Abstract
Research on RNA-associated interactions has exploded in recent years, and increasing numbers of studies are not limited to RNA–RNA and RNA–protein interactions but also include RNA–DNA/compound interactions. To facilitate the development of the interactome and promote understanding of the biological functions and molecular mechanisms of RNA, we updated RAID v2.0 to RNAInter (RNA Interactome Database), a repository for RNA-associated interactions that is freely accessible at http://www.rna-society.org/rnainter/ or http://www.rna-society.org/raid/. Compared to RAID v2.0, new features in RNAInter include (i) 8-fold more interaction data and 94 additional species; (ii) more definite annotations organized, including RNA editing/localization/modification/structure and homology interaction; (iii) advanced functions including fuzzy/batch search, interaction network and RNA dynamic expression and (iv) four embedded RNA interactome tools: RIscoper, IntaRNA, PRIdictor and DeepBind. Consequently, RNAInter contains >41 million RNA-associated interaction entries, involving more than 450 thousand unique molecules, including RNA, protein, DNA and compound. Overall, RNAInter provides a comprehensive RNA interactome resource for researchers and paves the way to investigate the regulatory landscape of cellular RNAs.
INTRODUCTION
RNA-associated interactions involve many physiological and pathological processes, such as cell growth and development, cell differentiation and inflammation (1–4). With the rapid development of biotechnology techniques, new RNA-associated interactions are being discovering continuously. These new techniques include Degradome-seq (5), LIGR-seq (6), MARIO (7) and PARIS (8) for the detection of RNA–RNA interactions (RRIs); dCLIP (9), PAR-CLIP (10), RIP-seq (11) and uvCLAP (12) for the detection of RNA–protein interactions (RPIs) and ChIRP-seq (13), ChOP-seq (14), diMARGI (15) and GRO-seq (16) for the detection of RNA–DNA interactions (RDIs) (see description in Supplementary Table S1). Recently, the regulatory roles of drug-associated miRNAs and lncRNAs in drug resistance have been a research focus (17–19). Transcription factors (TFs) and histone modifications contribute to the transcriptional regulation of RNA, which participates in various biological processes (20,21). The integration of these is therefore a prerequisite for RNA-related biomarker or mechanistic studies. However, many databases have manually collected and identified RNA-associated interactions through experimental validation and computational prediction from the literature and high-throughput sequencing. The majority of these resources focus on certain types of interactions with insufficient molecular information. Thus, numbers of annotations about RNA and other interactors, such as target sites, RNA editing and RNA modification, should be included. Currently, a global view of the RNA interactome with comprehensive annotations is not available across most species.
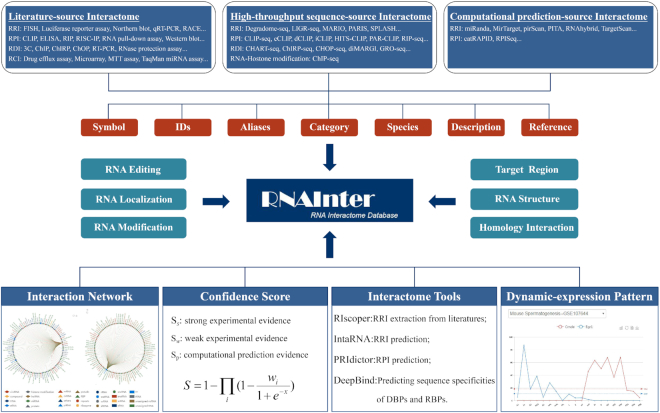
Here, we updated RAID v2.0 (22) to RNAInter (RNA Interactome Database, http://www.rna-society.org/rnainter/ or http://www.rna-society.org/raid/) to address these challenges. RNAInter establishes a repository of integrated experimentally validated and computationally predicted RNA-associated interactions through manual curation of the literature, along with another 35 resources under one common framework (Figure 1, Table 1). It also supports interaction network, RNA dynamic expression and four RNA interactome tools: RIscoper (23), IntaRNA (24), PRIdictor (25) and DeepBind (26) (Figures 1 and 4). In total, RNAInter integrated >41 million RNA-associated interactions across 154 species. It will provide a valuable resource for better understanding the RNA interactome.
Figure 1.
Overview of the RNAInter database.
Table 1.
