Abstract
Objective:
To demonstrate feasibility of performing geriatric assessment (GA) in the National Clinical Trials Network (NCTN) and to explore the utility of GA to characterize treatment tolerance.
Materials and Methods:
We conducted a multisite companion study (CALGB 361006) to CALGB 11001, a phase 2 trial of adults ≥60 years old with newly diagnosed FLT3- mutated AML, testing the efficacy of adding sorafenib to intensive chemotherapy. On 361006, a GA was administered prior to induction and prior to post-remission therapy. The GA is divided into items requiring administration by a health care professional (HCP) and patient self-administered questionnaires. Feasibility outcomes were recruitment rate, time to GA completion, difficulty with GA administration, percent of patients requiring assistance, and satisfaction. Change in GA measures pre- and post-induction were compared using Wilcoxon signed rank test and McNemar’s tests.
Results:
The recruitment rate was 80% (N=43, median age 68 years). Median completion time of the GA was 30 minutes; (10 and 21 minutes for HCP and patients, respectively). HCP reported no difficulty completing assessments (100%). Most patients completed questionnaires without assistance (77%), and were satisfied with the length (89%). Self-reported physical function, mental health, social activity and nutritional parameters worsened after induction.
Conclusion:
GA is feasible to administer in the setting of intensive induction for older adults with AML in the NCTN and provides evidence of the impact of induction therapy on physical and emotional health.
Keywords: acute myeloid leukemia, leukemia, older, age, geriatric assessment, feasibility, hematology, hematologic malignancy
Introduction:
Acute myeloid leukemia (AML) is a disease most commonly diagnosed in older adults. Survival for patients with AML is age dependent, with significantly lower survival rates reported for older adults.1,2 While selected older adults may benefit from aggressive therapies, older adults, as a group, are at risk for increased toxicity from treatment. 1,3,4 Assessment tools are needed to better characterize fitness in the context of therapy and to capture how older adults feel and function prior to and after therapy. This information could assist clinicians in making treatment decisions, inform future trial design, and identify potentially modifiable risk factors for development of interventions to improve treatment outcomes and the quality of survivorship.
Geriatric assessment (GA) is a strategy that can characterize the heterogeneity of aging and capture important functional outcomes for older adults. Based on single institution data, pretreatment GA for older adults with newly diagnosed AML appears feasible.5 In this setting GA detects significant, potentially unrecognized, impairments in the majority of patients scheduled to receive intensive therapy despite good performance status. 5 Importantly, GA measures such as objectively measured impaired physical function and cognitive performance are independently associated with worse survival among intensively treated patients.6 Similarly, a fitness score derived from clinical and GA parameters has also been associated with survival among older adults with AML or myelodysplastic syndrome receiving non-intensive therapies.7 Retrospective studies evaluating specific patient characteristics such as comorbidity, polypharmacy, and symptoms further support the importance of comprehensive and standardized risk assessment strategies for older adults.7-11 However, the feasibility and utility of GA has not been demonstrated in multisite AML National Cancer Institute (NCI) National Clinical Trials Network (NCTN) studies.
A brief, comprehensive, standardized GA to characterize a patient’s “functional age” was developed in the Alliance for Clinical Trials in Oncology (Alliance) Cancer in the Older Adult Committee and was previously piloted in a multisite trial among older adults scheduled to receive chemotherapy predominantly for solid tumors.12,13 The assessment evaluates functional status, comorbid medical conditions, cognition, nutritional and psychological status, social support and social activities and was feasible to administer to patients enrolling on non-AML NCTN trials. A toxicity risk score derived from this GA predicts chemotherapy toxicity.14,15 These published data, however, do not include patients with newly diagnosed AML. AML diagnosis and treatment represents a unique, often high-acuity setting, requiring rapid diagnosis and initiation of treatment commonly in the inpatient setting. It is uncertain whether data from non-AML studies can be extrapolated to the care paradigm for AML. To address this issue, the primary objective of this study was to evaluate the feasibility of performing GA in an AML NCTN treatment trial. Secondary objectives were to investigate the impact of induction chemotherapy on physical, cognitive, psychosocial factors and explore the association of baseline GA measures with overall survival.
Methods:
Cancer and Leukemia Group B (CALGB) 361006 was a prospective multisite embedded companion study offered to patients enrolled on CALGB 11001 at the 15 participating CALGB institutions. CALGB 11001 was a phase II study of adults 60 years of age or older with newly diagnosed FMS-like tyrosine kinase-3 (FLT3) mutated AML testing the efficacy of adding sorafenib to intensive chemotherapy with daunorubicin and cytarabine open between 2011 and 2014.16 Any site participating in CALGB 11001 could enroll on 361006. This study, with the 361006 companion, was approved by the NCI Central Institutional Review Board and by the institutional review board (IRB) at each participating institution. CALGB is now part of the Alliance for Clinical Trials in Oncology.
Eligibility criteria
Patients were eligible to enroll on the GA companion study (CALGB 361006) if they met eligibility criteria for and enrolled on CALGB 11001. Briefly, eligibility criteria for the treatment and companion study included: a new histologic diagnosis of AML excluding acute promyelocytic leukemia and core binding factor leukemia; documented FLT3 mutation determined by central laboratory; 60 years of age or older; and no prior chemotherapy for AML. The minimum acceptable performance status or laboratory parameters were not specified.
