Abstract
Campylobacter is a worldwide foodborne pathogen, associated with human gastroenteritis. The efficient translocation of Campylobacter and its ability to secrete toxins into host cells are the 2 key features of Campylobacter pathophysiology which trigger inflammation in intestinal cells and contribute to the development of gastrointestinal symptoms, particularly diarrhoea, in humans. The purpose of conducting this literature review is to summarise the current understanding of: i) the human immune responses involved in the elimination of Campylobacter infection and ii) the resistance potential in Campylobacter against these immune responses. This review has highlighted that the intestinal epithelial cells are the preliminary cells which sense Campylobacter cells by means of their cell-surface and cytosolic receptors, activate various receptors-dependent signalling pathways, and recruit the innate immune cells to the site of inflammation. The innate immune system, adaptive immune system, and networking between these systems play a crucial role in bacterial clearance. Different cellular constituents of Campylobacter, mainly cell membrane lipooligosaccharides, capsule, and toxins, provide protection to Campylobacter against the human immune system mediated killing. This review has also identified gaps in knowledge, which are related to the activation of following during Campylobacter infection: i) cathelicidins, bactericidal permeability-increasing proteins, chemokines, and inflammasomes in intestinal epithelial cells; ii) siglec-7 receptors in dendritic cell; iii) acute phase proteins in serum; and iv) T-cell subsets in lymphoid nodules. This review evaluates the existing literature to improve the understanding of human immunity against Campylobacter infection and identify some of the knowledge gaps for future research.
Keywords: Campylobacter, Lipooligosaccharides, Guillain-Barré Syndrome, Inflammasomes, Toll-like receptors, Antigen-presenting cells
INTRODUCTION
Campylobacter is commensal in poultry, but pathogenic in humans (1,2). The annual estimated number for Campylobacter infection cases is 96 million worldwide (2). Campylobacter is not harmless even for chickens as it stimulates the innate and adaptive immune responses in almost all types of chicken breeds. However, the extent of harm posed by Campylobacter may vary among different breeds of chickens (3). The differential susceptibility to Campylobacter across different breeds of chicken can be associated to the variation in their diet and gut microbiota composition (4,5). Chickens become colonised with Campylobacter at the age of 2–3 wk. Due to a lack of a fully developed adaptive immune system at this age, maternal Abs, already passed from hens to chicks, provide protection against Campylobacter (6,7). Maternal Abs against the Campylobacter flagellar proteins, outer membrane proteins and lipooligosaccharides (LOS) were observed in new-born chicks (7). After developing an adaptive immune system at the age of 6–7 wk, chickens produce Abs against Campylobacter cellular components, such as outer membrane proteins and flagellum (7,8). However, circulation of maternal Abs as well as development of adaptive immune B-cells play a limited role in the clearance of Campylobacter cells from the chicken intestines (6,9). It is proposed that Campylobacter avoids rapid clearance in the chicken intestine due to the adaptation to a novel colonisation mechanism, where it continues short-term invasion of chicken intestinal cells followed by escape from these cells (10). Clearance of Campylobacter from the chicken intestines may take many weeks, causing its persistence in chickens beyond slaughter age. Therefore, a poultry flock contaminated with Campylobacter is considered as a major source of Campylobacter transmission to humans (9,11).
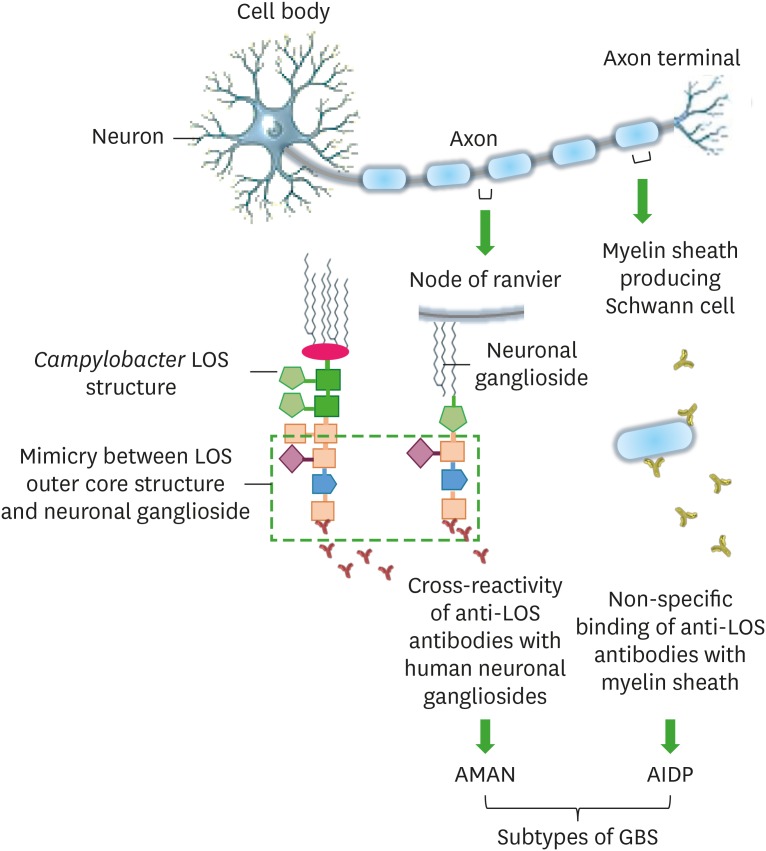
The gastrointestinal (GI) tract in humans structurally comprises of four cell layers: the mucosa, submucosa, muscularis externa, and serosa. The mucosa is the innermost layer which is further divided into the epithelium, lamina propria, and muscularis mucosae. The mucosal epithelium of the small intestine comprises of a single layer of intestinal epithelial cells (IECs), mucus secreting goblet cells, paneth cells, and M-cells. The epithelium forms villi or crypts (finger-like projections) which are covered by a highly viscous mucus layer (12). The Campylobacter adherence to the mucus layer as well as its invasion into the IECs and lamina propria occurs mainly due to its mucins (that are glycosylated proteins in the mucus layer) degrading enzymes, flagella, and adhesins (which primarily include Jejuni lipoprotein A, fibronectin [Fn], Fn-binding protein FlpA, cadherin-Fn binding protein [CadF], cell binding factor 1, and cell-surface glycans) (13,14,15,16,17). Following invasion into the host cells, Campylobacter reside into the LAMP-1 (late endosome marker) expressing Campylobacter-containing vacuoles (18,19). The adherence, invasion and intracellular survival facilitate the cellular translocation (endocytosis and exocytosis) of Campylobacter within the epithelial cells. Campylobacter translocation is coupled with the release of toxins in order to trigger the ion instability, cell apoptosis, and pore formation in host cells (20,21). The Campylobacter translocation and its potential to secrete toxins into host cells are the two main features of Campylobacter pathophysiology which enhance the inflammation and fluid secretion in intestinal cells and contribute to the development of infection in humans (16,20,21,22). Campylobacter infection in humans is characterised by an acute, self-limiting gastroenteritis which lasts for 5 to 7 days. The abdominal pain, watery or bloody diarrhoea, headache, fever, chills, and dysentery together with stools containing leukocytes and erythrocytes are the major signs of severe Campylobacter infection (23,24,25,26). Campylobacter infection can improve the progression of various persistent diseases including Guillain-Barré syndrome (GBS), Miller Fisher syndrome, Reiter's arthritis, and Irritable bowel syndrome (IBS) in humans. The LOS-outer core structures of Campylobacter are variable and mimic the structures of human neuronal gangliosides and for this reason, Abs produced against the LOS structural epitopes do not only bind to LOS structures, but also to human gangliosides. The cross-reactivity or non-specific binding of anti-LOS Abs with human neuronal gangliosides forms the basis of neural diseases specifically GBS development in humans (Fig. 1) (27,28,29,30).
