Abstract
The leaf senescence database (LSD) is a comprehensive resource of senescence-associated genes (SAGs) and their corresponding mutants. Through manual curation and extensive annotation, we updated the LSD to a new version LSD 3.0, which contains 5853 genes and 617 mutants from 68 species. To provide sustainable and reliable services for the plant research community, LSD 3.0 (https://bigd.big.ac.cn/lsd/) has been moved to and maintained by the National Genomics Data Center at Beijing Institute of Genomics, Chinese Academy of Sciences. In the current release, we added some new features: (i) Transcriptome data of leaf senescence in poplar were integrated; (ii) Leaf senescence-associated transcriptome data information in Arabidopsis, rice and soybean were included; (iii) Senescence-differentially expressed small RNAs (Sen-smRNA) in Arabidopsis were identified; (iv) Interaction pairs between Sen-smRNAs and senescence-associated transcription factors (Sen-TF) were established; (v) Senescence phenotypes of 90 natural accessions (ecotypes) and 42 images of ecotypes in Arabidopsis were incorporated; (vi) Mutant seed information of SAGs in rice obtained from Kitbase was integrated; (vii) New options of search engines for ecotypes and transcriptome data were implemented. Together, the updated database bears great utility to continue to provide users with useful resources for studies of leaf senescence.
INTRODUCTION
Plant leaves harvest light energy and fix CO2 to produce carbohydrates, and serve as a major food source on the earth (1). The leaf undergoes complex developmental and physiological transitions during their life history. Senescence is the final stage of leaf lifespan and is essential for plant fitness as nutrient remobilization from senescing leaves to developing organs through this process (2–4). Therefore, leaf senescence has been regarded as a genetically controlled biological process that was evolutionarily acquired for better fitness and survival (5). Efforts to understand the molecular regulatory mechanisms underlying leaf senescence have been largely made by genetic, genomic, transcriptomic, proteomic and metabolomic studies, revealing that leaf senescence is a highly coordinated process regulated by a large number of senescence-associated genes (SAGs) (5). Forward genetic studies of leaf senescence by screening senescence-related mutants and reverse genetic analyses of SAGs in plants have provided deep insights into the molecular basis of leaf senescence (6). To facilitate systematic and comparative studies of leaf senescence, we developed the leaf senescence database (LSD) in 2010 and updated to LSD 2.0 in 2014 with 5356 genes and 324 mutants from 44 species (7,8). These SAGs were manually retrieved based on experimental evidence and were categorized according to their functions in leaf senescence. We performed extensive curations through both manual and computational approaches to provide comprehensive annotations for SAGs. Currently, LSD has been widely used for functional studies of SAGs in Arabidopsis and systematic identification of SAGs in agronomically important plants (9–13).
In the past five years, continuously increasing efforts have been devoted to the field of leaf senescence studies, accordingly leading to the identification of a number of genes as functional SAGs. For example, circadian clock genes, such as EARLY FLOWERING 3 (ELF3), EARLY FLOWERING 4 (ELF4), LUX ARRHYTHMO (LUX) and PSEUDO-RESPONSE REGULATOR 9 (PRR9), affect both dark-induced and age-dependent leaf senescence (14,15). Specifically, PRR9 promotes leaf senescence through directly transcriptional activation of ORESARA1 (ORE1), an important positive regulator of senescence (16,17), and indirectly via suppressing miR164, a post-transcriptional repressor of ORE1. Recently, epigenetic regulation pathways have been found to be involved in regulating the leaf senescence process (18). The histone H3K4 demethylase JMJ16 negatively regulates age-dependent leaf senescence, and loss-of-function of JMJ16 increases H3K4me3 levels and induces the expression of numerous SAGs (13). The H3K27me3 demethylase REF6 positively regulates senescence process by directly upregulating SAGs, such as ETHYLENE INSENSITIVE 2 (EIN2) and ORE1 (19). Reverse genetic studies have also revealed that several senescence-associated transcription factors (Sen-TFs), for example, WRKY75, ANAC019, ANAC032, ANAC072 and OsNAC2, function as positive regulators of leaf senescence (11,20,21). The ABA receptor PYRABACTIN RESISTANCE 1-LIKE 9 (PYL9) accelerates leaf senescence but promotes extreme drought tolerance in Arabidopsis (22). Moreover, high-resolution time-course transcriptome analyses in Arabidopsis identified a large number of new SAGs (23) through small RNA-TF regulatory networks (24), especially miRNAs and transacting small interfering RNAs (tasiRNAs), providing new insights into the fine regulation of leaf senescence process.