Procedures
Before patients were enrolled in CALGB 361006, the study principal investigator (HDK) trained research staff at each participating site (via telephone). Training calls (approximately 15-30 minutes) in length reviewed written standardized administration and scoring instructions for each GA measure and provided an opportunity for staff to ask clarifying questions. Then, patients who were eligible to enroll on CALGB 11001 were offered the opportunity to enroll on CALGB 361006. All patients who chose to participate in the GA companion study (361006) completed an IRB-approved, protocol-specific informed consent at the time of consent to the treatment trial (CALGB 11001). The GA and a quality-of-life questionnaire were completed at baseline (prior to initiation of induction chemotherapy) and again after induction at the time of evaluation for consolidation therapy either in the inpatient or outpatient setting. Patient registration, data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. The study chair contacted site staff to obtain reasons for missing data when one was not provided.
Measures
The GA tool includes validated measures assessing the domains of physical function, comorbid medical conditions, psychological state, social activities and support, nutritional status, cognitive function and medications.12,13 It was developed for use in the NCTN setting with a full description of measures previously reported. Modifications to the GA tool for this study include the addition of the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI)17 and the substitution of the Mental Health Inventory-17 (MHI-17) to assess psychological health.18 The HCT-CI was added because it is well validated in leukemia and provides more detailed information on comorbidity burden than the patient-reported comorbidity assessment included in the GA.19-21 The MHI-17 was chosen as a substitute assessment for psychological health by the Alliance Cancer in the Older Adult Subcommittee due to the proprietary nature of the previously included measure.
The GA tool includes a healthcare provider (nurse or certified research associate) administered assessment and a self-administered patient questionnaire. The healthcare provider-administered questionnaire included the following 5 brief assessments: 1) HCT-CI (a validated comorbidity index associated with remission status and survival among older adults with AML)17,19,21; 2) Karnofsky performance status; 3) Timed up and Go (a performance based measure of physical function; time assessed in seconds for those who could complete the test or recorded as unable to perform)22; 4) Blessed Orientation Memory Concentration test (score ≥11 indicating impairment)23; 5) recording of height and weight (current and 6 months prior) to evaluate nutritional status including calculation of body mass index. Prior weight was collected by self-report if not recorded in the medical record. Intentionality of weight loss was not assessed.
The self-administered patient questionnaire included several validated surveys and demographic questions. The questionnaire included self-reported measures of physical function and activities (inclusive of activities of daily living, instrumental activities of daily living and mobility items), a patient-rated Karnofsky performance status, self-reported falls in the past 6 months, self-reported comorbid conditions and a rating of the degree to which each causes interference in activities, number and type of medications, assessment of psychological state (symptoms of anxiety and depression), social activity, and social support. A member of the health care team could assist those patients who needed help.
Health-related quality-of-life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30). 24,25 Domains assessed included general physical symptoms, fatigue and malaise, and physical, social and emotional functioning. Time points for assessment were the same as for the GA questionnaires.
Outcomes
The primary outcome was feasibility evaluated by recruitment of participants, implementation (time to completion, difficulty with administration, percent of participants requiring assistance), and patient satisfaction with the assessment. Time to completion of the battery of tests was estimated in minutes by the health care professional for both portions of the evaluation. The research team completed survey questions about difficulty with administration. Staff was asked whether any items were difficult to administer or difficult to complete by the staff or participant. To determine patient satisfaction with the assessment, the patient questionnaire surveyed participants regarding difficulty understanding questions, length of the assessment, whether items were upsetting and whether important questions were left out. Similar to prior feasibility work in the NCTN setting, feasibility thresholds were recruitment rate of 70%, no difficulty in implementation reported by ≥70% of participants and ≥ 80% of research staff and ≥80% of participants reporting satisfaction.12 A time to completion indicating feasibility of the entire assessment was ≤ 40 minutes.
Secondary outcomes included: 1) evaluation of the change in GA measures from baseline to after induction assessment; 2) exploration of relationships between specific baseline GA measures and overall survival to inform larger trials; and 3) description of health care utilization by capturing hospitalizations, oncology clinic visits and nursing home use during the study from the medical record. OS was estimated from the time of signing the consent form.
Statistical analyses
Descriptive statistics were used to summarize feasibility outcomes and GA measures. Continuous variables were described by mean (standard deviation) and median (range) and categorical variables by frequency and percentage. Changes from baseline to post-induction therapy were calculated using the Wilcoxon signed rank test for continuous variables and McNemar’s test for categorical variables. Survival probabilities were estimated using the Kaplan-Meier estimator.26 Associations between baseline GA measures and overall survival were explored by comparing survival probabilities between groups (dichotomized by the median score due to small sample size) using the log-rank test. All analyses were performed using SAS Version 9.3 (SAS Institute, Cary NC, USA) and had a two-sided alpha level of 0.05. Data lock for trial data was on May 11, 2016. Due to the exploratory nature of this analysis, there was no adjustment for multiple comparisons.
