Abstract
目的
研究三维可视化技术联合吲哚菁绿(ICG)荧光影像技术在原发性肝癌诊治的应用价值。
方法
收集2016年1月~2018年11月南方医科大学珠江医院肝胆外科收治的154例患者临床资料进行回顾性分析, 分为实验组与对照组。实验组57例患者术前完成CT、GD-EOB-DTPA增强MRI、三维可视化并进行手术规划, 术中使用ICG荧光影像实时侦测肿瘤部位、边界, 肝内卫星癌灶、转移癌灶, 根据术中荧光探查结果并联合三维可视化手术规划, 完成最终手术方案; 对照组97例患者进行常规手术评估及手术切除。对术前肿瘤的影像学信息、术中肿瘤的探测情况、术后实验室指标、病理信息及随访信息进行分析。
结果
实验组57例, 术前CT影像学检查发现63个病灶; MRI发现70个病灶; 术中ICG分子荧光发现80个病灶, 比CT多发现的17个病灶中, 病理证实10个为肝细胞癌, 7个为肝硬化结节。实验组术中中位出血量为300(250) mL[M(QR)], 少于对照组的400(390) mL (Z=2.291, P=0.022);两组患者均无严重并发症发生及围手术期死亡。实验组患者术后并发症发生率为21%(12/57), 与对照组的48.4%(47/97)相比, 差异有统计学意义(χ2=11.406, P=0.001)。实验组的24个月总体无瘤生存率为74.9%, 而对照组总体无瘤生存率为28.9%, 两组间的差异性具有统计学意义(P=0.022)。
结论
三维可视化技术联合ICG荧光影像能够为原发性肝癌治疗提供精确的术前诊断、手术规划及实施方案, 以及术中微小肝癌侦测、精准导航, 减少术后并发症发生, 提高术后无瘤生存时间。
Keywords: 原发性肝癌, 成像,三维, 吲哚菁绿, 导航, 肝切除术
Abstract
Objective
To explore the value of three-dimensional visualization technology (3DVT) combined with indocyanine green (ICG) fluorescence imaging in the diagnosis and treatment of primary hepatocellular carcinoma (HCC).
Methods
We retrospectively analyzed the clinical data of 154 patients with HCC admitted to the Department of Hepatobiliary Surgery, Zhujiang Hospital, Southern Medical University between January, 2016 and November, 2018. In 57 of the patients (3DVT group), preoperative CT and Gd-EOB-DTPA-enhanced MRI were performed and 3D visualization and surgical planning was carried out before the operation; intraoperative ICG florescence imaging was performed for real-time detection of the tumor location and demarcation, intrahepatic satellite lesions and metastases. According to the intraoperative fluorescent signals and 3D visualization-based surgical planning, the final surgical plan was determined. In the other 97 patients (control group), conventional surgical assessment and surgical resection of the tumor was carried out. The preoperative imaging findings, intraoperative tumor detection, postoperative laboratory results, pathological reports, and follow-up data of the patients were analyzed.
Results
In 3DVT group, 63 and 70 lesions were detected by preoperative CT and MRI, respectively; compared with CT examination, intraoperative ICG florescence imaging revealed additional 17 lesions, among which 10 were pathologically confirmed as HCC and 7 as cirrhosis nodules. The median volume of bleeding was 300 mL in 3DVT group, significantly less than that in the control group (400 mL; Z=2.291, P=0.022). In both groups, serious complications or perioperative death occurred in none of the patients. The incidence of postoperative complications was significantly lowed in 3DVT group than in the control group [21% (12/57) vs 48.4% (47/97); χ2=11.406, P=0.001]. The overall disease-free survival rate at 2 years after the operation was significantly higher in 3DVT group than in the control group (74.9% vs 28.9%, P=0.022).
Conclusion
3DVT combined with ICG fluorescence imaging allows precise preoperative diagnosis, surgical planning and implementation, intraoperative detection of small liver cancers and precise navigation for HCC treatment, thereby helping to reduce postoperative complications and improve the disease-free survival rate of the patients.
