Abstract
Cefepime is a fourth-generation cephalosporin antibiotic with an extended spectrum of activity against many Gram-positive and Gram-negative bacteria. There is a growing need to develop sensitive, small volume assays, along with less invasive sample collection to facilitate pediatric pharmacokinetic clinical trials and therapeutic drug monitoring. The volumetric absorptive microsampling (VAMS™) approach provides an accurate and precise collection of a fixed volume of blood (10 μL), reducing or eliminating the volumetric blood hematocrit assay-bias associated with the dried blood spotting technique. We developed a high-performance liquid chromatographic method with tandem mass spectrometry detection for quantification of cefepime. Sample extraction from VAMS™ devices, followed by reversed-phase chromatographic separation and selective detection using tandem mass spectrometry with a 4 minute runtime per sample was employed. Standard curves were linear between 0.1 – 100 μg/mL for cefepime. Intra- and inter-day accuracies were within 95.4 – 113% and precision (CV) was < 15% based on a 3-day validation study. Recoveries ranged from 40.8 – 62.1% and the matrix effect was within 89.5 – 96.7% for cefepime. Cefepime was stable in human whole blood under assay conditions (3 h at room temperature, 24 h in autosampler post-extraction). Cefepime was also stable for at least 1 week (7 days) at 4 °C, 1 month (39 days) at −20 °C and 3 months (91 days) at −78°C as dried microsamples. This assay provides an efficient quantitation of cefepime and was successfully implemented for the analysis of whole blood microsamples in a pediatric clinical trial.
Keywords: cefepime, volumetric absorptive microsampling (VAMS™), human whole blood, liquid chromatography, mass spectrometry
1. Introduction
Cefepime is a fourth-generation cephalosporin antibacterial agent with activity against a range of Gram-positive and Gram-negative bacteria [1], administered for the treatment of serious community- and hospital-acquired bacterial infections [2]. Due to its broad spectrum of activity, it is often used in patients suspected to have serious bacterial infections prior to microbiological confirmation of infection, most notably in critically ill and immunocompromised patients. It is also administered as definitive treatment for infections caused by Gram-negative bacilli resistant to narrower spectrum antibiotic agents [3]. Cefepime concentrations are typically measured in human plasma or serum using validated LC-MS/MS methods [4-9]. Cefepime quantitation in human plasma poses a challenge due to rapid ex-vivo degradation in plasma at room temperature, requiring that all sample processing occur at 4 °C [4].
Volumetric absorptive microsampling (VAMS™) is a promising sample collection technique [10] for quantitative analysis of drugs, especially in pediatrics where multiple collections of blood volume (1 mL per sample) represents a limitation in conducting clinical studies and routine therapeutic drug monitoring (TDM), due to the excessive blood drawn per volume of total body blood volume over multiple samples. VAMS™ devices enable the collection of an accurate volume of blood samples (10, 20, or 30 μL) and have been successfully implemented previously in the quantitative analysis of antibiotics [11, 12]. Other techniques, such as dried blood spot (DBS), also provide lesser volume sizes (30 μL per spot), but present limitations such as the impact of varying hematocrit (HCT) levels on the performance of the assay including spot size, sample homogeneity, drying time, and analyte recovery [13-17]. The VAMS™ approach eliminates the effect of HCT on analysis and other complications while also accomplishing small sample volumes (10 - 30 μL) accurately and robustly.
In the present study, we developed and validated the use of VAMS™ for the quantification of cefepime in human whole blood by LC–MS/MS. The accurate measurement of cefepime concentration is important in individualizing the treatment in critically ill children [15]. Knowledge of cefepime concentrations would provide clinicians with information about whether the administered dose is too low, leading to exposures below effectiveness thresholds, or too high with increased risk for adverse side effects. These measurements could prompt dose adjustments to improve both effectiveness and reduce toxicity. The present study also describes the in vitro comparison of the concentration of cefepime in blood, plasma and VAMS™, which provides further insight into the relationship between plasma (considered gold standard) and VAMS™ concentrations of cefepime. This is the first study to describe a VAMS™-LC-MS/MS assay for the quantification of cefepime in human whole blood and could help facilitate pediatric clinical pharmacology studies of cefepime.
2. Materials and Methods
2.1. Materials
The analytical standard, cefepime hydrochloride, was purchased from Sigma Aldrich (St. Louis, MO, USA) and the internal standard (IS), cefepime-d3 disulfate, was purchased from Toronto Research Chemicals (Toronto, Canada). Nanopure water from a Synergy® UV-R system was utilized for preparing solvents and sample preparation. ACS reagent-grade formic acid (98%) and ACS reagent-grade ammonium acetate were purchased from EMD Millipore Corporation (Billerica, MA, USA). Volumetric absorptive microsampling (VAMS™) devices were purchased from Neoteryx (Torrance, CA, USA). Pooled human whole blood was provided by BioIVT (Westbury, NY) or by healthy volunteers at CHOP (IRB protocol # 18-015852).
2.2. Stock Solutions, Calibration Standards, and Quality Controls
All whole blood samples were collected with lithium heparin (LiHep) as the anti-coagulant, with a measured HCT of 41% ± 2.0%. The blood was inverted in the collection tube 2-3 times immediately after collection and stored at 4 °C, and used up to 48 hours. Blood was pre-warmed at 37 °C and gently mixed by hand before spiking calibration standards and quality controls (QCs). Two independent primary stock solutions of cefepime were prepared in dimethyl sulfoxide (DMSO) for the calibration standards and QCs with a concentration of 10.0 mg/mL and stored at −20 °C in an amber glass vial. An intermediate stock solution was prepared at 1 mg/mL in human whole blood for the preparation of the calibration standards and QCs. The compound was allowed to equilibrate with the human whole blood for 15 minutes at room temperature and mixed every 5 minutes before continuing with further dilutions. Serial dilutions of the calibration standards were prepared in human whole blood at 0.1, 0.5, 1, 5, 10, 20, 50, and 100 μg/mL. QCs were also prepared in human whole blood at concentrations of 0.1, 0.25, 40, and 80 μg/mL. The primary stock solution of the IS, cefepime-d3, was prepared at a concentration of 1 mg/mL in DMSO. The working IS solution was prepared at a final concentration of 1 μg/mL by diluting the primary stock solution with acetonitrile and then stored at 4 °C.