Figure 1. An illustration of interactions of anti-LOS Abs with human neuronal cells, likely to cause GBS in humans post Campylobacter infection.
Cross-reactivity of anti-LOS Abs occurs due to the mimicry between Campylobacter cell surface LOS core structures and human neuronal (node of ranvier) gangliosides and it develops a GBS subtype, AMAN. In some cases, anti-LOS Abs non-specifically bind to the Schwann cells to develop another type of GBS known as AIDP.
AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy.
The scope of this review is to summarise available data on human immune responses involved in the defense against Campylobacter and how Campylobacter cells oppose these immune responses during infection. This review explains the role of human immune system in the elimination of Campylobacter infection by highlighting: i) induction of signalling pathways in intestinal mucosa for pathogen recognition; ii) influx of professional phagocytes into intestinal submucosa for bacterial clearance; iii) activation of adaptive immunity for persistent infection; iv) activation of serum proteins for persistent infection; and v) networking between human cells during Campylobacter infection.
INDUCTION OF SIGNALLING PATHWAYS IN INTESTINAL MUCOSA FOR PATHOGEN RECOGNITION
Campylobacter cells colonise the crypts in high numbers, rather than the intestinal lumen, due to the low concentration of oxygen and nutrients availability for maximal growth in crypts (17,31). The pathogen recognition receptors (PRRs) of IECs become activated to recognise the pathogen-associated molecular patterns (PAMPs) when pathogens develop interaction with IECs extracellularly or survive intracellularly after invasion. The signalling pathways downstream the PRRs produce inflammatory and anti-inflammatory cytokines to regulate the immune responses during infection (32,33,34). In addition to the activation of PRRs of IECs, increase in the number of mast cells and eosinophils has also been observed in lamina propria because they sense the Campylobacter cells as danger signals (35,36).
Stimulation of cell-surface receptors in IECs
TLRs are the cell membrane bound PRRs which recognise the PAMPs during Campylobacter infection and consequently, induce the secretion of different interleukins and chemokines from IECs (32). Different cellular constituents of Campylobacter such as lipoproteins (bind TLR-1/2/6), LOS (bind TLR4), DNA (bind TLR9), capsule, cell wall polysaccharides, flagella, and toxins can bind to TLRs in human IECs to activate them (37,38,39,40,41,42,43,44,45). The signalling pathway activates due to PAMPs binding to TLRs which recruits an adaptor protein, MyD88, to interact with IL-1 receptor-associated kinase complex and TNF receptor-associated factor 6 (TRAF6) (46,47). This interaction induces the mitogen activated protein kinase kinase kinase (MAP3K) which further stimulates the MAPK including ERK and p38 induction. These MAPK translocate to the nucleus, act as transcription factors, and regulate the transcription of NF-κB as well as synthesis of pro-inflammatory cytokines (IL-8 and TNFα) in IECs (37,41,48,49,50,51). The IECs TLRs (mainly TLR2) also involve the MyD88-independent signalling where they interact with adaptor proteins, Toll/IL-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF) and TRIF-related adaptor molecule (TRAM), and activate the IFN regulatory factor 3 (IRF-3) (52,53,54). IRF-3 accumulates in the nuclei and stimulates the synthesis of ILs (e.g. IL-1α and IL-6) via coordination with NF-κB (32,52). During infection with Campylobacter, IECs also secrete chemokines including growth related oncogene alpha (GROα), MIP-1, MCP-1, and IFN-γ-inducible protein 10 (IP-10). This is also known to occur by the regulation of NF-κB transcription (55,56,57); however, the role of specific TLRs in the induction of chemokines in IECs following infection with Campylobacter has not been yet investigated. Campylobacter jejuni (C. jejuni) can activate NF-κB independently of TLR signalling pathway (58), which might be linked to the chemokines production in Campylobacter infected IECs.
Stimulation of intracellular receptors in IECs
Campylobacter maintain their survival within the vacuoles (distinct from canonical endocytic vacuoles) inside of IECs (19). Direct invasion and subsequent intracellular survival of Campylobacter into IECs as well as the release of outer membrane vesicles (OMV) from extracellular Campylobacter cells cause delivery of the cellular constituents of Campylobacter into cytosol of host cells (18,59,60). The nucleotide-binding oligomerisation domain (NOD) are the intracellular PRRs in IECs which directly recognise the microbe-associated molecular patterns (MAMPs; Campylobacter toxins, flagella, muramyl dipeptides, and adhesins) of Campylobacter into cytosol and induce the release of antimicrobial peptides, particularly human β-defensins (hBD)-2 (61,62,63,64). The hBD-2 are bactericidal as they disrupt the Campylobacter cell wall integrity (65). In addition, NOD1 binding to Campylobacter MAMPs also promotes the secretion of IL-8 from human IECs (37,38,61) by activating the MAPK (19,51). The OMV enclosed LOS, toxins, and N-linked glycoproteins, after their delivery into host cells, can also activate MAPK, particularly p38, to induce the secretion of IL-8, IL-6, TNF-α, and hBD-3 from IECs (59). IL-8 subsequent to its release recruits the innate immune cells including neutrophils, macrophages, and dendritic cells at the site of infection (34,37,41,48,50,66). Inflammasome, a type of NOD-like receptor, is known to induce the pro-inflammatory cytokines (IL-1β and IL-18) in human cells (67). A recent study has demonstrated that inflammasome play a significant role in clearing the intracellular Campylobacter cells from human IECs (68), however, inflammasomes dependent specific mechanisms or signalling pathways involved in bacterial clearance are yet unknown.