Keywords: primary hepatocellular carcinoma; imaging, three-dimensional; indocyanine green; navigation; hepatectomy
原发性肝癌(PHC)是常见的恶性肿瘤,根治性手术为首选的治疗方式[1]。如何早期发现诊断肿瘤、精确定位肿瘤及边界、发现微小病灶、最大限度保留正常肝脏组织,从细胞功能水平进行导航根治性切除术,是肝脏外科医师追求的目标。自Ishizawa等[2]于2009年首次使用ICG分子荧光影像导航肝切除以来,该技术在肝胆胰肿瘤诊断与手术中得到广泛应用。Gotoh等[3]报道ICG可用于术中侦测微小肝癌病灶;Nishino等[4]将ICG应用于肝切除术边界界定的实时导航;Terasawa等[5]报道了ICG荧光影像技术在腹腔镜肝切除术中的应用疗效,但是既往研究多侧重于ICG在肝切除术术中的应用疗效,针对ICG荧光影像导航肝切除术预后情况及术后无瘤生存率的研究报道仍较少,且未见国内外文献报道解剖性、功能性、根治性肝切除术。
本团队在前期已将三维可视化技术应用于肝脏肿瘤的术前手术评估及术中导航,使得PHC患者的手术过程更为精确、安全、有效[6-9]。在此基础上我们将基于解剖形态学的三维可视化技术和分子功能诊断的ICG荧光影像技术相结合,应用于PHC的外科诊治,以期达到解剖性、功能性、根治性肝切除术,从而改善患者预后,提高术后无瘤生存率。本文总结分析联合应用两种技术的PHC患者临床资料,评价两者结合在原发性肝癌诊治中的应用价值。
1. 资料和方法
纳入标准:术前影像学检査发现肝脏占位性病变;术前肝功能Child-Pugh分级标准为A级或B级;无肝外远处转移;临床病例资料完整。排除标准:不能耐受手术的各种基础疾病者(如严重的心肺功能不全、肾功能不全、恶液质及血液系统疾病等);既往对ICG过敏或对碘过敏者。
1.1. 一般资料
回顾性分析2016年1月~2018年11月南方医科大学珠江医院肝胆外科收治的154例患者临床资料,分为实验组57例,行三维可视化评估、ICG荧光影像研究和肝切除手术;对照组97例,行常规肝切除手术。两组患者一般资料见表 1。本研究通过我院伦理委员会审批,批号为2014-GDYK-008。患者及家属术前均签署手术知情同意书,符合医疗护理常规操作规范。
1.
两组患者肝切除术前一般资料的比较
Comparison of general data before liver resection between the two groups
| Variables | Experimental group (n=57) | Control group (n=97) | Statistics | P |
| Age, year (x±s) | 50.16+11.38 | 50.05+12.51 | t=0.053 | 0.958 |
| Gender (Male/Female) | 43/14 | 79/18 | χ2=0.786 | 0.414 |
| HBsAg (+/-) | 42/15 | 59/38 | χ2=2.630 | 0.117 |
| Liver function Child-pugh grade (A/B) | 56/1 | 93/4 | χ2=0.642 | 0.652 |
| Preoperative AFP, ng/mL | ||||
| < 400 ng/mL | 45 | 69 | χ2=1.140 | 0.343 |
| > 400 ng/mL | 12 | 28 | ||
| Surgical procedures | ||||
| Laparoscopic surgery | 17 | 36 | χ2=0.845 | 0.835 |
| Laparotomy surgery | 40 | 61 |
1.2. 研究方法
将患者薄层CT数据导入腹部医学图像三维可视化系统(MI-3DVS,软件著作权号No:2008SR18798)进行三维重建,具体参数详见指南[10-12],根据三维可视化分析制定个体化手术方案及术前规划。手术前24 h静脉注射ICG,剂量为0.05~0.1 mg/kg,对有肝硬化患者提前至术前48~72 h[13]。术中充分游离肝脏后使用ICG分子荧光摄像头在适当距离扫描肝脏及其他腹腔脏器,侦测病灶及定位;对于行解剖性肝切除的患者,在目标肝段血管游离控制后使用正显法(门静脉)或反显法(外周静脉)注射ICG,侦测肝脏染色、肿瘤边界、左右半肝界线、卫星灶和肝脏其他部分微小癌灶;肝切除术后对剩余肝脏进行荧光侦测,检测有无残留病灶,肝断面有无胆漏侦测。
1.3. 观察指标
(1)术前CT、MRI影像学发现肿瘤数目;(2)术前三维可视化重建情况,发现血管变异数目;(3)术中荧光显像发现病灶情况;(4)实验组与对照组临床疗效对比分析:两组患者基线资料的对比分析,两组术中资料包括手术时间对比分析,两组术后资料包括术后1、3、5 d血常规、肝功能(POD1,3,5)、术后并发症(根据Clavien-Dindo并发症分级)发生率、术后24月的肿瘤复发率进行对比分析。血常规结果包括血红蛋白(Hb)及血小板(Plt)计数,肝功能结果包含谷丙转氨酶(ALT)、谷草转氨酶(AST)、总胆红素(Tbil)及白蛋白(Alb)指标;(5)随访情况:采用门诊、住院、网络和电话方式进行随访,记录患者术后肿瘤复发和生存情况。随访时间截至2018年12月。
1.4. 统计学方法
采用SPSS 22.0版本统计软件进行分析,计量资料表示:均数±标准差,服从正态分布的计量资料比较采用独立样本t检验;非正态分布的计量资料以M(QR)表示,采用Wilcoxon秩和检验比较,计数资料采用卡方检验或Fisher精确检验统计分析;术后肿瘤复发率和生存情况采用Kaplan-Meier法进行生存分析。双侧P < 0.05视为差异有统计学意义。
2. 结果
2.1. 实验组与对照组临床资料比较分析:
实验组与对照组间的基线资料比较分析详见表 1。两组的一般资料包括年龄、性别、肝炎病史、肝功能Child-pugh分级的差异性无明显统计学意义(P=0.652);两组术前AFP值比较,差异无明显统计学意义(P=0.343);实验组HBsAg(+)42例,对照组59例,两组HBsAg(+)患者比较,差异性无明显统计学意义(P=0.117);实验组腹腔镜手术17例,开腹手术40例;对照组有腹腔镜手术36例,开腹手术61例,两组的手术方式比较差异无明显统计学意义(P=0.835);两组的基线资料比较差异无明显统计学意义。
2.2. CT、MRI、ICG分子荧光侦测肿瘤比较
实验组患者术前成功完成增强CT及GD-EOBDTPA增强MRI检查,术中使用ICG荧光进行病灶侦测及导航切除。实验组57例患者中,术前增强CT共发现病灶63个;GD-EOB-DTPA增强MRI共发现病灶70个;术中ICG分子荧光共发现病灶80个,其中2个病灶由于距离肝脏表面>l cm,肉眼观察、扪诊和ICG分子荧光均未能发现,术中超声定位后,切开肝脏实质组织,ICG分子荧光影像识别。与术前增强CT结果相比,术前GD-EOB-DTPA增强MRI新发现病灶7个;术中ICG荧光新发现病灶17个,术后病理诊断肝细胞肝癌10个(58.8%)、肝硬化结节7个(41.1%)(表 2)。
2.
