Abstract
Maternal consumption of ethanol during pregnancy is known to increase the offspring’s risk for developing alcohol use disorders and associated behavioral disturbances. Studies in adolescent and adult animals suggest the involvement of neuroimmune and neurochemical systems in the brain that control these behaviors. To understand the origin of these effects during early developmental stages, we examined in the embryo and neonate the effects of maternal intraoral administration of ethanol (2 g/kg/day) from embryonic day 10 (E10) to E15 on the inflammatory chemokine C-C motif ligand 2 (CCL2) and its receptor CCR2 in a specific, dense population of neurons in the lateral hypothalamus (LH), where they are closely related to an orexigenic neuropeptide, melanin-concentrating hormone (MCH), known to promote ethanol consumption and related behaviors. We found that prenatal ethanol exposure increases the expression and density of CCL2 and CCR2 cells along with MCH neurons in the LH and the colocalization of CCL2 with MCH. We also discovered that these effects are sexually dimorphic, consistently stronger in female embryos, and are blocked by maternal administration of a CCL2 antibody (1 and 5 μg/day, i.p., E10-E15) that neutralizes endogenous CCL2 and of a CCR2 antagonist INCB3344 (1 mg/day, i.p., E10-E15) that blocks CCL2’s main receptor. These results, which in the embryo anatomically and functionally link the CCL2/CCR2 system to MCH neurons in the LH, suggest an important role for this neuroimmune system in mediating ethanol’s sexually dimorphic, stimulatory effect on MCH neurons that may promote higher level of alcohol consumption described in females.
Keywords: prenatal ethanol, embryo, hypothalamus, CCL2, CCR2, MCH
INTRODUCTION
Clinical studies have consistently demonstrated that maternal alcohol consumption during pregnancy increases the risk of alcohol use disorders (AUD) and related hazardous behaviors in the offspring (Baer et al., 2003; Alati et al., 2006; Malone et al., 2010), with these effects sometimes seen more strongly in females (Hommer et al., 2001; Squeglia et al., 2012; Alfonso-Loeches et al., 2013; Pascual et al., 2015). Animal models similarly support the positive relation between maternal consumption of moderate alcohol during pregnancy and the increased risk for alcohol use and abuse (Chotro et al., 2007; Youngentob and Glendinning, 2009; Chang et al., 2015; Chang et al., 2018). Persistent alterations in the neuroimmune systems induced by maternal ethanol exposure have been linked to these long-lasting behavioral outcomes, with ethanol intake found to stimulate inflammatory factors and cause perturbations in neuroimmune signaling that can invoke dysfunctional drinking and related behaviors in both rats and humans (He and Crews, 2008; Donzis and Tronson, 2014; Kane and Drew, 2016; Poon and Leibowitz, 2016; Abrahao et al., 2017; Crews et al., 2017; Roberto et al., 2017). With much of the existing literature focusing on post-weaning, adolescent and adult animals, there is little information concerning ethanol’s effects on neuroimmune systems in the embryo and how these in turn may impact the development of neurochemical systems that modulate behavior. Further, with animal studies using predominantly male subjects, there is little understanding of neural mechanisms underlying sex differences in brain development that may contribute to stronger behavioral disturbances induced by early ethanol exposure in females (Hommer et al., 2001; Squeglia et al., 2012; Alfonso-Loeches et al., 2013; Pascual et al., 2015; Chang et al., 2018).
To investigate these questions, we focused this study specifically on neuroimmune and neuropeptide systems in the lateral hypothalamus (LH) which may contribute to AUD. We examined neurons expressing the orexigenic peptide, melanin-concentrating hormone (MCH), which are particularly dense in the LH (Bittencourt et al., 1992). This neuropeptide is found to be strongly stimulated by low-to-moderate ethanol doses administered in utero as well as during adolescence and adulthood (Morganstern et al., 2010; Chang et al., 2015; Chang et al., 2018), and it is positively linked to ethanol consumption and behaviors such as anxiety associated with AUD (Duncan et al., 2005; Gonzalez-Burgos et al., 2006; Cippitelli et al., 2010; Morganstern et al., 2010). Of particular interest is that a large proportion of these MCH neurons in the adolescent and adult LH are found to co-localize with the inflammatory chemokine, C-C motif ligand 2 (CCL2), and its receptor CCR2 (Banisadr et al., 2005b; Banisadr et al., 2005c; Chang et al., 2015; Chang et al., 2018). Both CCL2 and CCR2 in different brain areas are shown to be stimulated by ethanol (He and Crews, 2008; Chang et al., 2015; Drew et al., 2015; Xu et al., 2016; Harper et al., 2017; Chang et al., 2018; Zhang et al., 2018), and they in turn are found to affect alcohol-associated behaviors (Breese et al., 2008; Valenta and Gonzales, 2016; Bray et al., 2018). Thus, with the CCL2/CCR2 system shown to be anatomically linked to MCH neurons in adolescent and adult rodents, we are encouraged to investigate this neuroimmune-neuropeptide relationship during embryonic development under conditions of ethanol exposure in utero.
