Abstract
Zika virus (ZIKV) is a mosquito-borne flavivirus associated with severe neurological disorders including Guillain-Barré syndrome and microcephaly. The host innate immune responses against ZIKV infection are essential for protection; however, ZIKV has evolved strategies to evade and antagonize antiviral responses via its nonstructural (NS) proteins. Here, we demonstrated that ZIKV infection unexpectedly inhibits NLRP3-dependent inflammasome activation in bone marrow-derived macrophages and mixed glial cells from mouse brain. ZIKV infection led to increased transcript levels of proinflammatory cytokines such as IL-1β and IL-6 via activating NF-κB signaling. However, ZIKV infection failed to trigger the secretion of active caspase-1 and IL-1β from macrophages and glial cells even in the presence of LPS priming or ATP costimulation. Intriguingly, ZIKV infection significantly attenuated NLRP3-dependent, but not absent in melanoma 2-dependent caspase-1 activation and IL-1β secretion from both cells. ZIKV infection further blocked apoptosis-associated speck-like protein containing a caspase recruitment domain oligomerization in LPS/ATP-stimulated macrophages. Interestingly, expression of ZIKV NS3 protein reduced NLRP3-mediated caspase-1 activation and IL-1β secretion in macrophages, whereas NS1 and NS5 proteins showed no effects. Furthermore, NLRP3 was found to be degraded by the overexpression of ZIKV NS3 in 293T cells. Collectively, these results indicate that ZIKV evades host NLRP3 inflammasome-mediated innate immune responses in macrophages and glial cells; this may facilitate ZIKV's ability to enhance the replication and dissemination in these cells.
Keywords: Zika virus, Caspase 1, Inflammasome, NLRP3, Macrophages, Glial cells
INTRODUCTION
Zika virus (ZIKV) is a single-stranded positive-sense RNA virus belonging to the family Flaviviridae and transmitted by mosquitoes (1). ZIKV genome encodes a single large polyprotein that is processed by viral and host protease into 3 structural proteins (capsid C, premembrane prM, and envelope E) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (2,3). ZIKV infection in humans is typically characterized by the absence or presence of only mild symptoms that are similar to mild dengue fever. However, in rare cases, severe neurological complications such as Guillain-Barré syndrome have been reported (4). Interestingly, a prenatal ZIKV infection in pregnant women can result in detrimental effects, ranging from congenital abnormalities to pregnancy loss and miscarriage (5,6).
Notably, macrophages are attractive viral targets and reservoirs. Several viruses, such as the respiratory syncytial virus, vesicular stomatitis virus, hepatitis C virus, and ZIKV, utilize macrophages as vessels for their dissemination, long-term persistence within tissues, and replication (7). ZIKV infects and replicates in human placental macrophages, called Hofbauer cells (8). Additionally, ZIKV reportedly targets decidual macrophages present in the maternal decidual tissues (9). ZIKV-infected macrophages have demonstrated a higher migration ability through suppression of migration inhibitory factor, and this phenomenon enable the ZIKV to cross the placental barrier and spread within the body (10). Unexpectedly, ZIKV-infected macrophages show a minimal increase in proinflammatory cytokine and chemokine production, with minimal cell death (8). Thus, ZIKV infection causes mild inflammation that prolongs the infection and persistence of the virus in the body (10). In addition, Foo et al. (11) identified CD14+ monocytes as the primary target for both the African and Asian lineage ZIKV infections in pregnant women. Notably, differential immunomodulatory responses have been observed in the monocytes of pregnant women, with the African lineage ZIKV infection resulting in M1-skewed inflammation, whereas the Asian lineage ZIKV infection led to M2-skewed immunosuppression (11).
Inflammasome is a cytosolic multiprotein complex that activates inflammatory cascades in response to pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs) (12). Inflammasome activation is initiated by a wide range of sensor proteins, such as absent in melanoma 2 (AIM2) and various nucleotide-binding oligomerization domain-like receptor (NLR) subsets (13). The activation of inflammasomes results in the activation of caspase-1, which then subsequently cleaves pro-IL-1β and pro-IL-18 into their active form. Additionally, inflammasome activation can cause lytic cell death called pyroptosis (14). Recent studies have suggested that ZIKV infection can activate NLRP3 inflammasome and the ZIKV NS5 protein has a critical role in ZIKV-mediated NLRP3 inflammasome activation (15,16,17). However, the effect of each ZIKV protein on the inflammasome signaling pathways needs further elucidation.
Viruses have evolved several strategies to evade the host immune system. Multiple viral proteins such as the flavivirus NS proteins are known to either prevent IFN induction or abrogate IFN signaling (18,19). In particular, ZIKV NS proteins reportedly evade the innate immune response via the suppression of type I and III IFN production and downstream signaling (20,21). ZIKV NS3 protein, a serine protease, inhibits both the JAK-STAT signaling pathway and cGAS-STING pathway in a protease activity-dependent manner (22,23). Here, we examined whether ZIKV infection modulates the activation of inflammasome pathways in macrophages and propose that ZIKV NS3 could contribute to the evasion of the innate immune response via targeting inflammasome activation.