Results:
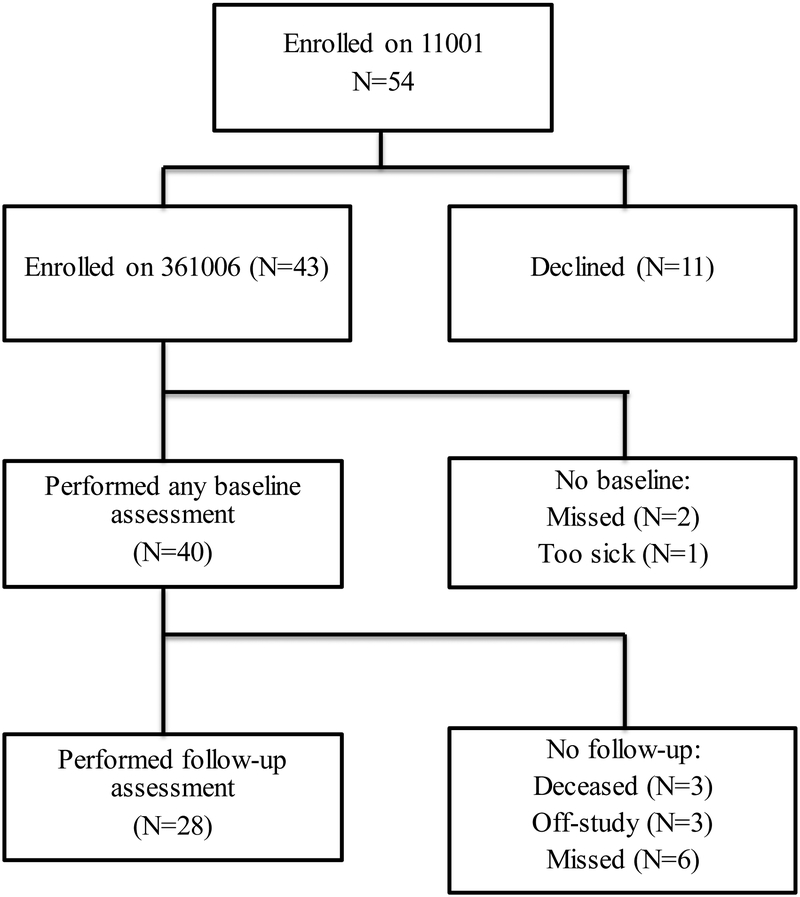
Among the 54 patients who enrolled on clinical trial CALGB 11001, 43 (80%) enrolled on the GA companion study (CALGB 361006) from 14 different institutions (Figure 1). Of these, 40 completed at least one baseline assessment (93%). Twenty-eight patients (70%) performed a follow-up assessment after induction therapy. Of those who did not complete follow-up assessment, 3 were deceased, 3 came off study treatment (1 for resistant disease) and 6 assessments were missed (reasons not documented).
Figure 1.
Consort Diagram of enrollment and follow-up on A361006
Baseline characteristics are presented in Table 1. Nearly 40% of participants were aged 70 years and above. Most were non-Hispanic, white males. The majority (75%) received one cycle of induction therapy. The remission rate was 78% and 64% received post-remission therapy. The early death rate was 10% and median overall survival was 14.9 months (95% C.I. 12.6-23.3 months). Tumor and treatment characteristics of the GA cohort were similar to the overall study cohort.16
Table 1.
Baseline characteristics of older adults with AML on A361006 (N=40)
| Baseline Characteristics | Median (range) or percent |
|---|---|
| Demographics | |
| Age (yrs) median (range) | 68 (61-83) |
| % 60-64 | 30 |
| % 65-69 | 30 |
| % 70-74 | 23 |
| % 75-79 | 10 |
| % ≥80 | 8 |
| % female | 40 |
| % white | 98 |
| % non-Hispanic | 85 |
| % married | 62 |
| % with ≥ college education | 39 |
| Clinical | |
| FLT3 mutation | |
| ITD | 75 |
| TKD | 25 |
| Onset of AML | |
| De novo | 83 |
| Therapy-related | 8 |
| MDS-related | 4 |
| ELN classification | |
| CN-AML | 55 |
| Intermediate II | 28 |
| ELN Adverse | 5 |
| Unconfirmed cytogenetics | 13 |
| Bone marrow blast percentage | 58 (0-96) |
| White blood cell count (x103/mm3) | 15 (1-344) |
| Lactate dehydrogenase (U/L) | 516 (103-2813) |
| Creatinine (mg/dl) | 0.9 (0.4-1.7) |
Abbreviations: AML: acute myeloid leukemia; FLT3; fms related tyrosine receptor kinase 3, ITD; internal tandem duplication, TKD; tyrosine kinase domain; AML; acute myeloid leukemia, MDS; myelodysplastic syndrome, ELN; European LeukemiaNet; CN; cytogenetically normal
The median time recorded for completion of the GA battery inclusive of both the health care professional and patient questionnaires was 30 minutes (Interquartile range 23-40 minutes). The median times recorded for completion of the health care professional questionnaire was 10 minutes (range 2-30 minutes) and for the patient questionnaire was 23 minutes (range 3-90 minutes). Among research staff, 100% reported no difficulty administering the healthcare professional component. The majority of patients reported no difficulty understanding questions (89%) and completed the assessment without assistance (77%). Patient satisfaction was high; 89% were satisfied with the length and no patients found the questionnaire upsetting.