INFLUX OF PROFESSIONAL PHAGOCYTES INTO SUBMUCOSA FOR BACTERIAL CLEARANCE
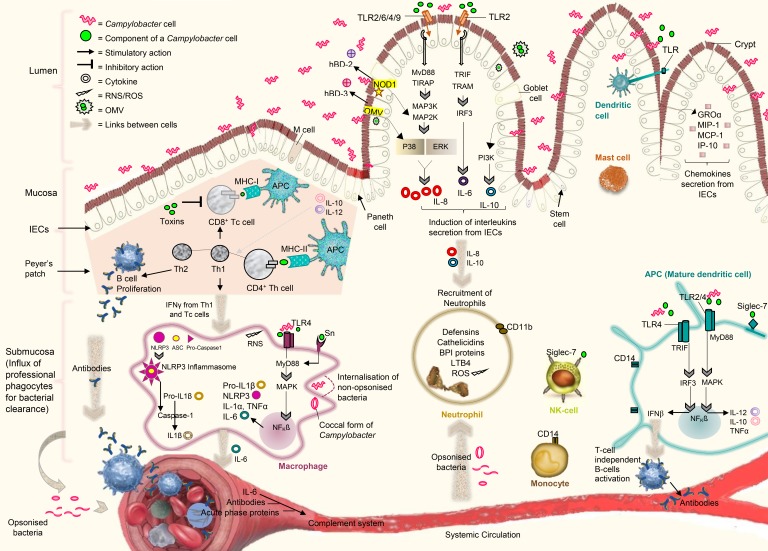
The intestinal crypts and IECs undergo the inflammation and severe damage during Campylobacter infection. In response to the inflammation and damage, IECs secrete cytokines and chemokines, which further recruit the professional mononuclear phagocytes including macrophages, neutrophils, NK cells and dendritic cells into the intestinal submucosa. These immune cells infiltrate the submucosal lining, interact with each other through cytokines, and help in clearing the bacterial cells from epithelium (23,25,66,69). A complex network of cytokines, linking IECs-to-immune cells and immune cells-to-each other, is presented in Fig. 2 together with key immune responses important for defense against Campylobacter.
Figure 2. A representation of key immune responses, important for defense in humans against Campylobacter infection. The MyD88 dependent and independent signalling pathways downstream the IECs TLRs are involved in the synthesis of interleukins and chemokines via coordination with NF-κB. The cytosolic receptors (NOD1) and OMV also contribute to the activation of NF-κB for the synthesis of β-defensins. IECs-derived cytokines recruit neutrophils, macrophages, NK cells, and dendritic cells into the intestinal submucosa. The stimulation of MyD88 dependent and independent signalling downstream the TLRs in dendritic cells lead to the secretion of different cytokines, which further activate B-cells in a T-cell independent manner. The activation of MyD88 dependent signalling downstream the cell-surface receptors (TLRs and Sn) and stimulation of cytosolic receptors (NLRP3 inflammasomes) occur in macrophages during Campylobacter infection in order to produce inflammatory cytokines. The mature dendritic cells present Ags by MHC type molecules and secrete interleukins (IL-10 and IL-12) to increase the proliferation of naïve T-cells into Tc and Th cells. The interaction of dendritic expressed siglec-7 with Campylobacter LOS promote the differentiation of Th cells into Th1 and Th2. T-cells activate B-cells to produce Abs specific to the Campylobacter cell constituents. Abs from B-cells, IL-6 from macrophages and high level of acute-phase proteins in serum contribute to the activation of complement systems. Cells are associated to each other by a complex network of cytokines to regulate the immune responses. Cell-to-cell association is demonstrated by large brown arrows.
ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; BPI, bactericidal permeability increasing; GROα, growth related oncogene alpha; IP-10, IFN-γ-inducible protein 10; MAP2K, mitogen-activated protein kinase kinases; MAP3K, mitogen activated protein kinase kinase kinase; RNS, reactive nitrogen species; Sn, sialoadhesin; TIRAP, Toll/IL-1 receptor domain-containing adapter protein.
Neutrophils
The IECs-derived IL-8 is a neutrophil chemotactic factor, which induces the influx of neutrophils into the intestinal submucosa and infiltration of these neutrophils to the site of inflammation. In Campylobacter, methionyl-tRNA formyltransferase (encoded by fmt gene) is involved in the production of n-formyl peptides. These Campylobacter n-formyl peptides along with a host cell enzyme, 12-lipoxygenase, direct the migration of neutrophils from the basolateral to apical surface of the epithelium (66). A recent study demonstrates that the secretion of IL-10 downstream of the PI3Kγ signalling pathway in IECs can also play an important role in the infiltration of neutrophils into intestinal crypts and submucosa (70). The accumulated neutrophils present CD11b (cell-surface markers), which is a sign of initiation of phagocytosis process (71). They phagocytose complement-opsonised bacteria more efficiently rather than the non-opsonised bacteria (72,73,74). They release toxic radicals or ROS (superoxide and hydrogen peroxide) for oxidative killing of bacteria and various inflammatory molecules including cationic antimicrobial peptides (CAPs), defensins, cathelicidins, bactericidal permeability increasing (BPI) protein (55 kDa), and leukotriene B4 (LTB4) for non-oxidative killing of bacteria (71,75,76). The production of ROS and inflammatory molecules from neutrophils damage IECs structurally and contribute to the abscesses formation and loss of function in crypts (71,77). This indicates that neutrophils are critical for the development of diarrhoea during Campylobacter infection. It is suggested that the non-invasion strains of Campylobacter can induce less concentration of IL-8 to further produce neutrophils and LTB4 in lesser amounts, which can lead to the development of non-inflammatory diarrhoea in humans rather than the inflammatory diarrhoea (78).
Monocytes/macrophages
Human monocytes with a range of cell-surface markers (CD14, CD11a) can be found in the intestinal mucosa following infection with Campylobacter for phagocytosis of Campylobacter cells (50,79). Human macrophages (differentiated monocytes) are more important than the complement system for Campylobacter infection (79) and have ability to phagocytose the whole-bacterial cells, unlikely to neutrophils (72,73,74). Monocytes undergo apoptosis following infection with Campylobacter, however, macrophages rapidly kill Campylobacter cells subsequent to their internalisation (19,73,80,81). Campylobacter cells in coccal or degenerative form can be observed in macrophages after 4–8 hours of infection (72). Human macrophages generally possess cell membrane bound receptors (TLR and lectin receptors [LRs]) and cytosolic receptors (inflammasomes) (33,44,82), which become activated to recognise the PAMPs when Campylobacter cell develops interaction with a macrophage or survive intramacrophage subsequent to the phagocytosis (33,83). Campylobacter LOS, cell wall polysaccharides, lipoproteins, and N-linked glycosylated proteins have been reported as ligands of macrophage TLR4, sialoadhesin (Sn; a type of LRs), and galactose-type lectin receptors (another type of LRs) (14,44,81,82,84,85). These cellular components detached from killed Campylobacter cells as well as viable cells of Campylobacter can bind to macrophage (specifically M1) receptors and induce TLR-MyD88 dependent signalling pathway in order to secrete the pro-inflammatory cytokines (IL-1α, TNF-α, pro-IL-1β, and IL-6) and proteins (such as NOD-like receptors with pyrin domain-containing 3 [NLRP3] proteins). M1-derived IL-6 helps in the activation of complement system while other cytokines recruit more innate immune cells to the site of inflammation. The NLRP3 proteins, after release into cytosol, combine with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and pro-caspase1 to assemble NLRP3-inflammasomes (protein complexes). Upon a second signal, NLRP3-inflammasomes activate caspase-1 to catalyse the 31 kDa pro-IL-1β (already located into cytosol) into mature, biologically functional 17 kDa IL-1β. The cleaved IL-1β is then secreted outside the cell to mediate the inflammatory responses (14,33,44,50,81,85). Further, IFN-γ (which is released from IECs and T-cells during actual infection) leads to the production of nitric oxide synthase 2 and reactive nitrogen species (RNS) in M1 macrophages, which are potent and effective towards killing of Campylobacter cells (34,86,87). During Helicobacter pylori infection, M2 macrophages and cytokines (IL-10, IL-12, and TGFβ) production from these M2 cells can be stimulated by T-cells derived anti-inflammatory cytokines (such as IL-4, IL-10, and IL-13) in order to suppress further activation of immune cells and inflammation (88,89). In the same way, M2 macrophage polarisation can play its role in immunity and inflammation suppression during Campylobacter infection, however, it needs to be confirmed by further research.
