Abstract
The serine/threonine protein kinase casein kinase 1α (CK1α) functions as a negative regulator of Wnt signaling, phosphorylating β-catenin at serine 45 (P–S45) to initiate its eventual ubiquitin-mediated degradation. We previously showed that the repurposed, FDA-approved anthelminthic drug pyrvinium potently inhibits Wnt signaling in vitro and in vivo. Moreover, we proposed that pyrvinium’s Wnt inhibitory activity was the result of its function as an activator of CK1α. An understanding of the mechanism by which pyrvinium activates CK1α is important because pyrvinium was given an orphan drug designation by the FDA to treat familial adenomatous polyposis, a precancerous condition driven by constitutive Wnt signaling. In the current study, we show that pyrvinium stimulates the phosphorylation of S45 β-catenin, a known CK1α substrate, in a cell-based assay, and does so in a dose- and time-dependent manner. Alternative splicing of CK1α results in four forms of the protein with distinct biological properties. We evaluated these splice products and identified the CK1α splice variant, CK1αS, as the form that exhibits the most robust response to pyrvinium in cells. Kinetic studies indicate that pyrvinium also stimulates the kinase activity of purified, recombinant CK1αS in vitro, increasing its catalytic efficiency (kcat/Km) toward substrates. These studies provide strong and clear mechanistic evidence that pyrvinium enhances CK1α kinase activity.
Casein kinase 1α (CK1α) negatively regulates the Wnt signaling pathway to suppress the initiation and progression of a subset of cancers.1 In the absence of Wnt, CK1α forms a “destruction complex” with other Wnt signaling components, including adenomatous polyposis coli (APC), axin, and glycogen synthase kinase 3 (GSK3), which recruits cytoplasmic β-catenin. Subsequent to recruitment, β-catenin is phosphorylated by CK1α on serine 45 (P-S45), which creates a phospho-substrate recognition site for GSK3.2–7 This dual phosphorylation of β-catenin results in its ubiquitin-mediated proteasomal degradation.8–10 Upon Wnt activation, the destruction complex is inhibited, and β-catenin accumulates and enters the nucleus.11 β-catenin associates with the T cell factor (TCF) family of transcription factors and lymphoid enhancer-binding factor 1 (LEF1) in the nucleus to drive a Wnt transcriptional program.12,13 A variety of other transcriptional cofactors, including B cell CLL/lymphoma 9 (BCL9) and pygopus, modulate this Wnt transcriptional response.14–16
The anthelmintic drug pyrvinium was initially identified as a small-molecule Wnt inhibitor in a large-scale screen of FDA-approved drugs capable of attenuating Wnt activity in Xenopus laevis egg extracts.17 Pyrvinium was shown to specifically activate CK1α to inhibit Wnt-driven activity in multiple colorectal cancer cell lines.18 This work suggested a model in which pyrvinium inhibited Wnt signaling by promoting the activity of CK1α, which resulted in the reduced stability of β-catenin and pygopus.17,18 Pyrvinium also attenuated Wnt activity in vivo, inhibiting the growth of intestinal adenomas driven by constitutive Wnt activity in a mouse model of familial adenomatous polyposis (FAP).18
Although numerous publications have now utilized pyrvinium as a Wnt inhibitor,19–22 the mechanism by which it does so has remained controversial. It was also suggested that pyrvinium functions as a Wnt inhibitor via a mechanism that does not involve activation of CK1α.23 This work was unable to show that pyrvinium could activate CK1α, using either a cell-based assay or an in vitro protein kinase assay. Thus, we determined to perform a more detailed biochemical analysis of the activation of CK1α by pyrvinium in order to try and resolve this controversy.