To cover the important progress achieved in the past several years and extend the web functionality of LSD, we upgraded it to a new version LSD 3.0 by extensive manual curation and annotation. LSD 3.0 integrates a comprehensive collection of 5853 genes and 617 mutants from 68 species (Table 1 and Supplementary Table S1), an extension from LSD 2.0 containing 5356 genes and 322 mutants from 44 species. To facilitate comparative study of the molecular regulatory mechanisms of leaf senescence in perennial and annual plants, we identified 678 SAGs in poplar leaves by high-resolution temporal transcriptome analysis of autumn leaf senescence. New features were included in the current version, including images of Arabidopsis ecotypes, senescence differentially expressed small RNAs (DEsmRNA), DEsmRNA–SenTFs interaction pairs, and implementation of new options of search engines for ecotypes and transcriptome data.
Table 1.
Statistics and comparisons of gene number among the three versions of LSD
| Species | LSD 1.0 | LSD 2.0 | LSD 3.0 |
|---|---|---|---|
| Grain Amaranths (Amaranthus hypochondriacus) | 0 | 1 | 1 |
| Arabidopsis lyrata | 0 | 2 | 2 |
| Arabidopsis thaliana | 949 | 3744 | 3852 |
| Chinese Milk Vetch (Astragalus sinicus) | 1 | 1 | 1 |
| Birch (Betula pendula) | 0 | 0 | 1 |
| Cabbage (Brassica campestris) | 0 | 2 | 2 |
| Rapeseed (Brassica napus) | 15 | 8 | 13 |
| Broccoli (Brassica oleracea) | 4 | 9 | 9 |
| Turnip (Brassica rapa) | 0 | 0 | 1 |
| Chinese cabbage (Brassica rapa subsp. Pekinensis) | 0 | 1 | 1 |
| Cabbage (Brassica rapa var. parachinensis) | 0 | 5 | 19 |
| Tea (Camellia sinensis) | 0 | 1 | 3 |
| Pepper (Capsicum annuum) | 0 | 1 | 3 |
| Red goosefoot (Chenopodium rubrum) | 1 | 1 | 1 |
| Chrysanthemum (Chrysanthemum morifolium) | 0 | 0 | 1 |
| Sweet Orange (Citrus sinensis) | 0 | 0 | 4 |
| Autumn Crocus (Crocus sativus) | 0 | 1 | 1 |
| Muskmelon (Cucumis melo) | 0 | 1 | 1 |
| Carrot (Daucus carota) | 0 | 1 | 1 |
| Carnation (Dianthus caryophyllus) | 0 | 1 | 1 |
| Persimmon (Diospyros kaki) | 0 | 0 | 2 |
| Erianthus arundinaceus | 0 | 0 | 1 |
| Tall fescue (Festuca arundinacea) | 0 | 1 | 1 |
| Fescue (Festuca pratensis Huds) | 1 | 1 | 1 |
| Strawberry (Fragaria x ananassa) | 0 | 1 | 1 |
| Soybean (Glycine max) | 4 | 12 | 20 |
| Cotton (Gossypium hirsutum) | 0 | 0 | 15 |
| Sunflower (Helianthus annuus) | 0 | 0 | 5 |
| Barley (Hordeum vulgare) | 3 | 14 | 19 |
| Sweet potato (Ipomoea batatas) | 0 | 4 | 8 |
| Japanese morning glory (Ipomoea nil) | 1 | 1 | 2 |
| Physic nut (Jatropha curcas) | 0 | 0 | 1 |
| Easter lily (Lilium longiflorum) | 0 | 0 | 1 |
| Litchi trees (Litchi chinensis) | 0 | 0 | 1 |
| Perennial ryegrass (Lolium perenne) | 0 | 4 | 5 |
| Apple (Malus domestica) | 0 | 0 | 3 |
| Chinese crabapple (Malus prunifolia) | 0 | 0 | 2 |
| Mango (Mangifera indica) | 0 | 1 | 1 |
| Alfalfa (Medicago sativa) | 1 | 2 | 3 |
| Medicago truncatula | 31 | 31 | 31 |
| Miscanthus lutarioriparius | 0 | 0 | 11 |