NK cells
NK cells could be a source of cytokines, such as IFN-γ, but its association to Campylobacter cells has not been yet investigated. Siglec 7 present on the cell surface of NK cells could be potential receptors involved in the development of pathogen-host cell interaction (74).
Dendritic cells
Dendritic cells reside under IECs and can extend themselves between IECs to sample the lumen, therefore, they are well positioned to interact with Campylobacter cells present inside of intestinal lumen and submucosa (90,91). Dendritic cells form an early line of defense within the submucosa against the invasive Campylobacter strains, as well as, in the intestinal lumen against the non-invasive Campylobacter strains (12,40,92). Dendritic cells readily internalise the Campylobacter cells, express cell surface co-stimulatory molecules (CD14, CD40, CD80, and CD86), and become mature (93,94). TLRs in mature dendritic cells (Ag-presenting cells; APCs) can become activated as a consequence of their internalisation of whole-bacterial cell or their interaction with bacterial cellular components. The MyD88-dependent signalling pathway downstream the TLR2 and TLR4 produce different pro-inflammatory cytokines including IL-1, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNF-α, while, the MyD88-independent signalling via TLR4-TRIF axis contributes to the production of IFN-β in mature dendritic cells (40,87,95). It is proposed that C. jejuni cell-surface sialylated LOS structures intensify the production of IFN-β and TNF-α in dendritic cells which further contribute to the proliferation of human mucosal B-cells in a T-cell independent manner. It may link the C. jejuni sialylated LOS structures with the initiation of B-cells mediated autoimmunity in GBS (93,94). Other cell membrane bound receptors, siglec-7, of mature dendritic cells can also interact with Campylobacter LOS to increase the cytokines production and uptake of Campylobacter cells into dendritic cells (74), but the mechanisms involved downstream of the siglec-7 activation in mature dendritic cells are not yet known.
ACTIVATION OF ADAPTIVE IMMUNITY FOR PERSISTENT INFECTION
The adaptive immunity develops typically to eradicate the persistent Campylobacter infection as well as to reduce the post-infection severe complications (96). It is supported by the presence of high amount of intraepithelial T-lymphocytes in patients with post-dysenteric IBS, acute inflammatory demyelinating polyneuropathy (AIDP), and colorectal cancer (97,98,99). The APCs after pathogen recognition migrate to the lymphoid nodules (Peyer's patches) where they present Ags via cell-surface molecules (MHC-I and MHC-II) to trigger the polarisation of naïve T-cells to CD8+ cytotoxic T (Tc) cells and CD4+ Th cells. Further, Campylobacter LOS structures bind with cell-surface siglec-7 receptors of APCs to mediate the differentiation of Th cells into the Th1 and Th2 cells (40,87). It has been reported that APC-expressed siglec-7 receptors interaction with α2, 8-linked sialylated LOS induces the Th1 polarisation, whereas, its interaction with α2, 3-linked sialic acid induces a Th2 development (87). Moreover, cytokines (IL-12 and IL-10) from APCs also stimulate the proliferation of Th1 cells and secretion of IFN-γ, TNF-α, IL-22, and IL-17 from these T-cells. Th1 cells activate more Tc cells and macrophages to enhance immunity against the invading or intracellular microbes, whereas, Th2 mediate class switching in B-cells to enhance immunity against the extracellular microbes. The cytokines from dendritic cells do not only induce the activation and proliferation of B-cells in a T-cell dependent manner, but also in T-cell independent manner. Hence, dendritic cells act as a bridge between innate and adaptive immune systems (40,87,100,101,102). Apart from Th1 and Th2 cells, frequency of other types of CD4+ Th cells including Th17, Th22, and Treg and their associated cytokines (IL-17, IL-18, IL-22, and IL-23) has also been observed elevated in patients serum following infection with Campylobacter (103,104). Subsequently, B-cells after activation produce Abs against the Campylobacter toxins, flagella, LOS, CadF, and major outer membrane proteins, and secrete them in human serum (7,29,105,106). In the acute phase of infection (7 days post-infection), the level of serum Abs, IgA, and IgM, increase in serum (107). In the convalescent phase of infection (1 wk to 2 months), IgG also begins to circulate in the blood (108,109). These serum Abs are detectable in the serum and faeces of Campylobacter infected patients (110). IgA in up to 20 days and IgM in 2 months attain their normal levels back. In contrast, IgG present in serum as well as serum IgG expelled into saliva, remain extant inside the host for long time period (1 year) and provide protection against subsequent Campylobacter infection (108,109).
The LOS-outer core structures (GM1, GM2, GM3, GD3, or GD1-like) present on the cell-surface of Campylobacter mimic the GM1, GM2, GM3, GD3, and GD1 containing human neuronal gangliosides. Campylobacter possess phase variation in LOS biosynthesis genes and therefore, can switch the LOS-outer core structures from one form to other (GM1⇔GM2; GM2⇔GM3; GD1⇔GD3). The LOS-outer core structures' mimicry with human neuronal gangliosides and their switching ability help Campylobacter to escape from the host immune system (30,111,112). It has been identified that Campylobacter toxins arrest human T-cells in the G2 phase of cell cycle and halt their development (113). The representation of mimics of human neuronal gangliosides on cell-surface, ability to vary these mimics, and toxins mediated inhibition of T-cells indicate that Campylobacter has evolved strategies to escape from the host adaptive immunity.
ACTIVATION OF SERUM PROTEINS FOR PERSISTENT INFECTION
Bacteria opsonised by the professional phagocytes (macrophages, neutrophils, dendritic cells) can enter into blood stream and can be circulated back to neutrophils as neutrophils mediate killing of opsonised bacteria more efficiently than the non-opsonised ones. Defense system in humans involving innate and adaptive immune responses limit the infection to the site of inflammation and does not allow live microbes to enter into bloodstream (72,73). However, during Campylobacter infection, bacteremia can be developed in those patients which are immunocompromised or have persistent post-infection complications. The acute phase proteins particularly C-reactive proteins have been found elevated in GBS patients and patients with weak immune system (such as Campylobacter infected children), which are further likely to activate the complement system (114,115). Serum proteins involved in both classical and alternative complement pathways are bactericidal and can facilitate direct killing of Campylobacter. Campylobacter capsule can provide protection to Campylobacter against killing mediated by serum or complement proteins (76,116,117,118). Human C3b proteins were found unable to bind to the encapsulated Campylobacter fetus previously, supporting the role of capsule S-layer proteins in the development of interaction with human serum proteins (119).
