Abstract
Purpose:
Critically ill pediatric allogeneic hematopoietic cell transplant (HCT) patients may benefit from early and aggressive interventions aimed at reversing the progression of multiorgan dysfunction. Therefore, we evaluated 25 early risk-factors for PICU mortality in order to improve mortality prognostication.
Methods:
We merged the Virtual Pediatric Systems (VPS, LLC) and Center For International Blood and Marrow Transplant Research (CIBMTR) databases and analyzed 936 critically ill patients ≤21 years of age who had undergone allogeneic HCT and subsequently required PICU admission between January 1, 2009 and December 31, 2014.
Results:
Of 1,532 PICU admissions, the overall PICU mortality rate was 17.4% (95% CI 15.6%−19.4%) but was significantly higher for patients requiring mechanical ventilation (44.0%), renal replacement therapy (56.1%), or extracorporeal life support (77.8%). Mortality estimates increased significantly the longer that patients remained in the PICU. Of 25 HCT- and PICU-specific characteristics available at/near the time of PICU admission, moderate/severe pre-HCT renal injury, pre-HCT recipient cytomegalovirus (CMV) seropositivity, <100 day interval between HCT and PICU admission, HCT for underlying Acute Myeloid Leukemia (AML), and greater admission organ dysfunction as approximated by the PRISM-3 score were each independently associated with PICU mortality. A multivariable model using these components identified that patients in the top quartile of risk had three times greater mortality than other patients (35.1% vs. 11.5%, p<0.001, classification accuracy 75.2%, 95% CI 73.0–77.4%).
Conclusions:
These data improve our working knowledge of the factors influencing the progression of critical illness in pediatric allogeneic HCT patients. Future investigation aimed at mitigating the effect of these risk-factors is warranted.
Keywords: Intensive Care Units, Pediatric, Hematopoietic Stem Cell Transplantation, Organ Dysfunction Scores, Prognosis, Survival Analysis
INTRODUCTION
Each year, hematopoietic cell transplantation (HCT) offers the potential for disease-free survival to over 2,500 children with malignant and non-malignant disorders worldwide [1]. Unfortunately, complications from HCT frequently lead to critical illness and necessitate pediatric intensive care unit (PICU) admission in 17–35% of children [2–4]. However, recent advances in the areas of donor selection, graft manipulation, preparative conditioning, infection prophylaxis, and intensive care have significantly improved outcomes, allowing for increasing complexity of patients eligible for HCT and increasing medical complexity of post-HCT care [5–7].
A primary focus of both transplant and intensive care physicians remains the prompt and accurate identification of high-risk patients in order to halt the progression of critical illness before irreversible organ failure ensues [8]. Central to this focus is the strong body of evidence demonstrating that organ failure is modifiable through early recognition and early intervention [9–13]. Among patients who become critically ill, severity of organ failure at the time of PICU admission remains one of the most useful prognostic indicators for mortality [14,15]. However, attempts to improve prognostic models by incorporating HCT-specific variables have been limited by sample size constraints. In addition, many investigators have identified conflicting results regarding the association between PICU mortality and HCT-specific characteristics such as underlying disease, degree of HLA mismatch, failure of neutrophil engraftment, and the presence and severity of acute graft versus host disease (GVHD) [7,16–21]. Ultimately, the medical complexity of critically ill pediatric allogeneic HCT patients necessitates a thorough understanding of HCT-specific risk factors that might modulate the development and progression of critical illness, as these data will be crucial for accurate mortality prognostication as well as the design of future patient-specific interventions aimed at reducing post-HCT mortality.
Therefore, we aimed to merge two large multicenter databases in order to complete a comprehensive assessment of HCT-specific risk-factors for PICU mortality and develop an improved prognostic model for early assessment of mortality-risk in critically ill pediatric allogeneic HCT patients. We specifically aimed to assess factors available at/near the time of PICU admission so as to capture high-risk patients during a window of opportunity where potential clinical interventions might have the best chance of success. We hypothesized that the information learned from this study might inform our understanding of the development and progression of critical illness in children after allogeneic HCT.
METHODS
Approach:
Two large de-identified administrative databases were selected for record matching based on complementary and partially overlapping documentation of PICU- and HCT-related data. VPS: The Virtual Pediatric Systems (VPS, LLC) Database documents consecutive PICU admissions across over 140 sites predominantly in the United States and Canada. Admission characteristics, severity of illness scores, critical care interventions, and critical care-related ICD-9 and other diagnosis codes are documented by trained analysts at each site, with >95% inter-rater reliability. CIBMTR: The Center For International Blood and Marrow Transplant Research (CIBMTR) is a collaboration between the National Marrow Donor Program®/Be The Match® and the Medical College of Wisconsin. It comprises over 450 transplant centers worldwide that contribute high-quality longitudinal data on consecutive allogeneic HCT patients. Additional description of each database is provided in Supplemental Text 1.
Patients:
To identify critically ill pediatric HCT patients for this study, VPS was queried for patients ≤21 years of age who were admitted to a PICU between 1/1/2009 and 12/31/2014 with a diagnosis code indicating prior HCT. To avoid analyzing low-risk patients, we excluded patients with short-term semi-elective PICU admissions, which we defined as any scheduled (>12 hours notice) or perioperative admission lasting <2 days. To obtain further descriptive HCT-related characteristics for these critically ill patients, CIBMTR was queried for patients ≤21 years of age at the time of receiving a first allogeneic HCT between 1/1/2008 and 12/31/2014. While the VPS query started with 1/1/2009, the CIBMTR query included the preceding year to capture post-HCT patients with PICU admissions in early 2009 who underwent HCT in 2008. Patients were excluded if they underwent HCT outside of the USA/Canada, had an identical twin, or lacked 100-day follow-up.
Matching:
Records from VPS were merged with records from CIBMTR according to identical sex, date of birth, and HCT indication. VPS records were excluded if (1) there was no HCT indication documented in VPS, (2) there was no matching record in CIBMTR, or (3) there were ≥2 matching records in CIBMTR. VPS patients with no matching record in CIBMTR were assumed to meet CIBMTR exclusion criteria, including receipt of autologous rather than allogeneic HCT, or receipt of allogeneic HCT outside of the USA/Canada and/or outside of the study interval (Supplemental Text 2). After records were merged, VPS records were excluded if the patient developed relapse of underlying malignancy after HCT but prior to PICU admission. Where patients had multiple PICU admissions in the study interval, PICU admissions were truncated after the 3rd PICU admission. This methodology was approved by the UCSF IRB (Study 18–26930). To confirm validity of matching, we performed an unblinded IRB-approved review of matching results from records at the University of California, San Francisco Benioff Children’s Hospital (Supplemental Text 3).
