Abstract
Inhalation of the toxic smoke produced by combusting tobacco products, primarily cigarettes, is the overwhelming cause of tobacco-related disease and death in the U.S. and globally. A diverse class of alternative nicotine delivery systems (ANDS) has recently been developed that do not combust tobacco and are substantially less harmful than cigarettes. ANDS have the potential to disrupt the 120-year dominance of the cigarette and challenge the field on how the tobacco pandemic could be reversed if nicotine is decoupled from lethal inhaled smoke. ANDS may provide a means to compete with, and even replace combusted cigarette use, saving more lives more rapidly than previously possible. Based on the scientific evidence on ANDS, we explore benefits and harms to public health to guide practice, policy, and regulation. A re-framing of nicotine’s use in society through the lens of harm minimization can enhance the impact of tobacco control efforts.
Keywords: Harm minimization, nicotine, e-cigarettes, smoking, tobacco
1. INTRODUCTION
The 50th Anniversary United States (U.S.) Surgeon General’s Report of 2014 concluded: “The burden of death and disease from tobacco use in the U.S. is overwhelmingly caused by cigarettes and other combusted tobacco products; rapid elimination of their use will dramatically reduce this burden” (p. 7) (102). Despite declines over the last 50 years, about 520,000 Americans annually die prematurely from smoking-related causes (101; 102). The Surgeon General concluded, “The current rate of progress in tobacco control is not fast enough. More needs to be done.” (p. 875) (102). It is imperative to find additional ways to accelerate the decline in smoking in the U.S. and globally, because, if nothing changes, a billion lives will be prematurely lost by 2100 (121). It is past time to add new and even “radical” approaches (114).
The term “alternative nicotine delivery systems” (ANDS) encompasses a diverse class of products, primarily exemplified by e-cigarettes that are “vaped,” not smoked. ANDS raises fundamental questions for society, namely: Could ANDS be leveraged to effectively compete with cigarettes, eventually making smoking obsolete sooner than would otherwise be possible (2; 25; 49)? Can many types of alternative modes of nicotine delivery, when decoupled from deadly toxins in combusted tobacco smoke, be accepted by the public and by its health, regulatory and advocacy bodies as an extraordinary opportunity to save lives rather than as a threat to the success of past tobacco control efforts? These questions are contentious and their answers are complicated. Addressing opportunities for ANDS requires re-examination of the role nicotine plays in sustaining smoking, and the role nicotine can play in reducing smoking when delivered in a safer, yet appealing manner (33; 66; 73).
Re-examination of nicotine’s role in society requires re-considering the harm minimization perspective within tobacco control (10). The primary goal of harm minimization is preventing the use of nicotine containing products among non-users, while pragmatically acknowledging that less harmful products can reduce risk for those who smoke any combusted form of tobacco product. As such, harm minimization is wholly consistent with tobacco control goals to prevent any use by underage youth and encourage complete smoking cessation in both youth and adults. While the term “harm reduction” implies any reduction in relative harm from a prior level, “harm minimization” strives to reduce harms to zero (i.e. no use and thus no exposure). Harm minimization is also responsive to the 50th anniversary Surgeon General’s admonition that more must be done now to eliminate the preventable deaths overwhelmingly caused by the smoking of combusted tobacco (102).
We suggest a fundamental science-based reframing of nicotine use to inform current and future U.S. and global tobacco control strategy. We often will use e-cigarettes as case examples of ANDS, but emerging types of ANDS (e.g., heat not burn tobacco, nicotine salts) (88; 98) and new scientific evidence will continue to force discussions about nicotine’s role in society. At times, ANDS may also encompass examples of products that deliver nicotine in less harmful alternative modalities than smoking (i.e., nicotine replacement therapy (NRT), low nitrosamine Swedish snus, or other smokeless tobacco) (26; 33; 36; 50; 52; 57).
The changing product landscape of existing reduced harm products and emerging ones calls for updating tobacco control strategies, some of which will continue to be effective while others may become ineffective or possibly iatrogenic (49) if a strategy slows rather than speeds the demise of smoking (2; 66). Herein, we integrate current science and policy analysis to address the critical questions that underpin public health practice, policy, regulation, advocacy and communication on nicotine containing products (110).
2. UPDATING TOBACCO CONTROL AND NICOTINE USE
Decades of tobacco control interventions (e.g., age purchasing restrictions, taxation, media campaigns, cessation services) have significantly decreased smoking prevalence in the U.S. (17; 32; 48); these efforts to prevent youth smoking and encourage cessation must be continued and strengthened. Regulatory changes in the U.S. have given the tobacco control community additional tools. In 2009, the Tobacco Control Act (TCA) (104) gave the U.S. Food and Drug Administration (FDA) authority to regulate tobacco. The TCA includes a public health standard that requires regulators to consider the net impact of the products they regulate on the population as a whole (38; 102; 110). Adding to FDA’s prior role (via the Center for Drug Evaluation Research – CDER) of approving medicinal products (e.g., NRT) for smoking cessation, the FDA established the Center for Tobacco Products (CTP) to regulate the manufacture, distribution, and marketing of tobacco products based on scientific evidence (112).
While the CTP’s authorities emphasize preventing the marketing of products that could harm public health, the CTP can also promote public health by supporting behaviors and products that reduce net population harm (104; 110). The FDA, through public education, must ensure users of nicotine containing products are accurately informed about harms. Ideally, harms should be compared to both deadly smoking (relative risk) and to no use (absolute risk) (2; 49).
Both the emergence of new ANDS products and the TCA provide an opportunity to enrich tobacco control efforts. The following sections use e-cigarettes as the main case example of the individual and the population health potential of ANDS. E-cigarettes represent a diverse class of devices with a variety of names and product designs, but with the common characteristic of delivering nicotine without any tobacco in an aerosol (commonly called “vapor”). We examine a selected number of concerns regarding harm minimization in the context of ANDS and other harm minimizing alternatives to deadly smoking (26; 49; 89).
2.1. Decoupling Nicotine from Inhaled Smoke for Harm Minimization
The logic of tobacco harm minimization is simple and compelling. As Michael Russell, a pioneer in the field, put it, “People smoke for nicotine but they die from the tar” (90). In getting the nicotine they seek, smokers are exposed to enormous harm due to inhalation of tobacco combustion products, including cardiovascular disease, cancer, and pulmonary diseases (102). For most smokers, there is little evidence that nicotine itself causes any of these classes of disease when decoupled from smoke (see details in Niaura et al., 2016 (73)). Although nicotine use poses some risk for cardiovascular disease, it is dwarfed by the risk posed by smoking cigarettes (8; 25; 26; 73). Nicotine itself does not appear to cause cancer, even in former smokers who use low nitrosamine snus for decades (26; 50; 52; 56–58; 73). Smokers switching to vaping have experienced improved lung capacity and less frequent asthma (83–85). Evidence also indicates that nicotine itself is relatively safe when obtained from CDER-approved over-the-counter NRT (73), widely used for smoking cessation. At the doses smokers experience, nicotine itself carries minimal harm (36; 73). Thus, if smokers could be shifted from smoking to consuming “clean” nicotine (i.e., without the smoke), many lives would be saved (26). The safest course is to stop smoking or, better, never to start. But a harm minimization approach recognizes that demanding absolute perfection is often counter-productive and that, when a harmful behavior cannot be eliminated, it is necessary to reduce its adverse health consequences (42). For those who are smoking and are unwilling to quit nicotine use, moving to cleaner nicotine – including ANDS, e-cigarettes, NRTs or low nitrosamine snus, – would reduce harm relative to smoking.