Overview of curated interaction data from 35 resources
| Evidence type | Interaction type | Interaction entry | Database resource | Reference |
|---|---|---|---|---|
| Experimental validation | RCI | 4525 | SM2miR | (27) |
| 4113 | ncDR | (28) | ||
| 822 | EmDL | (29) | ||
| RDI | 138 062 | LnChrom | (30) | |
| RPI | 1 530 693 | POSTAR2 | (31) | |
| 199 835 | TransmiR v2.0 | (32) | ||
| RRI | 258 818 | RISE | (33) | |
| 155 622 | LncRNA2Target v2.0 | (34) | ||
| 7904 | VIRmiRNA | (35) | ||
| 3028 | LncACTdb 2.0 | (36) | ||
| 2680 | NPInter v3.0 | (37) | ||
| 1846 | OncomiRDB | (38) | ||
| 1213 | ncRDeathDB | (39) | ||
| 559 | miR2Disease | (40) | ||
| 405 | sRNATarBase 3.0 | (41) | ||
| 81 | MNDR v2.0 | (42) | ||
| 60 | LncRNADisease 2.0 | (43) | ||
| RHI/RPI | 9 515 123 | ChIPBase v2.0 | (44) | |
| RPI/RRI | 1 246 631 | starBase v2.0 | (45) | |
| 737 835 | miRTarBase | (46) | ||
| Computational prediction | RPI | 23 304 537 | RNAct | (47) |
| RRI | 1 956 709 | miRDB | (48) | |
| 1 557 635 | miRanda | (49) | ||
| 547 003 | piRTarBase | (50) | ||
| 247 731 | RepTar | (51) | ||
| 191 123 | TargetScan | (52) | ||
| 149 817 | EIMMo | (53) | ||
| 106 471 | DroID | (54) | ||
| 74 884 | ZIKV - CDB | (55) | ||
| 243 | HumanViCe | (56) | ||
| 14 | miRcode | (57) | ||
| Experimental validation/ | RRI | 538 529 | VmiReg | (58) |
| Computational prediction | RPI/RRI | 5 272 396 | RAID v2.0 | (22) |
| 327 123 | RAIN | (59) | ||
| 110 293 | ViRBase | (60) |
Figure 4.
Snapshot of four RNA interactome tools in RNAInter: RIscoper, PRIdictor, IntaRNA and DeepBind (left: input option, right: result presentation).
DATA ORGANIZATION
Data collection
RNAInter integrated experimentally validated and computationally predicted RNA interactome data from the literature and another 35 resources (Table 1). Literature within PubMed (mainly from 2016 to 2019) was screened with the following keyword combinations: (RNA molecule) AND (other molecule) AND (interaction). The keyword in brackets represents (i) RNA molecule: RNA symbols or RNA category names and (ii) other molecule: RNA symbols or RNA category names, protein symbols or ‘transcription factor’ or ‘RNA-binding protein’ or ‘protein’, gene symbols or ‘chromosome’, compound symbols or ‘compound’ or ‘drug’, or histone modification symbols or ‘histone modification’; and (iii) interaction: ‘bind’ or ‘interact’ or ‘regular’ or ‘target’. Finally, we reviewed over 31 000 published studies that included 419 522 RNA-associated interactions. Diverse RNA-associated interactions were also integrated from 24 experimentally validated databases and 14 computationally predicted databases (22,27–60) (see details in Table 1).
To facilitate elucidating the role of RNA in molecular interactions, more annotation information for the interactors was collected, including RNA modification sites from RMBase v2.0 (61), RNA subcellular localization from RNALocate (62), and RNA editing sites from RADAR (63), DARNED (64) and Lncediting (65). Simultaneously, the transcript and protein sequences from Refseq (66) and miRBase (67) were included to visualize the structure of RNA and represent target sites by miRanda, RIsearch (68) (tools for predicting RRIs), or PRIdictor (tool for predicting RPIs). The experimentally verified RNA-binding sites in proteins documented in the RBPDB (69), RsiteDB (70) and PDB (71) databases were also incorporated. Furthermore, we integrated the orthology/paralogy gene sets from miRBase and NCBI Gene (72) to reveal the conservation of homologous RNA-associated interactions across species.
Data procession
Integrating multisource data requires unifying them into common reference databases to annotate various interactors. Four major types of interactor symbols were used: (i) miRNA symbols from the miRBase database, (ii) DNA, RNA and protein symbols from the NCBI Gene or Ensembl (73) database, (iii) compound symbols from the PubChem Compound (74) database and (iv) histone modification symbols from the ChIPBase v2.0 database. Notably, each histone undergoes various modifications, and we separated RNA–histone modification interactions (RHIs) from RPIs to specify the relationship between RNA and histone modification. Additionally, Entrez ID, Ensembl Gene ID, miRBase accession, PubChem Compound CID and their external links are also provided, which can efficiently retrieve a substantial amount of genome-associated information from external resources. For the convenience of users, interactor information also included NCBI Aliases, DrugBank Aliases, OMIM ID, HGNC ID, HPRD ID, UniprotKB protein accession, among others. The software ‘RNAstructure’ (75) was used to predict RNA secondary structure.