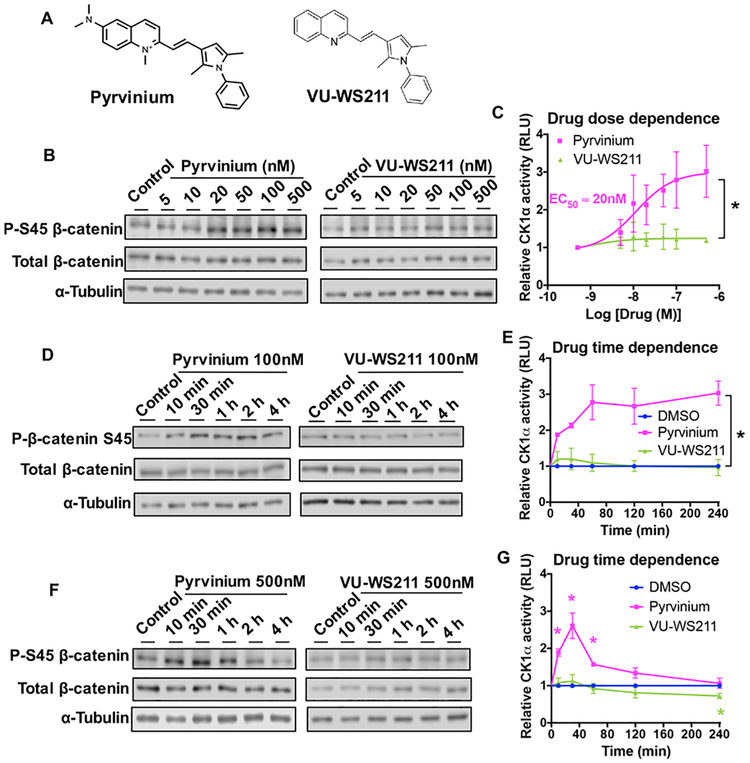
To examine the potential activation of cellular CK1α by pyrvinium, we first treated HEK293T cells with different concentrations of pyrvinium, or the chemically similar, inactive pyrvinium analogue VU-WS211 (Figure 1A),17 and evaluated the phosphorylation of a known CK1α substrate, S45 β-catenin, using a phospho-specific antibody. Pyrvinium increased P–S45 β-catenin levels relative to total β-catenin and did so in a dose-dependent manner (Figure 1B). Consistent with pyrvinium acting in a specific manner, VU-WS211 treatment did not increase P–S45 β-catenin levels. Following treatment with pyrvinium, the levels of P–S45 β-catenin plateaued at approximately 500 nM, with an EC50 of ~20 nM (Figure 1C). We next treated HEK293T cells with two different concentrations of pyrvinium or VU-WS211 for different periods of time and analyzed cellular lysates for P–S45 β-catenin. Pyrvinium increased P–S45 β-catenin levels in a time-dependent manner, which peaked at different times (30 or 60 min) depending on the dose of pyrvinium used, approximately 3-fold higher than the vehicle or VU-WS211 treated cells (Figure 1D–G). However, in the presence of the higher dose (500 nM) of pyrvinium, this increase in phosphorylated β-catenin was transient, returning to basal levels after 1 h.
Figure 1.
CK1α is activated by pyrvinium in HEK293T cells. (A) Chemical structures of pyrvinium and VU-WS211. (B) HEK293T cells were treated with increasing concentrations of pyrvinium or VU-WS211 for 30 min. (D, F) HEK293T cells were treated with pyrvinium or the inactive pyrvinium analogue VU-WS211 at 100 nM (D) or 500 nM (F) for the indicated time. Cell lysates were resolved by SDS-PAGE and subjected to immunoblotting for the indicated proteins. Representative immunoblots are shown (n = 3). (C, E, G) Quantification (mean ± S.E.M., n = 3) of panels B, D, and F, respectively, was performed using Li-Cor Image Studio software. CK1α activity indicates the level of P–S45 β-catenin normalized to that of total β-catenin. An asterisk indicates statistical significance (Student’s t test, p value ≤ 0.05).