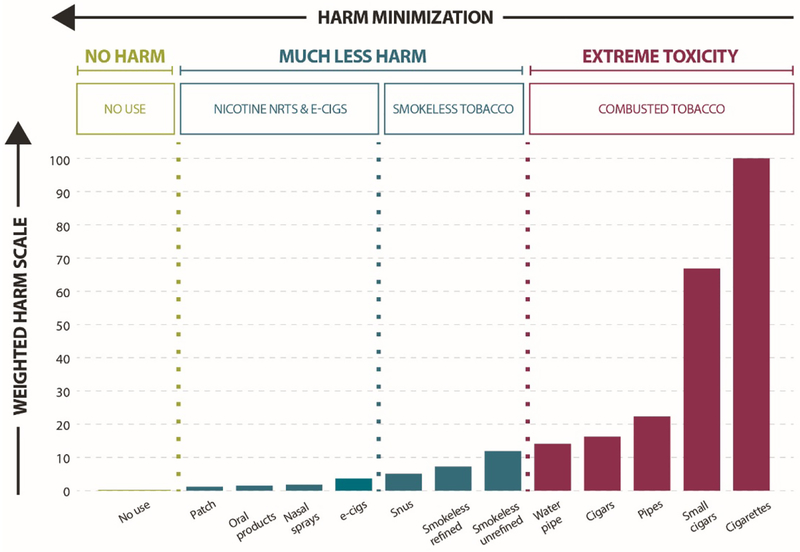
The harm minimization continuum (Figure 1) posits that all nicotine-containing products are not equally harmful and instead, range from exceptionally low harm (e.g., NRT) to exceptionally high harm (e.g., combusted cigarettes, cigars, hookah) (38–40; 43; 53; 73; 78; 89). Smokeless tobacco is also much lower on the risk continuum than cigarettes and varies in risk by type (e.g., low nitrosamine “Swedish” snus versus other smokeless products, some with much higher nitrosamine or other toxin levels than Swedish snus) (26).
Figure 1.
Products along the harm minimization continuum. Adapted from Nutt et al., 2014 (77).
2.2. ANDS and the Harm Continuum: How Harmful are E-cigarettes?
When nicotine is decoupled from the deadly toxins in inhaled smoke, it is substantially less harmful (8; 73; 89; 102). Most of the harm is due to the inhalation of combustion products (about 70 human carcinogens, other toxins, and carbon monoxide) (105). E-cigarette aerosol is very different. They do not contain any tobacco and do not produce carbon monoxide (89). The harm continuum (Figure 1) emphasizes a key point -- it is not that e-cigarettes are completely safe, or even the safest nicotine-containing product available, but that they are much safer than smoking. NRTs are safe enough that CDER approved them for consumer use over two decades ago, and long-term use of NRT has been endorsed as an acceptable strategy to reduce morbidity and mortality from smoking (20; 33; 34). CDER updated NRT labeling in 2013 to permit NRT use while smoking (also known as “dual use”) as part of the journey to cessation, and permits sustained use for relapse prevention for a lifetime if need be (36).
Most reviews of toxicological, clinical, and epidemiological evidence indicate the chemicals found in e-cigarettes, when used as intended, are far fewer and well below levels seen in cigarette smoke (8; 38; 39; 43; 73). According to the United Kingdom’s (U.K.) Royal College of Physicians summary of these studies: “the available data suggest that they are unlikely to exceed 5% of those associated with combusted tobacco products […]” (p. 87) (89). Among smokers who switched to e-cigarettes, studies have also documented improved physiological outcomes including reduced blood pressure, improved lung function, and lower disease symptoms (83–85). Data also indicate that e-cigarettes are much less dependence-producing than cigarettes (62; 94). Thus, the potential harm of e-cigarettes falls in the low range on the continuum. Harm levels do differ among e-cigarettes. Lab studies have documented some potentially toxic constituents in some devices, e-liquids, and flavors, especially when overheated to produce aldehydes (such as acrolein and formaldehyde) and an acrid “dry puff condition” unlikely to be tolerated by actual users (31). Nonetheless, prudent product standards can readily eliminate these unnecessary risks and ensure quality control over products and liquids (26).
2.3. Re-thinking Nicotine: A Three-Dimensional Framework for Harm Minimization
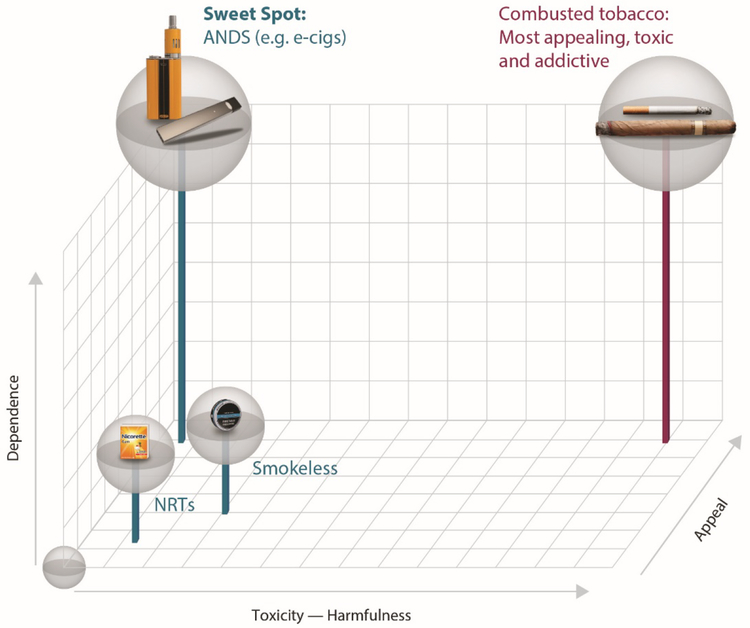
Nicotine and tobacco products can fit into a three-dimensional conceptual space (Figure 2): (1) harmfulness (x-axis), (2) appeal (z-axis) and (3) dependence (y-axis). Figure 2 provides a roadmap in which to envision how to optimize ANDS product use to successfully compete with and replace smoking - minimizing risk and making both an individual and a net population health impact.
Figure 2.
Multidimensional framework for nicotine containing products, considering (1) harmfulness, (2) appeal, and (3) dependence.
As already depicted in Figure 1 and described in section 2.1, nicotine products (ANDS, smokeless and NRTs) differ substantially from smoking in their toxicity and potential for harm (x-axis, Figure 2). Nicotine containing products also differ in their appeal and therefore their ability to displace smoking (z-axis, Figure 2), which contributes to the likelihood that the product will be adopted and its use sustained at a scale large enough to affect the population-level outcomes (21). Appeal is a complex function of attractiveness, sensory characteristics, and subjective satisfaction (including nicotine level, taste, and flavors) as well as cost, accessibility, and marketing practices (26; 29; 30; 91). A product with minimal appeal will not be adopted or used extensively; this proved to be the case with over-the-counter NRT (41; 116). Ideally, less harmful products must be sufficiently appealing to encourage switching from the high- to the low-harm products.