In particular, we collected and processed four single-cell RNA-seq (scRNA-seq) data sets from the Gene Expression Omnibus (GEO) (76) to visualize the RNA molecular dynamic expression pattern during diverse stages of human (or mouse) spermatogenesis and HSC lineage commitment (77,78). Firstly, scRNA-seq reads were downloaded and processed to remove adaptor contaminants and low-quality bases using trimmomatics v0.36 (79). The processed clean reads were aligned to the human and mouse reference genome (hg38 and mm10 from GENCODE) using TopHat v2.0.12 (80). The HTSeq v0.11.0 (81) was used to estimate the gene expression of each single-cell. The transcript copy number, counted by distinct unique molecular identifiers (UMIs), was obtained by removing duplicated transcripts according to the UMI information. For a given cell, the number of UMIs represents the transcript number of each gene. Secondly, we filtered out cells with fewer than 2000 genes and 10 000 transcripts to retain high-quality cells. In total, we obtained 2414 human bone marrow cells (GSE75478), 99 mouse precursor-haematopoietic stem cells (GSE67120), 2,435 human testicular cells (GSE106487) and 1136 mouse spermatogenic cells (GSE107644). The RNA expression levels were normalized by transcripts per million (TPM). Finally, we evaluated the correlation between two RNAs with the Pearson correlation coefficient (PCC) during human (or mouse) spermatogenesis and HSC lineage commitment.
RESULTS
RNAInter statistics
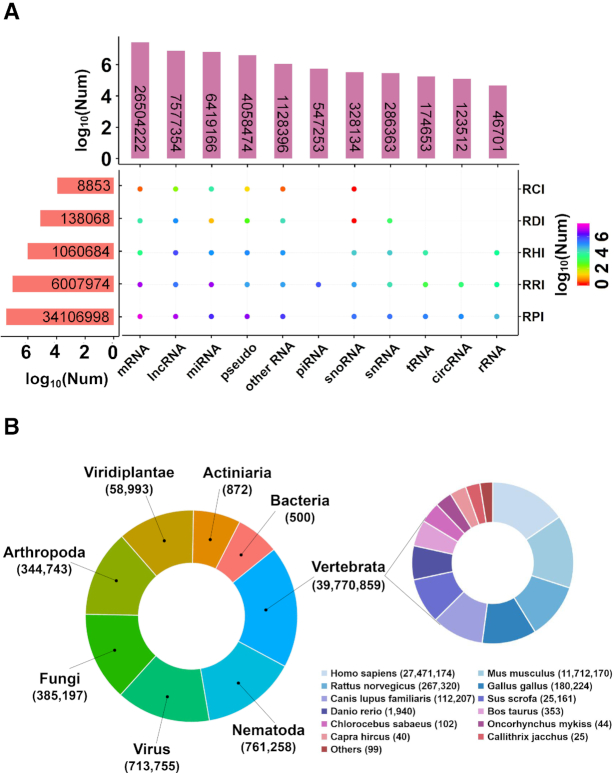
In summary, RNAInter contains 41 322 577 RNA-associated interactions, including 34 106 998 RPIs, 6 007 974 RRIs, 1 060 684 RHIs, 138 068 RDIs and 8853 RNA-compound interactions (RCIs) (Figure 2A, Table 1). These interactions involve 381 319 nonredundant RNAs and 42 215 nonredundant proteins, 33 970 newly added nonredundant DNAs, 425 nonredundant compounds and 61 nonredundant histone modifications. RNAInter involved 22 RNA types, eight of which added for the first time, including enhancer RNA (eRNA), Piwi-interacting RNA (piRNA), repeats, ribozyme, short hairpin RNA (shRNA), small Cajal body-specific RNA (scaRNA), small RNA (sRNA) and noncoding RNA (indefinite classified ncRNA) (Table 2). The distribution of the five types of interactions among different RNAs is shown in Figure 2A. The number of organisms in RNAInter increased from 60 to 154 compared with that in RAID v2.0 (Table 2). All the species covered nine categories (actiniaria, arthropoda, bacteria, fungi, mycetozoa, nematode, vertebrata, viridiplantae, virus). Homo sapiens and Mus musculus interactions took up the main part of the vertebrata (Figure 2B). Other model organisms, such as Drosophila melanogaster, Rattus norvegicus, Saccharomyces cerevisiae and zebrafish (Danio rerio), have also been documented in RNAInter.
Figure 2.
Statistics on RNAInter. (A) The distribution of five interaction types (RCI/RDI/RHI/RPI/RRI) in 22 RNA categories. The category ‘other RNA’ includes eRNA, ncRNA, others, repeats, ribozyme, scaRNA, scRNA, shRNA, sncRNA sRNA, unassigned RNA and unknown. (B) Number of interactions in vertebrata, nematoda, virus, arthropoda, fungi, viridiplantae, actiniaria, bacteria (left) and 28 species belonging to vertebrata (right).
Table 2.