MATERIALS AND METHODS
Mice
C57BL/6 mice were obtained from Orient Bio. All of the mice were maintained under specific pathogen-free conditions; 9−12-wk-old male mice were selected for use in the experiments. Protocols for the animal experiments were approved by the Institutional Ethical Committee, Yonsei University College of Medicine (2018-0099). All experiments were performed in accordance with the approved guidelines of the Institutional Ethical Committee.
Cell cultures
Mouse primary bone marrow-derived macrophages (BMDMs) were prepared from the femurs of C57BL/6 mice as previously described (24). All BMDMs were maintained in L929-conditioned DMEM supplemented with 10% FBS and antibiotics. Mouse brain mixed glial cells were isolated from the whole brain of mouse pups on postnatal day 1−3 and cultured for 3 wk as previously described (25). Mouse mixed glial cells were maintained in DMEM/F12 (1:1) supplemented 10% FBS and antibiotics. 293T cells were maintained in DMEM medium supplemented with 10% FBS and antibiotics.
Reagents and Abs
LPS, ATP, nigericin and poly dA:dT were purchased from Sigma-Aldrich. Mouse IL-1β, IL-6 and IFN-β ELISA kits were obtained from R&D Systems or Biolegend. Anti-mouse caspase-1 and anti-NLRP3 Abs were obtained from AdipoGen Life Sciences. Anti-mouse IL-1β Ab was obtained from R&D Systems. Anti-apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and anti-mouse β-actin Abs were purchased from Santa Cruz Biotechnology. Anti-IκB, anti-phospho-IκB and anti-IL-6 Abs were obtained from Cell Signaling Technology.
Expression constructs
The mammalian expression plasmids for GFP or ZIKV NS proteins (pLV-GFP, pLV-Zika-NS1-Flag [Variant: W98G], pLV-Zika-NS3-Flag and pLV-Zika-NS5-Flag) were obtained from Addgene (Watertown, MA, USA). The expression plasmid for NLRP3 (pcDNA-Flag-NLRP3) was described previously (26). Transfection of poly dA:dT and other plasmids were performed using Lipofectamine or Lipofectamine 2000 in accordance with the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA).
Virus infection
ZIKV MR766 strain was purchased from American Type Culture Collection. Viral titers were determined using a standard plaque assay. Viral supernatants were added to BMDMs or mixed glial cells at a multiplicity of infection (MOI) of 10. At 2-h post-infection (PI), cells were washed, replenished with culture medium and incubated for an additional 20 h. Then, cells were further treated with appropriate reagents.
Assay of inflammasome activation
To stimulate the activation of NLRP3 inflammasome, cells were treated with LPS (0.25 µg/ml, 3 h), followed by treatment with ATP (1–2 mM, 30 min) or nigericin (1–2.5 µM, 30 min) treatment. To induce AIM2 inflammasome activation, poly dA:dT (1 µg/ml) was transfected into BMDMs or mixed glial cells for 6 h. Inflammasome activation was determined by the presence of active caspase-1 p20 and active IL-1β from culture supernatants using immunoblotting, and by the extracellular IL-1β quantification using an ELISA kit. To determine the oligomerization of ASC, discuccinimidyl suberate (DSS, Thermo Scientific)-mediated cross-linking assay was performed as previously described (27).
Immunoblot analysis
Briefly, cells were lysed in a buffer containing 20 mM HEPES (pH 7.5), 0.5% Nonidet P-40, 50 mM KCl, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, and protease inhibitors. Soluble lysates were fractionated by SDS-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes. In some experiments, cell culture supernatants were precipitated by methanol/chloroform as previously described (28) and then immunoblotted. All blots shown are representative images of at least 3-independent experiments.
Quantification of mRNA production
To measure mRNA production, total RNA was isolated using the TRIzol reagent (Invitrogen) and reverse transcribed using PrimeScript RT Master Mix (Takara, Seoul, Korea). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (Takara). Primers were as follows: 5′-GCC CAT CCT CTG TGA CTC AT-3′ and 5′-AGG CCA CAG GTA TTT TFT CG-3′ (mouse Il-1β); 5′-AGT TGC CTT CTT GGG ACT GA-3′ and 5′-TCC ACG ATT TCC CAG AGA AC-3′ (mouse Il-6); 5′-TTC CTG CTG TGC TTC TTC AC-3′ and 5′-CTT TCC ATT CAG CTG CTC CA-3′ (mouse Ifn-β); 5′-CGC GGT TCT ATT TTG TTG GT-3′ and 5′-AGT CGG CAT CGT TTA TGG TC-3′ (mouse Rn18s); 5′-TTG GTC ATG ATA CTG CTG ATT GC-3′ and 5′-CCT CCA TGT TCC AAG ACA ACA TC-3′ (ZIKV); 5′-TGG AGG AAA CAT GTG GAA CA-3′ and 5′-CTT TCC TGG GCC TTA TCT CC-3′ (ZIKV NS1); 5′-CGT GAT CAA AAA CGG GAG TT-3′ and 5′-CTA AGG GCC TCC TCC ATT TC-3′ (ZIKV NS3); 5′-GAT ACC TGC AGC CCT ATG GA-3′ and 5′-ACC CAT AGC TTT ACA CCA AC-3′ (ZIKV NS5).