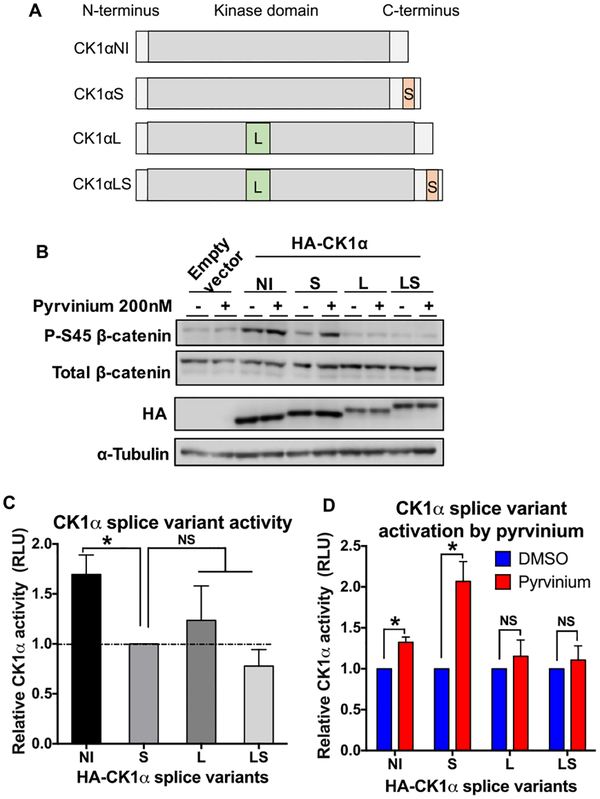
CSNK1α1 (the gene encoding CK1α) encodes two alternative exons, a long insert of 28 amino acids (L) within the kinase domain and a short insert of 12 amino acids (S) within the C-terminus. Thus, alternative splicing can result in four proteins with distinct biological properties: a form that lacks either insert (CK1αNI), a form expressing both inserts (CK1αLS), and two forms expressing only one of the two inserts (CK1αS or CK1αL) (Figure 2A).24—26 It was reported that CK1α containing the L insert is less sensitive to CKI-7, a small-molecule CK1 inhibitor, compared to CK1αNI and CK1αS.26 Thus, we also investigated whether the CK1α splice variants exhibit a differential response to pyrvinium. Plasmids encoding the four HA-tagged CK1α splice variants were transfected into HEK293T cells. We observed that CK1αS, CK1αL, and CK1αLS exhibited comparable abilities to phosphorylate β-catenin in cells, while CK1αNI was approximately 70% more active (Figure 2B,C). This finding is consistent with previous work showing that CK1αNI is the most active splice variant.25 These CK1α splice variant expressing cells were subsequently treated with pyrvinium for 30 min. Interestingly, only two of the four CK1α splice variants appeared to be pyrvinium-responsive in this assay, CK1αNI and CK1αS, with CK1αS exhibiting the most robust activation by pyrvinium (Figure 2B,D). However, the differential basal activity of CK1αNI and CK1αS may also contribute to this difference in pyrvinium fold activation.
Figure 2.
Pyrvinium activates the CK1α splice variant CK1αS. (A) Schematic of CK1α splice variants. (B) Plasmids expressing HA-tagged CK1α splice variants were transfected into HEK293T cells for 48 h, followed by treatment with DMSO or 200 nM pyrvinium for 30 min. Cell lysates were resolved by SDS-PAGE and subjected to immunoblotting for the indicated proteins. Representative immunoblots are shown (n = 3). (C, D) Quantification (mean ± S.E.M., n = 3) of CK1α activity in response to each indicated splice variant from cells treated with a vehicle (DMSO) or pyrvinium is shown. CK1α activity indicates the level of P–S45 β-catenin normalized to that of total β-catenin and then normalized to that of HA-CK1α. An asterisk indicates statistical significance (Student’s t test, p ≤ 0.05), and NS indicates no statistical significance (Student’s t test, p > 0.05).
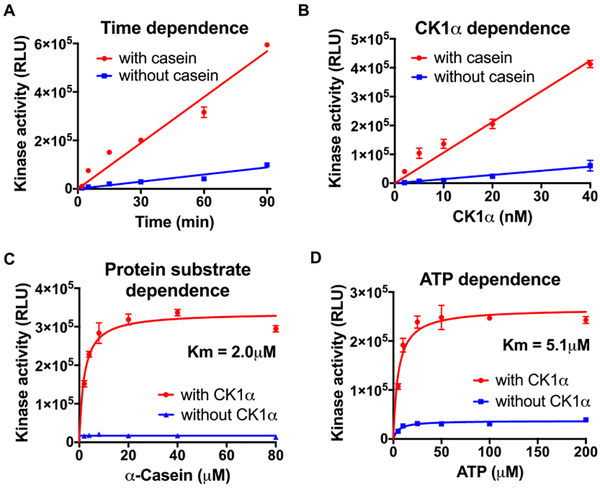
Since CK1αS was most responsive to pyrvinium in cell cultures, we used a commercially available recombinant, GST-tagged form of CK1αS to determine its mechanism of activation in vitro. We first determined the inherent enzymatic properties of this CK1αS protein and showed that the reactions proceeded in a linear fashion for at least 90 min (Figure 3A) when using up to 40 nM CK1αS (Figure 3B). We then determined the Km values for α-casein and ATP, which were 2.0 μM and 5.1 μM, respectively (Figure 3C,D), consistent with previous studies.27
Figure 3.