The features and developments of RNAInter
| Feature | RAID v1.0 | RAID v2.0 | RNAInter |
|---|---|---|---|
| Interaction entry* | 6112 (6112) | 5 272 396 (2 426 181) | 41 322 577 (13,653,108) |
| RNA symbol | 2070 | 118 878 | 381 319 |
| Species coverage | 1 | 60 | 154 |
| Interaction type | RNA-protein/RNA-RNA | RNA-Protein/RNA-RNA | RNA-Protein/RNA-RNA/RNA-Compound/RNA-DNA/RNA-Histone modification |
| RNA category | lncRNA/miRNA/mRNA/rRNA/snoRNA | circRNA/lncRNA/miRNA/miscRNA/mRNA/pseudogene/rRNA/scRNA/sncRNA/snoRNA/snRNA/tRNA | circRNA/lncRNA/miRNA/miscRNA/mRNA/pseudogene/rRNA/scRNA/sncRNA/snoRNA/snRNA/sRNA/tRNA/eRNA/ncRNA/piRNA/repeats/ribozyme/scaRNA/shRNA/sRNA |
| Detailed information | Basic annotations/Evidence support/Reference/Tissue or cell line | Basic annotations/Evidence support/Interactor homolog/Integrated confidence score/Reference/RNA-binding sites | Basic annotations/Evidence support/Interactor homolog/Integrated confidence score/Reference/RNA-binding sites/Homology interaction/RNA editing/RNA localization/RNA modification/Target region |
| Data visualization | Predicted binding sites/ Interaction network | Predicted binding sites | Predicted binding sites/Interaction network/RNA dynamic expression/RNA structure |
| Web application | - | Advanced filter search | Exact search/Batch search/Fuzzy search/Four interactome tools: RIscoper, IntaRNA, PRIdictor, DeepBind |
*The number in brackets counts interactions entries verified by experimental methods.
Data feature and utility
Then, we expanded the RNA-compound, RNA–DNA and RNA–histone modification interactions in RNAInter. Apart from basic annotation, support evidence, RNA-binding sites and references, we focused on the multifaceted supplementation of the details of RNA editing/localization/modification/structure/dynamic expression, the interaction network, the target region and the homology interaction in detail. ‘RNA editing’ provides editing position, editing type and genetic region. ‘RNA localization’ includes subcellular localization and the tissue or cell line. ‘RNA modification’ involves the modification position, modification type and genetic region. Moreover, ‘Homology interaction’ shows the conservative interactions across organisms documented in RNAInter. ‘Target region’ shows the target locus in RHI/RPI/RRI and data accession from the literature or high-throughput sequencing with their sample resources. All this information links to their corresponding databases.
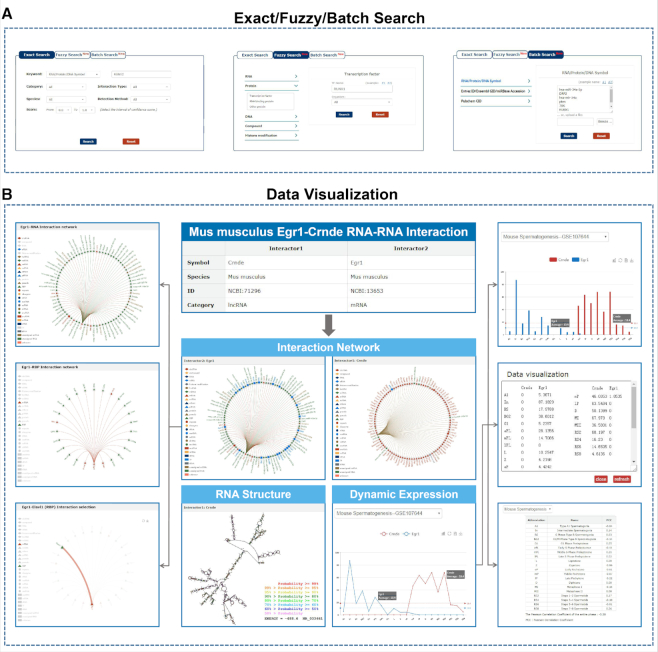
RNAInter provides a user-friendly platform for searching, browsing, visualizing and profiling RNA interactome data. To improve the search capability, RNAInter enables an optimized query with a new function of fuzzy and batch search. Fuzzy Search can help users to search interactions using unstandardized or uncertained interactor name under selected molecular category, then the result of interactions will be presented by selecting interactors in candidate list. Meanwhile, Batch Search supports for inputting a list of official symbols/IDs or uploading a file with text format to obtain multiple molecular categories associated interactions. Thus, users can select ‘Exact Search’ to filter the search results, or ‘Fuzzy Search’ to further focus on interactors of interest, or ‘Batch Search’ to customize their query content in batch (Figure 3A). Taking the load time into account, RNAInter offers the download option for over 2 million entries on the ‘Browse’ page. ‘RNA structure’ represents the putative RNA secondary structure for each transcript. In addition, ‘Interaction network’ is offered to picture the top 100 interactions ranked by integrative confidence score in RNAInter. Users can also select specific categories of RNA-associated interactions by clicking the different icons of interactor to conceal uninterested interactions for superior view. Click any edge of the network can jump to a detailed page of the corresponding entry (Figure 3B). To illustrate the RNA molecular dynamic expression pattern, ‘Dynamic expression’ shows the line chart of RNA expression values in each stage during human (or mouse) spermatogenesis and HSC lineage commitment and their expression correlation in each stage and entire phage with PCC (Figure 3B). The images of the interaction network and dynamic expression pattern can be downloaded.