Dependence (y-axis, Figure 2) refers to the potential for the product to provide satisfaction and relatedly possibly induce addiction, which is a function both of its pharmacological and its subjective rewarding and sensory properties. Dependence can reflect a response to negative consequences of stopping nicotine use (withdrawal), as well as to difficulty foregoing the positive desirable effects it can have for some users (i.e., self-medication to improve alertness, concentration, memory, or mood) (44; 95). Some degree of dependence upon a much less harmful ANDS product may have to be acceptable to society as a means of speeding the demise of smoking and its attendant massive harms.
Cigarettes and other combusted tobacco products are the most appealing, most addictive, and most toxic of all nicotine delivery products, and thus have dominated use for over a century. They are the perfect storm, occupying the space at the highest level on all three dimensions (highest on all axes, Figure 2). No use at all is depicted by an empty sphere in the bottom, left front corner of the space (zero on all 3 axes).
The question then arises: “where do other less harmful nicotine containing products fit?” The dimensional space depicted in Figure 2 can be helpful in locating what may be the “sweet spot” of an ideal e-cigarette or a future innovation of an ANDS. This “sweet spot” is depicted as a sphere that contains products that are both high in appeal (z-axis) and in desire to use instead of smoking (dependence on the y-axis), but is low in toxicity (x-axis). Flavors, efficient nicotine delivery, and lower cost than cigarettes all play an important role in improving the overall appeal of a product on a large-scale basis (29; 30). Smokers who have successfully completely switched to e-cigarettes also report anecdotally that being able to use flavors other than tobacco or menthol helped them to sustain e-cigarette use rather than dual use or going back to smoking their usual cigarette brand (30).
NRT, although presenting minimal harm, has little appeal and low dependence. For these reasons, NRT has demonstrated a weak ability to displace a large proportion of smokers, despite its evidence-based CDER approval as a cessation therapy and its strong support in tobacco control policy for over 20 years (97). In contrast to NRT, some e-cigarettes occupy the “sweet spot” in this three-dimensional space because some smokers have found an e-cigarette with sufficient appeal for them to sustain use and quit smoking (9; 12; 29; 30; 38; 45; 64). Scientific evidence is mounting that e-cigarettes are used by more smokers than NRT in quit attempts in both the U.S. and the U.K. (16; 89).
The three-dimensional space provides a road map to help inform a harm minimization framework and guide research, policy and practice. Different tobacco and nicotine products can be ordered in this space and be compared to one another. Classes of nicotine containing products (e.g., combustible vs. noncombustible; high vs. low nitrosamine; fast vs. slow nicotine delivery; flavored vs. non-flavored, etc.) can be evaluated for comparative safety, appeal, and impact on tobacco use. One challenge is to identify products that move the largest proportion of current nicotine users to a place along these three dimensions that minimizes net harm and maximize net benefits. Regulations and policy initiatives should be aligned so that less harmful products are able to compete with, and ultimately completely replace, smoking as a means of using nicotine for those adults who want to use it.
2.4. Systems Integration: Optimizing Population Benefits over Harms
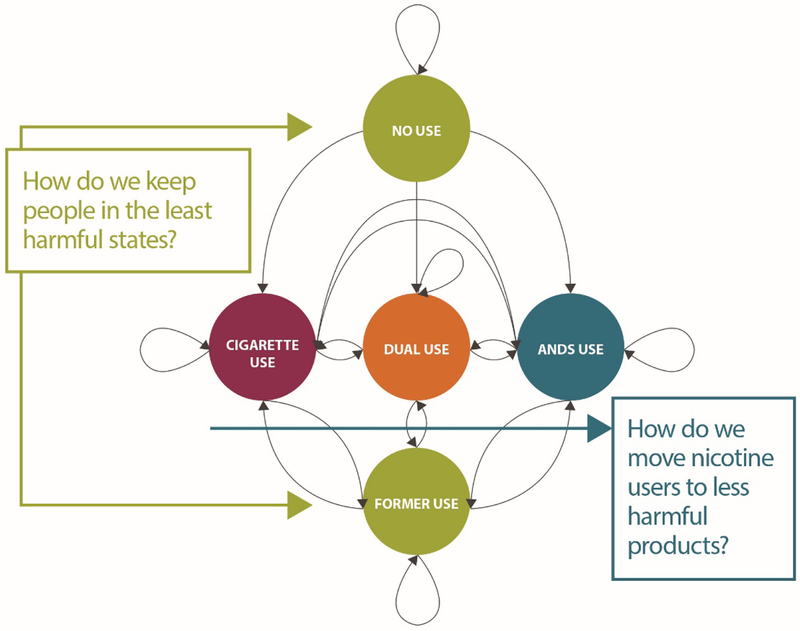
Population net exposure to harmful toxicants depends on the actual patterns and prevalence of product use that vary along the continuum of harm (Figures 1 and 2), as well as keeping as many non-users as possible, especially youth, from any use of nicotine. Figure 3 presents a state transition model using the example of cigarettes and ANDS to illustrate the possible states and pathways that must be considered to optimize a harm minimization strategy in tobacco control (20).
Figure 3.
Markov state transition model of cigarette and e-cigarette use. Adapted from Cobb et al., 2015 (20).
Directed arrows represent transitions, while looped arrows at each state represent maintenance of that state. Individuals begin in the non-current use state (a variant of never use) and can either remain in that state or transition to current exclusive use of cigarettes or ANDS or to dual use. Once in a current use state, individuals can maintain use, transition to one of two alternative states, or cease use of both products. Former users may also maintain no use or relapse to current exclusive or dual use. The CTP’s public health standard implies an integrated consideration of product harms and benefits at the individual and population levels (including likelihoods of initiation and cessation). Population health could be improved by changes in nicotine-containing product use that result in transitions to less harmful use states (20). These changes include limiting movement from non-current use (i.e., preventing initiation of any nicotine product use by non-users), and increasing movement away from cigarette use (perhaps via dual use) to exclusive use of less harmful ANDS and/or increased transition to former use and reduced relapse to smoking.
Each tobacco control strategy (e.g., taxes, media campaigns, treatment availability, accurate consumer knowledge of relative harms, regulations) will influence the flows from one state to another. Prevention of youth initiation and support for cessation will keep non-current and former users from starting or relapsing (tactics to reinforce the states depicted by green circles, Figure 3). Harm minimization strategies facilitate movement away from smoking (depicted by the blue arrow, Figure 3), by regulating and managing products according to their relative harms. Outcomes are determined empirically by estimating the prevalence rates within states and transition rates between states based on population surveillance. Simulation modeling of policies’ and regulations’ effects on transition rates can indicate where harms might exceed benefits, given different scenarios of product use (61).