2.3. Sample Preparation
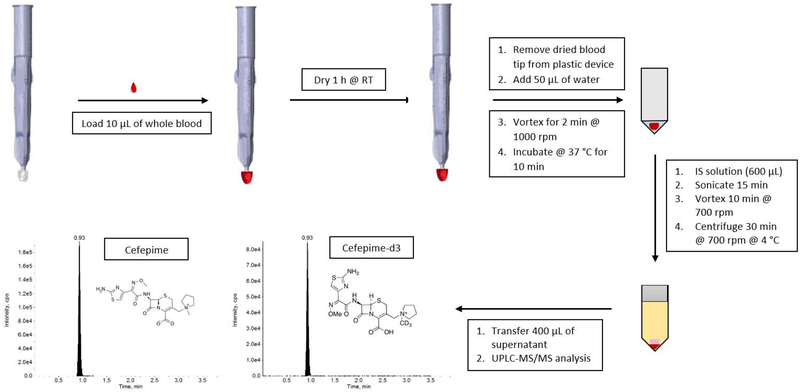
Before loading the VAMS™ devices, each sample was vortexed at a speed of 6.5 on a Fisher Scientific Vortexer for approximately 2-3 seconds and then inverted 3-4 times. Calibration standards, QCs, and blanks prepared in human whole blood were loaded onto VAMS™ devices at a fixed volume of 10 μL (Figure 1). The volume loaded onto a VAMS™ device is not exactly 10 μL, but depends on the exact lot of the VAMS™ devices used. The 10 μL Mitra tip has a <4% change in the volume that it loads [18]. The tips were loaded with whole blood by following the guidelines provided by Neoteryx [19] and other previously reported methods [12, 20]. VAMS™ devices were dipped into a 1.5 mL Eppendorf tube containing the spiked blood (or blanks) at a 45-degree angle ensuring only the surface of the blood was touched. After the tip of the VAMS™ device becomes fully red, it is held in the blood for an additional two seconds. The tip is then removed from the sample slowly and smoothly and placed on a rack for drying. The VAMS™ devices were loaded in the order of the batch sequence to minimize the variability resulting from the gradual separation of plasma from erythrocytes. For example, one calibration curve was loaded in sequential order, then each set of QCs, and then the final calibration curve. Loaded VAMS™ devices were dried at room temperature for 1 h in an enclosed plastic box containing 4 packs of desiccant (Whatman®) and then wrapped in aluminum foil. After 1 h of drying, the dried blood tip was removed from the plastic VAMS™ device and placed into a 96-well plate for extraction. Using a multi-channel pipette, water (50 μL) was added to all samples. The plate was then vortexed for 2 minutes at 1000 rpm, and then placed in the incubator for 10 minutes at 37 °C. Once the plate was removed from the incubator, 600 μL of the IS working solution (1 μg/mL cefepime-d3 in acetonitrile) was added to each sample. Double blanks received 600 μL of acetonitrile. The plate was sonicated for 15 minutes, vortexed at 700 rpm for 10 minutes and then centrifuged for 30 minutes at 3220 × g at 4 °C. The supernatant of each sample (400 μL) was transferred to a clean 96-well plate for LC-MS/MS analysis.
Figure 1.
Schematic of sample preparation for cefepime VAMS™-LC-MS/MS assay.
2.4. LC-MS/MS Analysis
A Waters Acquity UPLC I-class system (Milford, MA, USA) was utilized for the analysis of cefepime interfaced with an AB Sciex API 4000 triple quadrupole mass spectrometer equipped with a Turbo Ionspray source (Framingham, MA). Cefepime and cefepime-d3 were infused at a concentration of 100 ng/mL to optimize mass spectrometer conditions with a syringe pump at a flow rate of 10 μL/min. The electrospray ionization was employed in the positive mode with selective reaction monitoring (SRM) at the transitions of m/z 481.1 → 86.1 for cefepime and m/z 484.1 → 89.1 for cefepime-d3. A second product ion was determined for cefepime at a transition of m/z 481.1 → 166.8 for confirmation.
The optimized gas parameters included ion spray voltage (5000 V), curtain gas (30), GS1 (50), GS2 (50), collision gas (7) and source temperature (600 °C). Nitrogen (Airgas) was used as the nebulizer, collision, and curtain gases. Declustering potential (DP), entrance potential (EP), collision energy (CE) and collision cell exit potential (CXP) were optimized at 56, 10, 31 and 15, respectively, for both cefepime and cefepime-d3. All data acquisition and processing was performed using the Analyst Software (version 1.6.2).
A Phenomenex (Torrance, CA, USA) Kinetex F5 column (2.6 μm, 100 Å, 50 × 4.6 mm) was used for the chromatographic separation. A total of 3 μL of the supernatant was injected into the LC-MS/MS system at a flow ate of 0.500 mL/min. Consistent and robust chromatography for cefepime and cefepime-d3 was achieved with the mobile phases: A) 5 mM ammonium acetate in water (pH 5; adjusted with formic acid), and B) 5 mM ammonium acetate in 90:10 (v/v) acetonitrile: water. The isocratic solvent was maintained at 50% for the entire 4.00 minute run. A weak wash composed of 1% formic acid in water and a strong wash composed of a 1:1:1:1 (v/v/v/v) acetonitrile: methanol: water: IPA with 0.1% formic acid were employed to minimize carryover of cefepime and cefepime-d3.
2.5. Validation
VAMS™ devices were employed for a bioanalytical method validation for the quantitation of cefepime in human whole blood. Accuracy, precision, linearity, dilution integrity, carryover, stability, recovery, matrix effect, and blood to plasma partitioning were evaluated based on the FDA guidance for the bioanalytical validation [21].
2.5.1. Linearity and sensitivity
Linearity was assessed over the calibration range of 0.100 – 100 μg/mL in a 3-day core validation study. The LLOQ for this assay was 0.100 μg/mL since clinical concentrations for this drug are expected to be much higher than 0.100 μg/mL [6, 22]. Signal-to-noise ratios were also observed to determine the sensitivity of the assay.
2.5.2. Dilution integrity and carryover
Dilution integrity was evaluated by loading VAMS™ devices (n=6) with a known concentration of 200 μg/mL of cefepime in human whole blood. The extracted sample was diluted 40-fold with single blank extracts (prepared using control whole blood from VAMS™ devices) and then analyzed. Carryover was tested by analyzing the peak areas of an injected blank sample immediately following the highest calibration standard during each analytical run.
2.5.3. Accuracy and precision
Intra- and inter-day accuracies and precisions were assessed at the four QC concentrations of 0.1, 0.25, 40, and 80 μg/mL of cefepime in human whole blood (n=6) using the VAMS™ devices. The intra-day accuracy and precision was calculated using a single analytical validation run (n=6), whereas the inter-day accuracy and precision was calculated within the 3-day validation run (n=18).
2.5.4. Selectivity and specificity
Selectivity and specificity of cefepime and cefepime-d3 was evaluated using six individual lots and a pooled lot of human whole blood. The double blank (n=1) and the LLOQs (0.100 μg/mL; n=3) were prepared in the six individual lots and the pooled lot, loaded onto VAMS™ devices and extracted to ensure there was non-interference at the retention times of cefepime and cefepime-d3. These evaluations were also performed to ensure that there was no cross-interference between the analyte and the IS.