Figure 3.
New search function and data visualization of the RNA interactome. (A) Presentation of exact, fuzzy and batch search described in the search options. (B) Visualization of the interaction network, RNA structure and RNA dynamic expression.
Extended toolkit
In response to the diverse needs of users, RNAInter embeds four interactome tools: RIscoper, IntaRNA, PRIdictor and DeepBind. RIscoper is a tool for RNA–RNA interaction extraction from the literature. IntaRNA is a program for the fast and accurate prediction of interactions between two RNA molecules. PRIdictor is a protein–RNA interaction predictor. DeepBind predicted the sequence specificities of DNA- and RNA-binding proteins by deep learning (Figure 4).
CONCLUSIONS AND PERSPECTIVES
RNAInter is an update of RAID v2.0, a comprehensive resource for RNA interactome data obtained from the literature and other databases, containing over 41 million RNA-associated interactions of RCI, RDI, RHI, RPI and RRI. With detailed interactome information, visualized interaction network and RNA dynamic expression, enhanced search functions, and embedded RNA interactome tools, RNAInter depicts a system-level RNA interactome landscape with guides and help researchers to perform further studies. We expect RNAInter to update the manual curation of RNA interactome data and expand the available information about RNAs and other molecules in the future. Continuously integrating high-throughput data, including scRNA-seq, to provide more precise depiction of the dynamic expression pattern of RNAs illuminates the role of RNA across organisms. We may optimize the confidence score strategy with the emergence of new mass sequencing technologies, experimental methods and prediction algorithms. At the same time, more RNA-associated applications are docking with our database. Eventually, RNAInter will present the most comprehensive map of the RNA interactome to satisfy different requirements.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China [81770104]. Funding for open access charge: National Natural Science Foundation of China[81770104].
Conflict of interest statement. None declared.
REFERENCES
- 1. Lal A., Navarro F., Maher C.A., Maliszewski L.E., Yan N., O’Day E., Chowdhury D., Dykxhoorn D.M., Tsai P., Hofmann O. et al.. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol. Cell. 2009; 35:610–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nowakowski T.J., Rani N., Golkaram M., Zhou H.R., Alvarado B., Huch K., West J.A., Leyrat A., Pollen A.A., Kriegstein A.R. et al.. Regulation of cell-type-specific transcriptomes by microRNA networks during human brain development. Nat. Neurosci. 2018; 21:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumjohann D., Ansel K.M.. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 2013; 13:666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshikawa T., Wu J., Otsuka M., Kishikawa T., Suzuki N., Takata A., Ohno M., Ishibashi R., Yamagami M., Nakagawa R. et al.. Repression of MicroRNA function mediates Inflammation-associated colon tumorigenesis. Gastroenterology. 2017; 152:631–643. [DOI] [PubMed] [Google Scholar]
- 5. Shi M., Hu X., Wei Y., Hou X., Yuan X., Liu J., Liu Y.. Genome-Wide profiling of small RNAs and degradome revealed conserved regulations of miRNAs on Auxin-Responsive genes during fruit enlargement in peaches. Int. J. Mol. Sci. 2017; 18:E2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma E., Sterne-Weiler T., O’Hanlon D., Blencowe B.J.. Global mapping of human RNA–RNA interactions. Mol. Cell. 2016; 62:618–626. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen T.C., Cao X., Yu P., Xiao S., Lu J., Biase F.H., Sridhar B., Huang N., Zhang K., Zhong S.. Mapping RNA–RNA interactome and RNA structure in vivo by MARIO. Nat. Commun. 2016; 7:12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Z., Zhang Q.C., Lee B., Flynn R.A., Smith M.A., Robinson J.T., Davidovich C., Gooding A.R., Goodrich K.J., Mattick J.S. et al.. RNA duplex map in living cells reveals Higher-Order transcriptome structure. Cell. 2016; 165:1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg M., Blum R., Kesner B., Maier V.K., Szanto A., Lee J.T.. Denaturing CLIP, dCLIP, pipeline identifies discrete RNA footprints on Chromatin-Associated proteins and reveals that CBX7 targets 3′ UTRs to regulate mRNA expression. Cell Syst. 2017; 5:368–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M. Jr, Jungkamp A.C., Munschauer M. et al.. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010; 141:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J., Sarma K., Song J.J., Kingston R.E., Borowsky M., Lee J.T.. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010; 40:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maticzka D., Ilik I.A., Aktas T., Backofen R., Akhtar A.. uvCLAP is a fast and non-radioactive method to identify in vivo targets of RNA-binding proteins. Nat. Commun. 