Outcomes:
The primary outcome was PICU mortality, defined as death in the PICU during the study interval. In addition to assessing PICU mortality for all patients, we assessed PICU mortality for (1) patients using invasive mechanical ventilation (IMV), renal replacement therapy (RRT), or extracorporeal life support (ECLS), (2) patients with a diagnosis code of sepsis, Gram-positive, Gram-negative, viral, or fungal infection, as previously defined (Supplemental Text 4) [14], and (3) increasing duration of stay in the PICU. Use of vasoactive infusions is not documented by VPS.
Early Predictors:
Given their associations with HCT and/or intensive care mortality, we a priori selected 25 variables that were available to the clinical team at/near the time of PICU admission to test for an association with PICU mortality. We limited variable selection to only those available at/near the time of PICU admission in order to develop a model that could be implemented early in the course of PICU stay. Variables included metrics of demographics, pre-HCT morbidity, underlying disease, HCT characteristics, post-HCT course, and PICU Admission Illness Severity, which was estimated by the Pediatric Risk of Mortality (PRISM-3) score [22]. The PRISM-3 score ranges from 0–47 and is composed of 17 vital sign and laboratory derangements measured in the first 12 hours of PICU admission (Supplemental Table 1). In order to generate a prognostic model of PICU mortality using only variables available at/near the time of PICU admission, we did not include co-diagnosis codes such as presence/type of infection, as the timing of onset of these infections could not be determined using available data.
Statistical Approach:
Mortality rates were listed as percentages with 95% confidence intervals estimated by the binomial exact probability distribution. Generalized estimating equations (GEE) were used to model the odds of PICU mortality as a function of patient characteristics; GEE accounts for repeated PICU admissions by the same patient using a robust sandwich variance estimate. An appropriate working correlation matrix was selected by maximizing the Akaike Information Criterion (AIC). Stepwise variable selection was used to build the multivariate model and identify important patient characteristics for inclusion. Interactions between PICU visit number and patient characteristics were assessed to determine whether separate models were needed for 1st vs. 2nd vs. 3rd PICU admissions. Covariate effects were summarized using odds ratios for PICU mortality along with 95% confidence intervals. Receiver operating characteristics (AUROC) were generated using 20-fold internal cross-validation to reduce optimism. The distribution of probabilities of PICU mortality as predicted by the final GEE model was polychotomized into four quartiles and the cumulative incidence of PICU mortality was plotted over time.
RESULTS
Cohort:
2,319 pediatric HCT patients were identified in VPS (0.5% of all PICU patients) and were cross-referenced to 9,183 pediatric allogeneic HCT patients in CIBMTR (12.4% of all CIBMTR patients, Figure 1). 1,248 pediatric HCT patients in VPS were excluded due to lack of 1:1 match in CIBMTR; the majority of exclusions were due to patients having no matching record in CIBMTR, and therefore these VPS records were assumed to represent patients having undergone autologous HCT or allogeneic HCT outside of the US/Canada and/or outside of the study interval. Another 135 pediatric HCT patients were excluded due to relapsed malignancy after HCT and before PICU admission. The final cohort of 936 HCT patients accounted for 1,532 PICU admissions. Patient characteristics, including HCT-variables, use of critical care interventions, and presence of infections, are summarized in Tables 1 & 2 and presented in detail in Supplemental Tables 2–4.
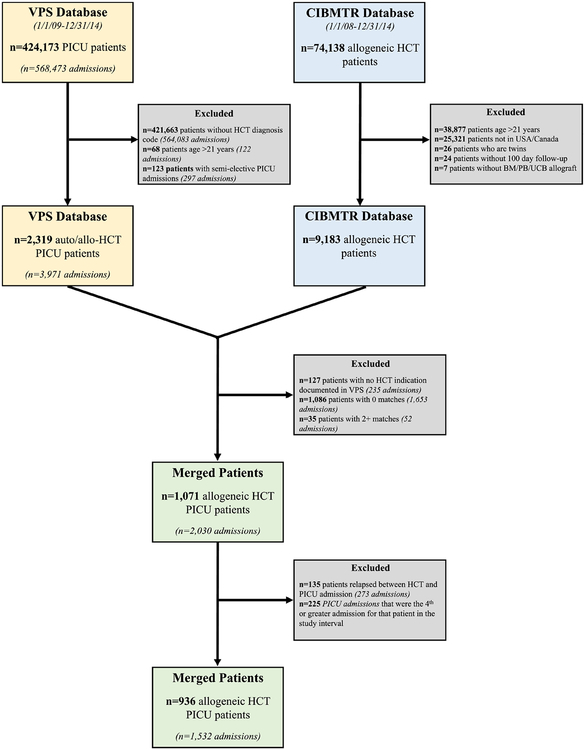
Figure 1. Inclusion / Exclusion Criteria.
The VPS and CIBMTR databases were queried and merged to produce the final study population of n=936 patients. 1,071 allogeneic HCT patients from VPS were successfully merged with CIBMTR records. Of these, 135 were excluded due to relapsed malignancy after HCT and prior to PICU admission. The final study population included 936 patients age <21 years admitted to a PICU between 1/1/2009–12/31/2014 each underwent a first allogeneic HCT in the USA/Canada between 1/1/2008–12/31/2014 and had not relapsed from primary disease at the time of PICU admission. These 936 patients accounted for 1,532 PICU admissions during the study interval.
Table 1.