NETWORKING BETWEEN HUMAN CELLS DURING CAMPYLOBACTER INFECTION
During Campylobacter infection, networking between IECs and immune cells as well as among different immune cells occurs with the help of cytokines (Fig. 2). In Fig. 2, it has been demonstrated that: i) the Campylobacter infected IECs release IL-8 and IL-10 to recruit neutrophils into lamina propria and submucosa; ii) different cytokines including IL-10, IL-12, TNF-α, and IFN-β from dendritic cells activate B-cells in both T-cell dependent and independent manners; iii) the IFN-γ secretion from Th1 and Tc cells stimulate more macrophages during infection; and iv) the production of IL-6 and Abs respectively from macrophages and B-cells as well as a high level of acute-phase proteins in serum contribute to the activation of complement system (40,66,70,72,86,87,94,105).
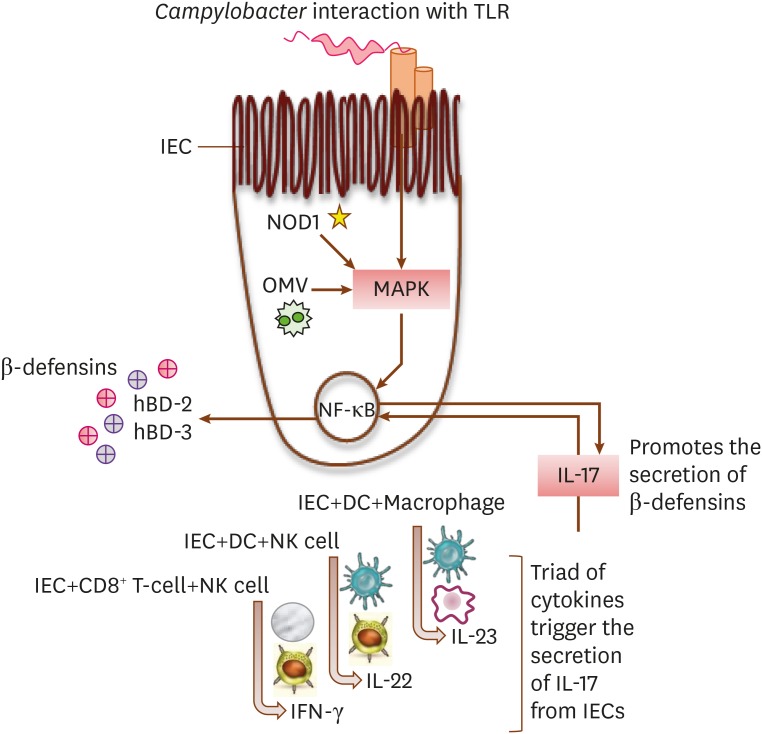
The GI tract in humans express hBD-1 constitutively, while, the production of other defensins (hBD-2 and hBD-3) involves cell receptors mediated signalling pathways, NF-κB transcription, and cytokines secretion (65). During Campylobacter infection, hBD-1 expression remains unchanged. However, expression of other defensins increases due to stimulation of IECs membrane bound receptors (TLR) and intracellular receptors (NOD1) following their interaction with Campylobacter cells or cellular constituents (61,65). The level of β-defensins in IECs further rises through networking between different immune cells (dendritic cells, CD14+ macrophages, Tc, and NK cells) and IECs (Fig. 3) (100,101).
Figure 3. Networking between human IECs and innate immune cells for maximal production of β-defensins in IECs.
The presence of Campylobacter OMV inside of an IEC, activation of intracellular NOD-like receptors (NOD1), and an interaction between the host IEC TLR and a Campylobacter cell, all lead to the activation of MAPK. The IECs and immune cells collaborate to secrete a triad of cytokines (IFN-γ, IL-22, and IL-23), which further elevates the expression of IL-17 in IECs. The activated MAPK and a high level of IL-17, both stimulate the production of β-defensins in IECs by the regulation of NF-κB transcription.
GAPS IN KNOWLEDGE FOR FUTURE RESEARCH
This review identifies that substantial gaps are present in that knowledge which relates the human protective immunity to Campylobacter infection. These knowledge gaps have not so far been investigated and can be filled by future research. The association of IECs-derived cathelicidins and bactericidal permeability-increasing proteins to Campylobacter infection has not been established to date (12). The IECs cell receptors and signalling pathways, important for the induction of chemokines during Campylobacter infection, have not been yet identified. The inflammasomes activation in IECs in response to Campylobacter infection as well as their functions have been reported previously (68), but further investigations are required to identify the stimulatory factors of inflammasomes and mechanisms involved subsequent to their stimulation. A link between the Campylobacter OMV and inflammasome induction inside of IECs might be present and can be focused in future studies. Similarly, Campylobacter OMV might also have implications for other NOD-like intracellular receptors (e.g. NOD1). In addition, the activation of siglec-7 receptors in dendritic cell, acute phase proteins in serum, and T-cell subsets in lymphoid nodules during Campylobacter infection and their related host responses are yet to be explored in detail. Moreover, a possible connection present between many acute phase proteins (alpha 1-antitrypsin, mannose-binding lactin, and serum amuloid A) and Campylobacter infection has never been investigated. An outer membrane protein of C. jejuni, CadF, facilitates interaction between the host cell fibronectin and Campylobacter cells (15), which might be a target of serum amyloid A (12). This prediction requires verification with further research.
Abbreviations
- AIDP
acute inflammatory demyelinating polyneuropathy
- APC
Ag-presenting cell
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- BPI
bactericidal permeability increasing
- CadF
cadherin-fibronectin binding protein
- CAP
cationic antimicrobial peptide
- Fn
fibronectin
- GBS
Guillain-Barré syndrome
- GI
gastrointestinal
- GROα
growth related oncogene alpha
- hBD
human β-defensins
- IBS
Irritable bowel syndrome
- IEC
intestinal epithelial cell
- IP-10
IFN-γ-inducible protein 10
- IRF-3
IFN regulatory factor 3
- LOS
lipooligosaccharides
- LR
lectin receptor
- LTB4
leukotriene B4
- MAMP
microbe-associated molecular pattern
- MAP3K
mitogen activated protein kinase kinase kinase
- NLRP3
NOD-like receptors with pyrin domain-containing 3
- NOD
nucleotide-binding oligomerisation domain
- OMV
outer membrane vesicle
- PAMP
pathogen-associated molecular pattern
- PRR
pathogen recognition receptor
- RNS
reactive nitrogen species
- Sn
sialoadhesin
- Tc
cytotoxic T
- TIR
Toll/IL-1 receptor
- TRAF6
TNF receptor-associated factor 6
- TRAM
Toll/IL-1 receptor-domain-containing adapter-inducing IFN-β-related adaptor molecule
- TRIF
Toll/IL-1 receptor-domain-containing adapter-inducing IFN-β
Footnotes
Conflict of Interest: The author declares no potential conflicts of interest.