Three examples of these approaches could be: (a) imposing a differential tax on nicotine-containing products that is proportional to their degree of harm, with less harmful products minimally taxed, and all combusted products very highly taxed (19); (b) reducing the addiction liability of combusted tobacco via nicotine reduction while ensuring adequate and satisfying nicotine delivery in ANDS (7; 24); and, (c) reducing the appeal of smoking by banning menthol and other flavors in smoked products (29; 30; 96; 107). Making combusted tobacco more expensive and less appealing along with more appealing, less harmful ANDS is consistent with embracing harm minimization to speed users away from smoking.
3. TWO MAJOR CHALLENGES TO ANDS AS A HARM MINIMIZATION STRATEGY
The concerns about a harm minimization strategy relying on ANDS derive from two concerns about unintended harmful consequences, and the fact that abstinence from all tobacco and nicotine products is safest. The concerns are that availability of e-cigarettes might lure some youth who would otherwise not smoke into smoking, and that smokers who adopt e-cigarettes, and who otherwise would have quit smoking altogether, might be led to continue smoking.
3.1. Do E-Cigarettes Attract Youth and Lead Them to Smoking and Lifelong Addiction?
Consistent with harm minimization, tobacco control should strive to prevent all youth initiation of nicotine, (e.g., prohibiting the sale of nicotine-containing products to those under legal purchase age, preventing predatory marketing to youth). This aspiration must be understood in the context of adolescent behavior. Risk-taking in adolescence is normative and results from competition between the strong socioemotional network in the brain and the immature cognitive-control network (93). Early risk-taking with any tobacco or nicotine product, like an e-cigarette, may result from social or emotional rewards from trying a product, including peer approval or mood enhancement. This suggests that eliminating all experimentation may not be a realistic goal, just as it has not been for cigarettes.
Existing studies show that current e-cigarette use by youth consists largely of experimentation, not long-term adoption (22; 109). As many as 70% of youth using e-cigarettes report only using flavors without nicotine (68). Poly-product use is common (22; 109). Findings are consistent with adolescent risk-taking (93) and shared vulnerabilities (22; 74; 106; 109). In the U.S., while past 30-day e-cigarette use in youth has risen between 2011 and 2014 (22; 109; 115), the prevalence of past 30-day cigarette smoking declined rapidly in youth (38; 113). These patterns are consistent with data from the U.K. (6).
Longitudinal studies of youth never cigarette users show that some ever e-cigarette users try cigarettes during a follow-up period (5; 47; 59; 60; 69; 86; 92; 118–120), which raises concern about so-called “gateway” effects (i.e., e-cigarette use leading directly to smoking) (55). But few studies examine the opposite transition – from cigarette use to e-cigarette use, a move towards less harm (blue arrow, Figure 3). Recent data show that 87% of past 30-day e-cigarette users have previously used a tobacco product, and 63% used a tobacco product in the past 30-days (109). Kozlowski and Warner (2017) concluded that while society must be vigilant in tracking youth use trends, fears of harms (103) due to gateway effects seem to be exaggerated and are unlikely to undermine the much larger potential benefits of discouraging smoking behavior in the whole population (55).
Jurisdictions have adopted bans on e-cigarette sales to youth. Studies comparing the rates of youth cigarette use in U.S. states with and without bans on sales to minors found that the prevalence of smoking was higher when youth access to e-cigarettes was restricted (35; 81; 82). This illustrates the potential for some well-intentioned “precautionary” policies to have harmful effects.
Simulation modeling with sensitivity analyses that examine all the states and transition pathways in the state transition model (Figure 3) show that the gateway effect would have to be implausibly large to increase the net public health harm (20; 61). Overall, the strongest science to date does not support the concerns that e-cigarettes are such a dire threat as to undermine 50 years of tobacco control success, renormalize smoking, and addict another generation of youth.
3.2. Do E-Cigarettes Help Current Smokers Quit or Do They Inhibit Cessation?
The public health benefits of e-cigarettes are enhanced if they promote complete cessation of smoking. Four randomized controlled trials (RCTs) and well-designed observational studies show that e-cigarettes are effective in helping some adult smokers successfully quit smoking (3; 13; 15; 38; 67; 79; 99). Rates of cessation using e-cigarettes are similar to or higher than rates of cessation from previous clinical trials of NRT (97). Although some studies with loosely-defined measures of use or comparison groups report that e-cigarette use may be associated with no change or negative correlations with cessation (38), studies with more robust measures of how e-cigarettes were used (e.g., duration of use, type of device, use specifically for cessation) suggest that daily vaping can facilitate quit attempts and cessation (9; 12; 45; 64). Newer e-cigarette models (e.g., tank, mod and pod systems) provide more effective nicotine delivery, so studies on earlier devices may not be as strong as recent evaluations of e-cigarette’s positive public health effect (80).
Smokers’ complete displacement of cigarettes can take time. For many, a period of dual use is expected and can be acceptable along the path to smoking cessation. A transitional period of dual use with e-cigarettes and cigarettes is consistent with CDER-approved dual-using of NRT (36). There is no evidence we are aware of that vaping has contributed to reduced interest in quitting smoking, slowed the rate of cessation, or promoted relapse in large numbers of long-term former smokers who had been quit for 5 years or longer (38). Surveys of e-cigarette users consistently indicate that, for most smokers, quitting cigarettes is one major reason for their use (38), even among youth (108). In the years when e-cigarette use increased the most, studies reveal a rise in quit attempts (4; 37), along with either a steady or faster drop in cigarette use among both youth and adults rather than a slowing of prevalence reduction (18; 70). Studies suggest daily users of e-cigarettes for a month or more are six times more likely to have quit two years later (9); former smokers who quit less than one year prior are four times more likely to be daily e-cigarette users compared to current smokers (23), and studies from the U.K. suggest e-cigarettes have increased quitting above what would have otherwise been expected (117).
Available scientific evidence does not support the contention that e-cigarettes when used daily specifically to quit smoking either inhibit cessation or are undermining historical tobacco control cessation efforts.
4. POLICY IMPLICATIONS
The harm minimization approach yields clear implications for tobacco control policies, demanding a reorientation of these policies, starting with a return to their harm minimization roots [insert SIDEBAR here].
4.1. Reaffirming Harm Minimization in Tobacco Control
Harm minimization was an accepted strategy at the beginning of tobacco control efforts in the 1960s (49). It was and still is implicit in tobacco control support for CDER-approved over-the-counter use of NRT as a safe nicotine product (36). Public health advocates are now often skeptical of reduced harm products because of mistrust of the tobacco industry and commercial entities more generally, given the experience of the highly misleading promotion of low tar “light” cigarettes (49; 51) that were not in fact reduced harm products (71). This skepticism has generalized, negating all harm minimization strategies and data, including the well-documented successful Swedish experience with snus. Smokeless tobacco is still viewed by the World Health Organization (WHO) and most countries as “not a safe alternative to smoking” even if much less harmful (49; 50; 52; 65) and e-cigarettes are also being banned in many countries (10).