2.5.5. Recovery and matrix effect
Recovery was assessed by comparing the peak areas of a normally extracted sample (containing cefepime and cefepime-d3) to double blank samples (no analyte or IS), which were back-spiked post-extraction with the cefepime (amount of analyte present in 10.0 μL of the microsample) and cefepime-d3 into the final matrix. Samples were evaluated at the low (0.25 μg/mL), medium (40 μg/mL), and high (80 μg/mL) QC concentrations at low (n=6), normal (n=11), and high (n=6) HCT values. A 10-day time-dependent recovery study was performed at the middle QC level (n=6) with normal HCT (41%) at 0, 5, 8 and 10 days to evaluate the recovery versus the stability of cefepime in dried microsamples. Matrix effect was evaluated by comparing the peak areas of the post-extracted samples (spiked with cefepime and cefepime-d3 post-extraction) with the peak areas of neat samples spiked at the same concentration levels. These samples were evaluated using five individual lots of human whole blood at (n=5) low, medium, and high QC levels.
2.5.6. Stability
The initial stability assessment of cefepime in phosphate-buffered saline (PBS), human plasma, blood, DBS, and VAMS™ was assessed by spiking cefepime at a single concentration (10 μg/mL; n=4) in each matrix. Each sample was aliquoted into a 1.5 mL Eppendorf tube (PBS, plasma, whole blood), or loaded onto a DBS card (DMPK-C) or VAMS™ device (whole blood) using 10 μL of each matrix. Extraction was performed in the same way as was done for the VAMS™ sample extraction method. Samples were stored at room temperature until the time indicated (0, 2, 3, 24, 50, and 70 h) and then placed in a −78°C freezer until the time of analysis. All samples were removed from the freezer and allowed to come to room temperature for approximately 1 h. The final time-point (70 h) was not placed in the freezer. Area ratios of cefepime to cefepime-d3 were compared to evaluate the stability. Post-extraction stability of cefepime in the autosampler (10 °C) was evaluated for 20 h.
Additional stability studies for cefepime were evaluated at the low, medium, and high QC concentrations (n=6) from loaded VAMS™ devices. Stability assessments were performed for 2 weeks at room temperature, 1 week at 4 °C, and 1 week and 1 month at −20 °C. Stability assessments at −78 °C were performed for 2 weeks, 1 month, and 3 months. Stability VAMS™ samples were placed in a plastic 96-rack box provided by Neoteryx with four packs of desiccant (Whatman®), wrapped in aluminum foil, dried for 1 hour at room temperature, and then stored at the designated temperature. After 1 week, 2 weeks, 1 month, and 3 months samples were taken out of the plastic box and were brought to room temperature unassisted for approximately 1 hour before beginning the extraction process. These samples were quantified using freshly prepared calibration curves and QCs employing VAMS™ devices.
2.5.7. Comparison of VAMS™, blood, and plasma concentrations
Whole blood to plasma partitioning was assessed using three different matrices (whole blood, plasma, and whole blood VAMS™) for the quantitation of cefepime at three QC levels (0.25, 40.0, and 80.0 μg/mL; n=6). First, whole blood samples were spiked, equilibrated at room temperature for 15 minutes, aliquoted (10 μL), processed through protein precipitation (same as the VAMS™ extraction), and measured against a freshly prepared blood curve. Unlike dried VAMS™ microsamples, the wet whole blood samples do not require reconstitution and were not incubated at 37 °C for 10 minutes after the addition of water. Using the same whole blood spikes, VAMS™ devices were loaded and extracted according to the optimized method. These samples were quantified against a calibration curve prepared using VAMS™ devices. The same blood spikes were then centrifuged at 2326 × g for 15 minutes at 4°C in order to collect the plasma of each working QC sample. These plasma samples were quantified against a freshly prepared plasma calibration curve as per previously validated method [22].
2.5.8. Clinical Sample Collection and Analysis
The principles outlined in the Declaration of Helsinki was followed for this study. For investigations involving human subjects, the Institutional Review Board (IRB) at the Children’s Hospital of Philadelphia approves all protocols and consent forms. Informed consent was obtained from all subjects enrolled. This validated method was evaluated for clinical sample analysis (IRB# 18-015558). A representative subject (11.0 kg) received an intravenous dose of cefepime (50 mg/kg). Blood samples (0.10 – 0.20 mL each) were collected from an arterial catheter into a 1 mL microtube at seven time points (pre-dose, end of infusion, 1, 2, 4, 6, and 8 h). Immediately, blood samples were loaded to VAMS™ devices (10 μL, n=2) following the optimized procedure described in Section 2.3 Sample Preparation, allowed to dry at room temperature for 1 hour, and stored at −78 °C until analysis. A VAMS™ sample was also collected at 4 hours by finger stick immediately after arterial sample collection, according to manufacturer’s instructions and processed as described above. VAMS™ clinical samples (n=1, at each time point) were analyzed as described in Section 2.3.
3. Results
3.1. Method Development
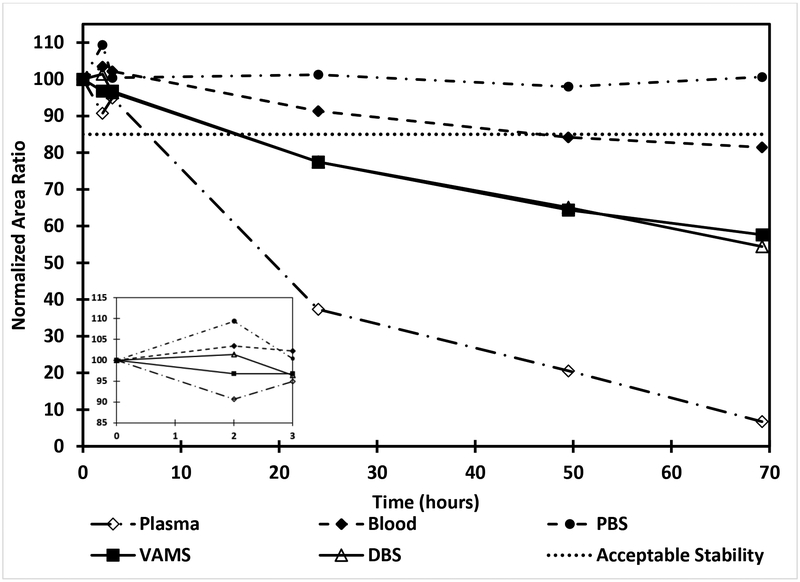
We have previously developed and validated a method for the analysis of cefepime in human plasma [22]. With the known instability of cefepime in biological matrices, an initial stability assessment of cefepime (10 μg/mL; n=4) was performed in PBS, human plasma, whole blood, DBS, and VAMS™ (Figure 2). Results show that cefepime is stable in all matrices for up to 3 h. Cefepime remains stable in PBS at room temperature for 70 h. However, it is less stable in the plasma matrix with only 37% of cefepime remaining after 24 h of storage. In whole blood samples 91% of cefepime remained after 24 h at room temperature. Both DBS and VAMS™ showed an identical stability profile with 77% of cefepime remaining after 24 h of storage at room temperature. Based on the limited stability and recovery of cefepime microsamples at room temperature, samples from remote sites need to be shipped on ice.
Figure 2.
Stability of cefepime (10 μg/mL) in PBS, plasma, blood, DBS and VAMS™ at room temperature. Expanded graph from 0 – 3 h is shown as an insert.