Statistical analysis
All values are expressed as the mean and SE of individual samples. Data were analyzed using the Student's t-test (unpaired) using GraphPad Prism 5. The level of statistical significance was set at p≤0.05.
RESULTS
ZIKV infection induces NF-κB-dependent production of IL-1β mRNA but not the secretion of IL-1β in BMDMs and mixed glial cells
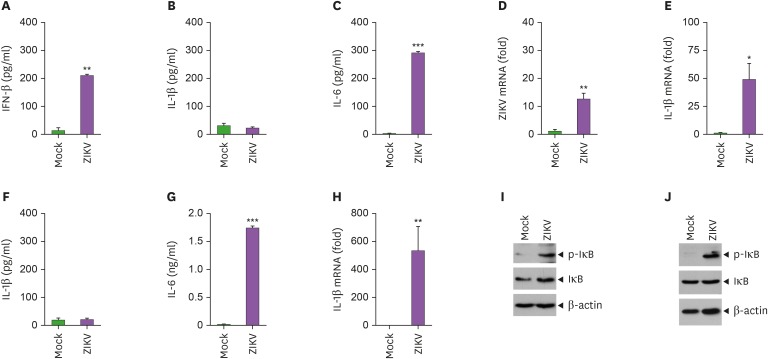
To examine whether ZIKV infection promotes innate immune responses, we analyzed the proinflammatory cytokines secreted from BMDMs following infection with ZIKV. ZIKV infection led to a robust secretion of IFN-β at 4-h PI (Fig. 1A). However, IL-1β was not detected in the culture supernatant of BMDMs at 24-h PI with ZIKV (Fig. 1B), whereas IL-6 was released from BMDMs upon infection with ZIKV (Fig. 1C). Interestingly, ZIKV infection induced significant production of IL-1β and IL-6 mRNA in BMDMs (Fig. 1D and E). These findings indicate that ZIKV infection triggers the transcription of proinflammatory cytokines but fails to induce IL-1β secretion from host macrophages.
Figure 1. ZIKV infection induces NF-κB-dependent mRNA production of IL-1β but not the secretion of IL-1β from bone marrow-derived macrophages and brain mixed glial cells. (A) Quantification of IFN-β in culture supernatants of BMDMs infected with mock or ZIKV (MOI=10) at 4-h post-infection (PI) (n=2). (B, C) Quantification of IL-1β (B) or IL-6 (C) in culture supernatants of BMDMs infected with mock or ZIKV (MOI=10) at 24-h PI (n=3). (D, E) Quantification of ZIKV (D) or IL-1β (E) mRNA levels in the mock- or ZIKV-infected (MOI=10) at 24-h PI (n=3). (F-H) Quantification of IL-1β (F) or IL-6 (G) in culture supernatants or IL-1β mRNA levels (H) of mixed glial cells infected with ZIKV as in (B-E) (n=3). (I, J) Immunoblots of cellular lysates from BMDMs (I) or mixed glial cells (J) infected with mock or ZIKV (MOI=10) at 20-h PI.
Asterisks indicate significant differences compared to mock-infected cells (*p<0.05; **p<0.01; ***p<0.001).
We then checked the production of proinflammatory cytokines from mouse brain mixed glial cells following infection with ZIKV. As observed in macrophages, mixed glial cells secreted a significant amount of IL-6 but not IL-1β at 24-h PI with ZIKV (Fig. 1F-G) despite the massive production of IL-1β mRNA (Fig. 1H). To further examine how ZIKV induces the transcription of proinflammatory cytokines, we investigated the phosphorylation of IκB, a critical phenomenon in the NF-κB signaling cascade. Indeed, ZIKV infection caused the robust phosphorylation of IκB in BMDMs and mixed glial cells (Fig. 1I-J), suggesting that ZIKV triggers the induction of proinflammatory cytokines in an NF-κB pathway-dependent manner.