Using a paradigm involving maternal intraoral administration of ethanol at a moderate dose (2 g/kg/day) during a period of peak neurogenesis from embryonic day 10 (E10) to E15 (Chang et al., 2015; Chang et al., 2018), we recently demonstrated in adolescent offspring that expression of CCL2, CCR2 and MCH mRNA and the density of CCL2-, CCR2- and MCH-immunoreactive and co-labeled neurons are increased in the LH by prenatal ethanol exposure, with little change in amniotic chemokine levels, and that these effects in adolescents occur in a sexually dimorphic manner, with females exhibiting a greater response to ethanol than males. Thus, with limited information regarding the mechanisms underlying ethanol’s effects in the embryo that may persist into adolescence, we investigated here the developmental origins of these ethanol-induced changes in the CCL2/CCR2 system and its relationship to MCH neurons in the LH. Our results here demonstrate that prenatal ethanol exposure profoundly influences the neuroimmune and neuropeptide systems in the LH early in embryonic development, and they reveal consistently stronger effects in female embryos, offering an explanation for the sexually dimorphic, neurobiological and behavioral consequences of prenatal ethanol that persist into adolescence.
METHODS AND PROCEDURES
All procedures were conducted in a fully accredited AAALAC facility (22°C, 12:12-h light-dark cycle with lights off at 8 am), in accordance with protocols approved by The Rockefeller University Animal Care and Use Committee and consistent with the NIH Guide to the Care and Use of Laboratory Animals.
Animals
Time-pregnant, Sprague-Dawley rats (220–240g) (Charles River Breeding Laboratories, Hartford, CT) arrived at the facility on embryonic day 5 (E5) and were acclimated to laboratory conditions until E10, at which time experiments began as described in detail below. In all experiments, rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO) and filtered water were available ad libitum. As described in the Experimental Design section below, female and male embryos were sacrificed before birth at E19, the age used in our prior study of the embryo (Chang et al., 2019) when MCH neurons in the LH first exhibit an adult-like pattern (Brischoux et al., 2001). For experiments conducted in neonatal offspring, the litters on postnatal day 0 (P0) were culled to n = 8, and both female and male offspring were sacrificed on P2, P7 or P15 when their MCH neurons are sufficiently dense to characterize their relationship with the local CCL2 neurons. In all experiments when both sexes were tested, 1 male and 1 female embryo or pup were taken from each litter while 1 female embryo or pup was taken from each litter when only females were tested, with the number of rats/sex/group (n = 6–7) equal to the number of litters/group for all experiments.
Maternal administration of ethanol
Pregnant rats (n = 6–7/experiment) were intraorally administered, from E10-E15 when MCH neurons develop in the hypothalamus (Brischoux et al., 2001), either a 2 g/kg/day ethanol solution (30% v/v) or a control solution of maltose-dextrin made isocaloric to the ethanol solution (Chang et al., 2012), with an additional group of pregnant rats assigned as Untreated controls. The daily dose of ethanol was split in half with all rats gavaged twice daily, with the first gavage occurring 2 h after start of the dark cycle and the second gavage occurring 7 h later. In blood collected from the tail vein at 2 h after the first ethanol gavage on E11, blood ethanol concentration (BEC) was measured using Analox GM7 Alcohol Analyzer (Lunenburg, MA, USA) and was elevated to ~80 mg/dL, consistent with previous reports (Qiang et al., 2002; Chang et al., 2012). There were no differences between the Untreated, Control, and Ethanol-treated groups, in terms of the dam’s body weight (238–256 g) and chow intake (60–80 kcal/day) and of the size (9–13) and body weight (5.5–7.0 g) of their litters, with no spontaneous abortions.