Initial method development for the quantitation of cefepime was performed with a simple protein precipitation using methanol containing cefepime-d3 [12], which showed no recovery of cefepime from the dried tips of the VAMS™ devices compared to the corresponding wet blood sample. However, the addition of 50 μL of water and 10 min incubation at 37 °C, as previously reported by Barco, et al, for antibiotics [11], significantly improved the recovery (40.8 - 62.1 %). The initial calibration standard range was tested from 0.100 – 200 μg/mL. There was signal saturation at 200 μg/mL, which resulted in lowering the upper limit of quantitation (ULOQ) to 100 μg/mL. Along with lowering the ULOQ, the addition of more extraction solvent (600 μL vs 250 μL) helped to eliminate the signal saturation. Despite improved linearity of the curve, carryover issues were observed following injection of the 100 μg/mL high standard. In an attempt to reduce the carryover, a mixture of water and methanol (1:1 v/v) with 0.1% formic acid was tested first as a possible wash solvent; however, this mixture did not resolve the issue. Therefore, a weak wash of 1% formic acid in water was employed along with a strong wash of 1:1:1:1 (v/v/v/v) of acetonitrile: methanol: water: IPA with 0.1% formic acid. The employment of these washes eliminated the carryover of cefepime.
3.2. Validation
3.2.1. Linearity and sensitivity
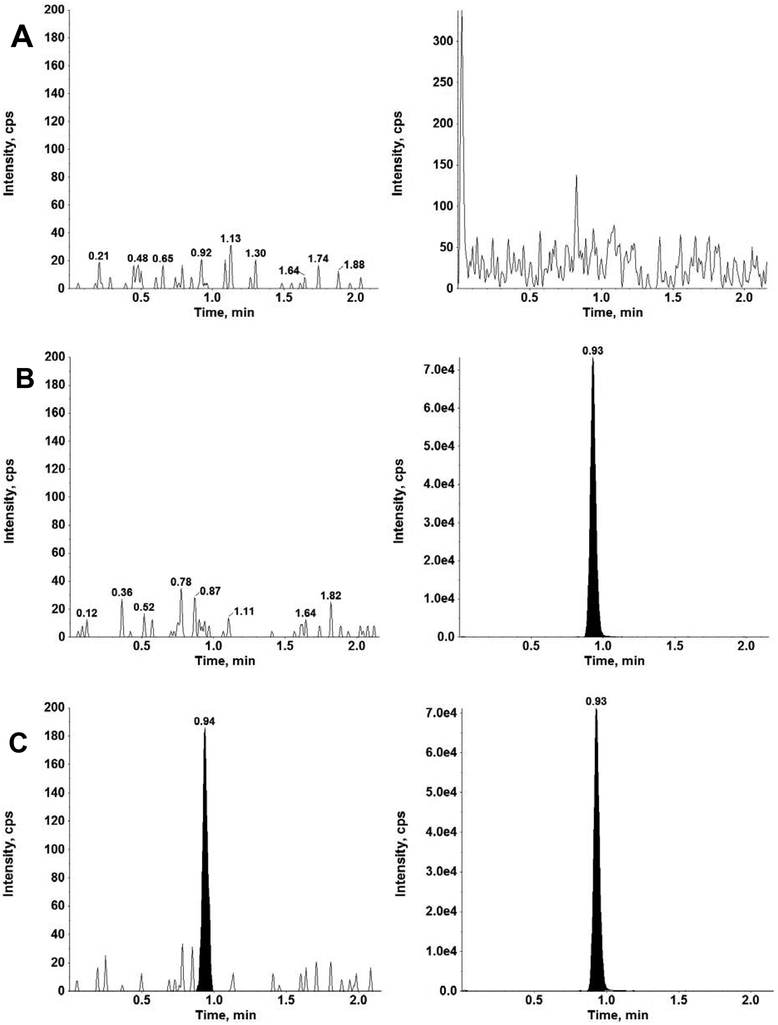
Linearity of cefepime calibration curves (n=2 per analytical run), over a range of 0.1 – 100 μg/mL, was determined from the results of the 3-day validation runs. The blood from a single donor was used for the 3-day validation study. Each analytical run contained a calibration curve at the beginning and the end of the batch with correlation coefficients (r2) ≥ 0.996 employing a weighted 1/x2 linear regression. The average (mean ± standard deviation) for the slope, y-intercept and r2 for the 3-day validation runs were 2.37×10−5 ± 8.72×10−7, 3.54×10−4 ± 5.86×10 −5 and 0.9973 ± 0.00106, respectively. Linearity was further evaluated as described by Jurado et al. [23] employing the principal graphical criteria of 1) visual inspection of the calibration plot, 2) the residuals plot, 3) response factors plot, and 4) percent relative error (%RE) of back-calculated concentration plot. Standardized residual plot, response factors plot and back-calculated relative errors (%RE) plot were all within the ±15% limits. Accuracies for all calibration standards ranged from 96.2% - 107%, including the LLOQ of 0.1 μg/mL, which showed a signal to noise ratio of at least 10. Representative chromatograms of cefepime and cefepime-d3 in a double blank, blank, and LLOQ are shown in Figure 3.
Figure 3.
Chromatograms for cefepime (left), and internal standard cefepime-d3 (right) in human whole blood using VAMS™ for A) double blank, B) single blank and c) LLOQ (0.100 μg/mL).
3.2.2. Accuracy and precision
Table 1 summarizes the intra- and inter-day accuracies and the coefficient of variation (%CV) for cefepime in human whole blood on VAMS™ devices (n=6) at the QC concentrations of 0.100, 0.250, 40.0, and 80.0 μg/mL. Intra- and inter-day accuracies were from 96.8% – 112% for the low, medium and high QC concentrations with %CV of 3.79% – 9.20%. The LLOQ had accuracies ranging from 95.4% – 113% with %CV from 6.00% – 14.4%.
Table 1.
Validation results for cefepime in human whole blood using VAMS™.