CT、MRI、ICG分子荧光侦测肿瘤情况
Detection of tumors by CT, MRI and ICG fluorescence imaging
| Factors | CT | Gd-EOB-DTPA-enhanced MRI | ICG fluorescence imaging |
| Number of tumors(n) | 63 | 70 | 80 |
| Pathology reports | |||
| Hepatocellular carcnoma | 52 | 56 | 62 |
| Cirrhosis nodules | 1l | 14 | 18 |
2.3. 三维可视化重建情况及血管变异情况[12]
57例患者均成功建立三维可视化模型。通过对每例三维模型进行拆分、移动、旋转,发现有25例患者存在肝内脉管走形变异,4例患者同时存在两种血管变异,其中肝动脉变异4例均为肝总动脉起源于肠系膜上动脉;门静脉变异9例,三维可视化门静脉Ⅰ型变异3例,三维可视化门静脉Ⅱ型变异6例;肝静脉变异16例,7例肝左静脉与肝中静脉共干,9例存在粗大的肝右后下静脉(表 3)。
3.
三维可视化25例血管变异情况
Vascular variations identified by 3D visualization in 25 cases
| Category | Case(n) |
| Hepatic artery variation | 4 |
| 3D-Portal vein variation | 9 |
| Type Ⅰ/type Ⅱ | 3/6 |
| Hepatic vein variation | 16 |
| Thick inferior right hepatic veins/ co-trunk of left and middle hepatic veins |
9/7 |
2.4. 术中及术后疗效比较
实验组与对照组在手术时间、术中失血量、术后住院天数及术后并发症(Clavien-Dindo并发症分级)的发生率对比分析详见表 4。实验组的手术时间长于对照组(6.45±2.45 vs 4.63±1.56,P < 0.001),差异具有统计学意义;对照组术中失血量400(390)mL高于实验组300(250)mL(Z=2.291,P=0.022),差异具有统计学意义;两组的术后住院天数之间的差异性无明显统计学意义(P=0.527);两组术后Clavien-Dindo并发症分级发生率有统计学差异(P=0.001)。
4.
实验组与对照组临床疗效分析比较
Comparison of the perioperative data of the patients between the two groups
| Variables | Experimental group (n=57) | Control group (n=97) | Statistics | P |
| Operative time, hour (x±s) | 6.45±2.45 | 4.63±1.56 | t=5.025 | < 0.001 |
| intraoperative blood loss, mL M (QR) | 300 (250) | 400 (390) | Z=2.291 | 0.022 |
| Postoperative length of hospitalization, d (x±s) | 12.25±4.69 | 11.76±4.48 | t=0.635 | 0.527 |
| Clavien-Dindo classification | 12 (21%) | 47 (48.4%) | χ2=11.406 | 0.001 |
| Grade Ⅰ, n(%) | 6(10.5%) | 7(7.2%) | χ2=0.509 | 0.553 |
| Grade Ⅱ, n (%) | 5 (8.7%) | 36 (37.1%) | χ2=14.377 | < 0.001 |
| Grade Ⅲ, n (%) | 1 (1.7%) | 4(4.1%) | χ2=0.642 | 0.652 |
| Grade Ⅳ, n (%) | 0 (0%) | 0 (0%) | N/A | N/A |
| Grade Ⅴ, n (%) | 0 (0%) | 0 (0%) | N/A | N/A |
实验组与对照组两组术后1、3、5 d临床指标比较详见图 1,提示两组患者肝功能(包括ALT、AST、Tbil)差异有统计学意义;术后1、3、5 d血常规(包括Hb及Plt)的差异性无明显统计学意义(P>0.05)。
1.