Baseline values for each GA measure and the quality-of-life questionnaire (EORTC CLC-Q30)are presented in Table 2. Most participants reported independence in instrumental activities of daily living, had good performance status, had no history of falls, screened negative for cognitive impairment, were taking on average 4.5 medications and had modest comorbidity burden. Mean scores were low for psychological state, social activity and social support scales, and global quality-of-life measures (indicating prevalent psychological symptoms, less social activity and support, and poor quality of life). Mean scores on the Timed Up and Go test indicated impaired mobility (≥11 seconds) although the majority (90%) were able to perform the test.
Table 2.
Baseline geriatric assessment and quality-of-life measures (N=40)
| Domain | Measure | No. of Items |
Range of Scores | Mean ± SD | Median (Range) |
|---|---|---|---|---|---|
| Functional Status | Activities of Daily Living (subscale of MOS Physical Health) | 10 | 0-100 (higher score: better physical function) | 72.1±25.8 | 83.3 (50-94.4) |
| Instrumental Activities of Daily Living (subscale of the OARS) | 7 | 0-14 (higher score: less need for assistance) | 13.3±1.8 | 14 (4-14) | |
| *Karnofsky Self-Reported Performance Rating Scale | 1 | 40-100 (higher score: better function) | 83.2±15.1 | 90 (80-100) | |
| Karnofsky Physician-Reported Performance Rating Scale | 1 | 10-100 (higher score: better function) | 84.9±13.5 | 90 (50-100) | |
| Number of falls in last 6 months | 1 | Higher score, worse performance | 0.4 ±1.2 | 0 (0-7) | |
| Timed up and Go (seconds) | 1 | Higher score: worse performance) | 15.1±10 | 13 (5-60) | |
| Cognition | Blessed Orientation Memory and Concentration Test (BOMC) | 6 | 0-28 (score≥11 indicates impairment) | 3.1±3.0 | 2 (0-12) |
| Comorbidity | Physician Health Section Survey (subscale of the OARS) | 13 | 0-39 (higher score: greater comorbidity) | 3.2±3.1 | 2.5 (0-16) |
| HCT-CI | 17 | 0-29 (higher score: greater comorbidity) | 1.8±1.9 | 1.0 (0-7) | |
| Medications | *Number of medications | 1 | 4.5±3.7 | 4.0 (0-13) | |
| Psychological State | Mental Health Inventory-17 | 17 | 0-100 (higher score: better mental health) | 56.4±4.0 | 56.5 (49.4-67.1) |
| Social Activity | MOS Social Activity Survey | 4 | 0-100 (higher score: better social activity) | 60.3±21.8 | 62.5 (6.3-93.8) |
| Social Support | MOS Social Support Survey: Emotional Information and Tangible Subscales | 12 | 0-100 (higher score: better social support) | 83.5±16.4 | 92.3 (15.4-92.3) |
| Nutrition | *Percent unintentional weight loss in last 6 months | 1 | −1.9±11.7 | −1.9 (−53.8-21.7) | |
| Global Quality of life | EORTC QLQ C30 Global | 30 | 0-100 (higher score: better QOL) | 46.8±30.7 | 50 (0-100) |
Abbreviations: MOS=Medical Outcomes Survey; OARS=Older American Resources and Services; HCT-CI=Hematopoietic cell transplantation comorbidity index; EORTC QLQ=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30; QOL=quality of life
Represents score for number of subjects completing this assessment: Patient reported KPS, N=37; Time up and go, N =35; number of falls, N = 36; number of medications, N = 33; percent weight loss, N=36.
For patients who completed post-induction assessment, change in GA measures and global quality-of-life is presented in Table 3. Receipt of induction therapy had a variable impact on GA measures for the 28 patients who survived induction therapy and returned for evaluation of post-remission therapy. Self-reported physical function (assessed by instrumental activities of daily living), psychological state, social activity, body mass index and unintentional weight loss significantly worsened. By contrast the global quality-of-life (EORTC CLC-Q30) score improved. Scores on the emotional, social, and several symptom-related subscales (pain, dyspnea, appetite) of the EORTC CLC-Q30 significantly improved after induction (p<0.05 for all) and none showed significant worsening (data not shown).
Table 3.