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to measure the gene expression of CCL2, CCR2, and MCH in the LH of E19 embryo after maternal administration of ethanol or the CCR2 antagonist INCB3344. The E19 embryos were sacrificed, their tails were collected for genotyping to determine sex, their brains were removed, and the LH was dissected as described (Chang et al., 2018). Total RNA was then extracted from each microdissected sample, cDNA was synthesized, and qRT-PCR was performed as previously described (Barson et al., 2009; Chang et al., 2012). The primers, designed with ABI Primer Express Version 3.0 software from published sequences, were: (1) cyclophilin: 5′-AATATGATCAAGCATTGGCTGATC-3′ (forward) and 5′-TTGTGC TTTTCGGTATAGTGCTTT-3′ (reverse); (2) CCL2: 5’-GTGCTGTCTCAGCCAGATGCAGTT −3’ (forward) and 5’-AGTTCTCCAGCCGACTCATTGGG-3’ (reverse); (3) CCR2: 5′-TACCTGTTCAACCTGGCCATCT-3′ (forward) and 5′-AGACCCACTCATTTGCAGCAT-3′ (reverse); and (4) MCH: 5′-CAAACAGGATGGCGAAGATGA-3’ (forward) and 5′-AGGCTTTCCCCATCCTGAAT-3′ (reverse). Concentration of the cyclophilin, CCL2 and CCR2 primers was 200 nM and of the MCH primer was 50 nM. As previously described in our publications (Chang et al., 2008; Barson et al., 2013) and others (West et al., 2017; Adachi et al., 2018), mRNA levels of the target gene in each rat were normalized within subject relative to mRNA levels of an internal housekeeping gene, cyclophilin, in the same sample, and this ratio of target gene expression to housekeeping gene expression was calculated in each rat using the standard delta-delta Ct method. The ANOVA was then run on this ratio calculated for each subject, with the effect of ethanol on target gene expression determined by comparing the average of the ratio in the experimental groups to the control groups, as well as the control groups to each other. These average ratio scores are represented in Tables 2 and 3 as “percent change” relative to the Untreated control group.
Table 2:
Prenatal ethanol exposure (2 g/kg/day, E10-E15) compared to control groups (n = 7/group) significantly increases the lateral hypothalamus the mRNA expression (measured using qRT-PCR) of CCR2 and MCH in both female and male embryos at E19, with the females showing a significantly greater effect, and of CCL2 mRNA in female embryos but not in male embryos. The data are represented here as mRNA percent change, based on average ratio scores of ethanol-treated embryos compared to the Untreated and Control groups. Data are mean ± SEM. *p < 0.05 versus control group. #, p < 0.05 versus males.
| mRNA expression | Female | Male | ||||
|---|---|---|---|---|---|---|
| Untreated | Control | Ethanol | Untreated | Control | Ethanol | |
| CCL2 | 100 ± 9.78 | 105 ± 5.07 | 173 ± 9.7*# | 100 ± 9.78 | 103 ± 10.01 | 88 ± 9.98 |
| CCR2 | 100 ± 7.19 | 107 ± 5.04 | 223 ± 4.71*# | 100 ± 3.42 | 112 ± 5.08 | 139 ± 8.38* |
| MCH | 100 ± 8.23 | 95 ± 7.27 | 191 ± 8.64*# | 100 ± 7.09 | 102 ± 8.57 | 134 ± 7.14* |
Table 3:
Maternal administration of a CCR2 antagonist INCB3344 (1 mg/kg/day) during the period of prenatal ethanol exposure (2 g/kg/day, E10-E15) prevents the stimulatory effect of ethanol on CCL2, CCR2, and MCH mRNA in the LH of female E19 embryos (n = 7/group) as measured using qRT-PCR. The data are represented here as mRNA percent change, based on average ratio scores of ethanol-treated embryos compared to the Control group. Data are mean ± SEM. *p < 0.05 versus control group.