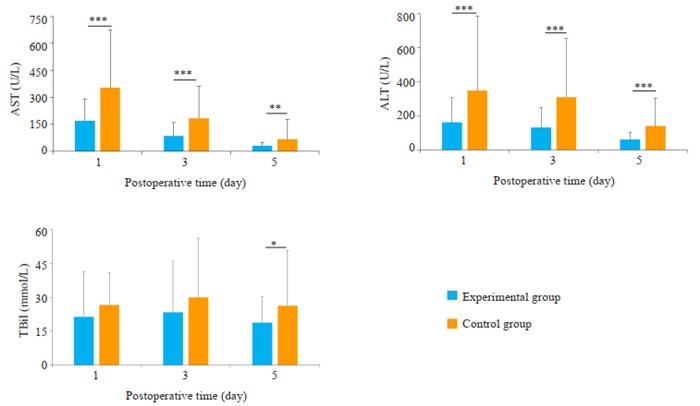
两组患者术后第1、3、5天ALT,AST,Tbil比较
Comparison of ALT, AST, Tbil on POD 1, 3, 5 between the two groups. *P < 0.05 vs Control group; **P < 0.01 vs Control group; ***P < 0.001 vs Control group.
实验组与对照组术后24月的肿瘤复发率比较分析详见图 2,实验组的总体无瘤生存率为74.9%,对照组总体无瘤生存率为28.9%,两组间的差异性具有统计学意义(P=0.022)。
2.
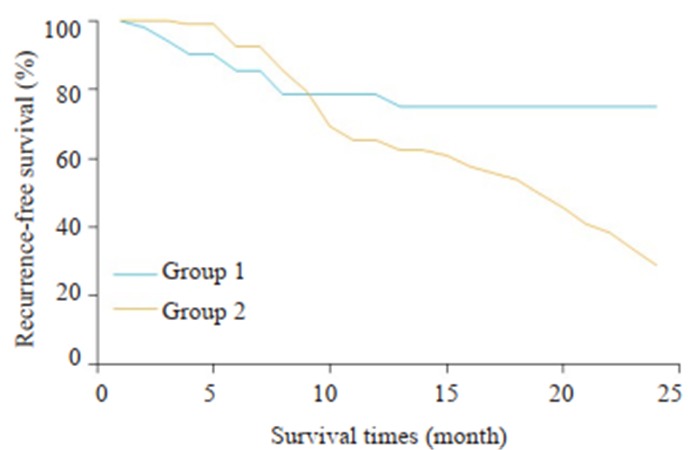
两组术后24月生存曲线比较
Comparison of survival curves at 24 months after the surgery between the two groups. 1: 3DVT group; 2: Control group.
3. 讨论
3.1. ICG荧光影像在PHC的应用
3.1.1. ICG荧光影像在肝断面边缘残余肿瘤、微小病灶的侦测
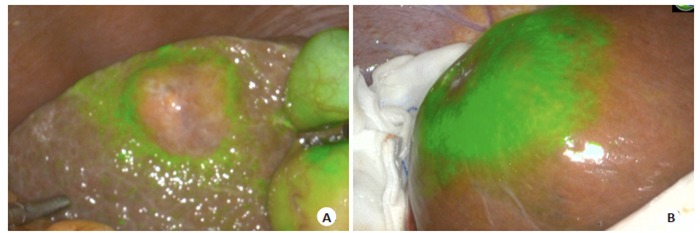
目前临床上肝胆外科医师术前多通过CT、MRI检查识别微小肝癌病灶[14],结合术中超声进行肿瘤定位。但对小于1 cm的微小肝癌,特别是伴有肝硬化的患者,侦测微小肝癌病灶的灵敏度较低,且术中定位不确切。因此,对于肝脏微小肝癌或切缘残留病灶的检测仍然是常规检测方法的盲点[15],ICG对于小肝癌和卫星病灶的识别有很高的灵敏度,能侦测术前CT、MRI所未发现的微小癌灶[16-17]。图 3为实验组术中ICG所侦测发现的荧光结节,予以切除后送病理,结果显示图 3A、B、D为肝细胞癌,图 3C为肝硬化结节。由于肝硬化结节、肝组织炎性改变等良性病变会导致肝组织内微胆管结构紊乱或缺失,ICG分子排出障碍,假阳性率较高。本组术中ICG侦测新发现的17个病灶中,病理诊断10个为癌组织,7个为肝硬化结节,假阳性率为41.1%。因此尽管存在较高的假阳性率,但ICG术中侦测微小癌灶对提高术后无瘤生存,实现肝癌根治性切除仍有重要意义[18-19]。
3.

ICG荧光影像术中侦测微小肝癌及残留肿瘤
Intraoperative detection of small hepatocellular carcinoma and residual tumors by ICG fluorescence imaging. A: ICG fluorescence imaging detects the demarcation of the massive hepatocellular carcinoma on the left lobe of the liver. On the right side, a fluorescence signal is detected in segment 8, and confirmed by intraoperative rapid pathology as hepatocellular carcinoma; B: ICG fluorescence imaging detects the tumor demarcation. Intraoperative detection and fluorescence signal are shown on the right side, and intraoperative rapid pathology confirmed hepatocellular carcinoma; C: ICG fluorescence imaging detects the fluorescence signal on the liver surface, and intraoperative rapid pathology confirmed the hepatic cirrhosis nodules; D: Intraoperative ICG detects fluorescence aggregation on the liver section, and the pathological result confirmed hepatocellular carcinoma. No fluorescence aggregation was detected after resection.