HCT Characteristics of Critically Ill Pediatric Allogeneic HCT Patients
| 1st PICU Admission (n=936) | 2nd PICU Admission (n=404) | 3rd PICU Admission (n=192) | |
|---|---|---|---|
| Age at transplant (median years, IQR) | 8 (2 – 14) | 9 (2 – 14) | 10 (2 – 14) |
| Sex (n, %) | |||
| Male | 548 (59) | 245 (61) | 112 (58) |
| Female | 388 (41) | 159 (39) | 80 (42) |
| Race/Ethnicity (n, %) | |||
| Caucasian, non-Hispanic | 475 (51) | 208 (51) | 108 (56) |
| Caucasian, Hispanic | 149 (16) | 53 (13) | 24 (13) |
| African-American | 176 (19) | 92 (23) | 41 (21) |
| Asian / Pacific Islander | 51 ( 5) | 24 ( 6) | 6 ( 3) |
| Native American | 15 ( 2) | 2 (<1) | 2 ( 1) |
| Unknown | 70 ( 7) | 25 ( 6) | 11 ( 6) |
| Pre-HCT Lansky/Karnofsky performance score (n, %) | |||
| <90% | 145 (15) | 69 (17) | 33 (17) |
| 90–100% | 777 (83) | 329 (81) | 155 (81) |
| Pre-HCT Comorbidity Index (HCT-CI) (n, %) | |||
| 0 | 598 (64) | 251 (62) | 116 (60) |
| 1–2 | 195 (21) | 94 (23) | 48 (25) |
| ≥3 | 142 (15) | 59 (15) | 28 (15) |
| HCT Indication (n, %) | |||
| Acute myelogenous leukemia (AML) | 144 (15) | 44 (11) | 14 ( 7) |
| Acute lymphoblastic leukemia (ALL) | 183 (20) | 69 (17) | 31 (16) |
| Chronic myelogenous leukemia (CML) | 18 ( 2) | 7 ( 2) | 3 ( 2) |
| Myelodysplastic Syndrome/Myeloproliferative Disorder (MDS/MPD) | 69 ( 7) | 35 ( 9) | 24 (13) |
| Hodgkin lymphoma (HL) or Non-Hodgkin lymphoma (NHL) | 33 ( 4) | 12 ( 4) | 8 ( 4) |
| Severe aplastic anemia | 61 ( 7) | 30 ( 7) | 12 ( 6) |
| Inherited abnormalities erythrocyte differentiation or function | 123 (13) | 61 (15) | 26 (14) |
| Severe Combined Immunodeficiency (SCID), other immune disorders | 129 (14) | 56 (14) | 31 (16) |
| Inherited disorders of metabolism | 66 ( 7) | 31 ( 8) | 10 ( 5) |
| Histiocytic disorders | 80 ( 9) | 40 (10) | 20 (10) |
| Other | 32 ( 3) | 19 ( 5) | 13 ( 7) |
| Graft source (n, %) | |||
| Bone marrow | 527 (56) | 225 (56) | 111 (58) |
| Peripheral blood | 126 (13) | 68 (17) | 34 (18) |
| Umbilical cord blood (UCB) | 283 (30) | 111 (27) | 47 (24) |
| Donor type/matching (n, %) | |||
| Related donor (HLA-matched) | 168 (18) | 59 (15) | 31 (16) |
| Related donor (HLA-mismatched) | 53 ( 6) | 28 ( 7) | 11 ( 6) |
| Unrelated donor (HLA-matched) | 257 (27) | 116 (29) | 54 (28) |
| Unrelated donor (HLA-mismatched) | 137 (15) | 68 (17) | 34 (17) |
| Umbilical cord blood donor (HLA-matched) | 54 ( 6) | 24 ( 6) | 12 ( 6) |
| Umbilical cord blood donor (HLA-mismatched) | 210 (22) | 83 (21) | 34 (18) |
| Other/Unknown | 56 ( 6) | 26 ( 6) | 16 ( 8) |
| Donor/recipient sex match (n, %) | |||
| Male-Male | 303 (32) | 140 (35) | 61 (32) |
| Male-Female | 193 (21) | 87 (22) | 39 (20) |
| Female-Male | 243 (26) | 103 (25) | 49 (26) |
| Female-Female | 195 (21) | 72 (18) | 41 (21) |
| Donor/recipient pre-HCT CMV Serostatus (n, %) | |||
| Negative/negative | 196 (21) | 86 (21) | 34 (18) |
| Negative/positive | 246 (26) | 107 (26) | 51 (27) |
| Positive/negative | 95 (10) | 44 (11) | 20 (10) |
| Positive/positive | 272 (29) | 122 (30) | 65 (34) |
| Unknown | 127 (14) | 45 (11) | 22 (11) |
| Conditioning regimen (n, %) | |||
| Busulfan/Cyclophosphamide ±others | 255 (27) | 110 (27) | 52 (27) |
| Busulfan/Fludarabine ±others | 65 ( 7) | 29 ( 7) | 14 ( 7) |
| Busulfan/Melphalan ±others | 18 ( 2) | 8 ( 2) | 4 ( 2) |
| Melphalan/Fludarabine ±others | 183 (20) | 88 (22) | 40 (21) |
| Total Body Irradiation (TBI)-based | 358 (38) | 144 (36) | 73 (38) |
| Immunosuppression only | 41 ( 4) | 16 ( 4) | 5 ( 3) |
| Other or unknown | 16 ( 2) | 9 ( 2) | 4 ( 2) |
| Serotherapy use (n, %) | |||
| Antithymocyte globulin (ATG) and/or alemtuzumab | 624 (67) | 289 (72) | 128 (67) |
| No ATG or alemtuzumab | 312 (33) | 115 (28) | 64 (33) |
Footnotes: The following variables had missing data for PICU admissions 1, 2, and 3, respectively: Lansky/Karnofsky score (n=14, 6, 4); HCT-CI (n=1, 0, 0); donor type/match (n=1, 0, 0);); donor/recipient sex match (n=2, 2, 2). All other missing data are noted in the table above.
Table 2.
PICU Characteristics of Critically Ill Pediatric Allogeneic HCT Patients
| 1st PICU Admission (n=936) | 2nd PICU Admission (n=404) | 3rd PICU Admission (n=192) | |
|---|---|---|---|
| Age at PICU admission (median years, IQR) | 8 (3 – 15) | 9 (3 – 15) | 11 (3 – 16) |
| Time from HCT to each PICU (median months, IQR) | 2 (1 – 6) | 4 (2 – 10) | 6 (3 – 12) |
| Time from HCT to each PICU group (n, %) | |||
| HCT same date as PICU | 15 ( 2) | 0 | 0 |
| PICU admission within one month post HCT | 339 (36) | 50 (12) | 7 (4) |
| PICU admission greater than 1 month but less than 100 days | 212 (23) | 123 (30) | 53 (28) |
| PICU admission after HCT ≥ 100 days | 370 (40) | 231 (57) | 132 (69) |
| Time from hospital admission to PICU admission (median days, IQR) | 15 (<1 – 30) | 19 (<1 – 58) | 23 (<1 – 81) |
| Neutrophil engraftment (n, %) | |||
| Neutrophil engrafted prior to 1st PICU admission | 636 (68) | 351 (87) | 177 (92) |
| Neutrophil engrafted after PICU admission, before PICU discharge | 89 (9) | 12 ( 3) | 2 (<1) |
| Neutrophil engrafted after PICU discharge | 122 (13) | 8 (2) | 5 ( 3) |
| No neutrophil engraftment achieved | 72 ( 8) | 30 (7) | 13 ( 7) |
| PRISM-3 score at each PICU admission (median score, IQR) | 9 (4 – 14) | 9 (4 – 13) | 9 (3 – 14) |
| Critical Care Interventions (n, %) | |||
| Invasive mechanical ventilation (IMV) | 346 (37) | 143 (35) | 77 (40) |
| Noninvasive mechanical ventilation (NIMV) | 64 ( 7) | 41 (10) | 26 (14) |
| Renal replacement therapy (RRT) | 110 (12) | 45 (11) | 18 ( 9) |
| Extracorporeal life support (ECLS) | 6 (<1) | 2 (<1) | 1 (<1) |
| Infections (n, %) | |||
| Any Infection | 574 (61) | 273 (68) | 140 (73) |
| Sepsis | 228 (24) | 91 (23) | 50 (26) |
| Gram-positive infection | 103 (11) | 47 (12) | 27 (14) |
| Gram-negative infection | 71 ( 8) | 41 (10) | 26 (14) |
| Fungal infection | 84 ( 9) | 51 (13) | 32 (17) |
| Viral infection | 255 (27) | 138 (34) | 62 (32) |
| PICU Mortality (n, %) | 149 (16) | 77 (19) | 41 (21) |
Footnotes: The following variables had missing data for PICU admissions 1, 2, and 3, respectively: neutrophil engraftment (n=17, 3, 0). All other missing data are noted in the table above.
