.5 D. Conversely, all three formulae produced their most accurate results in the medium AL subgroup: Holladay 2 had an MAE of 0.384 D, Hill-RBF had one of 0.370 D, and Barrett Universal II had one of 0.376 D. All three formulae produced their highest percentage of eyes with postoperative refraction within ±0.25 and ±0.5 D in the medium AL subgroup.
DISCUSSION
To our knowledge, this is the largest study to date comparing the accuracy of biometric formulae in cataract surgery in a teaching hospital setting. In our current sample size, we found no statistically significant difference between Holladay 2, Hill-RBF, and Barrett Universal II biometric formulae across multiple ALs. Kane et al[8] found that the Hill-RBF formula had a significantly lower MAE than the Barrett Universal II in short eyes, and that the Hill-RBF performed better in long eyes than in medium eyes. In our analysis, the Hill-RBF retained the lowest MAE for the entire AL range, though this was not statistically significant, most likely due to the smaller size of our study relative to that of Kane et al[8] (n=3241). In addition, we found that all three formulae produced their highest MAE in the short AL subgroup. This was consistent with previous studies that stratified predictive errors according to AL (Table 3)[10]–[11].
Table 3. Previous studies comparing biometric formulae.
| Study | No. of eyes | Formulae | MAE (D) |
Conclusions | |||
| Overall | Short AL | Medium AL | Long AL | ||||
| Melles et al, 2018[4] | 13301 | Barrett 2 | 0.311 | - | - | - | Barrett was most consistently accurate in different AL groups |
| Holladay 2 | 0.450 | - | - | - | |||
| Kane et al, 2017[8] | 3122 | Barrett 2 | 0.381 | 0.451 | 0.383 | 0.375 | Hill-RBF was more accurate than Barrett in short AL group; the Barrett was more accurate overall and in medium ALs |
| Holladay 2 | 0.410 | - | - | - | |||
| Hill-RBF | 0.407 | 0.423 | 0.412 | 0.373 | |||
| Cooke et al, 2016[11] | 1454 | Barrett 2 | 0.306 | 0.338 | - | 0.274 | All formulae were least accurate for short eyes |
| Holladay 2 | 0.346 | 0.426 | - | 0.394 | |||
| Gökce et al, 2017[2]a | 86 | Barrett 2 | - | 0.39 | - | - | Only short eyes were analyzed; no statistically significant difference in formula accuracy |
| Holladay 2 | - | 0.40 | - | - | |||
| Hill-RBF | - | 0.36 | - | - | |||
| Gökce et al, 2018[12]a | 270 | Barrett 2 | 0.29 | - | - | - | Compared formulae accuracy for patient groups with varying ACD; Barrett had lowest MAE for ACD<3.0 mm and ACD>3.5 mm |
| Holladay 2 | 0.31 | - | - | - | |||
| Hill-RBF | 0.28 | - | - | - | |||
| Carifi et al, 2015[21] | 28 | Holladay 2 | - | 0.82 | - | - | Only short eyes; no difference between formulae, but all with large MAE |
| Kane et al, 2016[7] | 3241 | Barrett 2 | 0.385 | 0.469 | 0.386 | 0.435 | All formulae were less accurate in short AL group; Barrett was most accurate for all other ALs |
| Holladay 2 | 0.420 | 0.466 | 0.416 | 0.544 | |||
MAE: Mean absolute prediction error; ACD: Anterior chamber depth; AL: Axial length; D: Diopters; Hill-RBF: Hill-Radial Basis Function. aStudy performed in a teaching hospital.
Only two previous studies by Gökce et al[2],[12] were done in a teaching hospital, but they were both limited by their small sample sizes and focus on short eyes (Table 3). These two studies did not evaluate the accuracy of the Holladay 2 or Hill-RBF formulae, but our MAE for the Barrett Universal II was consistent with theirs[2]. Our MAEs for each of the formulae were also consistent with values demonstrated in previous non-teaching hospital studies (Table 3)[4],[8],[11]. While this is not a one-to-one comparison between resident and attending surgical outcomes, it is a realistic representation of the differences between teaching (where a percentage of cases will still be performed by attending physicians) and non-teaching hospital settings.
This study has several limitations. First, we targeted patients receiving care in the Veterans Affairs teaching hospital; hence, our findings may not be generalizable to patients receiving cataract surgery elsewhere, including other teaching hospitals[19]. Second, our sample size may have precluded achieving statistically significant differences among the three biometric formulae. However, our findings confirm that the overall accuracy of biometric formulae in predicting refractive outcomes are comparable between teaching and non-teaching hospital settings. Third, we excluded 18.3% of patients due to lack of postoperative refractive follow-up within the designated timeframe. Some patients may have followed up with providers outside of the PVAMC, but others may have neglected to come to follow-up appointments due to satisfactory postoperative visual outcomes; this may have resulted in selection bias toward patients with worse refractive outcomes[4],[20].
In conclusion, this study found no difference in the accuracy of the Holladay 2, Hill-RBF, and Barrett Universal II formulae for cataract surgery in a US teaching hospital, although all three formulae were least accurate in short eyes.
Acknowledgments
Presented at the American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting, San Diego, CA USA–May 2019.