Harm minimization approaches have often been resisted in many areas of risky behavior because of fears of unintended harmful consequences. But when carefully implemented, these approaches have dramatically reduced harm at the individual and population levels (e.g., condom use (100) and needle exchange programs for human immunodeficiency virus (HIV) prevention (14; 73; 101; 111; 122)). In tobacco control, there is understandable trepidation in supporting alternatives that may risk undermining 50 years of tobacco control efforts given past tobacco industry behavior (for details, see Royal College of Physicians (2016), Chapter 9, pp.135–145 (89)).
However, while holding the industries strictly accountable, if, out of an abundance of caution, tobacco control strategies fail to fully embrace movement to less harmful products (or actively discourage such movement), the result could be iatrogenic for smokers who are unable to or who do not wish to quit nicotine use completely. A key provocative, or even radical question is raised: Can the combination of technological advances (ANDS) and regulation align makers of nicotine-containing products with public health advocates to eliminate combusted tobacco as a defective and unacceptable product for human use? (75; 76; 87). By not aligning regulations and policies with the principle of harm minimization, tobacco control could inadvertently perpetuate smoking by allowing this opportunity pass us by. Moreover, the precautionary principle invoked to support suppression of ANDS is violated rather than honored when a less harmful alternative is withheld or discouraged that significantly decreases the harms from a much more harmful behavior (27; 28).
A core harm minimization principle is that policy, regulation, and advocacy be science-based and proportional to the degree of product harm, with the most restrictive strategies applying to the most harmful product. For example, much less harmful products like e-cigarettes could help displace cigarettes on a larger scale than NRT, because of greater appeal, lower cost, and ease of access (19–21). A regulatory scheme that places the most burdensome standards on the most harmful products, while ensuring safety and quality of the least harmful products, supports harm minimization (123).
Accurate public information is a crucial part of tobacco control policy. The positive impact of e-cigarettes may have been slowed by exaggerated claims of their harms (54; 55) and the harms of nicotine in general. Only 5.3% of Americans correctly believe e-cigarettes are “much less harmful” than cigarettes, 37% believe they are the same or worse than smoking, and 34% don’t know (63; 72). Misperceptions of the harms of nicotine and e-cigarettes have increased in recent years, undermining their full potential to displace smoking (11; 46; 54; 63). A misinformed public deprives individuals of the opportunity to take health-protective action (52; 54). To counteract misperceptions of harm from nicotine and e-cigarettes, truthful and aggressive public education is needed to communicate the importance of smoking cessation and nicotine’s relative safety and efficacy when de-coupled from smoke.
5. CONCLUSIONS
Harm minimization is a pragmatic approach that can complement proven current tobacco control efforts of prevention and cessation (2; 49; 73; 89). Its primary goal is to move the whole population of smokers of toxic combusted tobacco products to exclusive use of much safer products and/or complete cessation as quickly as possible and as early as possible in their individual smoking careers. If prudently regulated, ANDS such as e-cigarettes and Swedish snus (56–58) provide a great opportunity to disrupt the U.S and global smoking-related disease pandemic, and offer a proof-of-principle for the role of ANDS in improving public health. This opportunity depends on encouraging rather than discouraging increased innovation of ANDS to find the appropriate balance between product safety and consumer appeal, as well as aggressive regulation to decrease use of combusted tobacco products.
Regulation, policy, practice, and advocacy for harm minimization approaches have the potential to align market forces and incentives for those responsibly manufacturing and marketing much less harmful nicotine delivery products. Even if the risk of harm to some youth who otherwise would not have smoked is marginally increased, such risks are substantially eclipsed among both youth and adults by the benefit of displacing smoking with safer nicotine products (55). Under all but the most implausible scenarios, population simulation modeling estimates millions of life years saved by employing the principles of harm minimization and switching smokers to safer nicotine products (61).
SUMMARY POINTS.
Inhaled tobacco smoke remains the single biggest threat to public health; it is widely used, highly appealing, addictive, and extremely toxic.
There is a continuum of harm of nicotine-containing products from the high harm of combusted tobacco to much lower harms of non-combustible nicotine delivery with or without tobacco, including NRT.
In considering how to maximize population benefit and minimize population harm, one must fully consider all three dimensions of nicotine products and locate the “sweet spot” (see Figures 2 and 3), which defines the characteristics of products most likely to displace smoking: (1) lower harm, (2) sufficient appeal, and (3) sufficiently satisfying nicotine delivery.
Tobacco control strategies should adopt the concept of harm minimization in developing coordinated regulations, policies, and interventions to rapidly move smokers toward less harmful nicotine delivery products, while preventing the adoption of regular nicotine-containing or tobacco product use among youth.
The public must be accurately educated about the relative harms of nicotine-containing products relative to smoking.
A harm minimization approach implies proportionality of harm based on each product class. Policies and regulations must be aligned based on proportionate harm.
Harm minimization is an evidence-based approach to tobacco control which, when complemented by other, proven tobacco control interventions, can simultaneously prevent youth from starting to smoke and help current smokers stop, saving many lives more quickly than would otherwise be possible.
FUTURE ISSUES.
Research is needed on the pathways by which ANDS can lead to displacement of smoking. Traditional smoking cessation treatment designs may not be optimal because they focus on near-term outcomes of focused quit efforts, whereas adoption of ANDS as an alternative to smoking may involve more of a gradual evolution in the smoker’s goals and behavior.
New and evolving ANDS products may raise new issues and data needs. For example, products that heat rather than burn tobacco, but still mimic smoking, may raise issues different from those raised by e-cigarettes.
Since not all effects of policies or products can be anticipated, frameworks for robust and responsive post-market population surveillance and for modeling of likely outcomes of ANDS use need to be established.
A regulatory framework that aligns business goals with public health goals will need to be developed. Absent regulation, ANDS have evolved very quickly towards more effective nicotine delivery. While regulation is necessary to ensure that product innovations are consistent with public health goals, it also has the potential to stifle innovation, and thus undermine the potential of ANDS as a public health success.
A harm minimization strategy acknowledges that nicotine use and even dependence may be acceptable in the interest of reducing tobacco-caused death and disease. This will require a focused, objective, evidence-based dialogue that separates concerns about nicotine use and dependence from concerns about medical harm, and implies a substantial shift in public, professional, and regulatory attitudes in the interest of eventually ending combusted tobacco use.
SIDEBAR.
The key challenge is to implement policies which maximize the net flow away from smoking and toward use of safer products or to no use. A balance can and must be found between protecting youth without discouraging cleaner nicotine use by smokers unable or not wishing to stop their nicotine use (1; 2). Considerations include: (1) devising a regulatory and policy framework that focuses on reducing smoking; (2) enabling the public to have accurate information about and incentives to adopt less harmful options of nicotine delivery; and, (3) allowing product innovation and market forces, as well as regulation proportionate to product harms, to contribute to the speedy demise of smoking. Delays in harm minimization may impede the end of smoking rather than complete switching to safer nicotine delivery products. Emergence and uptake of low-risk tobacco and nicotine products (ANDS), such as e-cigarettes, as alternatives to smoking creates the possibility of deep and rapid public health gains through the substitution of high-risk products by low-risk products.
ACKNOWLEDGEMENTS
All authors were supported by Truth Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration or the Truth Initiative.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
ACRONYMS AND DEFINITIONS
- U.S.