Change in geriatric assessment and global quality of life after induction (N=28)
| Measures | Baseline (mean±SD) |
Follow-up (mean±SD) |
Mean change from baseline±SD |
P-value |
|---|---|---|---|---|
| Activities of Daily Living (subscale of MOS Physical Health) | 69.5±27.8 | 60.5±26.6 | −9.0±33.3 | 0.13 |
| Instrumental Activities of Daily Living (subscale of the OARS) | 13.4±1.1 | 12.3±2.3 | −1.1±2.0 | 0.002 |
| *Karnofsky Self-Reported Performance Rating Scale | 81.9±15.9 | 82.6±17.9 | 0.7±21.3 | 0.66 |
| *Karnofsky Physician-Reported Performance Rating Scale | 82.8±13.1 | 84.8±9.6 | 2.0±13.2 | 0.52 |
| *No. of falls in last 6 months | 0.2±0.5 | 0.2±0.5 | 0±0.4 | 1.0 |
| *Timed Up and Go (seconds) | 13.5±4.3 | 12.1±3.9 | −1.4±3.7 | 0.06 |
| *Blessed Orientation Memory and Concentration Test (BOMC) | 4.0 ±3.2 | 3.2±4.2 | −0.6±3.6 | 0.18 |
| *Physician Health Section Survey (subscale of the OARS) | 3.4±3.5 | 3.7±4.6 | 0.3±5.0 | 0.80 |
| *HCT-CI | 1.9 (1.8) | 1.7 (1.6) | −0.2±1.2 | 0.51 |
| *Number of medications | 4.0±3.4 | 4.4±3.6 | 0.4±3.7 | 0.5 |
| Mental Health Inventory-17 | 56.1±3.4 | 54.2±3.2 | −1.9±4.3 | 0.04 |
| MOS Social Activity Survey | 62.3±22.1 | 42.4±23.1 | −19.9±24.7 | 0.0002 |
| MOS Social Support Survey: Emotional Information and Tangible Subscales | 94.3±10.5 | 91.7±15.2 | −2.4±13.5 | 0.63 |
| *Body Mass Index | 29.9±5.5 | 28.1±4.8 | −1.8±1.8 | <0.001 |
| *Percent unintentional weight loss in last 6 months | −2.7±12.5 | −8.2±13.4 | −5.6±17.3 | 0.03 |
| *EORTC QLQ C30 | 47.8±29.8 | 67.9±18.8 | 20.2±29.4 | 0.001 |
Abbreviations: MOS=Medical Outcomes Survey; OARS=Older American Resources and Services; HCT-CI=Hematopoietic cell transplantation comorbidity index; EORTC QLQ=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30; QOL=quality of life, KPS=Karnofsky performance status
Represents score for number of subjects completing this assessment: Patient reported KPS, N=27; Physician KPS, N=25; number of falls, N = 25; Time up and go, N =23; Blessed Orientation Memory Concentration Test, N=23, physical health survey, N=27, HCT-CI, N=25, number of medications, N = 21; body mass index, N= 25, percent weight loss, N=24.
Exploratory analyses investigating the relationship between baseline GA measures and overall survival did not show any statistically significant associations. Univariate results are shown in Table 4; they can be useful to estimate effect size for larger studies. For example, the hazard of mortality was higher for those with self-reported physical limitations (lower scores on MOS physical health subscale and Instrumental Activities of Daily Living) and for those reporting a fall. Similarly, there was no association between baseline global quality-of-life measured by EORTC CLC-Q30 and survival. However, the nausea/vomiting subscale of the EORTC CLC-Q30 was associated with worse survival; those with symptoms at baseline had an OS of 0.8 years compared to those without at 1.6 years (p=0.007).
Table 4.
Univariate association between baseline geriatric assessment measures and mortality
| Measure | Median Score Cut-point (N) | Hazard Ratio for Mortality (95% Confidence Interval) |
|---|---|---|
| Activities of Daily Living (subscale of MOS Physical Health) | ≤83.3 (18) | 1.6 (0.75-3.4) |
| >83.3 (21) | Reference | |
| Instrumental Activities of Daily Living (subscale of the OARS) | <14 (10) | 1.7 (0.75-3.7) |
| ≥ 14 (29) | Reference | |
| Number of falls in last 6 months | ≥1 (8) | 2.02 (0.87-4.69) |
| <1 (28) | Reference | |
| Karnofsky Self-Reported Performance Rating Scale | <90 (18) | 0.8 (0.4-1.7) |
| ≥90 (19) | Reference | |
| Karnofsky Physician-Reported Performance Rating Scale | <90 (15) | 0.7 (0.32-1.6) |
| ≥90 (24) | Reference | |
| Timed up and Go (seconds) | <13 (17) | 0.7 (0.32-1.53) |
| ≥ 13 (22) | Reference | |
| Blessed Orientation Memory and Concentration Test (BOMC) | ≤2 (24) | 0.8 (0.4-1.7) |
| > 2 (15) | Reference | |
| Physician Health Section Survey (subscale of the OARS) | ≤2.5 (19) | 0.9 (0.43-1.9) |
| >2.5 (19) | Reference | |
| HCT-CI | ≤1 (24) | 1.23 (0.6-2.7) |
| >1 (15) | Reference | |
| Number of medications | ≤4 (21) | 0.8 (0.3-1.8) |
| >4 (12) | Reference | |
| Mental Health Inventory-17 | <56.5 (24) | 0.7 (0.3-1.4) |
| ≥ 56.5 (15) | Reference | |
| MOS Social Activity Survey | ≤62.5 (22) | 0.7 (0.3-1.4) |
| >62.5 (17) | Reference | |
| MOS Social Support Survey | ≤92.3 (17) | 0.8 (0.4-1.6) |
| >92.3 (22) | Reference | |
| EORTC QLQ C30 Global | ≤50 (21) | 0.8 (0.3-1.7) |
| >50 (16) | Reference |
Abbreviations: MOS=Medical Outcomes Survey; OARS=Older American Resources and Services; HCT-CI=Hematopoietic cell transplantation comorbidity index; EORTC QLQ=European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30; QOL=quality of life
Health care utilization for participants who survived induction was high, with data available from 25 of 28 individuals. The median (range) number of days hospitalized for chemotherapy during the time between diagnosis and start of consolidation was 30 (7 – 61). In addition, roughly 27% were re-hospitalized for reasons other than chemotherapy during this period with a median of 25 (3 – 47) days. The median number of oncology clinic visits was 2.0 (0 – 4); 1 participant required care in a nursing facility for rehabilitation or long-term care.