| Mulberry (Morus alba) | 0 | 0 | 1 |
| Banana (Musa acuminata) | 0 | 882 | 882 |
| Banana (Musa x paradisiaca) | 0 | 0 | 1 |
| Bamboo (Neosinocalamus affinis) | 0 | 1 | 1 |
| Coyote tobacco (Nicotiana attenuata) | 0 | 1 | 1 |
| Tobacco (Nicotiana tabacum) | 5 | 9 | 18 |
| Rice (Oryza sativa) | 104 | 132 | 188 |
| Petunia (Petunia hybrida) | 0 | 1 | 1 |
| Picrorhiza (Picrorhiza kurrooa Royle ex Benth) | 0 | 0 | 1 |
| Pea (Pisum sativum) | 4 | 6 | 6 |
| Balloon flower (Platycodon grandiflorum) | 0 | 1 | 1 |
| Poplar (Populus tremula x Populus tremuloides) | 0 | 0 | 198 |
| Poplar (Populus trichocarpa) | 0 | 0 | 1 |
| Peach (Prunus persica L. Batsch) | 0 | 0 | 1 |
| Pear (Pyrus communis) | 0 | 0 | 1 |
| Radish (Raphanus sativus) | 0 | 0 | 1 |
| Rose (Rosa hybrida) | 1 | 1 | 1 |
| Foxtail millet (Setaria italica) | 0 | 0 | 2 |
| Tomato (Solanum lycopersicon) | 8 | 23 | 37 |
| Potato (Solanum tuberosum) | 3 | 3 | 6 |
| Sorghum (Sorghum bicolor) | 4 | 26 | 26 |
| Spinach (Spinacia oleracea) | 0 | 2 | 2 |
| Sugarcane | 0 | 0 | 1 |
| Wheat (Triticum aestivum) | 1 | 256 | 259 |
| Wheat (Triticum turgidum) | 1 | 65 | 65 |
| Cowpea (Vigna unguiculata) | 0 | 1 | 1 |
| Maize (Zea mays) | 3 | 94 | 98 |
| Total 68 | 1145 | 5356 | 5853 |
NEW FEATURES
Data collection
We collected all SAGs and mutants from published papers from January 2014 to April 2019 by searching the PubMed literature database with keywords ‘leaf senescence’, ‘leaf & senescence’, ‘plant senescence’ and ‘plant aging’, respectively. Then, we performed manual curation to retrieve a wide range of information, including gene name, locus name, GenBank ID, PubMed ID, mutant, species, senescence-associated phenotypes, the effect on leaf senescence and evidence. At last, we made extensive annotations for these SAGs through computational approaches (8).
Database access
To provide sustainable and reliable services to the plant leaf senescence research community, the website of LSD 3.0 has been moved to and maintained by the National Genomics Data Center (formerly named as BIG Data Center) (25) at Beijing Institute of Genomics, Chinese Academy of Sciences, and is publicly available at https://bigd.big.ac.cn/lsd/. Users can browse, search and download all the data through friendly web interfaces. A tree-like structure was designed for both species and phenotypes, and tables were also used to organize all relevant information for species, mutants, QTL, ecotypes, Arabidopsis and rice seeds, sen-smRNA, poplar transcriptome and public transcriptome data obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). In addition, the text search interface was updated and improved by allowing users to perform six types of queries: (i) GenBank ID, species, effects and description of genes; (ii) name and ecotype of mutants; (iii) title, author, journal and date of literature papers; (iv) locus name, alias and keywords; (v) miRNA name and (vi) locus name of poplar transcriptome.