Enzymatic characteristics of recombinant CK1αS. The protein kinase activity of recombinant GST-CK1αS (CK1αS) was determined as indicated in each panel. Kinase reactions were performed with 40 nM CK1αS, 50 μM α-casein, and 50 μM ATP at 30 °C unless otherwise indicated. (A) Time dependence. (B) CK1αS concentration dependence at 30 min. (C) α-Casein dependence at 30 min. (D) ATP dependence at 30 min. Representative figures are shown (n = 3). Error bars indicate the range of kinase activity in duplicate reactions. An asterisk indicates statistical significance (Two-way Anova, p ≤ 0.05).
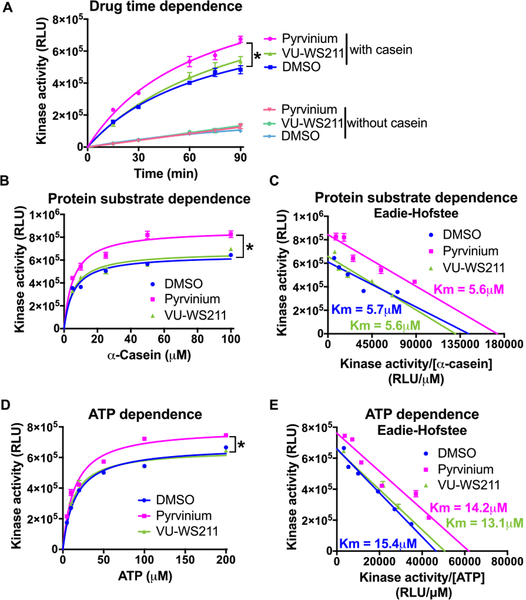
We next examined the kinetic mechanism of the action of pyrvinium. Pyrvinium increased CK1αS kinase activity in a time-dependent manner. In the presence of the inactive control analogue, VU-WS211, CK1αS showed activity similar to the vehicle control (Figure 4A). We also noted that the DMSO (5%) required to solubilize pyrvinium exhibited a small inhibition of CK1αS kinase activity (compare Figure 3A to 4A). We next performed the CK1αS kinase reaction with varying concentrations of α-casein or ATP, in the presence of DMSO, pyrvinium, or VU-WS211. Pyrvinium increased CK1αS kinase activity in a manner dependent on the concentration of α-casein or ATP, saturating at around 60 μM of α-casein and 100 μM of ATP, respectively (Figure 4B,D). To more accurately determine the effect of pyrvinium on the Km and Vmax of CK1α, we modeled the data using an Eadie-Hofstee analysis (Figure 4C,E). In the presence of pyrvinium, the Vmax of the CK1α reactions was increased by 50% with α-casein and 15% ATP, the Km of both substrates was not changed. We then summarized CK1α enzymatic parameters from three independent experiments (Table 1). These results suggest that pyrvinium enhances CK1α’s catalytic efficiency (kcat/Km) by increasing the Vmax and kcat of the reaction, without altering the Km. VU-WS211 exhibited no significant effect on kinase activity.
Figure 4.
Pyrvinium increases the enzymatic efficiency of CK1αS. The protein kinase activity of recombinant CK1αS (40 nM) was determined in the presence of DMSO, 200 nM pyrvinium, or 200 nM VU-WS211 at 30 °C (A) at the indicated time points. (B, D) At the indicated concentrations of α-casein (B) or ATP (D) at 60 min. (C, E) The data generated in panels B and D was transformed into an Eadie-Hofstee plot, respectively. Representative figures are shown (n = 3). Error bars indicate the range of kinase activity in duplicate reactions. An asterisk indicates statistical significance (Two-way Anova, p ≤ 0.05).
Table 1.