| mRNA expression |
Control | Ethanol | Ethanol+INCB3344 | Control+INCB3344 |
|---|---|---|---|---|
| CCL2 | 100 ± 5.08 | 175 ± 6.98* | 118 ± 7.27 | 108 ± 4.06 |
| CCR2 | 100 ± 4.11 | 226 ± 5.81* | 107 ± 3.79 | 102 ± 4.61 |
| MCH | 100 ± 9.02 | 198 ± 8.99* | 111 ± 3.25 | 117 ± 3.41 |
Single- and double-labeling immunofluorescence histochemistry
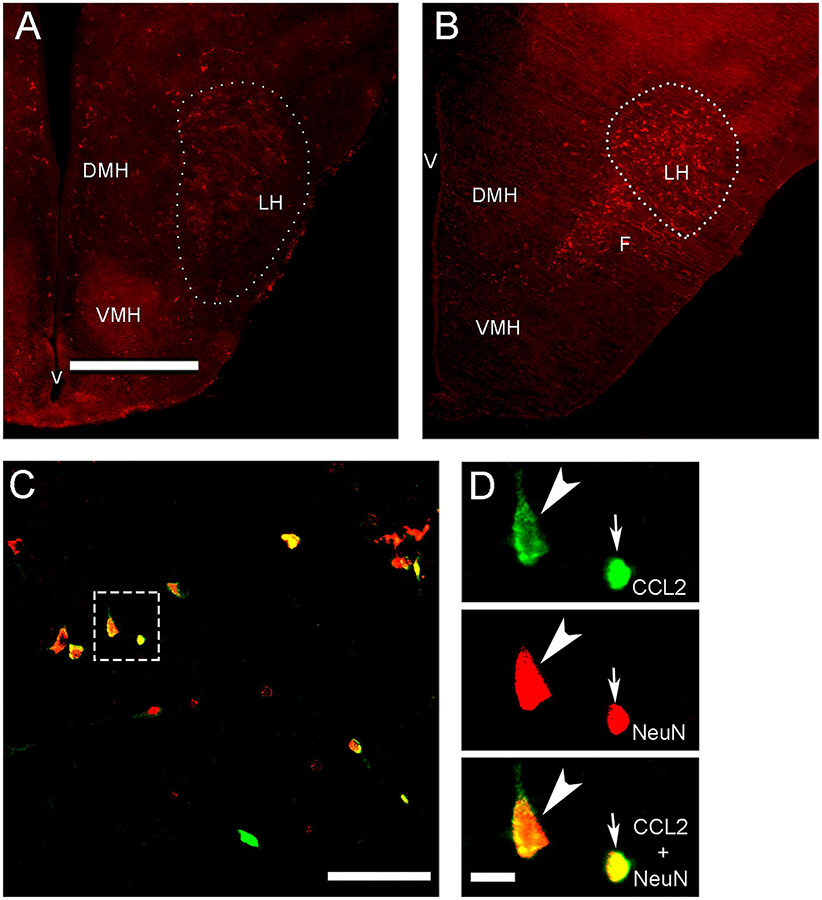
Immunofluorescence histochemistry (IF) as previously described (Chang et al., 2013, 2015) was used to characterize the CCL2-immunoreactive (CCL2+) and MCH-immunoreactive (MCH+) cells and their distribution pattern, to determine if they are neurons as shown in adolescent rats (Chang et al., 2018), and to quantify their density in the embryo at E19, neonate at P2, and postnatal offspring at P7 and P15. At these ages, we found both the CCL2+ and MCH+ cells to be densely concentrated in the same area of the LH, as illustrated by the MCH+ cells at E19 (Fig. 1A) and P2 (Fig. 1B). Further, almost all of these CCL2+ cells similar to MCH+ cells were found to be neurons, exhibiting co-labeling of the neuronal marker NeuN (Fig. 1C) as demonstrated in weanling and adolescent rats (Chang et al., 2015; Chang et al., 2018) but not of the markers of astrocytes, microglia and oligodendrocytes (Banisadr et al., 2005b; Chang et al., 2018). While all CCL2+ cells in the LH of the embryo and P2 neonate were small and similar in size, we detected in postnatal offspring starting at P5 and more clearly at P7 two distinct subtypes of CCL2+ neurons, larger ones with short proximal processes that colocalize MCH and smaller ones with minimal processes that do not, similar to that described in adolescent rats (Chang et al., 2018). To analyze these CCL2+ and MCH+ neurons using IF, the E19 embryos were killed by perfusing their dams intracardially with 0.9% normal saline followed by 4% paraformaldehyde in phosphate buffer (PB), and their brains were removed and post-fixed for 4 h in the same fixative at 4° C, cryo-protected in 25% sucrose at 4° C for 72–96 h, and then frozen and stored at −80° C. The P2 neonates and P7 and P15 offspring were directly perfused, and their brains were collected and processed as with the embryos.