ZIKV infection does not promote caspase-1 activation in macrophages and glial cells
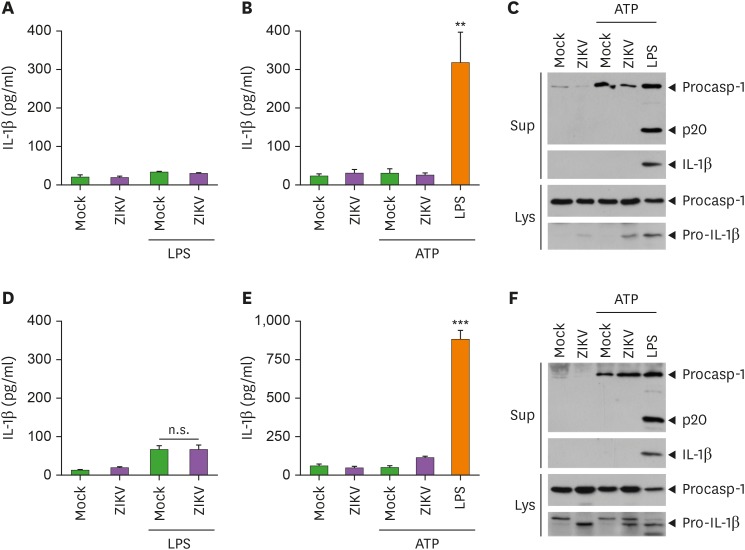
As shown above, ZIKV infection resulted in the IL-1β mRNA production, but not secretion. Unlike other conventional cytokines, IL-1β secretion requires the activation of inflammasome and caspase-1. Recent studies have shown that ZIKV can trigger NLRP3-dependent inflammasome activation in macrophages (16,17). Hence, we further examined whether ZIKV is able to promote inflammasome activation under different conditions. However, ZIKV infection failed to promote IL-1β secretion from LPS-primed macrophages (Fig. 2A). In addition, ZIKV infection, followed by ATP treatment, did not trigger the release of IL-1β in BMDMs (Fig. 2B). Accordingly, ZIKV infection was incapable of inducing caspase-1 activation in the culture supernatants of BMDMs as determined by immunoblotting (Fig. 2C). Furthermore, as observed in macrophages, ZIKV infection with LPS priming and ATP costimulation failed to induce a considerable IL-1β secretion and caspase-1 activation in mixed glial cells (Fig. 2D-F). These results indicate that ZIKV infection is not likely to promote inflammasome-mediated immune response in macrophages and glial cells.
Figure 2. ZIKV infection fails to induce inflammasome activation in bone marrow-derived macrophages and brain mixed glial cells. (A) Quantification of IL-1β in culture supernatants of BMDMs primed with LPS (0.25 µg/ml, 3 h) and further infected with mock or ZIKV (MOI=10) for 24 h (n=3). (B, C) Quantification of IL-1β in culture supernatants (B) or immunoblots in culture supernatants (Sup) or cellular lysates (Lys) (C) of BMDMs infected with mock or ZIKV (MOI=10) for 20 h or treated with LPS (0.25 µg/ml, 3 h), followed by ATP treatments (2.5 mM, 30 min). (D) Quantification of IL-1β in culture supernatants of LPS-primed mixed glial cells infected with mock or ZIKV (MOI=10) for 24 h (n=3). (E, F) Quantification of IL-1β in culture supernatants (E) or immunoblots in culture supernatants (Sup) or cellular lysates (Lys) (F) of mixed glial cells treated as in (B, C) (n=3).
Asterisks indicate significant differences compared to mock-infected control (**p<0.01; ***p<0.001; n.s., not significant).
ZIKV infection inhibits NLRP3-dependent caspase-1 activation and ASC oligomerization
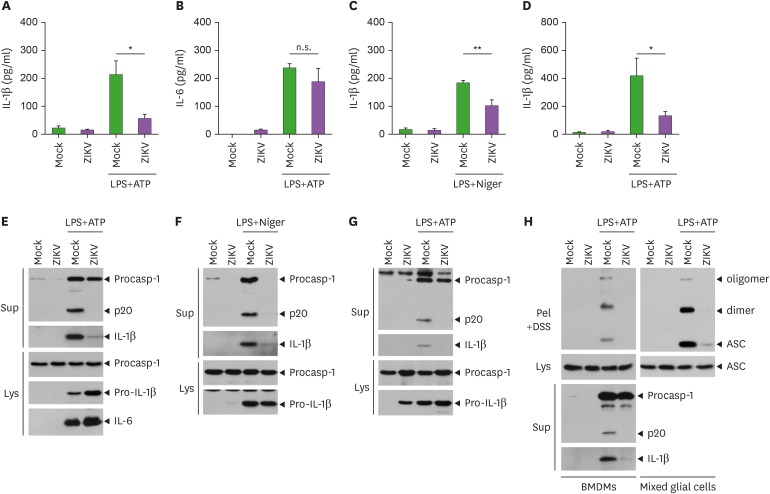
Next, we examined whether ZIKV infection could enhance NLRP3 inflammasome activation triggered by conventional stimulations. LPS priming with ATP costimulation, the most well-studied condition for NLRP3 activation, induced a significant IL-1β production in the culture supernatant of BMDMs (Fig. 3A). Notably, ZIKV infection before LPS priming significantly inhibited IL-1β release triggered by LPS/ATP stimulation in BMDMs (Fig. 3A). In contrast, ZIKV-infected macrophages produced a similar amount of IL-6 in response to LPS/ATP stimulation compared to the control macrophages (Fig. 3B). ZIKV infection also impaired IL-1β release from BMDMs in response to LPS/nigericin stimulation, another NLRP3-activating condition (Fig. 3C). Similarly, LPS/ATP stimulation led to a reduced IL-1β secretion from ZIKV-infected mixed glial cells compared to the control mock-infected cells (Fig. 3D). These observations clearly indicate that ZIKV infection can attenuate NLRP3 inflammasome activation mediated by conventional stimulants. Accordingly, ZIKV infection dampened the caspase-1 cleavage triggered by LPS/ATP or LPS/nigericin stimulations in BMDMs (Fig. 3E and F, Supplementary Fig. 1A) and mixed glial cells (Fig. 3G and Supplementary Fig. 1B). Furthermore, ZIKV infection clearly inhibited LPS/ATP-promoted ASC oligomerization, an essential phenomenon of inflammasome assembly, in BMDMs and mixed glial cells (Fig. 3H). Collectively, these results suggest that ZIKV infection could abrogate NLRP3-mediated caspase-1 activation and the subsequent IL-1β secretion.