Summary of CK1αS Enzymatic Parameters
| Enzymatic parameters | α-Caaein | ATP | ||||
|---|---|---|---|---|---|---|
| DMSO | Pyrvinium | VU-WS211 | DMSO | Pyrvinium | VU-WS211 | |
| Vmax (pmol·min−1) | 9.39 ± 0.49 | 13.62 ± 0.51 * |
9.91 ±0.22 | 11.35 ± 0.14 NS |
12.87 ±0.25 * |
11.47 ± 0.20 |
| Km (μM) | 5.26 ± 0.84 | 6.51 ± 0.95 | 8.83 ± 1.18 | 14.02 ± 0.86 | 15.50 ± 0.18 | 14.12 ± 0.52 |
| kcat(min−1) | 4.69 ± 0.24 | 6.81 ± 0.26 * |
4.95 ± 0.11 | 5.68 ± 0.07 NS |
6.44 ±0.13 * |
5.73 ± 0.10 |
| kcat/Km (μM−1·min−1) | 0.88 ± 0.12 | 1.15 ± 0.13 * |
0.66 ± 0.18 | 0.38 ± 0.01 | 0.43 ± 0.01 * |
0.40 ± 0.01 |
p value ≤ 0.05, compared to Group “α-Casein, DMSO”,
p value >0.05, compared to Group “α-Casein, DMSO”,
p values ≤ 0.05, compared to Group “ATP, DMSO”, Student’s t-test.
Summarized enzymatic parameters of CK1αS (mean ± S.E.M.) were calculated from the mean ± SD (n = 2) of three independent biological replicates. Representative data of such experiments are shown in Figure 4. An asterisk indicates statistical significance compared to the relevant DMSO control (Student’s t test, p ≤ 0.05), and NS indicates no statistical significance (Student’s t test, p > 0.05).
During our studies of CK1α activation by pyrvinium, we identified several potential reasons for the discrepancies between our findings and the contrary findings of Venerando et al.23 (1) We found that the activation of CK1α by pyrvinium in cells is a rapid event, and Venerando et al. only examined pyrvinium’s ability to activate CK1α after 16 h.23 (2) We observed that pyrvinium’s ability to induce β-catenin phosphorylation varied depending on the confluence of HEK293T cells, consistent with previous work examining β-catenin activation,28 and note that Venerando et al. did not mention the confluence status of their cells. (3) Our work indicated that CK1α splice variants exhibit differential responses to pyrvinium in cellular assays. Although Venerando et al. did not specify the CK1α spliced variant used in their protein kinase assays, it remains possible that the spliced variant of CK1α they used is not activated by pyrvinium (see Figure 2).
While the number of small-molecule protein kinase activators described is limited, their ability to activate key regulatory enzymes, whose activity is down-regulated under pathological circumstances, highlights their therapeutic potential.18,29–32 Several of these protein kinase activators are already in clinical use, including metformin, a 5′ AMP-activated protein kinase (AMPK) activator, and bryostatin, a protein kinase C (PKC) activator.33,34 As a class, these small-molecule protein kinase activators typically function by improving the catalytic efficiency of their target kinase, changing its Vmax, Km, or both.35 For example, the synthetic AMPK activator A-769662 enhances its catalytic efficiency by increasing its Vmax and decreasing its Km for its peptide substrate. Alternatively, the endogenous AMPK activator AMP increases the Vmax of AMPK without altering its Km.36 Similarly, we show that pyrvinium increases the Vmax of CK1α but does not modulate its Km for either α-casein or ATP. This suggests that pyrvinium is activating CK1α by inducing a conformational change. In our previous work, we showed that pyrvinium-bound CK1α exhibits a distinct pattern upon a limited trypsin digest, consistent with pyrvinium inducing a conformational change in CK1α upon binding.17
We show here that CK1α splice variants without the L insert exhibit greater sensitivity to pyrvinium than those with the L insert. The L sequence inserts after amino acid 152 of CK1α, between β-strands 7 and 8, which form the back of the active site, and very close to the activation loop (DFG—SIN, aa 156–190).25,37 The reduced effect of pyrvinium on forms with the L insert suggests that the L insert blocks access of the small molecule to the active site. The β-strands and activation loop appear pivotal to the function of pyrvinium through either a direct interaction or via an allosteric mechanism.
Taken together, our work shows that pyrvinium potently activates CK1α in cells and does so by enhancing the catalytic efficiency (kcat/Km) of CK1α. The mechanism of action of pyrvinium will provide the rationale for the development of other CK1α activators that target Wnt signaling in various human diseases. On the basis of the findings presented here, we suggest that Wnt-dependent tumors or cell lines expressing the CK1αS variant may be the most vulnerable to treatment with pyrvinium.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Drs. Jun Long and Jezabel Rodriguez-Blanco for their insightful discussions during the course of this work. We also thank the other members of the Capobianco and Robbins laboratories for their insightful advice.
Funding
This work was supported by the National Institutes of Health [R01CA219189 (D.J.R.) and R35GM122516 (E.L.)] and funds from the Sylvester Comprehensive Cancer Center (D.J.R).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.9b00891.