Example of annotation
LSD 3.0 features comprehensive collection and extensive annotations of SAGs. A typical example is NOE1 (LOC_Os03g03910) encoding a rice catalase. Loss-of-function of NOE1 promotes leaf senescence by increasing the production of H2O2 in the leaves (26). Accordingly, the current release of LSD provided a wealth of information for NOE1 obtained by both manual and computational approaches (Figure 1). We performed detailed annotations and organized all relevant information in terms of basic information (locus name, organism, function category, effect for senescence, evidence, references, protein–protein interactions and sequence) (Figure 1A), mutant information (Figure 1B), miRNA interaction (Figure 1C), ortholog group (Figure 1D), cross link (Figure 1E) as well as newly added mutant seed information (Figure 1F).
Figure 1.
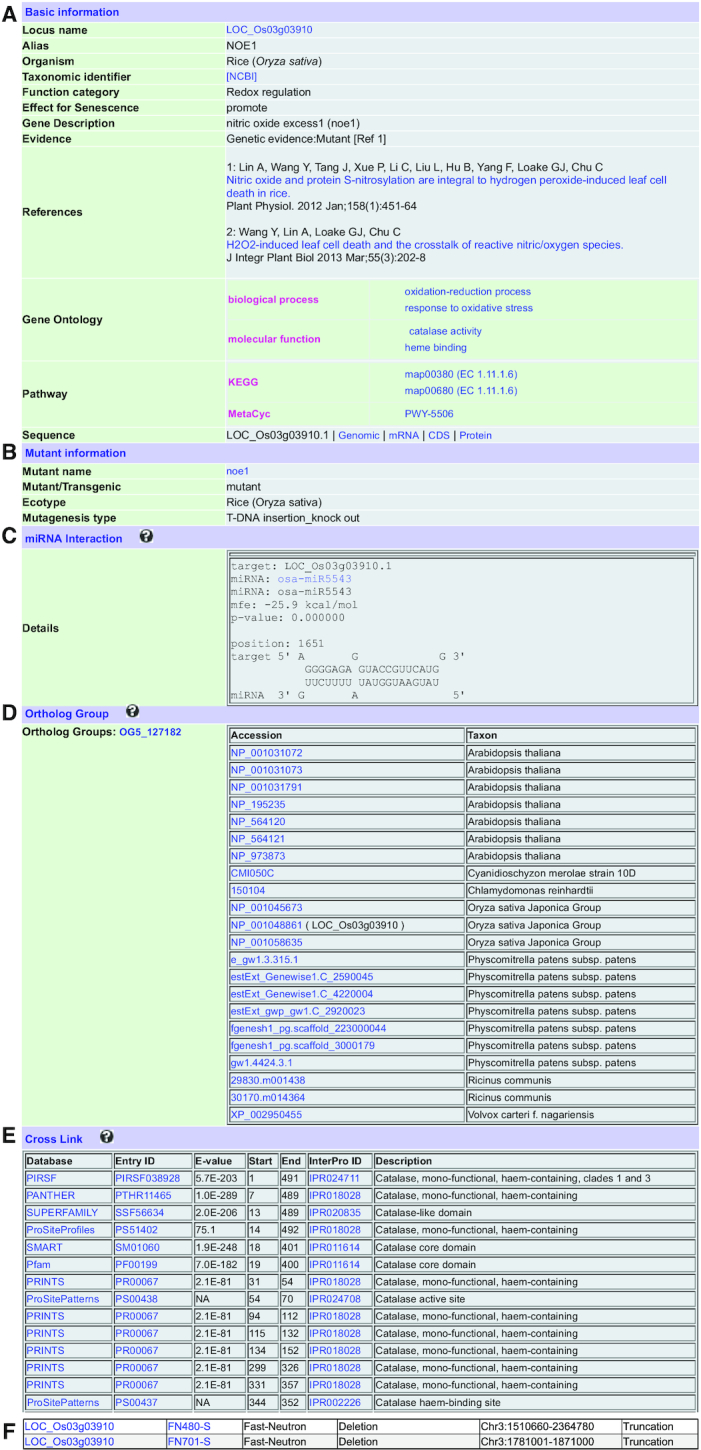
A typical entry for the rice NOE1 gene (LOC_Os03g03910) in LSD 3.0. (A) Basic information, (B) Mutant information, (C) miRNA interaction, (D) Ortholog group, (E) Cross link to other databases and (F) Newly added mutant seed information.