Primary Outcome:
PICU mortality occurred in 17.4% of admissions (267/1,532, 95% CI 15.6%−19.4%), and because many patients had more than one PICU admission, 28.5% of patients died in the PICU during the study interval (267/936, 95% CI 25.7%−31.5%). Mortality rates for 1st, 2nd, and 3rd PICU admissions were not statistically significantly different (15.9% vs. 19.1% vs. 21.3%, p=0.173). Stratified Outcomes: PICU mortality rates for patients using IMV, RRT, and ECLS were 44.0%, 56.1%, and 77.8%, respectively (n=249/566, n=97/173, n=7/9, respectively). Of note, 23/936 patients (2.5%) died in the PICU without the use of IMV. PICU mortality rates for admissions associated with a Gram-positive, Gram-negative, viral, and fungal infection were 18.6%, 21.7%, 25.2%, and 38.3%, respectively (n=33/177, n=30/138, n=115/456, and n=64/167). Each additional day that patients remained in the PICU was associated with significantly increased rates of PICU mortality (Figure 2). For example, among 1st PICU admissions, mortality for patients still in the PICU after 1 week was 17.4% (95% CI 14.7–20.1), whereas mortality for patients still in the PICU after 2 weeks was 32.0% (95% CI 26.8–37.2) and mortality for patients still in the PICU after 4 weeks was 45.0% (95% CI 35.9–54.1).
Figure 2. PICU Mortality Increases with Increasing Length of Stay.
Patients have increasing mortality risk the longer they are unable to be discharged from the PICU. Many patients are discharged from the PICU within the first week of PICU stay. Mortality point estimates with 95% confidence intervals for patients still requiring the PICU after 1 week are 17.4% (14.7–20.1), 20.5% (16.2–24.8), and 23.6% (17.0–30.3) for 1st, 2nd, and 3rd PICU admissions, respectively. Mortality estimates for patients still requiring the PICU after 2 weeks increase to 32.0% (26.8–37.2), 37.9% (30.1–45.8), and 47.8% (35.6–60.0) for 1st, 2nd, and 3rd PICU admissions, respectively. Mortality estimates for patients still requiring the PICU after 4 weeks increase to 45.0% (35.9–54.1), 54.6% (41.1–68.0), and 66.7% (50.3–83.1) for 1st, 2nd, and 3rd PICU admissions, respectively. These estimates pertain to each independent PICU admission, not to cumulative days in the PICU over the study interval.
Risk Factors Available at the Time of PICU Admission:
Demographics:
Neither HCT center, PICU center, age at PICU admission, sex, nor race/ethnicity were associated with PICU mortality (p>0.05).
Pre-HCTMorbidity:
The HCT-Comorbidity Index (HCT-CI) was ≥1 in 36% of patients and ≥3 in 15% of patients; there was a non-significant trend towards increased PICU mortality in patients with an HCT-CI ≥3 (OR 1.41, 95% CI 0.98–2.03, p=0.063). On analysis of each individual component of the HCT-CI, only moderate/severe pre-HCT renal injury (defined as serum creatinine >2 mg/dL or >177 μmol/L and/or use of dialysis and/or prior renal transplantation), was associated with significantly increased PICU mortality, and therefore this risk factor was selected for inclusion in the multivariable model. In addition, there was no observed association between PICU mortality and pre-HCT Lansky/Karnofsky score or a history of IMV or invasive fungal infection (IFI, p>0.05).
Underlying Disease:
Approximately half of the cohort underwent HCT for malignant disease, and the most common HCT indications were ALL, AML, and primary immunodeficiency (PID). Patients with AML had significantly increased odds for mortality (OR 1.79, 95% CI 1.16–2.78, p=0.008 relative to patients with ALL). There were no other statistically significant differences in outcomes for other HCT indications, including subtypes of non-malignant disease (p>0.05). Among patients with hematologic malignancies, there was no evidence of increased PICU mortality for patients with advanced disease or greater time interval between disease diagnosis and date of HCT (p>0.05).
HCT Type:
Allograft HLA match was associated with mortality, with significantly greater PICU mortality among patients having received HCT from mismatched unrelated donor or mismatched UCB allografts (OR 1.74, 95% CI 1.15–2.65, p=0.009 relative to matched related or matched UCB allografts). In addition, donor/recipient cytomegalovirus (CMV) serostatus mismatch was significantly associated with PICU mortality, with pre-HCT CMV seropositivity strongly associated with PICU mortality regardless of CMV serostatus of the allograft donor. Allograft source, preparative conditioning regimen, use of serotherapy, GVHD prophylaxis, donor/recipient sex mismatch, and year of HCT varied significantly across the cohort but were not associated with PICU mortality (p>0.05).
Post-HCT Course:
Patients admitted to the PICU before day +100 had greater PICU mortality than patients admitted to the PICU on or after day +100. However, lack of neutrophil engraftment at the time of PICU admission was not associated with mortality, nor was the presence of acute/chronic GVHD or VOD (p<0.05).
PICU Admission Illness Severity:
The median PRISM-3 score for 1st PICU admissions was 9 of a maximum 47 points (IQR 4–14), suggesting that most patients had mild-to-moderate multiorgan dysfunction in the first 12 hours of PICU admission. Greater multiorgan dysfunction in the first 12 hours of PICU admission, as measured by the PRISM-3 score, was strongly associated with PICU mortality (p<0.001).
Multivariable Adjusted Analysis:
On multivariable analysis of factors available at or near the start of PICU admission, PICU mortality was independently associated with PRISM-3 score, moderate/severe pre-HCT renal comorbidity, pre-HCT recipient CMV seropositivity, <100 day interval between HCT and PICU admission, and HCT for underlying AML (Table 3). Use of an allograft from an unrelated donor and an elevated total HCT-CI were not associated with PICU mortality independent of these factors. To determine whether HCT characteristics might influence the association between PRISM-3 and PICU mortality, we tested for but did not detect any interactions between PRISM-3 score and moderate/severe pre-HCT renal comorbidity (p=0.539), pre-HCT recipient CMV seropositivity (p=0.282), <100 day interval between HCT and PICU admission (p=0.604), and HCT for underlying AML (p=0.450).