Figure 1:
Photomicrographs illustrate in the anterior-posterior level of the lateral hypothalamus (LH) the MCH+ neurons (A, B outlined by dotted line) and CCL2+ neurons (C, D located in box). A. Image shows MCH+ neurons at 2.5x in E19 embryo brain at coronal plate 12 to 13 at E20 embryo brain. B. The MCH+ neurons at 2.5x are shown in P2 neonatal brain at coronal plates A2.9 to 2.3 mm at P10. C. This image illustrates at 20x both large and small CCL2+ neurons (green) in P7 brain exposed in utero to a moderate dose of ethanol from E10-E15 showing that almost all (~95%) colabel NeuN (red) and appear yellow or red/yellow, similar to results obtained in adolescent offspring (Chang et al., 2018). D. CCL2+ neurons at 40x in box from C, showing both the large (arrowhead) and small (arrow) CCL2+ single-labeled neurons (green), NeuN+ single-labeled neurons (red), and CCL2+/NeuN+ double-labeled neurons (yellow). Abbreviations: DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; F, fornix; LH, lateral hypothalamus; V, ventricle. Scale bar: 500 μm in A and B, 100 μm in C and D.
Brains were cut with cryostat in serial coronal sections at 30 μm, a total 18–20 sections (approximately every other one) were taken for immunofluorescence staining in the LH, and these free-floating sections were processed with primary antibodies and their corresponding secondary antibodies listed in Table 1. Sections were viewed, and fluorescence images were captured using a Zeiss fluorescence microscope with MetaVue software. 8–12 of these sections were collected in each animal to provide images of the LH along the same anterior-posterior level. The fluorescence images of the entire LH for quantitation were captured using a 10x objective, while the images for Figs. 1A and 1B were captured with a 2.5x objective to illustrate and outline the LH area in the embryo and neonate. For analysis in each animal, images of the LH were taken at the same anterior-posterior level of coronal plates 12 to 13 in E20 for the E19 embryo brains (Altman and Bayer, 1995) and coronal plates A2.9 to 2.3 mm in P10 for the P2, P7 and P15 postnatal brains (Sherwood, 1970). The density of single immunofluorescent cells in this area was then quantified using Image-Pro Plus software (Version 4.5; Media Cybernetics), as previously described (Chang et al., 2013, 2015). Only intact cells with an area of 100–200 μm2 were counted with the 10x objective, and the population density was used to determine the density reported as cells/μm2. The measure of cell density, defined by the total cell number/area of LH, was calculated by Image-plus software, which after revealing no change across groups in the size of the LH area indicates that a change in cell density reflects a change in total cell number. To examine the small and large CCL2+ cells in the same area, the image was further captured with 20x and 40x objectives and analyzed. Those CCL2+ cells with an area of ≤100 μm2 at 20x or 100–200 μm2 at 40x are defined as small cells, while those cells with an area of ≥200 μm2 at 20x or ≥400 μm2 at 40x are defined as large cells. The density of small and large CCL2 cells was quantified with 20x images and reported as cells/μm2.
Table 1:
Antibodies used for single- and double-labeled immunofluorescence histochemistry.
| Primary antibody | Dilution | Vendor | Secondary antibody | Dilution | Vendor |
|---|---|---|---|---|---|
| Single-labeling | |||||
| Rabbit anti-CCL2 | 1:100 | Cat#:orb36895, Biorbyt | Bio-Horse anti-Rabbit IgG +TSA Fluorescin | 1:100 | Vector |
| Rabbit anti-MCH | 1:200 | Cat#:H-070–47, Phoenix Pharmaceuticals | Cy3-Donkey anti-Rabbit IgG | 1:100 | Jackson ImmunoResearch Laboratories |
| Double-labeling | |||||
| Rabbit anti-CCL2 | 1:200 | Cat#:orb36895, Biorbyt | Bio-Horse anti-Rabbit IgG + TSA Fluorescein | 1:100 | Vector |
| Goat anti-pro-MCH | 1:200 | Cat#:sc-14509, Santa Cruz Biotechnology | Cy3-Donkey anti-Goat IgG | 1:100 | Jackson ImmunoResearch Laboratories |
Double-labeling IF was used to examine the co-existence of CCL2 with MCH in the LH. This was performed using a combination of primary antibodies and their corresponding secondary antibodies as listed in Table 1, based on our previous procedures (Chang et al., 2013, 2015). For analysis of the double-labeled CCL2+ cells with MCH, the images were captured by a Zeiss LSM 880 confocal microscope with 20x objective, and the double-labeled cells were further confirmed by Z-stack sectioning with a 40x oil-immersion lens, with the Z-stacks 30 μm thick and the step size of 0.44–0.50 μm for optimal stack collection and analysis. Double-labeled cells were counted in 20x images and are reported here as the percentage of total, single-labeled cells. In all analyses, the cells were counted only on one plane in each section, and only intact cells of a designated size were counted, thus eliminating any objects that were part of a cell or any small cell that might be an edge of a larger cell. The distance between the small and large cells was also measured, and in some cases it ranged from 10–30 μm when the cells were particularly close while in other cases it was up to 150 μm. To confirm that the small CCL2+ cells were not debris or edges of larger cells, DAPI was used to stain the nucleus, confocal Z-Stack sectioning (30μM thick and step size of 1.02μM) of the double-labeling DAPI and CCL2 at 20x was collected, 3D images were then reconstituted, and DAPI/CCL2 co-labeled cells were analyzed using Imaris image analysis software (Version 9.12, 2018). This analysis demonstrated that almost all of the small CCL2+ cells were labeled with DAPI, indicating that they were independent, intact cells.