3.1.2. 术中界定肿瘤边界
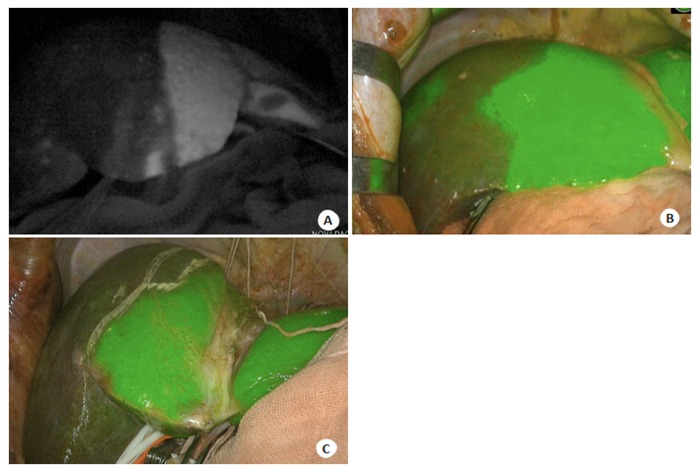
临床上肝胆外科医师通常通过术中肉眼观察和触诊来判断有无残余肿瘤。切除范围过小,不能达到R0切除;切除范围过大,增加血管损伤及术后肝功能衰竭的风险。对于可切除的肝癌患者,传统观点认为最理想的切除范围是距离肿瘤1.5~2.0 cm,但对于巨块型肝癌,考虑残肝体积和术后肝功能,难以达到此要求[20]。ICG荧光影像中可清楚界定肿瘤边界,三维可视化定位肿瘤与大血管关系,结合术中快速病理诊断,在确保切缘阴性前提下,最大限度保留更多残肝组织。ICG荧光可反映活体状态下生物体内细胞分子水平的病变状态,因此实现了细胞功能层面的边界界定。如低分化肝癌组织细胞摄取ICG功能低下,但由于对周围肝脏组织的压迫,导致排泄障碍,使得呈现环形荧光信号,高分化肝癌组织对ICG仍有一定的摄取能力,但其胆道排泄功能异常,因而可较长时间显示荧光,表现为全荧光型信号(图 4)。与其他成像技术相比,可以在术中精确定位肿瘤组织,客观显示肿瘤边界信息[21],从而实现解剖性、功能性、根治性肝切除术。
4.
ICG荧光影像显示肿瘤边界
ICG fluorescence imaging showed tumor demarcation. A: Intraoperative ICG fluorescence imaging revealed that the tumor is projected onto the surface of the liver. The surrounding liver tissue shows circular fluorescence due to tumor compression; B: Intraoperative ICG fluorescence imaging shows all fluorescent modules of the tumors, which were confirmed by frozen pathology as well-differentiated hepatocellular carcinoma.
3.1.3. 左右半肝边界界定
传统理论认为,左半肝切除线为肝上下腔静脉左缘到胆囊底部,右半肝切除线为肝上下腔静脉右缘到胆囊底部。本研究实验组中,ICG荧光显示左右半肝界线呈多形状(图 5A直线;B:驼峰状;C:不规则状)。通过ICG荧光染色能够裸眼看到个体化的左右半肝界线,对比传统定义的直线分界线,沿ICG染色修正的左右半肝预切线进行肝切除能够保留更多的有效剩余肝体积,这能够有效降低术后肝功能衰竭风险。在本组行解剖性肝切除术病人中,应用ICG进行目标肝段染色,术中进行动态立体观察,根据肝实质的荧光界线对肝切除的范围作出相应的调整及纠正,实时导航肝切除术,术后无发生肝功能衰竭及围手术期死亡。
5.
ICG荧光显示左右半肝分界线
ICG fluorescent images showed the lines dividing left and right lobes of the liver. A: The straight line; B: The hump-shaped line; C: The irregular line.
3.2. ICG荧光影像技术提高术后无瘤生存率
术中结合ICG荧光影像技术实时导航修正肝预切线,可提高手术的安全性及最大限度保留正常肝脏组织,提高术后无瘤生存率,降低术后并发症发生[11, 22]。有Meta分析研究报道将ICG分子荧光影像应用于肝脏肿瘤的精准诊疗,可有效降低输血率,术后并发症发生率及提高切缘阴性率[23]。ICG荧光影像能够发现术前CT、MRI等影像学所没发现的微小癌灶,如本研究中ICG相较于CT所新发现的17个结节中有10个术后病理证实为肝细胞癌,这对于提高肝切除术后患者的预后有较好的效果。肝癌根治性切除后,术后高复发率仍是影响远期生存率的重要因素。PHC的早期复发定义为切除后2年内(≤2)肿瘤再发[24]。与对照组相比,实验组术后24月的无瘤生存率(69.4%),高于对照组总体无瘤生存率(28.9%)及文献报道的40.1%~56.9%[25-26]。此外,实验组肝切除术后Ⅲ级以上并发症为1.7%,也低于国外文献同类研究报道的14.9%~17.4%[4, 27]。
三维可视化技术实现了PHC术前精确形态学诊断[28],ICG荧光影像技术实现了术中微小癌灶侦测、肿瘤边界界定、导航手术,提高手术精度[29-30],二者联合真正实现了解剖性、功能性、根治性肝切除术,能够有效降低手术并发症,提高术后无瘤生存率。ICG分子荧光成像仅局限于肝脏的表浅部位,而且存在较高的假阳性率,有待进一步研究。
Biography
曾思略,硕士,E-mail: 1130186845@qq.com
Funding Statement
“十三五”国家重点研发计划数字诊疗装备研发重点专项(2016YFC0106500);国家自然科学基金重大科研仪器研制项目(81627805);NSFC-广东联合基金项目(U1401254);广东省科技计划项目(2017ZC0110);广州市科技计划项目(201604020144)
Supported by National Key R&D Program (2016YFC0106500) and Major Instrument Project of National Natural Science Fund (81627805)
Contributor Information
曾 思略 (Silue ZENG), Email: 1130186845@qq.com.