Table 3.
Multivariable Model of PICU Mortality in Pediatric Allogeneic HCT
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Moderate/Severe Pre-HCT Renal Injury | |||
| None/mild | 1.00 | ||
| Moderate/severe | 3.39 | 1.88–6.13 | p<0.001 |
| Recipient CMV Status | |||
| Negative | 1.00 | ||
| Positive | 1.59 | 1.09–2.09 | p=0.013 |
| Unknown | 1.39 | 0.87–2.21 | p=0.166 |
| Interval from HCT to PICU Admission | |||
| < 100 days | 1.39 | 1.04–1.82 | p=0.026 |
| ≥100 days | 1.00 | ||
| HCT Indication | |||
| ALL/Other acute leukemia | 1.00 | ||
| AML | 1.94 | 1.24–3.05 | p=0.004 |
| CML/MDS/MPD | 0.84 | 0.46–1.51 | p=0.551 |
| NHL/HL | 0.97 | 0.47–2.03 | p=0.942 |
| Non-malignant disease | 1.24 | 0.86–1.79 | p=0.256 |
| PRISM-3 Score (continuous*) | 1.11 | 1.09–1.13 | p<0.001 |
Footnotes: Generalized estimating equations (GEE) with clustering at the patient level were used to account for repeat PICU admissions of the same patient during the study interval.
Odds ratio for PRISM-3 score reflect increased mortality odds for each additional point of the score (range 0–47).
Mortality Prognostication:
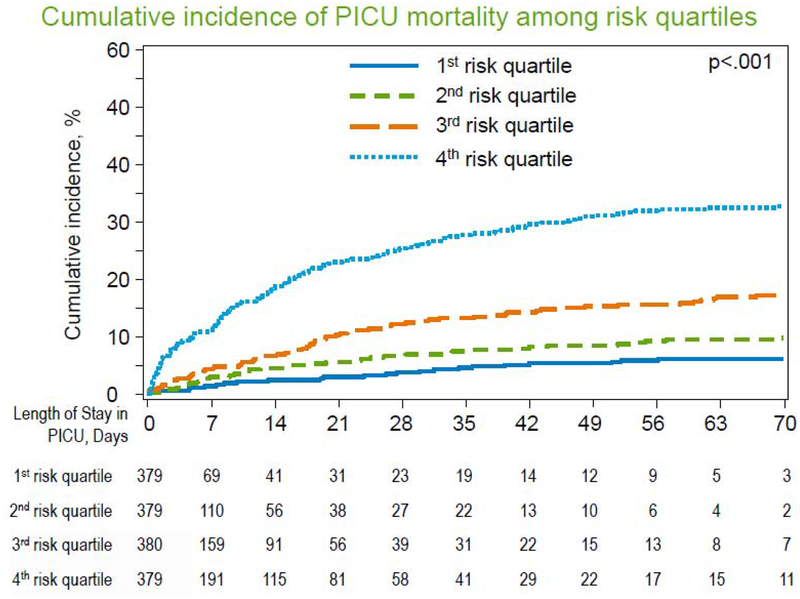
Having identified 5 factors available at/near the time of PICU admission that were each independently associated with PICU mortality, we then aimed to assess how well the combination of these 5 factors could predict PICU mortality. We first randomly divided our dataset into 20 sets of approximately 5% of patients. Each set served as a holdout validation set, where we fit a GEE multivariable model using the 5 factors above to the remaining 19 sets (95% of cases), and then tested the AUROC on the holdout set of 5%. Repeating this procedure 19 additional times (20-fold internal cross-validation) generated an AUROC of 0.69 (95% CI 0.66–0.72). The model provided a probability estimate for the outcome of mortality for each patient; these estimates were ordered lowest-to-highest and patients were separated into 4 equally-sized groups (quartiles) of increasing risk. We demonstrate that patients in the top quartile of risk have significantly greater PICU mortality (35.1% vs. 11.5%, p<0.001) which develops cumulatively over the PICU course (Figure 3, Gray’s p<0.001). Scoring in the top quartile of risk was associated with high specificity (80.4%, 95% CI 78.1–82.6%), high positive likelihood ratio (2.6, 95% CI 2.2–3.0), high negative predictive value (88.6%, 95% CI 87.3–89.8%) and high overall classification accuracy (75.2%, 95% CI 73.0–77.4%) for the outcome of PICU mortality; sensitivity for PICU mortality was modest (50.6%, 95% CI 44.4–56.8%). Compared to the model with PRISM-3 alone, implementation of the multivariable model resulted in the greatest change in the probability of PICU mortality amongst patients with higher PRISM-3 scores (Supplemental Figure 1). In order to contextualize how the multivariable model might change early prognostication, we selected two example patient cases from this cohort, presented in Supplemental Figure 2.
Figure 3. PICU Mortality Stratified by Multivariate Risk Group.
A multivariable model using 5 clinical variables accurately cumulative incidence of PICU mortality over time. Cumulative incidence of mortality for patients in the 1st, 2nd, 3rd, and 4th quartiles of risk according to the multivariable model are plotted across PICU length of stay. Patients who were discharged alive are censored at the time of PICU discharge. Comparison of cumulative incidence curves using Gray’s test demonstrated significant differences in curves (p<0.001).
DISCUSSION
In this study, we comprehensively evaluated 25 potential risk-factors for PICU mortality and identified 5 variables independently associated with intensive care mortality: moderate/severe pre-HCT renal comorbidity, pre-HCT recipient CMV seropositivity, <100 day interval between HCT and PICU admission, HCT for underlying AML, and admission illness severity, as approximated by the PRISM-3 score. By combining these four HCT-specific variables with the PRISM-3 score, we demonstrate significantly improved mortality prognostication at the time of PICU admission for patients with the highest levels of early organ dysfunction. These results provide new insights into factors involved in the evolution of critical illness in pediatric allogeneic HCT patients.