Experimental Design and Statistical Analysis
Effect of prenatal ethanol exposure on CCL2, CCR2 and MCH mRNA in LH of the embryo.
To determine the effect of moderate prenatal ethanol exposure on mRNA levels of CCL2, CCR2 and MCH in the LH of the developing embryo at E19, the dams were either untreated (Untreated) or received daily intraoral infusions from E10-E15 of either 2 g/kg/day of ethanol (Ethanol) or an isocaloric maltose-dextrin control solution (Control). The gene expression of CCL2 and MCH as well as CCR2 in the LH of E19 embryos (n = 7/group/sex) was measured using qRT-PCR, and the data analysis was performed using a two-way ANOVA and paired t-test.
Effect of prenatal ethanol exposure on CCL2+ and MCH+ neurons in LH of the neonate.
The density of single-labeled CCL2+ and MCH+ neurons, both heavily concentrated in the LH, were examined using IF in female and male neonatal offspring at P2 (n = 7/group/sex) from Untreated, Control or Ethanol dams, and the data analysis was performed using a two-way ANOVA and paired t-test.
Effect of prenatal ethanol exposure on large and small CCL2+ single-labeled neurons and large CCL2+/MCH+ double-labeled neurons.
Density of the total population of CCL2+ neurons, of the specific large and small CCL2+ single-labeled neurons, and of the large CCL2+ neurons that co-label MCH were examined using IF in female and male postnatal offspring at P7 (n = 6/group/sex) from Untreated, Control or Ethanol dams, and the data analysis was performed using a two-way ANOVA and paired t-test.
Maternal CCL2 antibody and its effect on ethanol-induced changes in expression and density of CCL2+ and MCH+ neurons in the embryo and postnatal offspring.
Based on the stronger effects of prenatal ethanol exposure observed in females compared to males, we tested the CCL2 antibody here only in female offspring. With no studies to date involving administration of anti-CCL2 neutralizing antibody to pregnant rats and examining its impact on the offspring, we first tested the effect of CCL2 antibody injection in non-pregnant females to determine if it has any adverse effects on their daily food and water intake. Non-pregnant female rats (n = 4/group) received at 4 h after dark onset a single intraperitoneal (i.p.) injection of the vehicle (sterile water) or CCL2 antibody at 1 or 5 μg/kg, doses based on prior rat studies of pain management (Thacker et al., 2009; Bell et al., 2013). Analysis of their 24 h food and water intake using a one-way ANOVA showed no main effect of treatment on their food intake (F(2,18) = 4.05, p = 0.425; 62 ± 6.2 vs 69 ± 7.1 kcal/day) or water intake (F(2,18) = 3.814, p = 0.643; 22 ± 5 vs 18 ± 4 ml/day). Then, to determine the effect of maternal CCL2 antibody administration on the density of CCL2 and MCH cells in the female LH, we gave two sets of pregnant dams daily injections from E10-E15 of the CCL2 antibody (Ab) at one or two doses (1 and 5 μg/rat/day) 30 min after ethanol gavage, creating the following groups: Isocaloric Control + Vehicle (sterile water) (Control); Ethanol + Vehicle (Ethanol); Ethanol + 1 μg CCL2 Ab; Ethanol + 5 μg CCL2 Ab; Control + 1 μg CCL2 Ab; and Control + 5 μg CCL2 Ab. In Set 1, embryos at E19 (n = 7/group) were sacrificed, the two doses of CCL2 antibody were administered, and their effect on the density of CCL2+ and MCH+ in the LH was measured using single-label IF. In Set 2, postnatal offspring at P15 (n = 7/group) were sacrificed, one dose of the CCL2 antibody (1 ug/rat/day) was administered, and its effect on the density of MCH+ neurons was measured using single-label IF.