| Intra-Assay and Inter-Assay Accuracy and Precision for Cefepime in Human Whole Blood using VAMS™ Devices | ||||||
|---|---|---|---|---|---|---|
| Nominal Conc. (μg/mL) |
Day 1 Intra-Assay (n=6 replicates) | Day 2 Intra-Assay (n=6 replicates) | ||||
| (Mean ± SD) (μg/mL) |
CV (%) | Accuracy (%) |
(Mean ± SD) (μg/mL) |
CV (%) | Accuracy (%) | |
| 0.100 | 0.113 ± 0.007 | 6.00 | 113 | 0.0954 ± 0.010 | 10.3 | 95.4 |
| 0.250 | 0.266 ± 0.025 | 9.20 | 107 | 0.264 ± 0.019 | 7.02 | 106 |
| 40.0 | 38.7 ± 2.71 | 7.00 | 96.8 | 40.0 ± 1.51 | 3.79 | 100 |
| 80.0 | 84.3 ± 7.46 | 8.86 | 105 | 81.9 ± 3.24 | 3.95 | 102 |
| Nominal Conc. (μg/mL) |
Day 3 Intra-Assay (n=6 replicates) | Inter-Assay (3 days, n = 18 replicates) | ||||
| (Mean ± SD) (μg/mL) |
CV (%) | Accuracy (%) |
(Mean ± SD) (μg/mL) |
CV (%) | Accuracy (%) | |
| 0.100 | 0.102 ± 0.015 | 14.4 | 102 | 0.103 ± 0.013 | 12.2 | 103 |
| 0.250 | 0.281 ± 0.011 | 3.81 | 112 | 0.270 ± 0.019 | 7.10 | 108 |
| 40.0 | 41.9 ± 2.70 | 6.47 | 104 | 40.2 ± 2.58 | 6.42 | 100 |
| 80.0 | 82.9 ± 3.17 | 3.83 | 104 | 82.2 ± 3.51 | 4.27 | 103 |
3.2.3. Dilution Integrity and Carry-Over
Dilution integrity was evaluated by preparing a known QC concentration of cefepime (200 μg/mL) in whole blood, loading it to a VAMS™ device and diluting the extract 40-fold with single blank extracts. The integrity of the dilutions was confirmed showing an acceptable accuracy and precision of 106% ± 6.77% for cefepime (n=6). The signal for the analyte peak in the single blank samples following the injection of the upper limit of quantitation (ULOQ) did not exceed 20% of the LLOQ, and therefore no significant carry-over was observed.
3.2.4. Selectivity and specificity
Double blank VAMS™ devices loaded with 6 individual lots and a pooled lot of human whole blood were extracted with blank acetonitrile (no analyte or IS). The results showed no interfering peaks at the retention times of cefepime and cefepime-d3. LLOQ samples were prepared using 6 individual lots and a pooled lot of human whole blood and were loaded onto VAMS™ devices along with freshly prepared calibration curves and QCs. All samples were extracted using the validated extraction procedure and the IS working solution in acetonitrile. LLOQ samples were quantified against the fresh calibration curve and QCs. Accuracies for these LLOQ samples ranged from 96.7 – 105%, which was within acceptable range.
3.2.5. Recovery and matrix effect
Cefepime was analyzed at the low, medium, and high QC concentration levels for the assessment of recovery (n ≥ 6) and matrix effect (n=6). The recovery for cefepime ranged from 40.8% – 62.1% across the three concentrations (Table 2). These results indicate that the recovery for cefepime decreases as HCT level increases. The recovery remains consistent across each QC concentration level of cefepime within each HCT value. Cefepime in whole blood VAMS™ were analyzed in a 10-day time dependent experiment. Normalized recovery (mean ± %CV) at 0, 5, 8 and 10 days were 100 ± 2.64%, 59.3 ± 5.08%, 42.2 ± 7.61%, and 38.9 ± 4.31%, respectively, based on the recovery at the first time point (0 days) set to 100%. Matrix effect results showed minimal effect when extracting cefepime in human whole blood from the VAMS™ devices. The matrix effect was calculated using 1) the peak area of cefepime and 2) the peak area ratio of cefepime to the internal standard. The matrix effect across the three concentration levels ranged from 89.5% – 96.7% based on the cefepime peak area and 100% – 107% based on the peak area ratio of cefepime to the internal standard (Table 2). Minimal matrix effect was observed based on the cefepime peak area measurements, which was further compensated for by the inclusion of the stably labeled internal standard. Results of matrix effect in the 6 different lots of human whole blood spiked at the LLOQ also showed very minimal matrix effect on the analyte and its stably labeled internal standard. These results indicate that cefepime has a minimal matrix effect in human whole blood employing VAMS™ devices, further demonstrating that the conditions for this assay have been optimized.
Table 2.
Mean recovery (n ≥ 6) and matrix effect (n=5) of cefepime in human whole blood using VAMS™.
| Cefepime (μg/mL) |
Average Recoverya (%) ± %CV | Matrix Effect at Normal HCT (%)± %CV |
|||
|---|---|---|---|---|---|
| Low HCT (n=6) |
Normal HCT (n=11) |
High HCT (n=6) |
Peak Areab (n=5) |
Area Ratioc (n=5) |
|
| 0.250 | 60.2 ± 6.50 | 55.7 ± 14.5 | 40.8 ± 9.99 | 96.7 ± 14.5 | 107 ± 14.1 |
| 40.0 | 57.8 ± 8.13 | 49.9 ± 10.9 | 43.4 ± 10.2 | 89.5 ± 8.68 | 101 ± 4.46 |
| 80.0 | 62.1 ± 4.09 | 48.9 ± 11.5 | 48.2 ± 12.1 | 89.8 ± 2.34 | 100 ± 2.58 |
Recovery was calculated based on analyte peak area.
Matrix effect was calculated based on analyte peak area.
Matrix effect was calculated based on analyte peak area/internal standard ratio.
3.2.6. Stability
Cefepime showed stability when loaded onto VAMS™ devices and stored for 2 weeks (14 days), 1 month (28 days), and 3 months (91 days) at −78 °C (Table 3). Stability was also established for 1 week (7 days) at 4 °C and 1 month (39 days) at −20 °C (Table 3). Stability samples were analyzed and quantified against a freshly prepared calibration curve and QCs employing VAMS™ devices. Cefepime showed instability at room temperature after 2 weeks with accuracies of 36.5% and 40.9% at the low and high QC concentrations, respectively, when measured against freshly prepared calibration curves and QCs. Cefepime remained stable in the autosampler (10 °C) after 20 hours in the final matrix (post-extraction).
Table 3.
Stability of cefepime (n=6) at 4 , −20 and −78°C in human whole blood using VAMS™ devices.
| Cefepime Conc. (μg/mL) |
Mean Accuracy of cefepime ± %CV | |||||
|---|---|---|---|---|---|---|
| 1 week at 4 °C | 1 week at −20 °C |
1 month at −20 °C |
2 weeks at −78 °C |
1 month at −78 °C |
3 months at −78 °C |
|
| 0.250 | 97.7 ± 7.73 | 106 ± 7.46 | 97.3 ± 13.2 | 102 ± 12.6 | 102 ± 9.17 | 97.0 ± 8.00 |
| 40.0 | 97.5 ± 4.68 | 105 ± 5.13 | 88.8 ± 2.37 | 97.2 ± 5.78 | 106 ± 3.86 | 107 ± 3.59 |
| 80.0 | 93.8 ± 8.91 | 107 ± 2.02 | 95.9 ± 6.86 | 104 ± 7.60 | 112 ± 11.2 | 114 ± 8.66 |
3.2.7. Comparison of VAMS™, blood, and plasma concentrations
In vitro comparison of the concentrations of cefepime in VAMS™ (whole blood), whole blood, and plasma samples at three QC concentration levels (0.25, 40.0, and 80.0 μg/mL; n=6) are summarized in Table 4. The results for the whole blood and VAMS™ devices were similar and consistent with each other. However, results in plasma showed a 2-fold increase compared to the whole blood and VAMS™ device values. The blood to plasma partitioning ratios for cefepime range from 0.524 – 0.578 and VAMS™ to plasma partitioning ratio for cefepime range from 0.529 – 0.570.