2018; 9:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu C., Qu K., Zhong F.L., Artandi S.E., Chang H.Y.. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell. 2011; 44:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akhade V.S., Arun G., Donakonda S., Rao M.R.. Genome wide chromatin occupancy of mrhl RNA and its role in gene regulation in mouse spermatogonial cells. RNA Biol. 2014; 11:1262–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sridhar B., Rivas-Astroza M., Nguyen T.C., Chen W., Yan Z., Cao X., Hebert L., Zhong S.. Systematic mapping of RNA-Chromatin interactions in vivo. Curr. Biol.: CB. 2017; 27:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L., Lin C., Jin C., Yang J.C., Tanasa B., Li W., Merkurjev D., Ohgi K.A., Meng D., Zhang J. et al.. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013; 500:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin A., Hu Q., Li C., Xing Z., Ma G., Wang C., Li J., Ye Y., Yao J., Liang K. et al.. The LINK-A lncRNA interacts with PtdIns(3,4,5)P3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat. Cell Biol. 2017; 19:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malek E., Jagannathan S., Driscoll J.J.. Correlation of long non-coding RNA expression with metastasis, drug resistance and clinical outcome in cancer. Oncotarget. 2014; 5:8027–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez-Barrueco R., Nekritz E.A., Bertucci F., Yu J., Sanchez-Garcia F., Zeleke T.Z., Gorbatenko A., Birnbaum D., Ezhkova E., Cordon-Cardo C. et al.. miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy. Genes Dev. 2017; 31:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H.M., Kuang S., Xiong X., Gao T., Liu C., Guo A.Y.. Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases. Brief. Bioinform. 2015; 16:45–58. [DOI] [PubMed] [Google Scholar]
- 21. Elsheikh S.E., Green A.R., Rakha E.A., Powe D.G., Ahmed R.A., Collins H.M., Soria D., Garibaldi J.M., Paish C.E., Ammar A.A. et al.. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009; 69:3802–3809. [DOI] [PubMed] [Google Scholar]
- 22. Yi Y., Zhao Y., Li C., Zhang L., Huang H., Li Y., Liu L., Hou P., Cui T., Tan P. et al.. RAID v2.0: an updated resource of RNA-associated interactions across organisms. Nucleic Acids Res. 2017; 45:D115–D118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Liu T., Chen L., Yang J., Yin J., Zhang Y., Yun Z., Xu H., Ning L., Guo F. et al.. RIscoper: a tool for RNA–RNA interaction extraction from the literature. Bioinformatics. 2019; 35:3199–3202. [DOI] [PubMed] [Google Scholar]
- 24. Mann M., Wright P.R., Backofen R.. IntaRNA 2.0: enhanced and customizable prediction of RNA–RNA interactions. Nucleic Acids Res. 2017; 45:W435–W439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuvshinjargal N., Lee W., Park B., Han K.. PRIdictor: protein-RNA Interaction predictor. Biosystems. 2016; 139:17–22. [DOI] [PubMed] [Google Scholar]
- 26. Alipanahi B., Delong A., Weirauch M.T., Frey B.J.. Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015; 33:831–838. [DOI] [PubMed] [Google Scholar]
- 27. Liu X., Wang S., Meng F., Wang J., Zhang Y., Dai E., Yu X., Li X., Jiang W.. SM2miR: a database of the experimentally validated small molecules' effects on microRNA expression. Bioinformatics. 2013; 29:409–411. [DOI] [PubMed] [Google Scholar]
- 28. Dai E., Yang F., Wang J., Zhou X., Song Q., An W., Wang L., Jiang W.. ncDR: a comprehensive resource of non-coding RNAs involved in drug resistance. Bioinformatics. 2017; 33:4010–4011. [DOI] [PubMed] [Google Scholar]
- 29. Xie W., Yan H., Zhao X.M.. EmDL: extracting miRNA-drug interactions from literature. IEEE/ACM Trans. Comput. Biol. Bioinf. 2017; doi:10.1109/TCBB.2017.2723394. [DOI] [PubMed] [Google Scholar]
- 30. Yu F., Zhang G., Shi A., Hu J., Li F., Zhang X., Zhang Y., Huang J., Xiao Y., Li X. et al.. LnChrom: a resource of experimentally validated lncRNA-chromatin interactions in human and mouse. Database. 2018; 2018:bay039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu Y., Xu G., Yang Y.T., Xu Z., Chen X., Shi B., Xie D., Lu Z.J., Wang P.. POSTAR2: deciphering the post-transcriptional regulatory logics. Nucleic Acids Res. 2019; 47:D203–D211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tong Z., Cui Q., Wang J., Zhou Y.. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019; 47:D253–D258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gong J., Shao D., Xu K., Lu Z., Lu Z.J., Yang Y.T., Zhang Q.C.. RISE: a database of RNA interactome from sequencing experiments. Nucleic Acids Res. 2018; 46:D194–D201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng L., Wang P., Tian R., Wang S., Guo Q., Luo M., Zhou W., Liu G., Jiang H., Jiang Q.. LncRNA2Target v2.0: a comprehensive database for target genes of lncRNAs in human and mouse. Nucleic Acids Res. 2019; 47:D140–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qureshi A., Thakur N., Monga I., Thakur A., Kumar M.. VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database. 