Maternal CCR2 antagonist and its effect on ethanol-induced changes in CCL2 and MCH mRNA and cell density of the embryo.
Again, with females exhibiting stronger effects of ethanol, we tested here only female embryos at E19 from 2 sets of pregnant rats given intraoral administration of ethanol (2 g/kg/day, E10-E15) or its isocaloric control and injected 30 minutes later with either INCB3344 (1 mg/kg/day, i.p.) or its vehicle. This paradigm yielded the following 4 groups/set: Isocaloric Control + Vehicle (Control); Ethanol + Vehicle (Ethanol); Ethanol + INCB3344; and Control + INCB3344. As indicated in our recent study (Chang et al., 2018), comparing the effect of INCB3344 vs Control revealed no effect on the dam’s food intake (t(6) = 1.25, p = 0.514, 70 ± 6 vs 65 ± 7 kcal/day, respectively), water intake (t(6) = 2.04, p = 0.712, 21 ± 4 vs 19 ± 2 ml/day, respectively), and body weight (t(6) = 0.99, p = 0.609, 444 ± 5.0 vs 459 ± 5.61 g), and on the offspring’s body weight at birth (t(6) = 2.36, p = 0.425, 8.9 ± 1.9 vs 6.9 ± 1.8 g), with no spontaneous abortions observed. The embryos from Set 1 (n = 7/group) were sacrificed and their brains analyzed using qRT-PCR for gene expression of CCL2, CCR2 and MCH in the LH, and those from Set 2 (n = 7/group) were examined for the density of CCL2+ and MCH+ neurons in the LH using single-label IF.
Statistical analysis.
All data were presented as mean ± SEM and were analyzed using SPSS (Version 24), for normality using Shapiro-Wilk test, and homogeneity of variance using Levene’s test. After finding all data sets to be normally distributed and to have equal variances, we then determined significance using ANOVA. Data in experiments using female and male offspring were analyzed using a two-way ANOVA, which tested within-subject main effects of prenatal ethanol exposure and their respective controls, between-subject main effects of sex, and the interactions between prenatal treatment and sex. A significant interaction was interpreted using simple main effect analyses to test the differences between females and males as well as differences within each sex. In experiments that tested females only, a one-way ANOVA was used to evaluate the effect of maternal administration of anti-CCL2 neutralizing antibody on CCL2+ and MCH+ single-labeled neurons in the LH and the effect of maternal CCR2 antagonist (INCB3344) administration on gene expression of CCL2, CCR2 and MCH and the density of CCL2+ and MCH+ neurons, followed by Sidak post-hoc test, with multiple comparisons corrected using Sidak Method. Paired t-tests were performed to directly compare between sexes the effects of maternal treatment vs control groups. All graphs were prepared using the GraphPad Prism software (Version 6).
RESULTS
Prenatal ethanol exposure increases the expression of CCL2, CCR2 and MCH in LH of female and male embryos
We first tested whether maternal intraoral administration of ethanol (from E10-E15) alters the gene expression of CCL2, CCR2 and MCH in the developing LH of the embryo at E19 and, if so, whether the effects of ethanol differ between the female and male embryos. Analysis via a two-way ANOVA revealed a significant main effect of maternal ethanol treatment on mRNA levels of both CCL2 (F(2,36) = 5.046, p = 0.012) and CCR2 (F(2,36) = 64.14, p = 0.000) and also of MCH (F(2,36) = 25.20, p = 0.000) in the LH, at 4 days after stopping in utero ethanol exposure (Table 2). In addition to a significant main effect of sex on CCL2 (F(1,36) = 9.873, p = 0.003) and CCR2 (F(1,36) = 37.81, p = 0.003) but not MCH (F(1,36) = 0.128, p = 0.722), it