Table 4.
Comparison of cefepime concentrations in VAMS™, whole blood and plasma (n=6).
| Whole Blood | VAMS™ | Plasma | Blood to Plasma ratio |
VAMS™ to Plasma ratio |
||||
|---|---|---|---|---|---|---|---|---|
| Cefepime Conc. (μg/mL) |
Measured Conc. ± SD (μg/mL) |
%CV | Measured Conc. ± SD (μg/mL) |
%CV | Measured Conc. ± SD (μg/mL) |
%CV | ||
| 0.250 | 0.285 ± 0.019 | 6.49 | 0.281 ± 0.011 | 3.81 | 0.493 ± 0.028 | 5.70 | 0.578 | 0.570 |
| 40.0 | 42.1 ± 1.55 | 3.68 | 41.8 ± 2.70 | 6.47 | 74.3 ± 1.67 | 2.24 | 0.566 | 0.562 |
| 80.0 | 82.0 ± 2.75 | 3.35 | 82.9 ± 3.17 | 3.83 | 157 ± 5.32 | 3.39 | 0.524 | 0.529 |
3.2.8. Clinical application
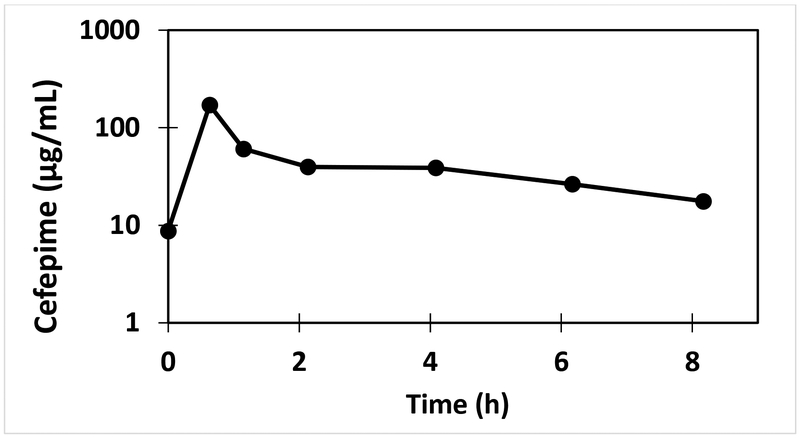
This validated method has been successfully utilized for cefepime analysis in VAMS™ samples from a subject (11.0 kg) following an intravenous dose of cefepime (50 mg/kg) and the resulting pharmacokinetic profile is shown in Figure 4. The results demonstrate that the assay range and sensitivity is appropriate for clinical pharmacokinetic analysis of cefepime employing the VAMS™ approach. The concentration of cefepime measured from the finger stick VAMS™ (36.6 μg/mL) and arterial VAMS™ (38.8 μg/mL) samples at 4 h following cefepime dose were similar.
Figure 4.
Pharmacokinetic profile of cefepime in a subject (11 kg) following intravenous infusion of cefepime over 30 minutes (50 mg/kg).
4. Discussion
Antibiotic treatment requires attainment of specific concentrations to achieve optimal outcomes, but there is often a balance between efficacy and toxicity that requires the use of TDM [11, 24]. Sample collection for TDM is limited in pediatric patients for multiple reasons. In children with indwelling venous catheters, there is risk of introducing infection and contamination of samples from the IV tubing when attempting to draw from existing catheters. In the outpatient setting, timing of sampling relative to doses and travel to collection sites imposes challenges. Along with these obstacles, shipping and handling of liquid samples to testing facilities can be costly and complicated, due to the need for dry ice and constant temperature monitoring [24].
There are several reported LC-MS/MS methods for the analysis of cefepime in human plasma or serum [4-9]. However, there are no methods available for analysis of cefepime in whole blood, DBS, or VAMS™ devices. In the present study, we have developed and validated an assay for cefepime with an LLOQ of 0.1 μg/mL using 10 μL of whole blood employing VAMS™ devices. While it was possible to further enhance the sensitivity to 0.01 μg/mL by reducing extraction volume and increasing injection volume, it resulted in signal saturation at the higher end of the calibration curve and increased carryover would be present. Our previous studies with effects of extracorporeal membrane oxygenation on pharmacokinetics of cefepime in critically ill pediatric patients employing plasma assays [22] showed that it was not necessary to have a sensitivity lower than 0.1 μg/mL. However, it is essential to have a higher limit of quantitation of at least 100 μg/mL to minimize the number of clinical samples that need to be diluted and reanalyzed given the expected high concentrations of cefepime in clinical samples. Two techniques in particular, DBS and VAMS™, present a way in which samples can be collected, dried and shipped under ambient temperatures without the predicaments found in liquid sample shipment [11, 24]. Although both DBS and VAMS™ have several advantages, they still have some limitations. For example, Andriguetti et al. [24] showed that DBS extraction recovery depends on the time at which the sample was applied to the DBS paper, whereas, extraction recovery using VAMS™ devices did not depend on application time of the sample. Barco et al. and others [11, 15, 16, 25], have presented issues in HCT when using DBS compared to VAMS™. For the four antibiotics (piperacillin-tazobactam, meropenem, linezolid and ceftazidime), DBS showed unacceptable bias and accuracy at different HCT levels (30%, 40%, 50% and 60%) when analyzed using a calibration curve prepared in HCT of 45%. Consistent with previously reported assays for other antibiotics [11], a rehydration step is critical for enhancing the recovery of cefepime from the dried VAMS™ devices. DBS demonstrated a dependency of HCT levels on the concentrations of antibiotics showing a negative bias in results using HCT of 30 and 40% and a positive bias using HCT of 50% and 60%. The same method of testing was performed using VAMS™, and all levels of antibiotics showed to be independent of HCT levels (within 15% of normal HCT level of 45%). Given these findings, the microsampling approach employing VAMS™ devices was determined to be the best method of collection and testing for the validation of cefepime in human whole blood for the production of robust and reproducible results.