References
- 1.Skirrow MB. Campylobacter enteritis: a “new” disease. BMJ. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio. 2014;5:e01364-14. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Z, Willer T, Pielsticker C, Gerzova L, Rychlik I, Rautenschlein S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016;8:56. doi: 10.1186/s13099-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Z, Willer T, Li L, Pielsticker C, Rychlik I, Velge P, Kaspers B, Rautenschlein S. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect Immun. 2017;85:e00380-17. doi: 10.1128/IAI.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin O, Luo N, Huang S, Zhang Q. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl Environ Microbiol. 2003;69:5372–5379. doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoaf-Sweeney KD, Larson CL, Tang X, Konkel ME. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl Environ Microbiol. 2008;74:6867–6875. doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawthraw S, Ayling R, Nuijten P, Wassenaar T, Newell DG. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 1994;38:341–349. [PubMed] [Google Scholar]
- 9.Lacharme-Lora L, Chaloner G, Gilroy R, Humphrey S, Gibbs K, Jopson S, Wright E, Reid W, Ketley J, Humphrey T, et al. B lymphocytes play a limited role in clearance of Campylobacter jejuni from the chicken intestinal tract. Sci Rep. 2017;7:45090. doi: 10.1038/srep45090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Deun K, Pasmans F, Ducatelle R, Flahou B, Vissenberg K, Martel A, Van den Broeck W, Van Immerseel F, Haesebrouck F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Berndtson E, Danielsson-Tham ML, Engvall A. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int J Food Microbiol. 1996;32:35–47. doi: 10.1016/0168-1605(96)01102-6. [DOI] [PubMed] [Google Scholar]
- 12.Iovine NM. Innate immunity in Campylobacter infections. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd ed. Washington, D.C.: ASM Press; 2008. pp. 337–344. [Google Scholar]
- 13.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin S, Joe A, Lynett J, Hani EK, Sherman P, Chan VL. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol. 2001;39:1225–1236. doi: 10.1111/j.1365-2958.2001.02294.x. [DOI] [PubMed] [Google Scholar]
- 15.Monteville MR, Yoon JE, Konkel ME. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- 16.Tu QV, McGuckin MA, Mendz GL. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J Med Microbiol. 2008;57:795–802. doi: 10.1099/jmm.0.47752-0. [DOI] [PubMed] [Google Scholar]
- 17.Stahl M, Vallance BA. Insights into Campylobacter jejuni colonization of the mammalian intestinal tract using a novel mouse model of infection. Gut Microbes. 2015;6:143–148. doi: 10.1080/19490976.2015.1016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Tall BD, Curtis SK, Kopecko DJ. Enhanced microscopic definition of Campylobacter jejuni 81-176 adherence to, invasion of, translocation across, and exocytosis from polarized human intestinal Caco-2 cells. Infect Immun. 2008;76:5294–5304. doi: 10.1128/IAI.01408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson RO, Galán JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008;4:e14. doi: 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrant RL, Wanke CA, Pennie RA, Barrett LJ, Lima AA, O'Brien AD. Production of a unique cytotoxin by Campylobacter jejuni . Infect Immun. 1987;55:2526–2530. doi: 10.1128/iai.55.10.2526-2530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O Cróinín T, Backert S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Infect Microbiol. 2012;2:25. doi: 10.3389/fcimb.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985;26:945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 25.Perkins DJ, Newstead GL. Campylobacter jejuni enterocolitis causing peritonitis, ileitis and intestinal obstruction. Aust N Z J Surg. 1994;64:55–58. doi: 10.1111/j.1445-2197.1994.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 26.Samie A, Ramalivhana J, Igumbor EO, Obi CL. Prevalence, haemolytic and haemagglutination activities and antibiotic susceptibility profiles of Campylobacter spp. isolated from human diarrhoeal stools in Vhembe District, South Africa. J Health Popul Nutr. 2007;25:406–413. [PMC free article] [PubMed] [Google Scholar]
- 27.Endtz HP, Ang CW, van Den Braak N, Duim B, Rigter A, Price LJ, Woodward DL, Rodgers FG, Johnson WM, Wagenaar JA, et al. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barré and Miller Fisher syndromes. J Clin Microbiol. 2000;38:2297–2301. doi: 10.1128/jcm.38.6.2297-2301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy N, Giesecke J. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni . Am J Epidemiol. 2001;153:610–614. doi: 10.1093/aje/153.6.610. [DOI] [PubMed] [Google Scholar]
- 29.Godschalk PC, Kuijf ML, Li J, St Michael F, Ang CW, Jacobs BC, Karwaski MF, Brochu D, Moterassed A, Endtz HP, et al. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barre and Miller Fisher syndromes. Infect Immun. 2007;75:1245–1254. doi: 10.1128/IAI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houliston RS, Vinogradov E, Dzieciatkowska M, Li J, St Michael F, Karwaski MF, Brochu D, Jarrell HC, Parker CT, Yuki N, et al. Lipooligosaccharide of Campylobacter jejuni: similarity with multiple types of mammalian glycans beyond gangliosides. J Biol Chem. 2011;286:12361–12370. doi: 10.1074/jbc.M110.181750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apel D, Ellermeier J, Pryjma M, Dirita VJ, Gaynor EC. Characterization of Campylobacter jejuni RacRS reveals roles in the heat shock response, motility, and maintenance of cell length homogeneity. J Bacteriol. 2012;194:2342–2354. doi: 10.1128/JB.06041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friis LM, Keelan M, Taylor DE. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect Immun. 2009;77:1553–1560. doi: 10.1128/IAI.00707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouwman LI, de Zoete MR, Bleumink-Pluym NM, Flavell RA, van Putten JP. Inflammasome activation by Campylobacter jejuni . J Immunol. 2014;193:4548–4557. doi: 10.4049/jimmunol.1400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang Y, Ren F, Song Z, Li Q, Zhou X, Wang X, Xu Z, Bao G, Wan T, Lei T, et al. Insights into Campylobacter jejuni colonization and enteritis using a novel infant rabbit model. Sci Rep. 2016;6:28737. doi: 10.1038/srep28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson L, Wennerås C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7:720–728. doi: 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 36.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. doi: 10.1186/1756-0500-6-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun. 2000;68:6535–6541. doi: 10.1128/iai.68.12.6535-6541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin S, Song YC, Emili A, Sherman PM, Chan VL. JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90α and triggers signalling pathways leading to the activation of NF‐κB and p38 MAP kinase in epithelial cells. Cell Microbiol. 2003;5:165–174. doi: 10.1046/j.1462-5822.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- 39.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu L, Bray MD, Osorio M, Kopecko DJ. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun. 2006;74:2697–2705. doi: 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng J, Meng J, Zhao S, Singh R, Song W. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-κB. Infect Immun. 2008;76:4498–4508. doi: 10.1128/IAI.01317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Zoete MR, Keestra AM, Roszczenko P, van Putten JP. Activation of human and chicken Toll-like receptors by Campylobacter spp. Infect Immun. 