Materials and methods (PDF)
Accession Codes
Casein kinase 1α: P48729–1, P48729–2, P48729–3, Q8JHD9
The authors declare the following competing financial interest(s): D.J.R., A.J.C., and E.L. are founders of StemSynergy Therapeutics Inc., a company commercializing small-molecule cell signaling inhibitors, such as pyrvinium.
REFERENCES
- (1).Knippschild U, Kruger M, Richter J, Xu P, Garcia-Reyes B, Peifer C, Halekotte J, Bakulev V, and Bischof J (2014) The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol 4, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, and Alkalay I (2002) Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Munemitsu S, Albert I, Souza B, Rubinfeld B, and Polakis P (1995) Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci U. S. A 92, 3046–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hagen T, Di Daniel E, Culbert AA, and Reith AD (2002) Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J. Biol. Chem 277, 23330–23335. [DOI] [PubMed] [Google Scholar]
- (5).Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, and He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847. [DOI] [PubMed] [Google Scholar]
- (6).Stamos JL, and Weis WI (2013) The beta-catenin destruction complex. Cold Spring Harbor Perspect. Biol 5, a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hart MJ, de los Santos R, Albert IN, Rubinfeld B, and Polakis P (1998) Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr. Biol 8, 573–581. [DOI] [PubMed] [Google Scholar]
- (8).Orford K, Crockett C, Jensen JP, Weissman AM, and Byers SW (1997) Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem 272, 24735–24738. [DOI] [PubMed] [Google Scholar]
- (9).Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, and Polakis P (1999) The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol 9, 207–210. [DOI] [PubMed] [Google Scholar]
- (10).Aberle H, Bauer A, Stappert J, Kispert A, and Kemler R (1997) beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Henderson BR (2000) Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat. Cell Biol. 2, 653–660. [DOI] [PubMed] [Google Scholar]
- (12).Daniels DL, and Weis WI (2005) Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol 12, 364–371. [DOI] [PubMed] [Google Scholar]
- (13).Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, and Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382, 638–642. [DOI] [PubMed] [Google Scholar]
- (14).Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, and Basler K (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109, 47–60. [DOI] [PubMed] [Google Scholar]
- (15).Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, and Hahn WC (2012) beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, and Bienz M (2002) A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 4, 367–373. [DOI] [PubMed] [Google Scholar]
- (17).Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM 3rd, and Lee E (2010) Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol 6, 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Li B, Flaveny CA, Giambelli C, Fei DL, Han L, Hang BI, Bai F, Pei XH, Nose V, Burlingame O, Capobianco AJ, Orton D, Lee E, and Robbins DJ (2014) Repurposing the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic agent for intestinal polyposis. PLoS One 9, No. e101969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Cui L, Zhao J, and Liu J (2018) Pyrvinium Sensitizes Clear Cell Renal Cell Carcinoma Response to Chemotherapy Via Casein Kinase 1alpha-Dependent Inhibition of Wnt/beta-Catenin. Am. J. Med. Sci 355, 274–280. [DOI] [PubMed] [Google Scholar]
- (20).Stoddart A, Wang J, Hu C, Fernald AA, Davis EM, Cheng JX, and Le Beau MM (2017) Inhibition of WNT signaling in the bone marrow niche prevents the development of MDS in the Apc(del/+) MDS mouse model. Blood 129, 2959–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Xiang W, Cheong JK, Ang SH, Teo B, Xu P, Asari K, Sun WT, Than H, Bunte RM, Virshup DM, and Chuah C (2015) Pyrvinium selectively targets blast phase-chronic myeloid leukemia through inhibition of mitochondrial respiration. Oncotarget 6, 33769–33780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Polosukhina D, Love HD, Moses HL, Lee E, Zent R, and Clark PE (2017) Pharmacologic Inhibition of beta-Catenin With Pyrvinium Inhibits Murine and Human Models of Wilms Tumor. Oncol. Res 25, 1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Venerando A, Girardi C, Ruzzene M, and Pinna LA (2013) Pyrvinium pamoate does not activate protein kinase CK1, but promotes Akt/PKB down-regulation and GSK3 activation. Biochem. J 452, 131–137. [DOI] [PubMed] [Google Scholar]