Figure 3. ZIKV infection attenuates NLRP3-dependent inflammasome activation in bone marrow-derived macrophages and brain mixed glial cells. (A-D) Quantification of IL-1β or IL-6 in culture supernatants of BMDMs (A-C) or mixed glial cells (D) infected with mock or ZIKV (MOI=10, 20 h), washed and treated with LPS (0.25 µg/ml, 3 h), followed by the treatment with ATP (1.5 mM, A, B, D) or nigericin (Niger, 1 µM, C) for 30 min. (n=4, A-C; n=3, D). (E-H) Representative immunoblots from BMDMs (E, F, left panel of H) or mixed glial cells (G, right panel of H) treated as in (A-D). Culture supernatants (Sup), disuccinimidyl suberate (DSS)-crosslinked pellets (Pel+DSS) or cellular lysates (Lys) were immunoblotted with the indicated Abs.
Asterisks indicate significant differences (*p<0.05; **p<0.01; n.s., not significant).
ZIKV infection impairs NLRP3 inflammasome activation in an NS3-dependent manner
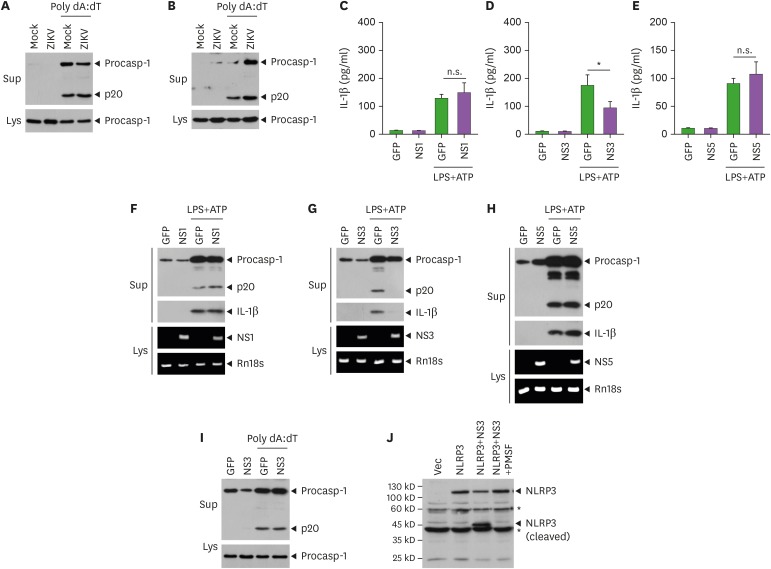
Next, we evaluated the impact of ZIKV infection on inflammasome signaling pathways other than NLRP3. Reportedly, the transfection of poly dA:dT, a synthetic double-stranded DNA, induces AIM2-dependent caspase-1 activation (28). Consequently, ZIKV infection did not affect AIM2-mediated caspase-1 activation in BMDMs and mixed glial cells (Fig. 4A and B), indicating that the inhibitory role of ZIKV on inflammasome activation was restricted to NLRP3.
Figure 4. ZIKV NS3 inhibits NLRP3-dependent caspase-1 activation and IL-1β secretion from bone marrow-derived macrophages and brain mixed glial cells. (A, B) Immunoblots from BMDMs (A) or mixed glial cells (B) infected with mock or ZIKV (MOI=10, 20 h), washed, and then transfected with poly dA:dT (1 μg/ml, 6 h). (C-E) Quantification of IL-1β in culture supernatants of BMDMs transfected with GFP control, ZIKV NS1 (C), ZIKV NS3 (D) or ZIKV NS5 (E) expression plasmid (0.5 µg) for 40 h, then treated with LPS (0.25 µg/ml, 3 h) and ATP (2 mM, 1 h) (n=5, C; n=6, D-E). (F-H) Immunoblots in culture supernatants (Sup) or RT-PCR for the mRNA expression of target genes in the cellular extracts from BMDMs treated as in (C-E). (I) Immunoblots from BMDMs transfected with GFP or ZIKV NS3 expression plasmid for 6 h, washed, incubated for additional 40 h, and then transfected with poly dA:dT (1 μg/ml, 6 h). (J) Immunoblots of the cell lysates of 293T cells transfected with NLRP3 or NS3 plasmids for 6 h, washed, and further incubated for 40 h with or without serine protease inhibitor (0.5 µM, PMSF).
Asterisks indicate significant differences (*p<0.05; n.s., not significant).