2014; 2014:bau103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang P., Li X., Gao Y., Guo Q., Wang Y., Fang Y., Ma X., Zhi H., Zhou D., Shen W. et al.. LncACTdb 2.0: an updated database of experimentally supported ceRNA interactions curated from low- and high-throughput experiments. Nucleic Acids Res. 2019; 47:D121–D127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hao Y., Wu W., Li H., Yuan J., Luo J., Zhao Y., Chen R.. NPInter v3.0: an upgraded database of noncoding RNA-associated interactions. Database. 2016; 2016:baw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang D., Gu J., Wang T., Ding Z.. OncomiRDB: a database for the experimentally verified oncogenic and tumor-suppressive microRNAs. Bioinformatics. 2014; 30:2237–2238. [DOI] [PubMed] [Google Scholar]
- 39. Wu D., Huang Y., Kang J., Li K., Bi X., Zhang T., Jin N., Hu Y., Tan P., Zhang L. et al.. ncRDeathDB: A comprehensive bioinformatics resource for deciphering network organization of the ncRNA-mediated cell death system. Autophagy. 2015; 11:1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang Q., Wang Y., Hao Y., Juan L., Teng M., Zhang X., Li M., Wang G., Liu Y.. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009; 37:D98–D104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J., Liu T., Zhao B., Lu Q., Wang Z., Cao Y., Li W.. sRNATarBase 3.0: an updated database for sRNA-target interactions in bacteria. Nucleic Acids Res. 2016; 44:D248–D253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cui T., Zhang L., Huang Y., Yi Y., Tan P., Zhao Y., Hu Y., Xu L., Li E., Wang D.. MNDR v2.0: an updated resource of ncRNA-disease associations in mammals. Nucleic Acids Res. 2018; 46:D371–D374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bao Z., Yang Z., Huang Z., Zhou Y., Cui Q., Dong D.. LncRNADisease 2.0: an updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019; 47:D1034–D1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou K.R., Liu S., Sun W.J., Zheng L.L., Zhou H., Yang J.H., Qu L.H.. ChIPBase v2.0: decoding transcriptional regulatory networks of non-coding RNAs and protein-coding genes from ChIP-seq data. Nucleic Acids Res. 2017; 45:D43–D50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H.. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chou C.H., Shrestha S., Yang C.D., Chang N.W., Lin Y.L., Liao K.W., Huang W.C., Sun T.H., Tu S.J., Lee W.H. et al.. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018; 46:D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang B., Armaos A., Tartaglia G.G.. RNAct: Protein-RNA interaction predictions for model organisms with supporting experimental data. Nucleic Acids Res. 2019; 47:D601–D606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong N., Wang X.. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015; 43:D146–D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Betel D., Koppal A., Agius P., Sander C., Leslie C.. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010; 11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu W.S., Brown J.S., Chen T.T., Chu Y.H., Huang W.C., Tu S., Lee H.C.. piRTarBase: a database of piRNA targeting sites and their roles in gene regulation. Nucleic Acids Res. 2019; 47:D181–D187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elefant N., Berger A., Shein H., Hofree M., Margalit H., Altuvia Y.. RepTar: a database of predicted cellular targets of host and viral miRNAs. Nucleic Acids Res. 2011; 39:D188–D194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agarwal V., Bell G.W., Nam J.W., Bartel D.P.. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015; 4:e05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gaidatzis D., van Nimwegen E., Hausser J., Zavolan M.. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinformatics. 2007; 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murali T., Pacifico S., Yu J., Guest S., Roberts G.G. 3rd, Finley R.L. Jr. DroID 2011: a comprehensive, integrated resource for protein, transcription factor, RNA and gene interactions for Drosophila. Nucleic Acids Res. 2011; 39:D736–D743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pylro V.S., Oliveira F.S., Morais D.K., Cuadros-Orellana S., Pais F.S., Medeiros J.D., Geraldo J.A., Gilbert J., Volpini A.C., Fernandes G.R.. ZIKV - CDB: A collaborative database to guide research linking SncRNAs and ZIKA virus disease symptoms. PLoS Negl. Trop. Dis. 2016; 10:e0004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ghosal S., Das S., Sen R., Chakrabarti J.. HumanViCe: host ceRNA network in virus infected cells in human. Front. Genet. 2014; 5:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeggari A., Marks D.S., Larsson E.. miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012; 28:2062–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shao T., Zhao Z., Wu A., Bai J., Li Y., Chen H., Jiang C., Wang Y., Li S., Wang L. et al.. Functional dissection of virus-human crosstalk mediated by miRNAs based on the VmiReg database. Mol. Biosyst. 2015; 11:1319–1328. [DOI] [PubMed] [Google Scholar]