- (24).Zhang J, Gross SD, Schroeder MD, and Anderson RA (1996) Casein kinase I alpha and alpha L: alternative splicinggenerated kinases exhibit different catalytic properties. Biochemistry 35, 16319–16327. [DOI] [PubMed] [Google Scholar]
- (25).Yong TJ, Gan YY, Toh BH, and Sentry JW (2000) Human CKIalpha(L) and CKIalpha(S) are encoded by both 2.4- and 4. 2-kb transcripts, the longer containing multiple RNA-destablising elements. Biochim. Biophys. Acta, Gene Struct. Expression 1492, 425–433. [DOI] [PubMed] [Google Scholar]
- (26).Burzio V, Antonelli M, Allende CC, and Allende JE (2002) Biochemical and cellular characteristics of the four splice variants of protein kinase CK1alpha from zebrafish (Danio rerio). J. Cell. Biochem 86, 805–814. [DOI] [PubMed] [Google Scholar]
- (27).Pulgar V, Tapia C, Vignolo P, Santos J, Sunkel CE, Allende CC, and Allende JE (1996) The recombinant alpha isoform of protein kinase CK1 from Xenopus laevis can phosphorylate tyrosine in synthetic substrates. Eur. J. Biochem 242, 519–528. [DOI] [PubMed] [Google Scholar]
- (28).Steel MD, Puddicombe SM, Hamilton LM, Powell RM, Holloway JW, Holgate ST, Davies DE, and Collins JE (2005) Beta-catenin/T-cell factor-mediated transcription is modulated by cell density in human bronchial epithelial cells. Int. J. Biochem. Cell Biol. 37, 1281–1295. [DOI] [PubMed] [Google Scholar]
- (29).Serova M, Ghoul A, Benhadji KA, Cvitkovic E, Faivre S, Calvo F, Lokiec F, and Raymond E (2006) Preclinical and clinical development of novel agents that target the protein kinase C family. Semin. Oncol 33, 466–478. [DOI] [PubMed] [Google Scholar]
- (30).Kim J, Yang G, Kim Y, Kim J, and Ha J (2016) AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med 48, No. e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li B, Orton D, Neitzel LR, Astudillo L, Shen C, Long J, Chen X, Kirkbride KC, Doundoulakis T, Guerra ML, Zaias J, Fei DL, Rodriguez-Blanco J, Thorne C, Wang Z, Jin K, Nguyen DM, Sands LR, Marchetti F, Abreu MT, Cobb MH, Capobianco AJ, Lee E, and Robbins DJ (2017) Differential abundance of CK1alpha provides selectivity for pharmacological CK1alpha activators to target WNT-dependent tumors. Sci. Signaling 10, eaak9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rodriguez-Blanco J, Li B, Long J, Shen C, Yang F, Orton D, Collins S, Kasahara N, Ayad NG, McCrea HJ, Roussel MF, Weiss WA, Capobianco AJ, and Robbins DJ (2019) A CK1alpha Activator Penetrates the Brain and Shows Efficacy Against Drug-resistant Metastatic Medulloblastoma. Clin. Cancer Res. 25, 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Foretz M, Guigas B, Bertrand L, Pollak M, and Viollet B (2014) Metformin: from mechanisms of action to therapies. Cell Metab. 20, 953–966. [DOI] [PubMed] [Google Scholar]
- (34).Kollar P, Rajchard J, Balounova Z, and Pazourek J (2014) Marine natural products: bryostatins in preclinical and clinical studies. Pharm. Biol 52, 237–242. [DOI] [PubMed] [Google Scholar]
- (35).Zorn JA, and Wells JA (2010) Turning enzymes ON with small molecules. Nat. Chem. Biol 6, 179–188. [DOI] [PubMed] [Google Scholar]
- (36).Calabrese MF, Rajamohan F, Harris MS, Caspers NL, Magyar R, Withka JM, Wang H, Borzilleri KA, Sahasrabudhe PV, Hoth LR, Geoghegan KF, Han S, Brown J, Subashi TA, Reyes AR, Frisbie RK, Ward J, Miller RA, Landro JA, Londregan AT, Carpino PA, Cabral S, Smith AC, Conn EL, Cameron KO, Qiu X, and Kurumbail RG (2014) Structural basis for AMPK activation: natural and synthetic ligands regulate kinase activity from opposite poles by different molecular mechanisms. Structure 22, 1161–1172. [DOI] [PubMed] [Google Scholar]
- (37).Xu RM, Carmel G, Sweet RM, Kuret J, and Cheng X (1995) Crystal structure of casein kinase-1, a phosphate-directed protein kinase. EMBO J. 14, 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.