United States of America
- Combusted/combustible tobacco
burning tobacco products (cigarettes, cigars, pipe, roll your own products, and hookah)
- Smoking
the inhalation of the smoke from any combustible tobacco product
- ANDS
Alternative Nicotine Delivery Systems (e.g., e-cigarettes, heat-not-burn tobacco)
- E-cigarettes
also called vape pens, personal vaporizers, e-hookahs, e-pipes, and e-cigars, among other names, are battery-operated and produce an aerosol instead of smoke.
- Vaping
the inhalation of e-cigarette aerosol
- Harm minimization
or reduction, aims to reduce health consequences without necessarily eliminating the behavior itself
- NRT
Nicotine Replacement Therapy
- TCA
The Family Smoking Prevention and Tobacco Control Act
- FDA
Food and Drug Administration
- CDER
Center for Drug Evaluation Research
- U.K.
United Kingdom
- Dry puff
conditions when vaping with a high wattage, too much airflow, old coils, or no liquid; not normally used
- RCT
Randomized Controlled Trial
- WHO
World Health Organization
- HIV
human immunodeficiency virus
- Precautionary principle
resisting a new product with little known effects
- Non-combusted/non-combustible tobacco
non-burning tobacco products (smokeless tobacco, snus)
LITERATURE CITED
- 1.Abrams DB. 2014. Potential and pitfalls of e-cigarettes--reply. JAMA 311:1922–3 [DOI] [PubMed] [Google Scholar]
- 2.Abrams DB. 2014. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA 311:135–6 [DOI] [PubMed] [Google Scholar]
- 3.Adriaens K, Van Gucht D, Declerck P, Baeyens F. 2014. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health 11:11220–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. 2017. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 65:1457–64 [DOI] [PubMed] [Google Scholar]
- 5.Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Cruz TB, et al. 2016. E-Cigarettes and Future Cigarette Use. Pediatrics 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauld L, MacKintosh AM, Ford A, McNeill A. 2016. E-Cigarette Uptake Amongst UK Youth: Experimentation, but Little or No Regular Use in Nonsmokers. Nicotine Tob Res 18:102–3 [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL, Donny EC, Hatsukami DK. 2017. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction 112:6–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Fraiman JB. 2017. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biener L, Hargraves JL. 2015. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res 17:127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton J, Bogdanovica I, McNeill A, Bauld L. 2016. Commentary on WHO Report on Electronic Nicotine Delivery Systems and Electronic Non-Nicotine Delivery Systems. http://ukctas.net/pdfs/UKCTAS-response-to-WHO-ENDS-report-26.10.2016.pdf
- 11.Brose LS, Brown J, Hitchman SC, McNeill A. 2015. Perceived relative harm of electronic cigarettes over time and impact on subsequent use. A survey with 1-year and 2-year follow-ups. Drug Alcohol Depend 157:106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brose LS, Hitchman SC, Brown J, West R, McNeill A. 2015. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 110:1160–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, et al. 2013. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 382:1629–37 [DOI] [PubMed] [Google Scholar]
- 14.Canadian Paediatric Society. 2008. Harm reduction: An approach to reducing risky health behaviours in adolescents. Paediatr Child Health 13:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, et al. 2013. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 8:e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caraballo RS, Shafer PR, Patel D, Davis KC, McAfee TA. 2017. Quit Methods Used by US Adult Cigarette Smokers, 2014–2016. Prev Chronic Dis 14:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2014. Best Practices for Comprehensive Tobacco Control Programs -- 2014, U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2016. Early Release of Selected Estimates Based on Data From the National Health Interview Survey, January–March 2016, U.S Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics [Google Scholar]
- 19.Chaloupka FJ, Sweanor D, Warner KE. 2015. Differential Taxes for Differential Risks--Toward Reduced Harm from Nicotine-Yielding Products. N Engl J Med 373:594–7 [DOI] [PubMed] [Google Scholar]
- 20.Cobb CO, Villanti AC, Graham AL, Pearson JL, Glasser AM, et al. 2015. Markov Modeling to Estimate the Population Impact of Emerging Tobacco Products: A Proof-Of-Concept Study . Tobacco Reg Sci 1:121–41 [Google Scholar]
- 21.Cobb NK, Abrams DB. 2014. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med 371:1469–71 [DOI] [PubMed] [Google Scholar]
- 22.Collins LK, Villanti AC, Pearson JL, Glasser AM, Johnson AL, et al. 2017. Frequency of Youth E-Cigarette, Tobacco, and Poly-Use in the United States, 2015: Update to Villanti et al., “Frequency of Youth E-Cigarette and Tobacco Use Patterns in the United States: Measurement Precision Is Critical to Inform Public Health”. Nicotine Tob Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delnevo CD, Giovenco DP, Steinberg MB, Villanti AC, Pearson JL, et al. 2016. Patterns of Electronic Cigarette Use Among Adults in the United States. Nicotine Tob Res 18:715–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, et al. 2015. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 373:1340–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagerstrom K, Etter JF, Unger JB. 2015. E-cigarettes: a disruptive technology that revolutionizes our field? Nicotine Tob Res o 17:125–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagerstrom KO, Bridgman K. 2014. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addict Behav 39:507–11 [DOI] [PubMed] [Google Scholar]
- 27.Fairchild AL, Bayer R. 2015. Public health. Smoke and fire over e-cigarettes. Science 347:375–6 [DOI] [PubMed] [Google Scholar]
- 28.Fairchild AL, Bayer R, Colgrove J. 2014. The renormalization of smoking? E-cigarettes and the tobacco “endgame”. N Engl J Med 370:293–5 [DOI] [PubMed] [Google Scholar]
- 29.Farsalinos KE, Poulas K, Voudris V, Le Houezec J. 2017. Prevalence and correlates of current daily use of electronic cigarettes in the European Union: analysis of the 2014 Eurobarometer survey. Intern Emerg Med [DOI] [PubMed] [Google Scholar]
- 30.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. 2013. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health 10:7272–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farsalinos KE, Voudris V, Poulas K. 2015. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 110:1352–6 [DOI] [PubMed] [Google Scholar]
- 32.Feirman SP, Glasser AM, Rose S, Niaura R, Abrams DB, et al. 2017. Computational Models Used to Assess US Tobacco Control Policies. Nicotine Tob Res [DOI] [PubMed] [Google Scholar]
- 33.Fiore MC, Schroeder SA, Baker TB. 2014. Smoke, the chief killer--strategies for targeting combustible tobacco use. N Engl J Med 370:297–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. 2013. Nicotine Replacement Therapy Labels May Change. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm345087.htm
- 35.Friedman AS. 2015. How does electronic cigarette access affect adolescent smoking? J Health Econ 44:300–8 [DOI] [PubMed] [Google Scholar]