The present assay employing VAMS™ devices demonstrates accurate quantitation of cefepime with its fixed volume of 10 μL for sample collection. This assay has minimal matrix effect and shows HCT dependence recovery over the broad HCT range of 18.5% – 64.5%. These results are consistent with previous reports in literature [16, 17, 26, 27]. Normal HCT varies by age. As a result, normal HCT varies from 30-50% in children. Among hospitalized children, lower HCT values (in the 20-30% range) are frequently encountered. This is because anemia is a common condition among hospitalized children, particularly in those with serious or chronic illnesses. Since cefepime is a broad-spectrum antibiotic, it is typically administered to the sickest of hospitalized children: critically ill or immunocompromised children, or in those with suspected serious or resistant gram-negative infections. As a result, the subset of children who are treated with cefepime are more likely to have an HCT value below the lower limit of normal than other children. While the specific HCT value among children treated with cefepime has not been reported, low HCT values (20-30%) are probably common. On the other hand, extremely low HCT values (<20%) are generally only encountered in cases of severe anemia, which is uncommon, while abnormally high HCT values are even rarer. The vast majority of hospitalized children treated with cefepime will have HCT values available from their clinical records as part of routine performance of a complete blood count (CBC). However, collecting additional blood samples to measure HCT values during PK studies and preparing calibrants and QCs at a particular HCT level for each subject will be challenging. The average recovery at the normal HCT level (41.0%) was 51.5%. At low (18.5%) and high HCT (64.5%) levels the average recoveries were 60.0 and 44.1%, respectively (Table 2). When normalized to the recovery at the normal HCT level, the recovery at the low and high HCT levels are 117% and 85.7%, respectively. This demonstrates an effect of HCT on cefepime recovery from VAMS™, however the impact of the HCT on recovery was within 17%, even at extreme HCT levels (18.5% and 64.5%). These results suggests that the impact of HCT variability on recovery will be minimal in subjects with normal HCT levels. However, in the clinical subjects that may have extreme HCT values, VAMS™ data should be interpreted with caution.
Stability for cefepime was established for at least 1 week at 4 °C, 1 month at −20 °C, and three months at −78 °C. The validation of these temperatures creates ease of sample collection when a −78 °C freezer may not be immediately available. Although one of the advantages for utilizing VAMS™ devices for sample collection is their capability of storing and shipping at room temperature, this does not hold true for cefepime. Based on our present studies, the recovery of cefepime decreases with the increase in drying time. Our current study also shows that compared to wet whole blood and buffer, cefepime has limited stability in dried blood samples (Figure 2). Taken together, a combination of lower extraction efficiency and the instability over drying time of cefepime can contribute to reduced recovery.
Cefepime stability is known to be temperature- and matrix-dependent [4, 6, 28]. Published methods have shown that cefepime is stable in serum at room temperature for up to 6 h, in methanol at −20 °C for up to six weeks, and in both plasma and serum when stored at −78 °C for up to 3 months [7, 29]. D’Cunha, et al. [29], have performed stability studies of cefepime in whole blood samples and demonstrated stability in whole blood for up to 4 h at room temperature. The limited stability of cefepime in plasma may be due to the degradation to release N-methylpyrrolidine under physiological conditions [4, 30].
In this present study, the stability experiments for cefepime in PBS, whole blood, and plasma show similar results to the experiments performed by D’Cunha, et al. [29]. Additionally, we show that VAMS™ devices and DBS samples loaded with 10 μL of cefepime in human whole blood behave alike under the same storage conditions. While these samples showed a decline in cefepime after 24 h, cefepime in VAMS™ devices and DBS was stable for up to 3 h at room temperature. However, in our validation studies, we have shown that cefepime in dried whole blood microsamples is stable for 3 months at −78 °C allowing for samples to be collected over a period of 3 months to be batched and analyzed. For the present study, samples were collected only on-site. However, if remote sites were to be included in future analyses, then we intend on only shipping samples on ice or frozen because of the instability of cefepime.
Our in vitro study comparing the concentrations of cefepime in human whole blood, plasma, and VAMS™ devices provides some useful insights in interpreting and comparing previously reported plasma pharmacokinetic parameters to future VAMS™ data. We have recently described how the concentration of voriconazole in human whole blood, plasma, and VAMS™ devices compared using a similar approach [12]. The ratios of VAMS™ to plasma concentrations for cefepime ranged from 0.529 – 0.570, suggesting that measured concentrations in VAMS™ will be lower than those detected in human plasma. D’Cunha, et al. [29] have reported RBC to plasma ratios of cefepime, meropenem, piperacillin, and tazobactam. They have reported an RBC to plasma ratio of 0.954 for cefepime at 5 μg/mL. They have estimated the RBC concentration and method plasma concentrations to arrive at the RBC to plasma ratio. In the present study, we measure blood, VAMS™, and plasma concentrations using calibration curves in the corresponding matrices. Though the measurements of the cefepime concentration in various matrices are robust, there is a limitation and uncertainty whether spiked samples behave in a similar manner as the authentic clinical samples. In addition, further clinical validation with analysis of cefepime in paired clinical samples in VAMS™, venous blood, and plasma samples is required to examine any difference between capillary blood from finger or heel pricks and venous blood.
5. Conclusions
A robust VAMS™-LC-MS/MS assay for measuring cefepime in small volumes (10 μL) of human whole blood employing VAMS™ has been developed, validated, and applied for clinical sample analysis. The validated method demonstrated accuracy and precision in the assay range of 0.1 – 100 μg/mL, which can be applied to clinical samples allowing the accurate quantification of cefepime. However, extreme HCT levels can affect the accurate quantitation of cefepime in clinical samples. Additional studies are warranted to evaluate the effect of HCT levels on cefepime quantitation in clinical VAMS™ samples. The established 3 month stability of cefepime as dried microsamples at −78 °C allows for batching and analysis of the clinical samples within this time-frame. The present method provides an alternative sampling strategy to collect samples from neonates with heel pricks, and children and adults with finger pricks. Blood sampling with VAMS™ devices has several advantages and is expected to be used extensively for pediatric clinical pharmacology studies in the future.
Highlights.
Patient-centric volumetric absorptive microsampling approach
Development and validation of cefepime assay in human whole blood
In vitro comparison of human whole blood, plasma and volumetric absorptive microsampling
Application of the assay for pharmacokinetic analysis of cefepime in pediatric clinical samples
Acknowledgement:
This work was supported by NIH (1K99HD096123-01, Zane). KJD is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365. Authors thank Brandon Milan (Neoteryx®) for helpful discussions.
This research was supported by the National Institutes of Health (1K99HD096123-01, Zane).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Authors declare that there is no conflict of interest regarding the publication of this paper.