Furthermore, we considered the mechanism by which ZIKV infection could regulate the NLRP3 inflammasome activation. Recent studies demonstrated that NS1 and NS5 proteins can trigger or enhance the NLRP3 inflammasome activation (16,17). Thus, we evaluated the effect of ZIKV NS proteins in NLRP3 inflammasome signaling. Of interest, ZIKV NS3 expression significantly attenuated IL-1β secretion from macrophages upon stimulation with LPS/ATP, whereas neither NS1 nor NS5 proteins affected the LPS/ATP-induced IL-1β production in BMDMs (Fig. 4C-E). Consistent with these findings, only NS3, but not NS1 nor NS5, inhibited LPS/ATP-mediated caspase-1 activation in BMDMs (Fig. 4F-H and Supplementary Fig. 2A-C), indicating that ZIKV NS3 negatively regulates NLRP3 inflammasome signaling. Additionally, ZIKV NS3 has no effect on the AIM2-mediated caspase-1 activation in BMDMs (Fig. 4I). As ZIKV NS3 contains a protease domain, we examined whether NS3 can degrade NLRP3. Of interest, the overexpression of NS3 caused a marked degradation of NLRP3, which was protected by a serine protease inhibitor PMSF (Fig. 4J). Although we failed to detect an endogenous degraded NLRP3, these findings suggest that the protease activity of ZIKV NS3 contributes to the attenuation of NLRP3 inflammasome activation.
DISCUSSION
Viruses have evolved various strategies to evade the host immune system (29). Previous studies have suggested that NS proteins of ZIKV impair the antiviral pathways, such as cGAS-STING dependent signaling and type I IFN production (20,21,23). However, viruses can trigger diverse pattern-recognition receptor signaling including inflammasome pathways in terms of host defense (30). Therefore, in the present study, we attempted to examine the effect of ZIKV infection on the TLR- or inflammasome-mediated signaling pathways using BMDM and brain glial cell model.
Reportedly, ZIKV infects and replicates in human placental macrophages. Moreover, macrophages are considered to play a key role as vessels for virus dissemination, long-term persistence of virus-infected cells within tissues and virus replication (7,8). The expression levels of proinflammatory cytokines are mildly increased in ZIKV-infected macrophages, with the ZIKV-mediated inflammatory response demonstrating an up-regulation in macrophage mobility and dissemination of ZIKV (8,10). In agreement with earlier studies, our data indicated that ZIKV infection slightly induced proinflammatory cytokine production in both BMDMs and mixed glial cells. However, ZIKV infection did not alter the LPS-mediated proinflammatory cytokine production in BMDMs and mixed glial cells. These findings demonstrate that ZIKV infection can induce only mild inflammation and does not significantly alter TLR4 signaling pathways in macrophages.
Notably, our data indicated that ZIKV infection alone promoted a production of inactive pro-IL-1β in cytosol, but failed to induce the extracellular release of active IL-1β. These results suggest that ZIKV infection is not likely to trigger IL-1β-dependent proinflammatory responses in macrophages and glial cells including microglia. Furthermore, our results clearly demonstrated that ZIKV-infected cells do not properly respond to NLRP3-activating stimulations leading to the attenuation of NLRP3-mediated inflammasome signaling. These findings are not consistent with the recent studies demonstrating that ZIKV infection causes NLRP3 inflammasome activation (16,17,31). In particular, Wang et al. (16) reported that the NS5 protein of ZIKV is essential for NLRP3 inflammasome activation and directly interacts with the NLRP3 protein, facilitating NLRP3 inflammasome activation. In contrast, our data clearly indicated that the ZIKV infection failed to induce NLRP3 inflammasome activation. One possible explanation could be the different cells used. The previous study mainly used THP-1 cells or PBMCs to support a NLRP3-dependent inflammasome activation potential of ZIKV (16), whereas mouse macrophages and glial cells were employed in the present study.
Another possible explanation for this discrepancy could be the experimental use of different ZIKV lineages. While previous studies involving ZIKV-mediated inflammasome activations used the Asian (ZIKVAS) lineage (z16006, KU820898 and KU866423), we used the African (ZIKVAF) lineage (MR766) (15,16,31). The ZIKVAS lineage is known to cause microcephaly and other fetal developmental defects including severe brain damage and postnatal lethality (32,33). Recently, Dowall et al. (34) suggested that the expression patterns of ZIKV-induced cytokines differed between ZIKVAF and ZIKVAS infected groups. In this regard, the differential effect of ZIKV on NLRP3 inflammasome activation may be lineage-dependent. Nevertheless, further studies are crucial to for a detailed explanation of this discrepancy.
ZIKV encodes a single polypeptide that is proteolytically processed by viral and host proteases to produce 3 structural proteins and seven NS proteins. Among the NS proteins, NS3 is a proteolytic enzyme, which is a serine protease highly conserved in multiple strains of ZIKV (35). Recent studies suggested that ZIKV NS3 impairs the cGAS-STING pathway by triggering the cleavage of STING (23) and the JAK-STAT pathway by degrading JAK1 (22). Furthermore, ZIKV NS3 reportedly antagonizes RIG-I- and MDA5-mediated RLR signaling by binding to the 14-4-4 protein (36). Interestingly, our data demonstrated that ZIKV NS3 inhibited the NLRP3-dependent inflammasome activation possibly via the cleavage of NLRP3. These findings collectively suggest that ZIKV NS3 plays a crucial role in the evasion of the host innate immune responses.