方 驰华 (Chihua FANG), Email: fangch_dr@163.com.
References
- 1.中华人民共和国卫生和计划生育委员会医政医管局 原发性肝癌诊疗规范(2017年版) http://d.old.wanfangdata.com.cn/Periodical/crbxx201703001. 中华消化外科杂志. 2017;16(7):635–47. [中华人民共和国卫生和计划生育委员会医政医管局.原发性肝癌诊疗规范(2017年版)[J].中华消化外科杂志, 2017, 16(7):635-47.] [Google Scholar]
- 2.Ishizawa T, Fukushima N, Shibahara JA, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d6fdd8dc523709a82b6ca33fb477e61d. Cancer. 2009;115(11):2491–504. doi: 10.1002/cncr.24291. [Ishizawa T, Fukushima N, Shibahara JA, et al.Real-time identification of liver cancers by using indocyanine green fluorescent imaging[J].Cancer, 2009, 115(11):2491-504.] [DOI] [PubMed] [Google Scholar]
- 3.Gotoh K, Yamada T, Ishikawa O, et al. HOW I DO IT A novel Image-Guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100(1):75–9. doi: 10.1002/jso.21272. [Gotoh K, Yamada T, Ishikawa O, et al.HOW I DO IT A novel Image-Guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation[J].J Surg Oncol, 2009, 100(1):75-9.] [DOI] [PubMed] [Google Scholar]
- 4.Nishino H, Hatano E, Seo S, et al. Real-time navigation for liver surgery using projection mapping with indocyanine green fluorescence development of the novel medical imaging projection system. Ann Surg. 2018;267(6):1134–40. doi: 10.1097/SLA.0000000000002172. [Nishino H, Hatano E, Seo S, et al.Real-time navigation for liver surgery using projection mapping with indocyanine green fluorescence development of the novel medical imaging projection system[J].Ann Surg, 2018, 267(6):1134-40.] [DOI] [PubMed] [Google Scholar]
- 5.Terasawa M, Ishizawa T, Mise Y, et al. Applications of fusionfluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31(12):5111–8. doi: 10.1007/s00464-017-5576-z. [Terasawa M, Ishizawa T, Mise Y, et al.Applications of fusionfluorescence imaging using indocyanine green in laparoscopic hepatectomy[J].Surg Endosc, 2017, 31(12):5111-8.] [DOI] [PubMed] [Google Scholar]
- 6.Fang CH, Zhang P, Qi XL. Digital and intelligent liver surgery in the new era:Prospects and dilemmas. EBioMedicine. 2019;41:693–701. doi: 10.1016/j.ebiom.2019.02.017. [Fang CH, Zhang P, Qi XL.Digital and intelligent liver surgery in the new era:Prospects and dilemmas[J].EBioMedicine, 2019, 41:693-701.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu M, Hu HY, Cai W, et al. The safety and feasibility of threedimensional visualization technology assisted right posterior lobe allied with part of Ⅴ and Ⅷ sectionectomy for right hepatic malignancy therapy. http://www.ncbi.nlm.nih.gov/pubmed/29172950. J Laparoendosc Adv Surg Tech A. 2018;28(5):586–94. doi: 10.1089/lap.2017.0479. [Hu M, Hu HY, Cai W, et al.The safety and feasibility of threedimensional visualization technology assisted right posterior lobe allied with part of Ⅴ and Ⅷ sectionectomy for right hepatic malignancy therapy[J].J Laparoendosc Adv Surg Tech A, 2018, 28(5):586-94.] [DOI] [PubMed] [Google Scholar]
- 8.方 驰华, 刘 星星, 范 应方, et al. 3D技术在复杂性肝切除术中的安全性评价. http://www.j-smu.com/oa/pdfdow.aspx?Sid=2012081116. 南方医科大学学报. 2012;32(8):1116–21. [方驰华, 刘星星, 范应方, 等.3D技术在复杂性肝切除术中的安全性评价[J].南方医科大学学报, 2012, 32(8):1116-21.] [PubMed] [Google Scholar]
- 9.方 驰华, 张 鹏, 刘 允怡, et al. 肝胆胰疾病数字智能化诊疗核心技术, 体系构建及其应用. 中华外科杂志. 2019;57(4):253–7. doi: 10.3760/cma.j.issn.0529-5815.2019.04.003. [方驰华, 张鹏, 刘允怡, 等.肝胆胰疾病数字智能化诊疗核心技术, 体系构建及其应用[J].中华外科杂志, 2019, 57(4):253-7.] [DOI] [Google Scholar]