The medical fragility and propensity for rapid physiologic decompensation of critically ill HCT patients has motivated the search for early prognostic warning signs since the advent of HCT. A recent meta-analysis by Saillard et al demonstrated that among 2,342 critically ill adult HCT patients from 18 contemporary cohorts, cumulative failures of the hemodynamic, respiratory, renal, and hepatic organ systems remain strongly associated with poor outcome [23]. Ample single-center data in pediatrics have demonstrated similar findings, although both adult and pediatric studies typically define organ failures by the use of late-stage interventions such as invasive positive pressure ventilation, vasoactive infusions, and hemodiafiltration [14,15,24]. This study confirms these findings and offers the further ability to stage organ dysfunction using a continuous metric of vital signs and laboratory values (PRISM-3 score) that is applicable prior to patient reliance on intensive medical and mechanical support. Future expansion of the PRISM-3 components to include HCT-relevant variables such as absolute neutrophil and lymphocyte counts, B-type natriuretic peptide, and measures of fluid overload may prove useful in further quantifying early organ dysfunction. These data strongly support a multi-subspecialty approach to prevention and early detection of organ toxicity, with the goal to arrest the development of irreversible organ toxicity as early as possible in order to prevent the development of critical illness and transplant-related mortality (TRM).
This study adds to the literature by identifying several novel risk-factors for PICU mortality. Whereas previous adult and pediatric studies have not associated HCT indication with mortality, this study strongly associated underlying AML with poor survival. Multiple studies show that relative to other oncology patients, children with AML have high risk for hospital mortality, even after controlling for use of HCT, rates and type of infection, and the PRISM-3 score [15,25,26]. This study furthers our knowledge by showing that the association between AML and PICU mortality is robust to adjustment for a comprehensive list of HCT-specific risk factors. While AML patients used more Busulfan-based conditioning (51% [74/144] vs. 6% [10/183], p<0.001) and less TBI-based conditioning (42% [60/144] vs. 91% [167/183], p<0.001) than ALL patients, we did not detect a univariate association between certain conditioning regimens (ie: Busulfan-based) and PICU mortality, nor did we detect an interaction between conditioning regimen and disease type (ie: AML) with respect to PICU mortality. Nonetheless, the safety and potential non-inferiority of reduced intensity conditioning for AML transplants merits further investigation [27]. In addition, we speculate that other factors relating to cumulative duration of pre-HCT neutropenia and the effects of pre-HCT chemotherapy likely play a role in the unique vulnerability of these particular patients [28].
While previous data have shown the renal component of the HCT-CI to be associated with mortality in adults with AML, and to be associated with post-HCT AKI in children, this is to our knowledge the first study to show the independent prognostic utility of the renal component of the HCT-CI in critically ill children [29,30]. This finding is particularly significant given that the association was independent of the PRISM-3 score, which accounts for both creatinine and blood urea nitrogen in the first 12 hours of PICU admission. Potential mechanisms by which impaired pre-HCT renal function might influence survival include impaired clearance of conditioning chemotherapy, propensity towards fluid overload, and modulation of systemic inflammation [31,32]. As nearly two-thirds of children develop AKI after allogeneic HCT, and increasing severity of AKI is strongly associated with TRM, novel strategies to minimize renal toxicity are crucial for reducing mortality [30,33]. Current strategies for early surveillance and prevention involve early pediatric nephrology consultation, include the use of urinary neutrophil gelatinase-associated lipocalin (NGAL), serum cystatin C, and kidney injury molecule-1, and merit further study [34–36]. Of note, the HCT-CI defines moderate/severe renal impairment as a serum creatinine >2 mg/dL or >177 μmol/L, which is likely of inadequate sensitivity to identify milder forms of AKI and CKD in the pediatric population [37].
A novel finding of this work is the association between pre-HCT CMV seropositivity and post-HCT mortality in the PICU. Recent data show that post-HCT CMV reactivation occurs in approximately one-third of CMV seropositive patients and is strongly associated with mortality [38,39]. Post-HCT CMV reactivation likely contributes to increased mortality risk through organ-specific and treatment-related cytotoxicity, by facilitating co-infections, and by triggering GVHD and other alloreactive syndromes [40]. While the use of serotherapy and the use of UCB allografts have been associated with increased risk of CMV reactivation [41], neither of these risk factors were associated PICU mortality in this cohort, again suggesting that while many patients with these risk factors were quite sick, many patients without these risk-factors were comparably critically ill for other reasons. Future investigations aimed at minimizing the risk of CMV reactivation, improving early CMV detection in end-organs such as the lungs and intestine, and reducing the toxicity of anti-CMV therapies may improve outcomes for this subset of patients [42].
Our finding that PICU admission prior to day +100 was associated with mortality can be interpreted in several ways. First, it provides indirect evidence of a temporal link between acute regimen-related toxicity and TRM, which would be congruous with recent reports of improved outcomes among critically ill adults who received reduced intensity conditioning regimens [23]. Second, it may also provide indirect evidence for the role of impaired innate, cellular and humoral immunity in the pathogenesis of critical illness [43,44]. However, lack of neutrophil engraftment at PICU admission was not associated with PICU mortality, and hence we speculate that more sensitive measures of immune function may be required to necessary to capture an accurate profile of immunologic risk.
A number of other factors typically associated with mortality in the HCT setting showed no association with PICU mortality in this cohort. These included advanced malignancy status and presence of acute GVHD, among others. Specific components of the HCT-CI such as pulmonary and hepatic dysfunction were also not associated with PICU mortality on univariate analysis. We speculate that while each of these may be a risk-factor for the development of critical illness relative to HCT patients who are doing well, they may not account for the heterogeneity in outcomes among an intensive care cohort, wherein patients are all critically ill for varied reasons. For example, while VOD is associated with mortality in the general HCT population, in this study, patients with VOD were compared to patients who were critically ill for some other reason (rather than being compared to healthy controls); this may have contributed to the lack of univariate association between VOD and PICU mortality. Our finding that HLA mismatch was associated with PICU mortality on univariate analysis, but not after controlling for PRISM-3 score, suggests that HLA mismatch may result in organ dysfunction that is largely captured by the PRISM-3 score. While many variables of traditional prognostic importance were not associated with PICU mortality in this cohort, we were unable to assess other important clinical outcomes such as the development of complications not requiring critical care.
This study has several strengths. First, our approach of merging two independent yet complimentary databases facilitated the creation of a significantly more robust dataset with both HCT - and PICU-specific variables. We were, however, limited in the retrospective nature of the databases, and hence efforts to develop prospective databases remain ongoing. Second, the sample size afforded by merging databases allowed for high-powered analyses.