Transcriptome data of leaf senescence in Poplar
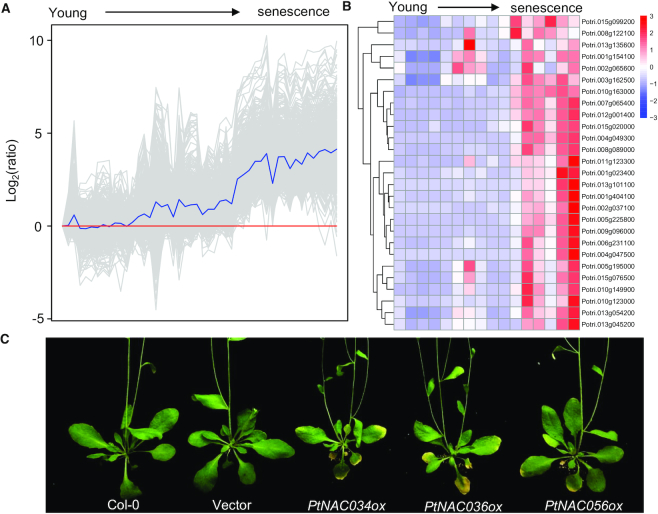
At present, thousands of SAGs have been identified and functionally studied in annual plants such as Arabidopsis, rice, maize or sorghum (5), while fewer SAGs have been identified in perennial woody plants due to the lack of well-annotated whole genomes (27). Poplar (Populus trichocarpa) is the first sequenced genome of the forest tree because of its modest genome size, rapid growth rate and relative ease of experimental manipulation (27). To provide the transcriptomic picture of leaf senescence in perennial plants, we performed high-resolution time-course profiling of gene expression during autumn leaf senescence in field-grown poplar by RNA sequencing (Figure 2). In total, 678 SAGs were identified according to their increased expression levels as leaves age (Figure 2A). Given that leaf senescence is finely tuned by many regulatory factors such as TFs, the senescence-associated TF (Sen-TFs) in poplar were identified and functionally characterized (Figure 2B). As shown in Figure 2C, overexpression of three poplar Sen-TFs (PtNAC034, PtNAC036 and PtNAC056) accelerates leaf senescence process in Arabidopsis demonstrated by the earlier leaf yellowing, suggesting that these genes are positive regulators of leaf senescence. Additionally, we identified the senescence-downregulated genes and integrated them in the updated database to provide comprehensive gene expression profiles during autumn leaf senescence.
Figure 2.
Identification and functional analysis of SAGs in poplar. (A) Identification of SAGs by high-resolution temporal transcriptome of autumn leaf senescence in poplar. (B) Heat map showing the expression pattern of several Sen-TFs as leaves age in poplar. (C) Functional analysis of poplar Sen-TFs in Arabidopsis reveals that PtNAC034, PtNAC036 and PtNAC056 positively regulate leaf senescence.
Newly added annotations
To help researchers study the function of SAGs in rice, the mutant seed information obtained from Kitbase (https://kitbase.ucdavis.edu/) was integrated into LSD 3.0. As leaf senescence is influenced by numerous environmental factors such as photoperiod and temperature under natural growth conditions, natural accessions (ecotypes) provide valuable materials to understand the regulatory mechanisms underlying leaf senescence (28,29). To this end, the senescence phenotype information of 90 ecotypes was added in the updated version. High-resolution and multi-dimensional analyses of transcriptome during leaf senescence provide a wealth of information to understand leaf senescence at the molecular level (24). To facilitate researchers to quickly obtain these resources, we searched the relevant transcriptome information from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) and integrated them into LSD 3.0. Given that small RNAs (smRNA) have been demonstrated to be involved in leaf senescence by regulating their target genes, we added the senescence DEsmRNAs as well as DEsmRNA–Sen-TFs interaction pairs into LSD 3.0.