- 10.Fang CH, Tao HS, Yang J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=83616bdc247bb2c913a290f4832dc44c. J Am Coll Surg. 2015;220(1):28–37. doi: 10.1016/j.jamcollsurg.2014.09.023. [Fang CH, Tao HS, Yang J, et al.Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma[J].J Am Coll Surg, 2015, 220(1):28-37.] [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Tao HS, Cai W, et al. Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. J Surg Oncol. 2018;118(7):1081–7. doi: 10.1002/jso.25258. [Yang J, Tao HS, Cai W, et al.Accuracy of actual resected liver volume in anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging[J].J Surg Oncol, 2018, 118(7):1081-7.] [DOI] [PubMed] [Google Scholar]
- 12.中华医学会数字医学分会, 中国医师协会肝癌专业委员会, 中国医师协会临床精准医学专业委员会, et al. 复杂性肝脏肿瘤三维可视化精准诊治指南(2019版) http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsywkzz201908002. 中国实用外科杂志. 2019;39(8):766–74. [中华医学会数字医学分会, 中国医师协会肝癌专业委员会, 中国医师协会临床精准医学专业委员会, 等.复杂性肝脏肿瘤三维可视化精准诊治指南(2019版)[J].中国实用外科杂志, 2019, 39(8):766-74.] [Google Scholar]
- 13.中华医学会数字医学分会, 中国研究型医院学会数字智能化外科专业委员会, 中国医师协会肝癌专业委员会, et al. 计算机辅助联合ICG分子荧光影像技术在肝脏肿瘤诊断和手术导航中应用指南(2019版) http://www.cnki.com.cn/Article/CJFDTotal-ZGWK201907001.htm. 中国实用外科杂志. 2019;39(7):641–50. [中华医学会数字医学分会, 中国研究型医院学会数字智能化外科专业委员会, 中国医师协会肝癌专业委员会, 等.计算机辅助联合ICG分子荧光影像技术在肝脏肿瘤诊断和手术导航中应用指南(2019版)[J].中国实用外科杂志, 2019, 39(7):641-50.] [Google Scholar]
- 14.International Consensus Group for Hepatocellular neoplasia the international consensus group for hepatocellular neoplasia. Pathologic diagnosis of early hepatocellular carcinoma:a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49(2):658–64. doi: 10.1002/hep.22709. [International Consensus Group for Hepatocellular neoplasia the international consensus group for hepatocellular neoplasia.Pathologic diagnosis of early hepatocellular carcinoma:a report of the international consensus group for hepatocellular neoplasia[J].Hepatology, 2009, 49(2):658-64.] [DOI] [PubMed] [Google Scholar]
- 15.Fomer A, Llovet JM, Bruix J. Hepatocellular carcinoma. http://d.old.wanfangdata.com.cn/Periodical/zhsywk201401026. Lancet. 2012;379(9822):1245–55. doi: 10.1016/S0140-6736(11)61347-0. [Fomer A, Llovet JM, Bruix J.Hepatocellular carcinoma[J].Lancet, 2012, 379(9822):1245-55.] [DOI] [PubMed] [Google Scholar]
- 16.Zhang YM, Shi R, Hou JC, et al. Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0c6a09ecf50132d79867b5048d7b5230. J Cancer Res Clin Oncol. 2017;143(1):51–8. doi: 10.1007/s00432-016-2267-4. [Zhang YM, Shi R, Hou JC, et al.Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging[J].J Cancer Res Clin Oncol, 2017, 143(1):51-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.方 驰华, 梁 洪玻, 迟 崇巍, et al. 吲哚氰绿介导的近红外光技术在微小肝脏肿瘤识别, 切缘界定和精准手术导航的应用. http://d.old.wanfangdata.com.cn/Periodical/zhwk201606012. 中华外科杂志. 2016;54(6):444–50. [方驰华, 梁洪玻, 迟崇巍, 等.吲哚氰绿介导的近红外光技术在微小肝脏肿瘤识别, 切缘界定和精准手术导航的应用[J].中华外科杂志, 2016, 54(6):444-50.] [Google Scholar]
- 18.Masuda K, Kaneko J, Kawaguchi Y, et al. Diagnostic accuracy of indocyanine green fluorescence imaging and multidetector row computed tomography for identifying hepatocellular carcinoma with liver explant correlation. Hepatol Res. 2017;47(12):1299–307. doi: 10.1111/hepr.12870. [Masuda K, Kaneko J, Kawaguchi Y, et al.Diagnostic accuracy of indocyanine green fluorescence imaging and multidetector row computed tomography for identifying hepatocellular carcinoma with liver explant correlation[J].Hepatol Res, 2017, 47(12):1299-307.] [DOI] [PubMed] [Google Scholar]
- 19.Li CX, Li RT, Zhang W. Progress in non-invasive detection of liver fibrosis. http://d.old.wanfangdata.com.cn/Periodical/zgzllc-e201802003. Cancer Biol Med. 2018;15(2):124–36. doi: 10.20892/j.issn.2095-3941.2018.0018. [Li CX, Li RT, Zhang W.Progress in non-invasive detection of liver fibrosis[J].Cancer Biol Med, 2018, 15(2):124-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki K, Shindoh J, Margonis GA, et al. Effect of background liver cirrhosis on outcomes of hepatectomy for hepatocellular carcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7e8315ac69bf436d00977c90e85a34de. JAMA Surg. 2017;152(3):e165059. doi: 10.1001/jamasurg.2016.5059. [Sasaki K, Shindoh J, Margonis GA, et al.Effect of background liver cirrhosis on outcomes of hepatectomy for hepatocellular carcinoma[J].JAMA Surg, 2017, 152(3):e165059.] [DOI] [PubMed] [Google Scholar]