Conflicts of Interest: Tang KS, None; Tran EM, None; Chen AJ, None; Rivera DR, None; Rivera JJ, None; Greenberg PB, None.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs or the United States government.
REFERENCES
- 1.Department of Veterans Affairs. Evaluation of Cataract Surgeris and Outcomes in the Veterans Health Administration Facilities. Veterans Health Administration; Mar 28, 2013. 2013. 11-02487-158. [Google Scholar]
- 2.Gökce SE, Zeiter JH, Weikert MP, Koch DD, Hill W, Wang L. Intraocular lens power calculations in short eyes using 7 formulas. J Cataract Refract Surg. 2017;43(7):892–897. doi: 10.1016/j.jcrs.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Brandsdorfer A, Kang JJ. Improving accuracy for intraocular lens selection in cataract surgery. Curr Opin Ophthalmol. 2018;29(4):323–327. doi: 10.1097/ICU.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 4.Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169–178. doi: 10.1016/j.ophtha.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Hodge C, McAlinden C, Lawless M, Chan C, Sutton G, Martin A. Intraocular lens power calculation following laser refractive surgery. Eye Vis (Lond) 2015;2:7. doi: 10.1186/s40662-015-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts TV, Hodge C, Sutton G, Lawless M, contributors to the Vision Eye Institute IOL outcomes registry Comparison of Hill-radial basis function, Barrett Universal and current third generation formulas for the calculation of intraocular lens power during cataract surgery. Clin Exp Ophthalmol. 2018;46(3):240–246. doi: 10.1111/ceo.13034. [DOI] [PubMed] [Google Scholar]
- 7.Kane JX, van Heerden A, Atik A, Petsoglou C. Intraocular lens power formula accuracy: comparison of 7 formulas. J Cataract Refract Surg. 2016;42(10):1490–1500. doi: 10.1016/j.jcrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Kane JX, van Heerden A, Atik A, Petsoglou C. Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg. 2017;43(3):333–339. doi: 10.1016/j.jcrs.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Wang QW, Jiang W, Lin T, Zhu Y, Chen C, Lin HT, Chen WR. Accuracy of intraocular lens power calculation formulas in long eyes: a systematic review and meta-analysis. Clin Exp Ophthalmol. 2018;46(7):738–749. doi: 10.1111/ceo.13184. [DOI] [PubMed] [Google Scholar]
- 10.Wang QW, Jiang W, Lin T, Wu XH, Lin HT, Chen WR. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356–363. doi: 10.1111/ceo.13058. [DOI] [PubMed] [Google Scholar]
- 11.Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157–1164. doi: 10.1016/j.jcrs.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Gökce SE, Montes De Oca I, Cooke DL, Wang L, Koch DD, Al-Mohtaseb Z. Accuracy of 8 intraocular lens calculation formulas in relation to anterior chamber depth in patients with normal axial lengths. J Cataract Refract Surg. 2018;44(3):362–368. doi: 10.1016/j.jcrs.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Kansal V, Schlenker M, Ahmed IIK. Interocular axial length and corneal power differences as predictors of postoperative refractive outcomes after cataract surgery. Ophthalmology. 2018;125(7):972–981. doi: 10.1016/j.ophtha.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Corey RP, Olson RJ. Surgical outcomes of cataract extractions performed by residents using phacoemulsification. J Cataract Refract Surg. 1998;24(1):66–72. doi: 10.1016/s0886-3350(98)80076-x. [DOI] [PubMed] [Google Scholar]
- 15.Smith JH, Seiff SR. Outcomes of cataract surgery by residents at a public County hospital. Am J Ophthalmol. 1997;123(4):448–454. doi: 10.1016/s0002-9394(14)70170-9. [DOI] [PubMed] [Google Scholar]
- 16.Martín-Serrano MJ, Roman-Ortiz C, Villa-Sáez ML, Labrador-Castellanos MP, Blanco-Carrasco R, Lozano-Ballesteros F, Pedraza-Martín C, José-Herrero MT, López-Ropero AM, Tenías Burillo JM. Concordance and interchangeability of biometric measurements of ocular axial length in patients awaiting cataract surgery. Eur J Ophthalmol. 2014;24(1):29–34. doi: 10.5301/ejo.5000318. [DOI] [PubMed] [Google Scholar]
- 17.Hoffer KJ, Aramberri J, Haigis W, Olsen T, Savini G, Shammas HJ, Bentow S. Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol. 2015;160(3):403–405.e1. doi: 10.1016/j.ajo.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Joint Commission on Allied Health Personnel in Ophthalmology. 2018. [Accessed on August 11, 2018]. http://www.jcahpo.org/.
- 19.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 20.Haneuse S. Distinguishing selection bias and confounding bias in comparative effectiveness research. Med Care. 2016;54(4):e23–e29. doi: 10.1097/MLR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carifi G, Aiello F, Zygoura V, Kopsachilis N, Maurino V. Accuracy of the refractive prediction determined by multiple currently available intraocular lens power calculation formulas in small eyes. Am J Ophthalmol. 2015;159(3):577–583. doi: 10.1016/j.ajo.2014.11.036. [DOI] [PubMed] [Google Scholar]