DISCUSSION AND FUTURE DIRECTIONS
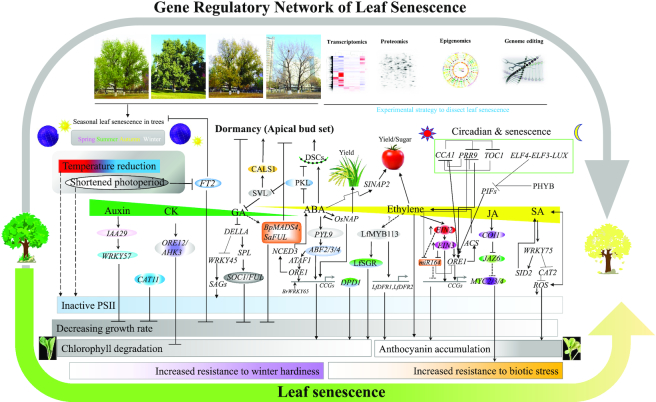
In the updated version, we have collected the SAGs from 68 species, including annual herbaceous plants such as Arabidopsis, rice, maize or sorghum, as well as perennial woody plants such as poplar. To our knowledge, LSD 3.0 is the only available resource specialized in leaf senescence, providing a convenient way to study leaf senescence via comparative biological strategy and construction of gene regulatory network (Figure 3). For example, the Arabidopsis NAC TF AtNAP has been demonstrated to be a key positive regulator of leaf senescence (30). Interestingly, a forward genetic screen shows that OsNAP, a homolog of AtNAP, also promotes leaf senQ'1escence in rice. More importantly, the silencing of OsNAP leads to an extension of the grain filling period and significantly increases grain yield (31). Because a lot of important breakthroughs for leaf senescence have been achieved in the model plant Arabidopsis (5), it is reasonable to translate these findings to guide senescence research in other plants. Toward this end, we listed the functional SAGs (delay or promote) as well as their curated annotations in Arabidopsis to help researchers identify the candidate SAGs in crops (Supplementary Tables S2 and 3), as testified by the fact that SAGs in maize, sorghum and cotton have been identified by using the LSD data (9,32,33). In addition, transcriptome data of leaf senescence in poplar deposited in LSD 3.0 could be helpful for us to perform a comparative analysis of leaf senescence between annual and perennial plants and explore the difference and/or similarity of their regulatory mechanisms.
Figure 3.
A gene regulatory network of leaf senescence with the integrated data from multiple species such as Arabidopsis, rice, rapeseed, tomato and poplar through an extensive literature survey.
To better serve the plant senescence research community, we plan to improve the database from the following aspects: (i) To integrate newly identified SAGs and mutant information via manual curation and computational annotation; (ii) To collect the senescence-associated phenotypes of ∼1150 ecotypes in the future because the ecotypes could help us better understand the relationship between senescence and environmental factors or other developmental traits such as flowering (Supplementary Figure S1); (iii) To collect worldwide publicly available publications (not limited to PubMed) related to leaf senescence; (iv) To update and improve web interfaces according to the suggestions from users; and (v) To develop online tools to facilitate comparative analysis of leaf senescence between annual and perennial plants. Taken together, considering that leaf senescence is a crucial biological process that exerts considerable influences on crop yield and quality, the updated LSD 3.0 would be of great help and broad utility for the plant research community.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Xiaochuan Liu for his contribution to the design of the first version of LSD. We are also grateful to LSD users for their valuable suggestions or notification of problems with the website. We also would like to thank the Arabidopsis Biological Resource Center (ABRC) for propagating the ecotype lines.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Strategic Priority Research Program of the Chinese Academy of Sciences [XDA19050302, XDB13040500 to Z.Z.]; National Natural Science Foundation of China [31970196 to Z.L., 31570286 to H.G., 31900173 to H.W.]; Chinese Postdoctoral Science Foundation [2019M650514 to Y. Z., 2019M650516 to H.W]; Beijing Advanced Innovation Center for Tree Breeding by Molecular Design, Beijing Forestry University’, National Key Research and Development Program of China, Startup Funding [2017YFC0907502 to Z.Z.]; 13th Five-year Informatization Plan of Chinese Academy of Sciences [XXH13505-05 to Z.Z.]; International Partnership Program of the Chinese Academy of Sciences [153F11KYSB20160008].