Discussion:
This is the first multisite NCTN study to demonstrate that GA is feasible and useful for older adults treated intensively for AML, despite the acuity and complexity of the disease setting. The wide range of scores for individual GA measures support the role of using this tool to better categorize the heterogeneity of aging and to identify potentially unrecognized vulnerabilities that can impact treatment outcomes and survivorship. GA measures post induction highlight the impact of treatment on functional domains and quality of life and provide potential targets for interventions to improve them. These findings support further investigation of GA in the context of clinical trials for AML to inform risk prediction and tailored supportive care.27
This study is consistent with others demonstrating the feasibility of incorporating GA strategies into multisite trials in the non-AML setting.12,28 Our results support the generalizability of incorporating GA into the evaluation of older adults receiving intensive therapy for AML, previously shown in a single institution trial.5 Assessments were well received by both patients and research staff, and the time required was considered acceptable, with no reported concerns about participant or staff burden. Compared to implementation of the same GA in a predominantly solid tumor population, the median time required was slightly longer (30 vs. 22 minutes) with a small increase in the proportion of AML patients requiring some assistance (77% vs. 87%) and with similar high degrees of patient satisfaction.12
This pilot also identifies opportunities to enhance efficiency, further minimize potential burden, and maximize value. First, training on measure administration using online educational modules that are now available for NCTN trials could enhance efficiency for sites when opening trials that include GA. Second, the follow-up GA can be further streamlined by tailoring assessments to those measures demonstrating change over time. Use of tablets for direct data capture can further minimize missing data and staff time.29,30 Attrition is a particular challenge in a dynamic patient population with high morbidity. While some attrition is unavoidable, collecting the GA/patient-reported outcome data as an integrated part of the treatment trial, rather than as a companion study requiring separate consent, can maximize participation and further minimize opportunities for missed assessments.
Similar to studies using GA in other settings, we demonstrate that older adults deemed fit for intensive chemotherapy have significant heterogeneity in physical function, cognition, comorbidity, emotional health, rates of polypharmacy, nutritional status, social activities and social support.31 This observation provides proof-of-principle that incorporating GA into AML therapy can help characterize the heterogeneity of aging in this context. Compared to results from an observational study of older adults with predominantly solid tumor malignancy using the same GA, patients in the current study had slightly higher levels of self-reported physical function at baseline with higher levels of psychological distress.15 However, similar to results among solid tumor patients, more than half had impaired objectively measured physical function (Timed Up and Go score >10 seconds) reinforcing the importance of capturing objective measures to characterize vulnerabilities.6,15,32
Our study adds to the literature by using GA and a global health-related quality-of-life measure concurrently as outcomes after intensive induction therapy to help characterize treatment tolerance. The impact of treatment on quality of life and independence is critical to inform decision-making and targeted supportive care for older AML patients. Yet only limited data have been collected in clinical trials for outcomes such as physical function, cognition, and psychological health. In a review of over 1000 clinical trials in hematologic malignancies, less than 10% collected endpoints related to quality of life, physical function or health care utilization.33
In this study, participants evaluated for post-remission therapy reported increased physical limitations, worse mental health, decreased social activities and experienced decline in nutritional parameters measured by GA. Short-term decline in physical function after intensive induction for older patients with AML has been reported previously34 although functional resilience may occur among longer term survivors.35 The relationship between receipt of induction therapy on emotional health is less clear, with some studies showing lower levels of depressive symptoms and distress after treatment among older survivors.34,35 Discrepant findings may relate to use of different measures versus varied timing of assessments or differences in patient populations.36 Importantly, declines in physical function, mental health, and nutritional status can be addressed with supportive care if recognized. Interventions targeting maintenance of physical function during and after treatment are a particularly promising area of research.37-39 Despite measured decrements in function by GA, global quality of life (QOL) scores were improved. Improved overall QOL after induction is consistent with results of other studies suggesting that achieving remission with improvements in symptoms has a positive effect on quality of life.34,35,40-43 Thus, information gained from validated GA measures is complementary to global health-related quality-of-life assessment and provides insight into specific domains such as function and nutritional status which may otherwise be missed.