- 36.Fucito LM, Bars MP, Forray A, Rojewski AM, Shiffman S, et al. 2014. Addressing the evidence for FDA nicotine replacement therapy label changes: a policy statement of the Association for the Treatment of Tobacco use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine Tob Res 16:909–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gitchell JG, Shiffman S, Sembower MA. 2017. Trends in serious quit attempts in the United States, 2009–14. Addiction 112:897–900 [DOI] [PubMed] [Google Scholar]
- 38.Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, et al. 2017. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am J Prev Med 52:e33–e66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. 2017. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob Res 19:160–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, et al. 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23:133–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond D, McDonald PW, Fong GT, Borland R. 2004. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 99:1042–8 [DOI] [PubMed] [Google Scholar]
- 42.Harm Reduction International. What is harm reduction? https://www.hri.global/what-is-harm-reduction
- 43.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, et al. 2015. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res 17:704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heishman SJ, Kleykamp BA, Singleton EG. 2010. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 210:453–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. 2015. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob Res 17:1187–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huerta TR, Walker DM, Mullen D, Johnson TJ, Ford EW. 2017. Trends in E-Cigarette Awareness and Perceived Harmfulness in the U.S. Am J Prev Med 52:339–46 [DOI] [PubMed] [Google Scholar]
- 47.Huh J, Leventhal AM. 2016. Progression of Poly-tobacco Product Use Patterns in Adolescents. Am J Prev Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute of Medicine. 2007. Ending the Tobacco Problem: A Blueprint for the Nation. Washington, D.C.: The National Academies Press [Google Scholar]
- 49.Kozlowski LT, Abrams DB. 2016. Obsolete tobacco control themes can be hazardous to public health: the need for updating views on absolute product risks and harm reduction. BMC Public Health 16:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozlowski LT, Edwards BQ. 2005. “Not safe” is not enough: smokers have a right to know more than there is no safe tobacco product. Tobacco control 14 Suppl 2:ii3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozlowski LT, Goldberg ME, Yost BA, White EL, Sweeney CT, Pillitteri JL. 1998. Smokers’ misperceptions of light and ultra-light cigarettes may keep them smoking. Am J Prev Med 15:9–16 [DOI] [PubMed] [Google Scholar]
- 52.Kozlowski LT, O’Connor RJ. 2003. Apply federal research rules on deception to misleading health information: an example on smokeless tobacco and cigarettes. Public Health Rep 118:187–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozlowski LT, Strasser AA, Giovino GA, Erickson PA, Terza JV. 2001. Applying the risk/use equilibrium: use medicinal nicotine now for harm reduction. Tob Control 10:201–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozlowski LT, Sweanor D. 2016. Withholding differential risk information on legal consumer nicotine/tobacco products: The public health ethics of health information quarantines. Int J Drug Policy 32:17–23 [DOI] [PubMed] [Google Scholar]
- 55.Kozlowski LT, Warner KE. 2017. Adolescents and e-cigarettes: Objects of concern may appear larger than they are. Drug Alcohol Depend 174:209–14 [DOI] [PubMed] [Google Scholar]
- 56.Lee PN. 2011. Summary of the epidemiological evidence relating snus to health. Regul Toxicol Pharmacol 59:197–214 [DOI] [PubMed] [Google Scholar]
- 57.Lee PN. 2013. Epidemiological evidence relating snus to health--an updated review based on recent publications. Harm Reduct J 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee PN, Hamling J. 2009. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leventhal AM, Stone MD, Andrabi N, Barrington-Trimis J, Strong DR, et al. 2016. Association of e-Cigarette Vaping and Progression to Heavier Patterns of Cigarette Smoking. JAMA 316:1918–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, et al. 2015. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. JAMA 314:700–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levy DT, Borland R, Villanti AC, Niaura R, Yuan Z, et al. 2017. The Application of a Decision-Theoretic Model to Estimate the Public Health Impact of Vaporized Nicotine Product Initiation in the United States. Nicotine Tob Res 19:149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu G, Wasserman E, Kong L, Foulds J. 2017. A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study. Prev Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majeed BA, Weaver SR, Gregory KR, Whitney CF, Slovic P, et al. 2017. Changing Perceptions of Harm of E-Cigarettes Among U.S. Adults, 2012–2015. Am J Prev Med 52:331–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, et al. 2015. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PLoS One 10:e0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marlatt GA, Witkiewitz K. 2010. Update on harm-reduction policy and intervention research. Annu Rev Clin Psychol 6:591–606 [DOI] [PubMed] [Google Scholar]
- 66.McNeill A, Brose LS, Calder R, Hitchman S, Hajek P, McRobbie H. 2015. E-cigarettes: an evidence update -- A report commissioned by Public Health England, Public Health England, London, England [Google Scholar]
- 67.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. 2014. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 12:Cd010216. [DOI] [PubMed] [Google Scholar]
- 68.Miech R, Patrick ME, O’Malley PM, Johnston LD. 2016. What are kids vaping? Results from a national survey of US adolescents. Tob Control [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miech R, Patrick ME, O’Malley PM, Johnston LD. 2017. E-cigarette use as a predictor of cigarette smoking: results from a 1-year follow-up of a national sample of 12th grade students. Tob Control [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monitoring the Future - The University of Michigan. 2017. Table 2: Trends in Prevalence of Use of Cigarettes in Grades, 8, 10, and 12. Ann Arbor [Google Scholar]
- 71.National Cancer Institute. Dispelling Myths about Nicotine Replacement Therapy http://smokefree.gov/sites/default/files/pdf/mythsaboutNRTfactsheet.pdf
- 72.National Cancer Institute. 2015. Health Information National Trends Survey: Compared to smoking cigarettes, would you say that electronic cigarettes are… https://hints.cancer.gov/question-details.aspx?PK_Cycle=8&qid=1282
- 73.Niaura R 2016. Re-thinking nicotine and its effects. https://truthinitiative.org/sites/default/files/ReThinking-Nicotine.pdf
- 74.Niaura RS, Glynn TJ, Abrams DB. 2014. Youth experimentation with e-cigarettes: Another interpretation of the data. JAMA Pediatr 312:1–2 [DOI] [PubMed] [Google Scholar]
- 75.Nocera J. Can E-Cigarettes Save Lives? 2015 https://www.nytimes.com/2015/10/17/opinion/can-e-cigarettes-save-lives.html?_r=0.