Conflict of interest statement. None declared.
REFERENCES
- 1. Zhu X.G., Long S.P., Ort D.R.. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010; 61:235–261. [DOI] [PubMed] [Google Scholar]
- 2. Lim P.O., Kim H.J., Nam H.G.. Leaf senescence. Annu. Rev. Plant Biol. 2007; 58:115–136. [DOI] [PubMed] [Google Scholar]
- 3. Guo Y., Gan S.. Leaf senescence: signals, execution, and regulation. Cur.r Top. Dev. Biol. 2005; 71:83–112. [DOI] [PubMed] [Google Scholar]
- 4. Avila-Ospina L., Moison M., Yoshimoto K., Masclaux-Daubresse C.. Autophagy, plant senescence, and nutrient recycling. J. Exp. Bot. 2014; 65:3799–3811. [DOI] [PubMed] [Google Scholar]
- 5. Kim J., Woo H.R., Nam H.G.. Toward systems understanding of leaf senescence: an integrated multi-omics perspective on leaf senescence research. Mol. Plant. 2016; 9:813–825. [DOI] [PubMed] [Google Scholar]
- 6. Woo H.R., Kim H.J., Lim P.O., Nam H.G.. Leaf senescence: systems and Dynamics aspects. Annu. Rev. Plant Biol. 2019; 70:347–376. [DOI] [PubMed] [Google Scholar]
- 7. Li Z., Zhao Y., Liu X., Peng J., Guo H., Luo J.. LSD 2.0: an update of the leaf senescence database. Nucleic Acids Res. 2014; 42:D1200–D1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X., Li Z., Jiang Z., Zhao Y., Peng J., Jin J., Guo H., Luo J.. LSD: a leaf senescence database. Nucleic Acids Res. 2011; 39:D1103–D1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu X.Y., Hu W.J., Luo H., Xia Y., Zhao Y., Wang L.D., Zhang L.M., Luo J.C., Jing H.C.. Transcriptome profiling of developmental leaf senescence in sorghum (Sorghum bicolor). Plant Mol. Biol. 2016; 92:555–580. [DOI] [PubMed] [Google Scholar]
- 10. Yang J., Worley E., Ma Q., Li J., Torres-Jerez I., Li G., Zhao P.X., Xu Y., Tang Y., Udvardi M.. Nitrogen remobilization and conservation, and underlying senescence-associated gene expression in the perennial switchgrass Panicum virgatum. New Phytol. 2016; 211:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo P., Li Z., Huang P., Li B., Fang S., Chu J., Guo H.. A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell. 2017; 29:2854–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grosskinsky D.K., Syaifullah S.J., Roitsch T.. Integration of multi-omics techniques and physiological phenotyping within a holistic phenomics approach to study senescence in model and crop plants. J. Exp. Bot. 2018; 69:825–844. [DOI] [PubMed] [Google Scholar]
- 13. Liu P., Zhang S., Zhou B., Luo X., Zhou X.F., Cai B., Jin Y.H., Niu, Lin J., Cao X. et al.. The histone H3K4 demethylase JMJ16 represses leaf senescence in Arabidopsis. Plant Cell. 2019; 31:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H., Kim H.J., Vu Q.T., Jung S., McClung C.R., Hong S., Nam H.G.. Circadian control of ORE1 by PRR9 positively regulates leaf senescence in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:8448–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y., Zhang Y., Wang L.. Cross regulatory network between circadian clock and leaf senescence is emerging in higher plants. Front. Plant Sci. 2018; 9:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J.H., Woo H.R., Kim J., Lim P.O., Lee I.C., Choi S.H., Hwang D., Nam H.G.. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science. 2009; 323:1053–1057. [DOI] [PubMed] [Google Scholar]
- 17. Li Z., Peng J., Wen X., Guo H.. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell. 2013; 25:3311–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ay N., Irmler K., Fischer A., Uhlemann R., Reuter G., Humbeck K.. Epigenetic programming via histone methylation at WRKY53 controls leaf senescence in Arabidopsis thaliana. Plant J. 2009; 58:333–346. [DOI] [PubMed] [Google Scholar]