The study also had several limitations. First, this study may have underreported mortality by excluding HCT patients transplanted before 2008 who died in a VPS PICU between 2009–2014. In addition, we could not identify critically ill patients admitted to ICUs not in the VPS network, including PICUs not participating in VPS and adult ICUs, which may have been relevant for some patients 18–21 years of age. We do note, however, that 1,071/9,138 pediatric allogeneic HCT patients in CIBMTR were matched 1:1 to a VPS record, suggesting PICU usage in at least 12%. Second, this study was not able to capture the exact reason for PICU in-transfer, the timing of onset of infectious comorbidities, the exact reason for PICU mortality, and the presence of limitations of care. We note that 2.5% of patients died in the PICU without initiation of IMV; these patients may have had a do-not-intubate limitation of care in place, although we cannot confirm this hypothesis. Third, we may have underestimated PICU mortality in cases where patients were transferred out of the PICU for end-of-life care and terminal extubation in another non-ICU location. Fourth, some HCT complications (ie: GVHD, VOD) were annotated for only a subset of patients, which limited statistical power for identifying associations with PICU mortality. Fifth, we were not able to assess the impact of 2nd or subsequent HCT on the outcome of PICU mortality. This remains an evolving area of interest, as an increasing number of patients with malignancies who relapse after a 1st allogeneic HCT may be candidates for a 2nd HCT due to novel bridging immunotherapies and cell-based therapies [45]. In addition, patients with non-malignant disorders with poor graft function after a 1st allogeneic HCT may be candidates for gene-modified autologous HCT or other novel treatments that were not captured in this study [46]. Sixth, despite an exhaustive effort to incorporate potential clinical risk-factors into this model, a significant portion of the variance in patient outcomes remains unexplained. Therefore, future directions to further improve prognostic modeling should include the incorporation of novel biomarkers that reflect critical illness pathobiology in pediatric allogeneic HCT [47,48]. This and any future models continue to require validation in external populations.
In conclusion, these data improve our working knowledge of the factors influencing the progression of critical illness in pediatric allogeneic HCT patients and highlight several high-risk subgroups for follow-up investigation, including patients with pre-HCT CMV seropositivity, moderate or severe renal injury, underlying AML, multi-organ dysfunction, and patients in the early post-HCT period. The multivariable modeling approach allows identification of high-risk patients early in the course of critical illness; however, future studies aimed at mitigating factors associated with the progression of multiorgan failure to mortality in the pediatric allogeneic HCT population are desperately needed and will require ongoing multidisciplinary collaboration between oncology, transplant, immunology, critical care, and other physicians and scientists.
Supplementary Material
Highlights:
Critically ill pediatric allogeneic hematopoietic cell transplant patients may benefit from early and aggressive interventions aimed at reversing the progression of multiorgan dysfunction.
Using a multicenter cohort of 936 critically ill pediatric allogeneic HCT patients across 77 intensive care units, this study identified that pre-HCT renal injury, pre-HCT CMV seropositivity, AML, post-HCT day, and a continuous metric of multiorgan dysfunction (the PRISM-3 score) prognosticated PICU mortality for the highest-risk patients.
A multivariable model using these components identified that patients in the top quartile of risk had three times greater mortality than other patients (35.1% vs. 11.5%, p<0.001).
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIH) National Institute of Child Health and Development K12HD000850 (to Zinter), the Pediatric Blood and Marrow Transplant Foundation (to Zinter), the National Marrow Donor Program Amy Strelzer Manasevit Grant (to Zinter), the NIH U24CA076518 (to CIBMTR), the NIH HL069294 (to CIBMTR), and the United States Health Resources and Services Administration (HRSA) HHSH250201200016C (to CIBMTR). VPS data were provided by the VPS, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest: Brent Logan, Caitrin Fretham, and Marcelo Pasquini are employed by the CIBMTR, which contributed data to this manuscript. There are no other conflicts of interest to disclose.
Statement of Prior Presentation: Presented in abstract form at the 2018 Annual Tandem Meeting of the American Society of Blood and Marrow Transplantation (ASBMT) and the Center for International Blood and Marrow Transplantation Research (CIBMTR) in Salt Lake City, UT, 22 February 2018.
REFERENCES
- 1.D’Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2017. 2017;2018. [Google Scholar]
- 2.Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr.Transplant 2006;10:299–303. [DOI] [PubMed] [Google Scholar]
- 3.Rowan CM, McArthur J, Hsing DD, et al. Acute Respiratory Failure in Pediatric Hematopoietic Cell Transplantation: A Multicenter Study. Crit.CareMed 2018;46:e967–e974. [DOI] [PubMed] [Google Scholar]
- 4.Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit.Care Med 2003;31:1299–1305. [DOI] [PubMed] [Google Scholar]
- 5.Duncan CN, Lehmann LE, Cheifetz IM, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr.Crit.Care.Med 2013;14:261–267. [DOI] [PubMed] [Google Scholar]
- 6.van Gestel JP, Bollen CW, van der Tweel I, Boelens JJ, van Vught AJ. Intensive care unit mortality trends in children after hematopoietic stem cell transplantation: a meta-regression analysis. Crit.Care Med 2008;36:2898–2904. [DOI] [PubMed] [Google Scholar]
- 7.Diaz MA, Vicent MG, Prudencio M, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87:292–298. [PubMed] [Google Scholar]
- 8.Saillard C, Blaise D, Mokart D. Critically ill allogeneic hematopoietic stem cell transplantation patients in the intensive care unit: reappraisal of actual prognosis. Bone Marrow Transplant. 2016;51:1050–1061. [DOI] [PubMed] [Google Scholar]
- 9.Lee DS, Suh GY, Ryu JA, et al. Effect of Early Intervention on Long-Term Outcomes of Critically Ill Cancer Patients Admitted to ICUs. Crit.Care Med 2015;43:1439–1448. [DOI] [PubMed] [Google Scholar]
- 10.Inwald DP, Tasker RC, Peters MJ, Nadel S, Paediatric Intensive Care Society Study Group (PICS-SG). Emergency management of children with severe sepsis in the United Kingdom: the results of the Paediatric Intensive Care Society sepsis audit. Arch.Dis.Child 2009;94:348–353. [DOI] [PubMed] [Google Scholar]
- 11.Song JU, Suh GY, Park HY, et al. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med. 2012;38:1505–1513. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Pickkers P, Soares M, et al. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017;43:1808–1819. [DOI] [PubMed] [Google Scholar]