- 76.Nocera J 2017. When Public Health and Big Tobacco Align. https://www.bloomberg.com/view/articles/2017-03-09/when-public-health-and-big-tobacco-align
- 77.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, et al. 2014. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res 20:218–25 [DOI] [PubMed] [Google Scholar]
- 78.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, et al. 2016. E-cigarettes are less harmful than smoking. Lancet 387:1160–2 [DOI] [PubMed] [Google Scholar]
- 79.O’Brien B, Knight-West O, Walker N, Parag V, Bullen C. 2015. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Leary R, MacDonald M, Stockwell T, Reist D. 2017. Clearing the Air: A systematic review on the harms and benefits of e-cigarettes and vapour devices, University of Victoria Centre for Addictions Research of BC, Victoria, British Columbia [Google Scholar]
- 81.Pesko M, Currie J. 2016. The Effect of E-cigarette Minimum Legal Sale Age Laws on Traditional Cigarette Use and Birth Outcomes Among Pregnant Teenagers, National Bureau of Economic Research, Cambridge, MA [Google Scholar]
- 82.Pesko MF, Hughes JM, Faisal FS. 2016. The influence of electronic cigarette age purchasing restrictions on adolescent tobacco and marijuana use. Prev Med 87:207–12 [DOI] [PubMed] [Google Scholar]
- 83.Polosa R, Campagna D, Sands MF. 2016. Counseling patients with asthma and allergy about electronic cigarettes: an evidence-based approach. Ann Allergy Asthma Immunol 116:106–11 [DOI] [PubMed] [Google Scholar]
- 84.Polosa R, Morjaria J, Caponnetto P, Caruso M, Strano S, et al. 2014. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. Int J Environ Res Public Health 11:4965–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, et al. 2016. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 21:99–108 [PubMed] [Google Scholar]
- 86.Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. 2015. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pediatr 169:1018–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reuters. 2016. Philip Morris CEO Sketches a Future Where the Company Doesn’t Sell Cigarettes. http://fortune.com/2016/11/30/philip-morris-ceo-future-without-cigarettes/
- 88.Rose JE, Turner JE, Murugesan T, Behm FM, Laugesen M. 2010. Pulmonary delivery of nicotine pyruvate: sensory and pharmacokinetic characteristics. Exp Clin Psychopharmacol 18:385–94 [DOI] [PubMed] [Google Scholar]
- 89.Royal College Physicians. 2016. Nicotine without smoke: Tobacco harm reduction, London [Google Scholar]
- 90.Russell MA. 1976. Low-tar medium-nicotine cigarettes: a new approach to safer smoking. Br Med J 1:1430–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smiley SL, DeAtley T, Rubin LF, Harvey E, Kierstead EC, et al. 2017. Early Subjective Sensory Experiences with “cigalike” E-cigarettes Among African American Menthol Smokers: A Qualitative Study. Nicotine Tob Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spindle TR, Hiler MM, Cooke ME, Eissenberg T, Kendler KS, Dick DM. 2017. Electronic cigarette use and uptake of cigarette smoking: A longitudinal examination of U.S. college students. Addict Behav 67:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steinberg L 2007. Risk Taking in Adolescence: New Perspectives from Brain and Behavioral Science. Curr Dir Psychol Sci 16:55–9 [Google Scholar]
- 94.Strong DR, Pearson J, Ehlke S, Kirchner TR, Abrams D, et al. In Press. Indicators of Dependence for Different Types of Tobacco Product Users: Descriptive Findings from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend [DOI] [PubMed] [Google Scholar]
- 95.Talati A, Keyes KM, Hasin DS. 2016. Changing relationships between smoking and psychiatric disorders across twentieth century birth cohorts: clinical and research implications. Mol Psychiatry 21:464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tobacco Products Scientific Advisory Committee. 2011. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations, U.S. Food and Drug Administration, Center for Tobacco Products, Rockville, MD [Google Scholar]
- 97.Tobacco Use and Dependence Guideline Panel. 2008. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): US Department of Health and Human Services [Google Scholar]
- 98.Trefis Team. 2016. FDA Approval For iQOS To Be A Game Changer For Altria. https://www.forbes.com/sites/greatspeculations/2016/12/30/fda-approval-for-iqos-to-be-a-game-changer-for-altria/#385ca26b1a36
- 99.Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, et al. 2016. A Randomized Trial Comparing the Effect of Nicotine Versus Placebo Electronic Cigarettes on Smoking Reduction Among Young Adult Smokers. Nicotine Tob Res 18:1937–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.U.S. Department of Health and Human Services. 2001. Workshop Summary: Scientific Evidence on Condom Effectiveness for Sexually Transmitted Disease (STD) Prevention (June 12–13, 2000), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Herndon, VA [Google Scholar]
- 101.U.S. Department of Health and Human Services. 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General., U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA [Google Scholar]
- 102.U.S. Department of Health and Human Services. 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA [Google Scholar]
- 103.U.S. Department of Health and Human Services. 2016. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General., U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA [Google Scholar]
- 104.U.S. Food and Drug Administration. 2009. Division A--Family Smoking Prevention and Tobacco Control Act In Public Law 111–31, ed. US Department of Health and Human Services [Google Scholar]
- 105.U.S. Food and Drug Administration. 2012. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. https://www.federalregister.gov/documents/2012/04/03/2012-7727/harmful-and-potentially-harmful-constituents-in-tobacco-products-and-tobacco-smoke-established-list
- 106.Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, et al. 2012. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 123 Suppl 1:S3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Villanti AC, Giovino GA, Burns DM, Abrams DB. 2013. Menthol cigarettes and mortality: keeping focus on the public health standard. Nicotine Tob Res 15:617–8 [DOI] [PubMed] [Google Scholar]
- 108.Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, et al. 2017. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am J Prev Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villanti AC, Pearson JL, Glasser AM, Johnson AL, Collins LK, et al. 2016. Frequency of youth e-cigarette and tobacco use patterns in the U.S.: Measurement precision is critical to inform public health. Nicotine Tob Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Villanti AC, Vargyas EJ, Niaura RS, Beck SE, Pearson JL, Abrams DB. 2011. Food and Drug Administration regulation of tobacco: integrating science, law, policy, and advocacy. Am J Public Health 101:1160–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vlahov D, Robertson AM, Strathdee SA. 2010. Prevention of HIV infection among injection drug users in resource-limited settings. Clin Infect Dis 50 Suppl 3:S114–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walton KM, Abrams DB, Bailey WC, Clark D, Connolly GN, et al. 2015. NIH Electronic Cigarette Workshop: Developing a Research Agenda. Nicotine Tob Res 17:259–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Warner K 2015. The remarkable decrease in cigarette smoking by American youth: further evidence. Preventive Medicine Reports 2:259–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warner KE. 2013. An endgame for tobacco? Tob Control 22 Suppl 1:i3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warner KE. 2016. Frequency of E-Cigarette Use and Cigarette Smoking by American Students in 2014. Am J Prev Med 51:179–84 [DOI] [PubMed] [Google Scholar]
- 116.West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. 2000. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 149:198–202 [DOI] [PubMed] [Google Scholar]
- 117.West R, Shahab L, Brown J. 2016. Estimating the population impact of e-cigarettes on smoking cessation in England. Addiction 111:1118–9 [DOI] [PubMed] [Google Scholar]
- 118.Wills TA, Gibbons FX, Sargent JD, Schweitzer RJ. 2016. How is the effect of adolescent e-cigarette use on smoking onset mediated: A longitudinal analysis. Psychol Addict Behav 30:876–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. 2017. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 26:34–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wills TA, Sargent JD, Gibbons FX, Pagano I, Schweitzer R. 2016. E-cigarette use is differentially related to smoking onset among lower risk adolescents. Tob Control [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization. 2008. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package., World Health Organization, Geneva, Switzerland [Google Scholar]
- 122.World Health Organization. 2016. HIV/AIDS: People who inject drugs. http://www.who.int/hiv/topics/idu/en/
- 123.Yong HH, Hitchman SC, Cummings KM, Borland R, Gravely SM, et al. 2017. Does the regulatory environment for e-cigarettes influence the effectiveness of e-cigarettes for smoking cessation?: Longitudinal findings from the ITC Four Country Survey. Nicotine Tob Res [DOI] [PMC free article] [PubMed] [Google Scholar]