NLRP3 inflammasome can be activated by host-derived DAMPs, such as extracellular ATP, cholesterol, and fatty acid (37) and inflammasome activation can lead to lytic cell death called pyroptosis (14). Notably, programmed cell death is an integral part of the host defense against invading intracellular pathogens and serves to limit virus replication in the infected cell, while simultaneously promoting the inflammatory responses that effectively modulate long-term host immunity (38). Hence, multiple viruses have developed mechanisms to suppress or modify the host cell death, such as apoptosis, necroptosis and pyroptosis, to enhance virus replication and dissemination (39,40). Similarly, our data demonstrated that the ZIKV infection inhibited NLRP3 inflammasome activation. Regardingly, it is highly possible that ZIKV might suppress NLRP3-dependent pyroptosis to facilitate its replication and dissemination in macrophages or glial cells. Collectively, our results indicate that ZIKV infection negatively regulates the NLRP3 inflammasome activation and ZIKV NS3 could play a crucial role in ZIKV-mediated evasion of the NLRP3 inflammasome signaling.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2015M3A9B6073856, 2017R1A2B2007467).
Abbreviations
- AIM2
absent in melanoma 2
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- BMDM
bone marrow-derived macrophage
- DAMP
damage-associated molecular pattern
- MOI
multiplicity of infection
- NLR
nucleotide-binding oligomerization domain-like receptor
- NLRP3
nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3
- NS
nonstructural
- PI
post-infection
- ZIKV
Zika virus
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Yu JW.
- Funding acquisition: Yu JW.
- Investigation: Gim E, Shim DW, Hwang I.
- Methodology: Gim E, Shim DW, Hwang I, Shin OS
- Resources: Shin OS.
- Supervision: Yu JW.
- Validation: Gim E, Shim DW.
- Writing - original draft: Shim DW, Yu JW.
- Writing - review & editing: Gim E, Shim DW, Shin OS, Yu JW.
SUPPLEMENTARY MATERIALS
ZIKV infection abolishes LPS/ATP-triggered inflammasome activation. (A, B) Immunoblots of BMDMs (A) or mixed glial cells (B) primed with LPS (0.25 µg/ml, 3 h), followed by the treatment with ATP (1.5 mM, 30 min) or transfection of poly dA:dT (1 µg/ml, 4.5 h), or treated with ATP (1.5 mM, 30 min), as indicated. Culture supernatants (Sup), or cellular lysates (Lys) were immunoblotted with the indicated Abs.
ZIKV NS3 inhibits LPS/ATP-triggered inflammasome activation. (A-C) Immunoblots in culture supernatants (Sup) or cellular lysates (Lys) and RT-PCR for the mRNA expression of target genes in the cellular extracts (B, Lys) from BMDMs transfected with GFP control, ZIKV NS1, ZIKV NS3 or ZIKV NS5 expression plasmid for 48 h, then treated with LPS (0.25 µg/ml, 3h) and ATP (2 mM, 30 min).
References
- 1.Pierson TC, Diamond MS. The emergence of Zika virus and its new clinical syndromes. Nature. 2018;560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 2.Pierson TC, Graham BS. Zika virus: immunity and vaccine development. Cell. 2016;167:625–631. doi: 10.1016/j.cell.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause KK, Azouz F, Shin OS, Kumar M. Understanding the pathogenesis of Zika virus infection using animal models. Immune Netw. 2017;17:287–297. doi: 10.4110/in.2017.17.5.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avelino-Silva VI, Martin JN. Association between Guillain-Barré syndrome and Zika virus infection. Lancet. 2016;387:2599. doi: 10.1016/S0140-6736(16)30843-1. [DOI] [PubMed] [Google Scholar]
- 5.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martínez-Vega R, Porgo TV, Haefliger A, Broutet NJ, Low N WHO Zika Causality Working Group. Zika virus infection as a cause of congenital brain abnormalities and Guillain-barré syndrome: systematic review. PLoS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabié A, Callier C, Carles G, Cassadou S, Césaire R, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378:985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 7.Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci. 2018;19:E2821. doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang J, Cheng Y, Rolfe A, Hammack C, Vera D, Kyle K, Wang J, Meissner TB, Ren Y, Cowan C, et al. An hPSC-derived tissue-resident macrophage model reveals differential responses of macrophages to ZIKV and DENV infection. Stem Cell Reports. 2018;11:348–362. doi: 10.1016/j.stemcr.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, Yoo JS, Ge J, Cheng G, Bonnin A, et al. Asian Zika virus strains target CD14+ blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol. 2017;2:1558–1570. doi: 10.1038/s41564-017-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 15.He Z, Chen J, Zhu X, An S, Dong X, Yu J, Zhang S, Wu Y, Li G, Zhang Y, et al. NLRP3 inflammasome activation mediates Zika virus-associated inflammation. J Infect Dis. 2018;217:1942–1951. doi: 10.1093/infdis/jiy129. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Li G, De Wu, Luo Z, Pan P, Tian M, Wang Y, Xiao F, Li A, Wu K, et al. Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin-1β secretion. Nat Commun. 2018;9:106. doi: 10.1038/s41467-017-02645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, Jin S, She Y, Li YP, Cui J. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 2018;37:37. doi: 10.15252/embj.201899347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia H, Luo H, Shan C, Muruato AE, Nunes BT, Medeiros DB, Zou J, Xie X, Giraldo MI, Vasconcelos PF, et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun. 2018;9:414. doi: 10.1038/s41467-017-02816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhary V, Yuen KS, Chan JF, Chan CP, Wang PH, Cai JP, Zhang S, Liang M, Kok KH, Chan CP, et al. Selective activation of type II interferon signaling by Zika virus NS5 protein. J Virol. 2017;91:e00163-17. doi: 10.1128/JVI.00163-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sánchez-Seco MP, Evans MJ, Best SM, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19:882–890. doi: 10.1016/j.chom.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016;17:1766–1775. doi: 10.15252/embr.201642627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Liu Q, Zhou J, Xie W, Chen C, Wang Z, Yang H, Cui J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro . Cell Discov. 2017;3:17006. doi: 10.1038/celldisc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Q, Gaska JM, Douam F, Wei L, Kim D, Balev M, Heller B, Ploss A. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc Natl Acad Sci U S A. 2018;115:E6310–E6318. doi: 10.1073/pnas.1803406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EH, Won JH, Hwang I, Yu JW. Cobalt chloride-induced hypoxia ameliorates NLRP3-mediated caspase-1 activation in mixed glial cultures. Immune Netw. 2013;13:141–147. doi: 10.4110/in.2013.13.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J, Fernandes-Alnemri T, Alnemri ES. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 27.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beachboard DC, Horner SM. Innate immune evasion strategies of DNA and RNA viruses. Curr Opin Microbiol. 2016;32:113–119. doi: 10.1016/j.mib.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Zhong B. Regulation of cellular antiviral signaling by modifications of ubiquitin and ubiquitin-like molecules. Immune Netw. 2018;18:e4. doi: 10.4110/in.2018.18.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tricarico PM, Caracciolo I, Crovella S, D'Agaro P. Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem Biophys Res Commun. 2017;492:597–602. doi: 10.1016/j.bbrc.2017.01.158. [DOI] [PubMed] [Google Scholar]
- 32.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Signor LC. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg Infect Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao Q, Herrlinger S, Zhu YN, Yang M, Goodfellow F, Stice SL, Qi XP, Brindley MA, Chen JF. The African Zika virus MR-766 is more virulent and causes more severe brain damage than current Asian lineage and dengue virus. Development. 2017;144:4114–4124. doi: 10.1242/dev.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowall SD, Graham VA, Hewson R. Lineage-dependent differences of Zika virus infection in a susceptible mouse model are associated with different profiles of cytokines, chemokines, growth factors and acute phase proteins. Cytokine. 2020;125:154864. doi: 10.1016/j.cyto.2019.154864. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, Ci Y, Wang L, Yang Y, Zhang L, Xu C, Qin C, Shi L. Zika virus NS3 is a canonical RNA helicase stimulated by NS5 RNA polymerase. Nucleic Acids Res. 2019;47:8693–8707. doi: 10.1093/nar/gkz650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedl W, Acharya D, Lee JH, Liu G, Serman T, Chiang C, Chan YK, Diamond MS, Gack MU. Zika virus NS3 mimics a cellular 14-3-3-binding motif to antagonize RIG-I- and MDA5-mediated innate immunity. Cell Host Microbe. 2019;26:493–503.e496. doi: 10.1016/j.chom.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim DW, Lee KH. Posttranslational regulation of the NLR family pyrin domain-containing 3 inflammasome. Front Immunol. 2018;9:1054. doi: 10.3389/fimmu.2018.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upton JW, Chan FK. Staying alive: cell death in antiviral immunity. Mol Cell. 2014;54:273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connolly PF, Fearnhead HO. Viral hijacking of host caspases: an emerging category of pathogen-host interactions. Cell Death Differ. 2017;24:1401–1410. doi: 10.1038/cdd.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, et al. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe. 2018;23:254–265.e257. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZIKV infection abolishes LPS/ATP-triggered inflammasome activation. (A, B) Immunoblots of BMDMs (A) or mixed glial cells (B) primed with LPS (0.25 µg/ml, 3 h), followed by the treatment with ATP (1.5 mM, 30 min) or transfection of poly dA:dT (1 µg/ml, 4.5 h), or treated with ATP (1.5 mM, 30 min), as indicated. Culture supernatants (Sup), or cellular lysates (Lys) were immunoblotted with the indicated Abs.
ZIKV NS3 inhibits LPS/ATP-triggered inflammasome activation. (A-C) Immunoblots in culture supernatants (Sup) or cellular lysates (Lys) and RT-PCR for the mRNA expression of target genes in the cellular extracts (B, Lys) from BMDMs transfected with GFP control, ZIKV NS1, ZIKV NS3 or ZIKV NS5 expression plasmid for 48 h, then treated with LPS (0.25 µg/ml, 3h) and ATP (2 mM, 30 min).