- 59. Junge A., Refsgaard J.C., Garde C., Pan X., Santos A., Alkan F., Anthon C., von Mering C., Workman C.T., Jensen L.J. et al.. RAIN: RNA–protein association and interaction networks. Database. 2017; 2017:baw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Y., Wang C., Miao Z., Bi X., Wu D., Jin N., Wang L., Wu H., Qian K., Li C. et al.. ViRBase: a resource for virus-host ncRNA-associated interactions. Nucleic Acids Res. 2015; 43:D578–D582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xuan J.J., Sun W.J., Lin P.H., Zhou K.R., Liu S., Zheng L.L., Qu L.H., Yang J.H.. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018; 46:D327–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang T., Tan P., Wang L., Jin N., Li Y., Zhang L., Yang H., Hu Z., Zhang L., Hu C. et al.. RNALocate: a resource for RNA subcellular localizations. Nucleic Acids Res. 2017; 45:D135–D138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramaswami G., Li J.B.. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res. 2014; 42:D109–D113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kiran A., Baranov P.V.. DARNED: a DAtabase of RNa EDiting in humans. Bioinformatics. 2010; 26:1772–1776. [DOI] [PubMed] [Google Scholar]
- 65. Gong J., Liu C., Liu W., Xiang Y., Diao L., Guo A.Y., Han L.. LNCediting: a database for functional effects of RNA editing in lncRNAs. Nucleic Acids Res. 2017; 45:D79–D84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haft D.H., DiCuccio M., Badretdin A., Brover V., Chetvernin V., O’Neill K., Li W., Chitsaz F., Derbyshire M.K., Gonzales N.R. et al.. RefSeq: an update on prokaryotic genome annotation and curation. Nucleic Acids Res. 2018; 46:D851–D860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019; 47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wenzel A., Akbasli E., Gorodkin J.. RIsearch: fast RNA–RNA interaction search using a simplified nearest-neighbor energy model. Bioinformatics. 2012; 28:2738–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cook K.B., Kazan H., Zuberi K., Morris Q., Hughes T.R.. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res. 2011; 39:D301–D308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shulman-Peleg A., Nussinov R., Wolfson H.J.. RsiteDB: a database of protein binding pockets that interact with RNA nucleotide bases. Nucleic Acids Res. 2009; 37:D369–D373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burley S.K., Berman H.M., Bhikadiya C., Bi C., Chen L., Di Costanzo L., Christie C., Dalenberg K., Duarte J.M., Dutta S. et al.. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019; 47:D464–D474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brown G.R., Hem V., Katz K.S., Ovetsky M., Wallin C., Ermolaeva O., Tolstoy I., Tatusova T., Pruitt K.D., Maglott D.R. et al.. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 2015; 43:D36–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cunningham F., Achuthan P., Akanni W., Allen J., Amode M.R., Armean I.M., Bennett R., Bhai J., Billis K., Boddu S. et al.. Ensembl 2019. Nucleic Acids Res. 2019; 47:D745–D751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., Han L., He J., He S., Shoemaker B.A. et al.. PubChem substance and compound databases. Nucleic Acids Res. 2016; 44:D1202–D1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bellaousov S., Reuter J.S., Seetin M.G., Mathews D.H.. RNAstructure: Web servers for RNA secondary structure prediction and analysis. Nucleic Acids Res. 2013; 41:W471–W474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M. et al.. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013; 41:D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou F., Li X., Wang W., Zhu P., Zhou J., He W., Ding M., Xiong F., Zheng X., Li Z. et al.. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016; 533:487–492. [DOI] [PubMed] [Google Scholar]
- 78. Wang M., Liu X., Chang G., Chen Y., An G., Yan L., Gao S., Xu Y., Cui Y., Dong J. et al.. Single-Cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 2018; 23:599–614. [DOI] [PubMed] [Google Scholar]
- 79. Bolger A.M., Lohse M., Usadel B.. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30:2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trapnell C., Pachter L., Salzberg S.L.. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009; 25:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Anders S., Pyl P.T., Huber W.. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015; 31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.