- 13.Fausser JL, Tavenard A, Rialland F, et al. Should We Pay Attention to the Delay Before Admission to a Pediatric Intensive Care Unit for Children With Cancer? Impact on 1-Month Mortality. A Report From the French Children’s Oncology Study Group, GOCE. J.Pediatr.Hematol.Oncol 2017;39:e244–e248. [DOI] [PubMed] [Google Scholar]
- 14.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New Insights Into Multicenter PICU Mortality Among Pediatric Hematopoietic Stem Cell Transplant Patients. Crit.CareMed 2015;43:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Garcia M, Gonzalez-Vicent M, Mastro-Martinez I, Serrano A, Diaz MA. Intensive Care Unit Admissions Among Children After Hematopoietic Stem Cell Transplantation: Incidence, Outcome, and Prognostic Factors. J.Pediatr.Hematol.Oncol 2015. [DOI] [PubMed] [Google Scholar]
- 17.Aspesberro F, Guthrie KA, Woolfrey AE, Brogan TV, Roberts JS. Outcome of pediatric hematopoietic stem cell transplant recipients requiring mechanical ventilation. J.Intensive Care Med 2014;29:31–37. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Vicent M, Marin C, Madero L, Sevilla J, Diaz MA. Risk score for pediatric intensive care unit admission in children undergoing hematopoietic stem cell transplantation and analysis of predictive factors for survival. J.Pediatr.Hematol.Oncol 2005;27:526–531. [DOI] [PubMed] [Google Scholar]
- 19.Faraci M, Bagnasco F, Giardino S, et al. Intensive care unit admission in children with malignant or nonmalignant disease: incidence, outcome, and prognostic factors: a single-center experience. J.Pediatr.Hematol.Oncol 2014;36:e403–9. [DOI] [PubMed] [Google Scholar]
- 20.Pillon M, Amigoni A, Contin A, et al. Risk Factors and Outcomes Related to Pediatric Intensive Care Unit Admission after Hematopoietic Stem Cell Transplantation: A Single-Center Experience. Biol.Blood Marrow Transplant 2017;23:1335–1341. [DOI] [PubMed] [Google Scholar]
- 21.Balit CR, Horan R, Dorofaeff T, Frndova H, Doyle J, Cox PN. Pediatric Hematopoietic Stem Cell Transplant and Intensive Care: Have Things Changed?. Pediatr.Crit.Care.Med 2016;17:e109–16. [DOI] [PubMed] [Google Scholar]
- 22.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit.Care Med 1996;24:743–752. [DOI] [PubMed] [Google Scholar]
- 23.Saillard C, Darmon M, Bisbal M, et al. Critically ill allogenic HSCT patients in the intensive care unit: a systematic review and meta-analysis of prognostic factors of mortality. Bone Marrow Transplant 2018;53:1233–1241. [DOI] [PubMed] [Google Scholar]
- 24.Ha EJ, Kim S, Jin HS, et al. Early changes in SOFA score as a prognostic factor in pediatric oncology patients requiring mechanical ventilatory support. J.Pediatr.Hematol.Oncol 2010;32:e308–13. [DOI] [PubMed] [Google Scholar]
- 25.Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr.Crit.Care.Med 2014;15:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foresto SA, Youlden DR, Baade PD, Hallahan AR, Aitken JF, Moore AS. The outcomes and treatment burden of childhood acute myeloid leukaemia in Australia, 1997–2008: A report from the Australian Paediatric Cancer Registry. Pediatr.BloodCancer 2015;62:1664–1666. [DOI] [PubMed] [Google Scholar]
- 27.Bitan M, He W, Zhang MJ, et al. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood. 2014;123:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolin JF. Molecular diagnosis and risk-adjusted therapy in pediatric hematologic malignancies: a primer for pediatricians. Eur.J.Pediatr 2011;170:419–425. [DOI] [PubMed] [Google Scholar]
- 29.Kagoya Y, Kataoka K, Nannya Y, Kurokawa M. Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol.BloodMarrow Transplant 2011;17:394–400. [DOI] [PubMed] [Google Scholar]
- 30.Koh KN, Sunkara A, Kang G, et al. Acute Kidney Injury in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation: Incidence, Risk Factors, and Outcomes. Biol.Blood Marrow Transplant 2017. [DOI] [PubMed] [Google Scholar]
- 31.Laskin BL, Nehus E, Goebel J, Furth S, Davies SM, Jodele S. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol.Blood Marrow Transplant 2014;20:2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brochner AC, Dagnaes-Hansen F, Hojberg-Holm J, Toft P. The inflammatory response in blood and in remote organs following acute kidney injury. APMIS. 2014;122:399–404. [DOI] [PubMed] [Google Scholar]
- 33.Kizilbash SJ, Kashtan CE, Chavers BM, Cao Q, Smith AR. Acute Kidney Injury and the Risk of Mortality in Children Undergoing Hematopoietic Stem Cell Transplantation. Biol.Blood Marrow Transplant 2016;22:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taghizadeh-Ghehi M, Sarayani A, Ashouri A, Ataei S, Moslehi A, Hadjibabaie M. Urine neutrophil gelatinase associated lipocalin as an early marker of acute kidney injury in hematopoietic stem cell transplantation patients. Ren.Fail 2015;37:994–998. [DOI] [PubMed] [Google Scholar]
- 35.McMahon KR, Rod Rassekh S, Schultz KR, et al. Design and Methods of the Pan-Canadian Applying Biomarkers to Minimize Long-Term Effects of Childhood/Adolescent Cancer Treatment (ABLE) Nephrotoxicity Study: A Prospective Observational Cohort Study. Can.J.KidneyHealth.Dis 2017;4:2054358117690338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benoit SW, Dixon BP, Goldstein SL, et al. A novel strategy for identifying early acute kidney injury in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin.J.Am.Soc.Nephrol 2015;10:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122:3359–3364. [DOI] [PubMed] [Google Scholar]
- 40.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol.BloodMarrow Transplant 2010;16:1309–1314. [DOI] [PubMed] [Google Scholar]
- 41.Rowe RG, Guo D, Lee M, Margossian S, London WB, Lehmann L. Cytomegalovirus Infection in Pediatric Hematopoietic Stem Cell Transplantation: Risk Factors for Primary Infection and Cases of Recurrent and Late Infection at a Single Center. Biol.Blood Marrow Transplant 2016;22:1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshikawa T Betaherpesvirus Complications and Management During Hematopoietic Stem Cell Transplantation. Adv.Exp.Med.Biol. 2018;1045:251–270. [DOI] [PubMed] [Google Scholar]
- 43.de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune Reconstitution after Allogeneic Hematopoietic Cell Transplantation in Children. Biol.Blood Marrow Transplant 2016;22:195–206. [DOI] [PubMed] [Google Scholar]
- 44.Toor AA, Sabo RT, Roberts CH, et al. Dynamical System Modeling of Immune Reconstitution after Allogeneic Stem Cell Transplantation Identifies Patients at Risk for Adverse Outcomes. Biol.Blood Marrow Transplant 2015;21:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund TC, Ahn KW, Tecca HR, et al. Outcomes after Second Hematopoietic Cell Transplantation in Children and Young Adults with Relapsed Acute Leukemia. Biol.Blood Marrow Transplant 2019;25:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mamcarz E, Zhou S, Lockey T, et al. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. N.Engl.J.Med 2019;380:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zinter MS, Delucchi KL, Kong MY, et al. Early Plasma Matrix Metalloproteinase Profiles: A Novel Pathway in Pediatric Acute Respiratory Distress Syndrome. Am.J.Respir.Crit.CareMed January 15 2019;199:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.