2010;78:1229–1238. doi: 10.1128/IAI.00897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hara JR, Feener TD, Fischer CD, Buret AG. Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice. Infect Immun. 2012;80:1563–1571. doi: 10.1128/IAI.06066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephenson HN, John CM, Naz N, Gundogdu O, Dorrell N, Wren BW, Jarvis GA, Bajaj-Elliott M. Campylobacter jejuni lipooligosaccharide sialylation, phosphorylation, and amide/ester linkage modifications fine-tune human Toll-like receptor 4 activation. J Biol Chem. 2013;288:19661–19672. doi: 10.1074/jbc.M113.468298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stahl M, Ries J, Vermeulen J, Yang H, Sham HP, Crowley SM, Badayeva Y, Turvey SE, Gaynor EC, Li X, et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog. 2014;10:e1004264. doi: 10.1371/journal.ppat.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 47.Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-κB proinflammatory responses. J Biol Chem. 2009;284:24192–24203. doi: 10.1074/jbc.M109.023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun. 1999;67:88–93. doi: 10.1128/iai.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mellits KH, Mullen J, Wand M, Armbruster G, Patel A, Connerton PL, Skelly M, Connerton IF. Activation of the transcription factor NF-κB by Campylobacter jejuni . Microbiology. 2002;148:2753–2763. doi: 10.1099/00221287-148-9-2753. [DOI] [PubMed] [Google Scholar]
- 50.Jones MA, Tötemeyer S, Maskell DJ, Bryant CE, Barrow PA, To S. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni . Infect Immun. 2003;71:2626–2633. doi: 10.1128/IAI.71.5.2626-2633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.John DA, Williams LK, Kanamarlapudi V, Humphrey TJ, Wilkinson TS. The bacterial species Campylobacter jejuni induce diverse innate immune responses in human and avian intestinal epithelial cells. Front Microbiol. 2017;8:1840. doi: 10.3389/fmicb.2017.01840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 54.Nilsen NJ, Vladimer GI, Stenvik J, Orning MP, Zeid-Kilani MV, Bugge M, Bergstroem B, Conlon J, Husebye H, Hise AG, et al. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J Biol Chem. 2015;290:3209–3222. doi: 10.1074/jbc.M114.593426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakhiet M, Al-Salloom FS, Qareiballa A, Bindayna K, Farid I, Botta GA. Induction of α and β chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni . J Infect. 2004;48:236–244. doi: 10.1016/j.jinf.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Hu L, Hickey TE. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun. 2005;73:4437–4440. doi: 10.1128/IAI.73.7.4437-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johanesen PA, Dwinell MB. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect Immun. 2006;74:3437–3447. doi: 10.1128/IAI.01740-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Sayeqh AF, Loughlin MF, Dillon E, Mellits KH, Connerton IF. Campylobacter jejuni activates NF-κB independently of TLR2, TLR4, Nod1 and Nod2 receptors. Microb Pathog. 2010;49:294–304. doi: 10.1016/j.micpath.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NF, Manson E, Imrie L, Bajaj-Elliott M, Wren BW, et al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun. 2012;80:4089–4098. doi: 10.1128/IAI.00161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konkel ME, Samuelson DR, Eucker TP, Shelden EA, O'Loughlin JL. Invasion of epithelial cells by Campylobacter jejuni is independent of caveolae. Cell Commun Signal. 2013;11:100. doi: 10.1186/1478-811X-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schüller S, Jones HE, Klein NJ, Núnez G, Wren BW, Bajaj-Elliott M. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni . Cell Microbiol. 2007;9:2404–2416. doi: 10.1111/j.1462-5822.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 63.Grimes CL, Ariyananda Lde Z, Melnyk JE, O'Shea EK. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc. 2012;134:13535–13537. doi: 10.1021/ja303883c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girardin SE, Travassos LH, Hervé M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 65.Zilbauer M, Dorrell N, Boughan PK, Harris A, Wren BW, Klein NJ, Bajaj-Elliott M. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun. 2005;73:7281–7289. doi: 10.1128/IAI.73.11.7281-7289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy H, Cogan T, Humphrey T. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect. 2011;13:42–48. doi: 10.1016/j.micinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 68.Stahl M, Hoang T, Knodler LA, Vallance B. The first line of defense: the role of epithelial cell inflammasomes in controlling Campylobacter jejuni infection. J Can Assoc Gastroenterol. 2018;1:401. [Google Scholar]
- 69.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun X, Liu B, Sartor RB, Jobin C. Phosphatidylinositol 3-kinase-γ signaling promotes Campylobacter jejuni-induced colitis through neutrophil recruitment in mice. J Immunol. 2013;190:357–365. doi: 10.4049/jimmunol.1201825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sørensen NB, Nielsen HL, Varming K, Nielsen H. Neutrophil activation by Campylobacter concisus . Gut Pathog. 2013;5:17. doi: 10.1186/1757-4749-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiehlbauch JA, Albach RA, Baum LL, Chang KP. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect Immun. 1985;48:446–451. doi: 10.1128/iai.48.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wassenaar TM, Engelskirchen M, Park S, Lastovica A. Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med Microbiol Immunol (Berl) 1997;186:139–144. doi: 10.1007/s004300050056. [DOI] [PubMed] [Google Scholar]
- 74.Avril T, Wagner ER, Willison HJ, Crocker PR. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun. 2006;74:4133–4141. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Purdy D, Cawthraw S, Dickinson JH, Newell DG, Park SF. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl Environ Microbiol. 1999;65:2540–2546. doi: 10.1128/aem.65.6.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keo T, Collins J, Kunwar P, Blaser MJ, Iovine NM. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence. 2011;2:30–40. doi: 10.4161/viru.2.1.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walan A, Dahlgren C, Kihlström E, Stendahl O, Lock R. Phagocyte killing of Campylobacter jejuni in relation to oxidative activation. APMIS. 1992;100:424–430. [PubMed] [Google Scholar]
- 78.Everest P. Campylobacter spp. and the ability to elicit intestinal inflammatory responses. In: Ketley JM, Konkel ME, editors. Campylobacter Molecular and Cellular Biology. Norfolk: Horizon Bioscience; 2005. p. 429. [Google Scholar]
- 79.Bär W. Role of murine macrophages and complement in experimental Campylobacter infection. J Med Microbiol. 1988;26:55–59. doi: 10.1099/00222615-26-1-55. [DOI] [PubMed] [Google Scholar]