This study provides additional information to support testing the role of GA to enhance risk prediction in the context of intensive AML therapy. Identifying characteristics of patients who are more or less likely to tolerate and benefit from intensive chemotherapy is critical for both treatment decision-making, trial design and supportive care.44 A single institution study showed that physical performance and cognition (measured by the Short Physical Performance Battery and Modified Mental State Exam) are associated with worse survival among older adults treated intensively, but confirmatory multisite studies are lacking. The GA measures included in this pilot study addressed the same domains but were less comprehensive to optimize implementation (i.e. use of the Timed Up and Go and a brief 6-item cognitive screen). Our exploratory analysis was not powered to demonstrate significant associations between GA measures and survival. However, promising candidate characteristics based on estimates of effect include measures of self-reported physical function and history of falls. Functional measures have been associated with survival in hematologic malignancies in many but not all studies investigating this domain.6,7,31,45 Data collected in this study can be used to support development of fully powered multisite studies investigating individual risk factors and risk profiles derived from GA. Larger studies will be needed to determine if more comprehensive measures of cognitive or physical function are needed to discriminate risk. Finally, the observed relationship between baseline nausea and vomiting and survival in this cohort may relate to the impact of symptom burden on outcomes which has been suggested in other studies although warrants confirmation in larger studies.7,8
This study has several strengths. This is the first multisite NCTN study to investigate feasibility and provide preliminary evidence for the utility of GA in the context of intensively treated patients with AML. All patients received the same therapy, limiting treatment confounding present in other studies. Collection of both GA and global quality-of-life measures provides an opportunity to better understand how to use these tools most effectively in future studies and highlights that they are not interchangeable. Finally, this study included a relatively high proportion of patients aged 70 or older (41%) which is uncommon in the literature.
This study also has limitations. The small sample size limits power to investigate the association of GA measures and treatment outcomes including overall survival and treatment-related mortality. Findings related to survival are exploratory and hypothesis generating. The sample size is inadequate to investigate which combinations of risk factors might optimally characterize fit or unfit individuals. However, the goal of this pilot was to provide preliminary data to support the feasibility of this line of investigation in larger studies. The optional nature of this ancillary study may have introduced some bias. Patients who opted not to enroll on the study may have been different in some way from those who enrolled. However, when comparing patient characteristics from those enrolled on the ancillary study to the entire clinical trial population, we see no significant differences in measurable baseline characteristics. Missing data is another limitation, with eight consented participants missing assessments without documentation of reason for missed visits. It is reasonable to expect that those who were missing data may have differed from those who provided data. In particular, if those 6 patients missing follow-up GA data had experienced worse treatment tolerance the negative impact on functional outcomes and quality of life may be under-represented. The optional nature of the study may have contributed to missing data. Finally, patients enrolled on this study were considered fit enough for intensive chemotherapy and feasibility results may differ among populations perceived to be less fit. For example, it would be expected that the time to administer this GA among frail patients may be longer regardless of the treatment setting. Results from the GA ancillary study included in Alliance 11002 clinical trial () which enrolled less fit patients will provide this information in the future and can help identify strategies to further streamline GA for frail populations.46 Similarly, feasibility results may differ in community practices compared to academic institutions. However, these results provide a framework to estimate resource/time allocation for patients and staff to conduct GA in the treatment evaluation of older adults, which is now guideline recommended.47 Use of a primarily self-administered GA enhances the usability in varied settings and minimizes staff resource requirements.
In summary we have demonstrated the feasibility of performing GA in the context of an NCTN AML intensive treatment trial for older adults. Our results provide evidence to support further investigation of the use of GA in this setting and highlight the role for use of post-treatment GA to understand the impact of treatment on multiple domains of function. Larger studies can confirm these findings and inform on clinically meaningful changes for older patients. We also highlight opportunities to streamline efficiency when integrating GA into multisite trials. Next steps include validation of the utility of GA to inform risk prediction in larger multisite studies, collection of GA measures in treatment trials to characterize survivorship, and testing of interventions targeting GA-identified vulnerabilities to decrease morbidity.
Acknowledgements:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers UG1CA189823, U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180833, U10CA180836, U10CA180850, U10CA180857, and U10CA180867. Dr. Klepin was funded by the American Society of Hematology (ASH)–Association of Specialty Professors (ASP) Junior Faculty ScholarAward in Clinical and Translational Research (supported by ASH, Atlantic Philanthropies, John A. Hartford Foundation, Association of Specialty Professors), the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG-021332), Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by National Institute on Aging, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies), and the Gabrielle’s Angel Foundation for Cancer Research. Also supported in part by funds from Bayer Healthcare/Berlex and Millennium for CALGB 11001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement:
The authors have no conflicts of interest to report.