- 19. Wang X., Gao J., Gao S., Song Y., Yang Z., Kuai B.. The H3K27me3 demethylase REF6 promotes leaf senescence through directly activating major senescence regulatory and functional genes in Arabidopsis. PLoS Genet. 2019; 15:e1008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mao C., Lu S., Lv B., Zhang B., Shen J., He J., Luo L., Xi D., Chen X., Ming F.. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017; 174:1747–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z., Woo H.R., Guo H.. Genetic redundancy of senescence-associated transcription factors in Arabidopsis. J. Exp. Bot. 2018; 69:811–823. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y., Chan Z., Gao J., Xing L., Cao M., Yu C., Hu Y., You J., Shi H., Zhu Y. et al.. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breeze E., Harrison E., McHattie S., Hughes L., Hickman R., Hill C., Kiddle S., Kim Y.S., Penfold C.A., Jenkins D. et al.. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011; 23:873–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woo H.R., Koo H.J., Kim J., Jeong H., Yang J.O., Lee I.H., Jun J.H., Choi S.H., Park S.J., Kang B. et al.. Programming of plant leaf senescence with temporal and inter-organellar coordination of transcriptome in Arabidopsis. Plant Physiol. 2016; 171:452–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. BIG Data Center Members Database Resources of the BIG Data Center in 2019. Nucleic Acids Res. 2019; 47:D8–D14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin A., Wang Y., Tang J., Xue P., Li C., Liu L., Hu B., Yang F., Loake G.J., Chu C.. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012; 158:451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tuskan G.A., Difazio S., Jansson S., Bohlmann J., Grigoriev I., Hellsten U., Putnam N., Ralph S., Rombauts S., Salamov A. et al.. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science. 2006; 313:1596–1604. [DOI] [PubMed] [Google Scholar]
- 28. He L., Wu W., Zinta G., Yang L., Wang D., Liu R., Zhang H., Zheng Z., Huang H., Zhang Q. et al.. A naturally occurring epiallele associates with leaf senescence and local climate adaptation in Arabidopsis accessions. Nat. Commun. 2018; 9:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyu J.I., Kim J.H., Chu H., Taylor M.A., Jung S., Baek S.H., Woo H.R., Lim P.O., Kim J.. Natural allelic variation of GVS1 confers diversity in the regulation of leaf senescence in Arabidopsis. New Phytol. 2019; 221:2320–2334. [DOI] [PubMed] [Google Scholar]
- 30. Guo Y., Gan S.. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006; 46:601–612. [DOI] [PubMed] [Google Scholar]
- 31. Liang C., Wang Y., Zhu Y., Tang J., Hu B., Liu L., Ou S., Wu H., Sun X., Chu J. et al.. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:10013–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin M., Lai D., Pang C., Fan S., Song M., Yu S.. Generation and analysis of a large-scale expressed sequence Tag database from a full-length enriched cDNA library of developing leaves of Gossypium hirsutum L. PLoS One. 2013; 8:e76443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sekhon R.S., Saski C., Kumar R., Flinn B., Luo F., Beissinger T.M., Ackerman A.J., Breitzman M.W., Bridges W.C., de Leon N. et al.. Integrated genome-scale analysis identifies novel genes and networks underlying senescence in maize. Plant Cell. 2019; 31:1968–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.