- 80.Hickey TE, Majam G, Guerry P. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun. 2005;73:5194–5197. doi: 10.1128/IAI.73.8.5194-5197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heikema AP, Koning RI, Duarte dos Santos Rico S, Rempel H, Jacobs BC, Endtz HP, van Wamel WJ, Samsom JN. Enhanced, sialoadhesin-dependent uptake of Guillain-Barre syndrome-associated Campylobacter jejuni strains by human macrophages. Infect Immun. 2013;81:2095–2103. doi: 10.1128/IAI.01437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Sorge NM, Bleumink NM, van Vliet SJ, Saeland E, van der Pol WL, van Kooyk Y, van Putten JP. N-glycosylated proteins and distinct lipooligosaccharide glycoforms of Campylobacter jejuni target the human C-type lectin receptor MGL. Cell Microbiol. 2009;11:1768–1781. doi: 10.1111/j.1462-5822.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 83.Day WA, Jr, Sajecki JL, Pitts TM, Joens LA. Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun. 2000;68:6337–6345. doi: 10.1128/iai.68.11.6337-6345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klaas M, Oetke C, Lewis LE, Erwig LP, Heikema AP, Easton A, Willison HJ, Crocker PR. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni . J Immunol. 2012;189:2414–2422. doi: 10.4049/jimmunol.1200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korneev KV, Kondakova AN, Sviriaeva EN, Mitkin NA, Palmigiano A, Kruglov AA, Telegin GB, Drutskaya MS, Sturiale L, Garozzo D, et al. Hypoacylated LPS from foodborne pathogen Campylobacter jejuni induces moderate TLR4-mediated inflammatory response in murine macrophages. Front Cell Infect Microbiol. 2018;8:58. doi: 10.3389/fcimb.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrauch Y. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni . Infect Immun. 2008;76:986–993. doi: 10.1128/IAI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, García-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun. 2011;79:2681–2689. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Fehlings M, Drobbe L, Moos V, Renner Viveros P, Hagen J, Beigier-Bompadre M, Pang E, Belogolova E, Churin Y, Schneider T, et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect Immun. 2012;80:2724–2734. doi: 10.1128/IAI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 91.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 92.Hu L, Kopecko DJ. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuijf ML, Samsom JN, van Rijs W, Bax M, Huizinga R, Heikema AP, van Doorn PA, van Belkum A, van Kooyk Y, Burgers PC, et al. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J Immunol. 2010;185:748–755. doi: 10.4049/jimmunol.0903014. [DOI] [PubMed] [Google Scholar]
- 94.Huizinga R, van Rijs W, Bajramovic JJ, Kuijf ML, Laman JD, Samsom JN, Jacobs BC. Sialylation of Campylobacter jejuni endotoxin promotes dendritic cell-mediated B cell responses through CD14-dependent production of IFN-β and TNF-α. J Immunol. 2013;191:5636–5645. doi: 10.4049/jimmunol.1301536. [DOI] [PubMed] [Google Scholar]
- 95.Rathinam VA, Appledorn DM, Hoag KA, Amalfitano A, Mansfield LS. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect Immun. 2009;77:2499–2507. doi: 10.1128/IAI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baqar S, Tribble DR, Carmolli M, Sadigh K, Poly F, Porter C, Larsson CJ, Pierce KK, Guerry P, et al. Campylobacter Study Team. Recrudescent Campylobacter jejuni infection in an immunocompetent adult following experimental infection with a well-characterized organism. Clin Vaccine Immunol. 2010;17:80–86. doi: 10.1128/CVI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 99.He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68:289–300. doi: 10.1136/gutjnl-2018-317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edwards LA, Nistala K, Mills DC, Stephenson HN, Zilbauer M, Wren BW, Dorrell N, Lindley KJ, Wedderburn LR, Bajaj-Elliott M. Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection. PLoS One. 2010;5:e15398. doi: 10.1371/journal.pone.0015398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol. 2014;7:802–817. doi: 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fimlaid KA, Lindow JC, Tribble DR, Bunn JY, Maue AC, Kirkpatrick BD. Peripheral CD4+ T cell cytokine responses following human challenge and re-challenge with Campylobacter jejuni . PLoS One. 2014;9:e112513. doi: 10.1371/journal.pone.0112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, Zhu J. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barré syndrome and downregulated by IVIg treatments. Mediators Inflamm. 2014;2014:740947. doi: 10.1155/2014/740947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bereswill S, Grundmann U, Alutis ME, Fischer A, Kühl AA, Heimesaat MM. Immune responses upon Campylobacter jejuni infection of secondary abiotic mice lacking nucleotide-oligomerization-domain-2. Gut Pathog. 2017;9:33. doi: 10.1186/s13099-017-0182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blaser MJ, Hopkins JA, Vasil ML. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984;43:986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kirimat M, Georges-Courbot MC, Georges AJ, Martin PM. Antibodies to Campylobacter flagellin recognize epitopes common to phase 1 and phase 2 flagella. Res Microbiol. 1989;140:645–651. doi: 10.1016/0923-2508(89)90196-4. [DOI] [PubMed] [Google Scholar]
- 107.Strid MA, Engberg J, Larsen LB, Begtrup K, Mølbak K, Krogfelt KA. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin Diagn Lab Immunol. 2001;8:314–319. doi: 10.1128/CDLI.8.2.314-319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cawthraw SA, Lind L, Kaijser B, Newell DG. Antibodies, directed towards Campylobacter jejuni antigens, in sera from poultry abattoir workers. Clin Exp Immunol. 2000;122:55–60. doi: 10.1046/j.1365-2249.2000.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cawthraw SA, Feldman RA, Sayers AR, Newell DG. Long-term antibody responses following human infection with Campylobacter jejuni . Clin Exp Immunol. 2002;130:101–106. doi: 10.1046/j.1365-2249.2002.01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lane EM, Batchelor RA, Bourgeois AL, Burr DH, Olson JG. Urine and faecal IgA response during naturally acquired infection with Campylobacter jejuni . Lancet. 1987;1:1141. doi: 10.1016/s0140-6736(87)91694-1. [DOI] [PubMed] [Google Scholar]
- 111.Linton D, Gilbert M, Hitchen PG, Dell A, Morris HR, Wakarchuk WW, Gregson NA, Wren BW. Phase variation of a β-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni . Mol Microbiol. 2000;37:501–514. doi: 10.1046/j.1365-2958.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- 112.Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro . Infect Immun. 2002;70:787–793. doi: 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mooney A, Clyne M, Curran T, Doherty D, Kilmartin B, Bourke B. Campylobacter upsaliensis exerts a cytolethal distending toxin effect on HeLa cells and T lymphocytes. Microbiology. 2001;147:735–743. doi: 10.1099/00221287-147-7-1815. [DOI] [PubMed] [Google Scholar]
- 114.Vaishnavi C, Kapoor P, Behura C, Singh SK, Prabhakar S. C-reactive protein in patients with Guillain Barré syndrome. Indian J Pathol Microbiol. 2014;57:51–54. doi: 10.4103/0377-4929.130897. [DOI] [PubMed] [Google Scholar]
- 115.Bae JY, Lee DH, Ko KO, Lim JW, Cheon EJ, Song YH, Yoon JM. Clinical manifestation of Campylobacter enteritis in children. Korean J Pediatr. 2018;61:84–89. doi: 10.3345/kjp.2018.61.3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blaser MJ, Black RE, Duncan DJ, Amer J. Campylobacter jejuni-specific serum antibodies are elevated in healthy Bangladeshi children. J Clin Microbiol. 1985;21:164–167. doi: 10.1128/jcm.21.2.164-167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernández H, Giusti G, Bertoglio JC. Effect of the complement system on the sensitivity of Campylobacter jejuni and Campylobacter coli to human blood serum. Braz J Med Biol Res. 1995;28:227–229. [PubMed] [Google Scholar]
- 118.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, et al. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun. 2013;81:665–672. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blaser MJ, Smith PF, Repine JE, Joiner KA. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Invest. 1988;81:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]