ClinicalTrials.gov Identifier: (CALGB 11001)
This study was presented in part at the Annual Meeting of the American Society of Clinical Oncology 2014, Chicago Illinois
References
- 1.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. [DOI] [PubMed] [Google Scholar]
- 3.Lowenberg B, Ossenkoppele GJ, van PW, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. NEnglJMed. 2009;361(13):1235–1248. [DOI] [PubMed] [Google Scholar]
- 4.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. NEnglJMed. 1994;331(14):896–903. [DOI] [PubMed] [Google Scholar]
- 5.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. JAmGeriatrSoc. 2011;59(10):1837–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman AE, Motyckova G, Fega KR, et al. Geriatric assessment in older patients with acute myeloid leukemia: a retrospective study of associated treatment and outcomes. Leukemia research. 2013;37(9):998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliot K, Tooze JA, Geller R, et al. The prognostic importance of polypharmacy in older adults treated for acute myelogenous leukemia (AML). Leukemia research. 2014;38(10):1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klepin HD, Pitcher BN, Ballman KV, et al. Comorbidity, Chemotherapy Toxicity, and Outcomes Among Older Women Receiving Adjuvant Chemotherapy for Breast Cancer on a Clinical Trial: CALGB 49907 and CALGB 361004 (Alliance). Journal of oncology practice / American Society of Clinical Oncology. 2014;10(5):e285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etienne A, Esterni B, Charbonnier A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109(7):1376–1383. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uy GL, Mandrekar SJ, Laumann K, et al. A phase 2 study incorporating sorafenib into the chemotherapy for older adults with FLT3-mutated acute myeloid leukemia: CALGB 11001. Blood Advances. 2017;1(5):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veit C, Ware JJ. The structure of psychological distress and well-being in general populations. In. Journal of Consulting and Clinical Psychology. Vol 511983:730–742. [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savic A, Kvrgic V, Rajic N, et al. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leukemia research. 2012;36(4):479–482. [DOI] [PubMed] [Google Scholar]
- 21.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. BrJHaematol. 2007;136(4):624–627. [DOI] [PubMed] [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. JAmGeriatrSoc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 23.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. AmJPsychiatry. 1983;140(6):734–739. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNatlCancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 25.Kornblith AB, Herndon JE, Silverman LR, et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a Cancer and Leukemia Group B study. JClinOncol. 2002;20(10):2441–2452. [DOI] [PubMed] [Google Scholar]
- 26.Therneau TM, Grambsch PM, eds. Modeling Survival Data: Extending the Cox Model. 1 ed. New York: Springer-Verland New York; 2000. Statistics for Biology and Health. [Google Scholar]
- 27.Hamaker ME, Wildes TM, Rostoft S. Time to Stop Saying Geriatric Assessment Is Too Time Consuming. J Clin Oncol. 2017;35(25):2871–2874. [DOI] [PubMed] [Google Scholar]
- 28.Corre R, Greillier L, Le Caer H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol. 2016;34(13):1476–1483. [DOI] [PubMed] [Google Scholar]
- 29.Hurria A, Akiba C, Kim J, et al. Reliability, Validity, and Feasibility of a Computer-Based Geriatric Assessment for Older Adults With Cancer. Journal of oncology practice / American Society of Clinical Oncology. 2016;12(12):e1025–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCleary NJ, Wigler D, Berry D, et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. The oncologist. 2013;18(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leukemia research. 2014;38(3):275–283. [DOI] [PubMed] [Google Scholar]
- 32.Goede V, Bahlo J, Chataline V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leukemia & lymphoma. 2016;57(4):789–796. [DOI] [PubMed] [Google Scholar]
- 33.Hamaker ME, Stauder R, van Munster BC. On-going clinical trials for elderly patients with a hematological malignancy: are we addressing the right end points? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(3):675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klepin HD, Tooze JA, Pardee TS, et al. Effect of Intensive Chemotherapy on Physical, Cognitive, and Emotional Health of Older Adults with Acute Myeloid Leukemia. J Am Geriatr Soc. 2016;64(10):1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alibhai SM, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 36.Pergolotti M, Langer MM, Deal AM, Muss HB, Nyrop K, Williams G. Mental status evaluation in older adults with cancer: Development of the Mental Health Index-13. J Geriatr Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klepin HD, Danhauer SC, Tooze JA, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. J Geriatr Oncol. 2011;2(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alibhai SM, O'Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leukemia research. 2012;36(10):1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alibhai SM, O'Neill S, Fisher-Schlombs K, et al. A pilot phase II RCT of a home-based exercise intervention for survivors of AML. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014;22(4):881–889. [DOI] [PubMed] [Google Scholar]
- 40.Alibhai SM, Leach M, Gupta V, et al. Quality of life beyond 6 months after diagnosis in older adults with acute myeloid leukemia. Crit RevOncolHematol. 2009;69(2):168–174. [DOI] [PubMed] [Google Scholar]
- 41.Alibhai SM, Leach M, Kermalli H, et al. The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol. 2007;64(1):19–30. [DOI] [PubMed] [Google Scholar]
- 42.Tinsley SM, Sutton SK, Thapa R, Lancet J, McMillan SC. Treatment Choices: A Quality of Life Comparison in Acute Myeloid Leukemia and High-risk Myelodysplastic Syndrome. Clinical lymphoma, myeloma & leukemia. 2017;17s:S75–s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timilshina N, Breunis H, Tomlinson GA, et al. Long-term recovery of quality of life and physical function over three years in adult survivors of acute myeloid leukemia after intensive chemotherapy. Leukemia. 2018. [DOI] [PubMed] [Google Scholar]
- 44.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2017. [DOI] [PubMed] [Google Scholar]
- 45.Timilshina N, Breunis H, Tomlinson G, Brandwein J, Alibhai SM. Do quality of life, physical function, or the Wheatley index at diagnosis predict 1-year mortality with intensive chemotherapy in older acute myeloid leukemia patients? Leukemia research. 2016;47:142–148. [DOI] [PubMed] [Google Scholar]
- 46.Roboz GJ, Mandrekar SJ, Desai P, et al. Randomized trial of 10 days of decitabine +/− bortezomib in untreated older patients with AML: CALGB 11002 (Alliance). Blood Adv. 2018;2(24):3608–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018:Jco2018788687. [DOI] [PMC free article] [PubMed] [Google Scholar]