References
- [1].Barradell LB, Bryson HM, Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use, Drugs 47(3) (1994) 471–505. [DOI] [PubMed] [Google Scholar]
- [2].Jan S, Ragunanthan B, DiBrito SR, Alabi O, Gutierrez M, Cefepime Efficacy and Safety in Children: A Systematic Review and Meta-analysis, Front Pediatr 6 (2018) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC, The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae, Clin Infect Dis 57(6) (2013) 781–8. [DOI] [PubMed] [Google Scholar]
- [4].Bugnon D, Giannoni E, Majcherczyk P, Glauser MP, Moreillon P, Pitfalls in cefepime titration from human plasma: plasma- and temperature-related drug degradation in vitro, Antimicrob Agents Chemother 46(11) (2002)3654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].D'Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, Winokur P, An G, Quantification of Cefepime, Meropenem, Piperacillin, and Tazobactam in Human Plasma Using a Sensitive and Robust Liquid Chromatography-Tandem Mass Spectrometry Method, Part 1: Assay Development and Validation, Antimicrob Agents Chemother 62(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moorthy GS, Downes KJ, Zane N, Zuppa AF, Liquid Chromatography-Tandem Mass Spectrometry Assays for Therapeutic Drug Monitoring of Cefepime, J Appl Bioanal 4(5) (2018) 144–156. [Google Scholar]
- [7].Paal M, Zoller M, Schuster C, Vogeser M, Schutze G, Simultaneous quantification of cefepime, meropenem, ciprofloxacin, moxifloxacin, linezolid and piperacillin in human serum using an isotope-dilution HPLC-MS/MS method, J Pharm Biomed Anal 152 (2018) 102–110. [DOI] [PubMed] [Google Scholar]
- [8].Rigo-Bonnin R, Ribera A, Arbiol-Roca A, Cobo-Sacristan S, Padulles A, Murillo O, Shaw E, Granada R, Perez-Fernandez XL, Tubau F, Alia P, Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of beta-lactam antibiotic concentration in human plasma, Clin Chim Acta 468 (2017) 215–224. [DOI] [PubMed] [Google Scholar]
- [9].Zander J, Maier B, Suhr A, Zoller M, Frey L, Teupser D, Vogeser M, Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation, Clin Chem Lab Med 53(5) (2015) 781–91. [DOI] [PubMed] [Google Scholar]
- [10].Spooner N, Denniff P, Michielsen L, De Vries R, Ji QC, Arnold ME, Woods K, Woolf EJ, Xu Y, Boutet V, Zane P, Kushon S, Rudge JB, A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit, Bioanalysis 7(6) (2015) 653–9. [DOI] [PubMed] [Google Scholar]
- [11].Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G, Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot, J Pharm Biomed Anal 145 (2017) 704–710. [DOI] [PubMed] [Google Scholar]
- [12].Moorthy GS, Vedar C, Zane N, Prodell JL, Zuppa AF, Development and validation of a volumetric absorptive microsampling assay for analysis of voriconazole and voriconazole N-oxide in human whole blood, J Chromatogr B Analyt Technol Biomed Life Sci 1105 (2019) 67–75. [DOI] [PubMed] [Google Scholar]
- [13].Antunes MV, Charao MF, Linden R, Dried blood spots analysis with mass spectrometry: Potentials and pitfalls in therapeutic drug monitoring, Clin Biochem 49(13-14) (2016) 1035–46. [DOI] [PubMed] [Google Scholar]
- [14].Evans C, Arnold M, Bryan P, Duggan J, James CA, Li W, Lowes S, Matassa L, Olah T, Timmerman P, Wang X, Wickremsinhe E, Williams J, Woolf E, Zane P, Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ Consortium Microsampling Working Group, AAPS J 17(2) (2015) 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guerra Valero YC, Wallis SC, Lipman J, Stove C, Roberts JA, Parker SL, Clinical application of microsampling versus conventional sampling techniques in the quantitative bioanalysis of antibiotics: a systematic review, Bioanalysis 10(6) (2018) 407–423. [DOI] [PubMed] [Google Scholar]
- [16].Kok MGM, Fillet M, Volumetric absorptive microsampling: Current advances and applications, J Pharm Biomed Anal 147 (2018) 288–296. [DOI] [PubMed] [Google Scholar]
- [17].Velghe S, Delahaye L, Stove CP, Is the hematocrit still an issue in quantitative dried blood spot analysis?, J Pharm Biomed Anal 163 (2019) 188–196. [DOI] [PubMed] [Google Scholar]
- [18].Neoteryx, Blood Collection Kit. https://cdn2.hubspot.net/hubfs/1806452/Content/Mitra_Product_Brochure.pdf, 2019).
- [19].Neoteryx, How to take a sample: The Do's And Don'ts of Using The Mitra Microsampler. https://www.neoteryx.com/how-to-properly-take-a-blood-sample-using-the-mitra-microsampler-vams, 2019).
- [20].Protti M, Mandrioli R, Mercolini L, Tutorial: Volumetric absorptive microsampling (VAMS), Anal Chim Acta 1046 (2019) 32–47. [DOI] [PubMed] [Google Scholar]
- [21].FDA, Guidance for Industry: Bioanalytical Methods Validation. https://www.fda.gov/media/70858/download,, 2018). [Google Scholar]
- [22].Zuppa AF, Zane NR, Moorthy G, Dalton HJ, Abraham A, Reeder RW, Carcillo JA, Yates AR, Meert KL, Berg RA, Sapru A, Mourani P, Notterman DA, Dean JM, Gastonguay MR, Eunice H Kennedy Shriver National Institute of Child, N. Human Development Collaborative Pediatric Critical Care Research, A Population Pharmacokinetic Analysis to Study the Effect of Extracorporeal Membrane Oxygenation on Cefepime Disposition in Children, Pediatr Crit Care Med 20(1) (2019) 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jurado JM, Alcazar A, Muniz-Valencia R, Ceballos-Magana SG, Raposo F, Some practical considerations for linearity assessment of calibration curves as function of concentration levels according to the fitness-for-purpose approach, Talanta 172 (2017) 221–229. [DOI] [PubMed] [Google Scholar]
- [24].Andriguetti NB, Lisboa LL, Hahn SR, Pagnussat LR, Antunes MV, Linden R, Simultaneous determination of vancomycin and creatinine in plasma applied to volumetric absorptive microsampling devices using liquid chromatography-tandem mass spectrometry, J Pharm Biomed Anal 165 (2019) 315–324. [DOI] [PubMed] [Google Scholar]
- [25].Capiau S, Veenhof H, Koster RA, Bergqvist Y, Boettcher M, Halmingh O, Keevil BG, Koch BCP, Linden R, Pistos C, Stolk LM, Touw DJ, Stove CP, Alffenaar JC, Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: Development and Validation of Dried Blood Spot-Based Methods for Therapeutic Drug Monitoring, Ther Drug Monit 41(4) (2019) 409–430. [DOI] [PubMed] [Google Scholar]
- [26].De Kesel PM, Lambert WE, Stove CP, Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study, Anal Chim Acta 881 (2015)65–73. [DOI] [PubMed] [Google Scholar]
- [27].Xie I, Xu Y, Anderson M, Wang M, Xue L, Breidinger S, Goykhman D, Woolf EJ, Bateman KP, Extractability-mediated stability bias and hematocrit impact: High extraction recovery is critical to feasibility of volumetric adsorptive microsampling (VAMS) in regulated bioanalysis, J Pharm Biomed Anal 156 (2018) 58–66. [DOI] [PubMed] [Google Scholar]
- [28].Nemutlu E, Kir S, Katlan D, Beksac MS, Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: application to validation and quantification of cefepime, cefixime and cefoperazone, Talanta 80(1) (2009) 117–26. [DOI] [PubMed] [Google Scholar]
- [29].D'Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, Winokur P, An G, Quantification of Cefepime, Meropenem, Piperacillin, and Tazobactam in Human Plasma Using a Sensitive and Robust Liquid Chromatography-Tandem Mass Spectrometry Method, Part 2: Stability Evaluation, Antimicrob Agents Chemother 62(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Forgue ST, Kari P, Barbhaiya R, N-oxidation of N-methylpyrrolidine released in vivo from cefepime, Drug Metab Dispos 15(6) (1987) 808–15. [PubMed] [Google Scholar]