- 21.张 雅敏, 侯 建存, 史 源, et al. 吲哚菁绿荧光显像技术在术中确定肝脏肿瘤边界的应用. http://d.old.wanfangdata.com.cn/Periodical/zhgdwk201607016. 中华肝胆外科杂志. 2016;22(7):487–8. [张雅敏, 侯建存, 史源, 等.吲哚菁绿荧光显像技术在术中确定肝脏肿瘤边界的应用[J].中华肝胆外科杂志, 2016, 22(7):487-8.] [Google Scholar]
- 22.Kobayashi Y, Kawaguchi Y, Kobayashi K, et al. Portal vein territory identification using indocyanine green fluorescence imaging:Technical details and short-term outcomes. J Surg Oncol. 2017;116(7):921–31. doi: 10.1002/jso.24752. [Kobayashi Y, Kawaguchi Y, Kobayashi K, et al.Portal vein territory identification using indocyanine green fluorescence imaging:Technical details and short-term outcomes[J].J Surg Oncol, 2017, 116(7):921-31.] [DOI] [PubMed] [Google Scholar]
- 23.张 玮琪, 卓 嘉明, 方 驰华. ICG分子荧光影像技术用于肝脏肿瘤手术安全性和有效性Meta分析. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgsywkzz201907023. 中国实用外科杂志. 2019;39(7):729–34. [张玮琪, 卓嘉明, 方驰华.ICG分子荧光影像技术用于肝脏肿瘤手术安全性和有效性Meta分析[J].中国实用外科杂志, 2019, 39(7):729-34.] [Google Scholar]
- 24.Vilarinho S, Calvisi DF. New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/hep.27311. Hepatology. 2014;60(6):1812–4. doi: 10.1002/hep.27311. [Vilarinho S, Calvisi DF.New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment[J].Hepatology, 2014, 60(6):1812-4.] [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Han J, Xing H, et al. Sex difference in recurrence and survival after liver resection for hepatocellular carcinoma:A multicenter study. Surgery. 2019;165(3):516–24. doi: 10.1016/j.surg.2018.08.031. [Zhang H, Han J, Xing H, et al.Sex difference in recurrence and survival after liver resection for hepatocellular carcinoma:A multicenter study[J].Surgery, 2019, 165(3):516-24.] [DOI] [PubMed] [Google Scholar]
- 26.Li SQ, Huang T, Shen SL, et al. Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=186086f14b39b3ccc80ee6cb56d9ad32. Br J Surg. 2017;104(1):118–27. doi: 10.1002/bjs.10311. [Li SQ, Huang T, Shen SL, et al.Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria[J].Br J Surg, 2017, 104(1):118-27.] [DOI] [PubMed] [Google Scholar]
- 27.Handgraaf H, Boogerd L, Hoppener D, et al. Long-term follow-up after near-infraied fluorescence-guided resection of colorectal liver metastases:A retrospective multicenter analysis. Eur J Surg Oncol. 2017;43(8):1463–71. doi: 10.1016/j.ejso.2017.04.016. [Handgraaf H, Boogerd L, Hoppener D, et al.Long-term follow-up after near-infraied fluorescence-guided resection of colorectal liver metastases:A retrospective multicenter analysis[J].Eur J Surg Oncol, 2017, 43(8):1463-71.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.方 驰华, 陈 康, 张 鹏. 智能化诊疗技术在普通外科中应用现状及前景. http://d.old.wanfangdata.com.cn/Periodical/zhwk201901001. 中华外科杂志. 2019;57(1):1–5. [方驰华, 陈康, 张鹏.智能化诊疗技术在普通外科中应用现状及前景[J].中华外科杂志, 2019, 57(1):1-5.] [Google Scholar]
- 29.Koch M, Ntziachristos V. Advancing surgical vision with fluorescence imaging. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a8140f07fa923b9f1afc9d45604af0f9. Annu Rev Med. 2016;(67):153–64. doi: 10.1146/annurev-med-051914-022043. [Koch M, Ntziachristos V.Advancing surgical vision with fluorescence imaging[J].Annu Rev Med, 2016(67):153-64.] [DOI] [PubMed] [Google Scholar]
- 30.张 树庚, 刘 连新. 吲哚菁绿荧光融合影像引导技术在腹腔镜肝切除中的应用及展望. http://d.old.wanfangdata.com.cn/Periodical/zhgdwk201902014. 中华肝胆外科杂志. 2019;25(2):129–31. [张树庚, 刘连新.吲哚菁绿荧光融合影像引导技术在腹腔镜肝切除中的应用及展望[J].中华肝胆外科杂志, 2019, 25(2):129-31.] [Google Scholar]