revealed a significant interaction between sex and maternal treatment for mRNA expression of CCL2 (F(2,36) = 9.224, p = 0.000) and CCR2 (F(2,36) = 9.224, p = 0.000) as well as MCH (F(2,36) = 4.803, p = 0.014). Simple main effect analyses of the data for the two control groups, Control and Untreated, showed no differences between the females and males in their mRNA levels of CCL2 (p = 0.284 for Control and p = 0.220 for Untreated), CCR2 (p = 0.376 for Control and p = 0.591 for Untreated), and MCH (p = 0.416 for Control and p = 0.208 for Untreated) and between the Control and Untreated groups for females for CCL2 (p = 0.978), CCR2 (p =00.872) and MCH (p = 0.985) and males for CCL2 (p = 0.996), CCR2 (p = 0.664) and MCH (p = 0.999) indicating that the intraoral infusion itself had little impact on gene expression in the embryo as previously shown in adolescent offspring (Chang et al., 2018). On the other hand, female and male embryos exposed to ethanol were clearly different, with the females having significantly higher mRNA levels of CCL2 (p = 0.000), CCR2 (p = 0.000), and MCH (p = 0.010) than the ethanol-exposed males (Table 2). Of particular note is the finding that ethanol exposure strongly increased CCL2 mRNA expression in the female embryos compared to the Control (p = 0.000) and Untreated (p = 0.000) groups, but had no impact on the male embryos compared to their Control (p = 0.869) or Untreated (p = 0.756) groups. Also, whereas maternal ethanol administration increased levels of CCR2 and MCH mRNA in both females (p = 0.000 and p = 0.000, respectively) and males (p = 0.009 and p = 0.047, respectively) compared to the Control and also for both female (p = 0.000 and p = 0.000, respectively) and males (p = 0.046 and p = 0.0501, respectively) compared to Untreated control groups, direct comparisons (via paired t-test) between the sexes exposed to ethanol showed that the ethanol-induced increase in CCR2 mRNA was significantly greater in females than males when compared to the Control (t(12) = 6.086, p = 0.000) and Untreated (t(12) = 5.869, p = 0.000) groups, similar to the ethanol-induced increase in MCH mRNA when compared to the Control (t(12) = 2.707, p = 0.019) and Untreated (t(12) = 3.066, p = 0.010) groups. Thus, in addition to confirming the results of our prior studies in adolescent offspring (Chang et al., 2015), these changes in the embryos 4 days after the ethanol treatment had stopped demonstrate that the stimulatory effects of in utero ethanol exposure on the CCL2/CCR2 system and MCH neurons in the developing LH are evident at this early age and even sexually dimorphic, with the effects on CCR2 and MCH significantly stronger in female than male embryos and the ethanol-induced increase in CCL2 mRNA not evident in male embryos.
Prenatal ethanol exposure increases the density of CCL2+ and MCH+ cells in female and male neonates
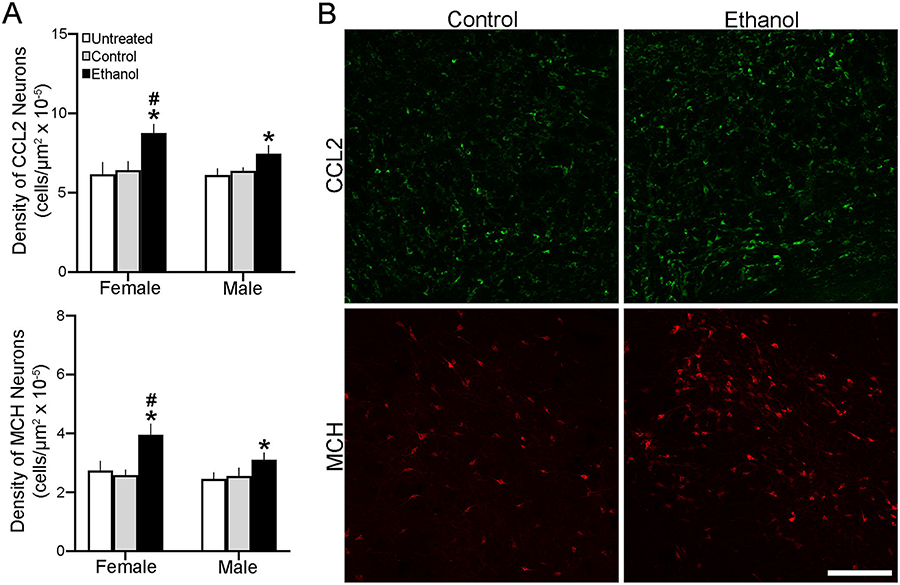
This experiment examined the effects of maternal ethanol administration on the density of CCL2+ and MCH+ neurons in the developing LH of female and male neonatal offspring, at P2 when these (but not CCR2+) neurons could be detected using IF. As with gene expression, analysis via a two-way ANOVA of the density of single-labeled neurons revealed a significant main effect of in utero ethanol exposure on the density of both CCL2+ (F(2,36) = 54.203, p = 0.000) and MCH+ (F(2,36) = 53.37, p = 0.000) cells in the LH compared to the Control group (Fig. 2A), as illustrated in the photomicrographs (Fig. 2B). It also showed a significant main effect of sex on the density of CCL2+ (F(1,36) = 7.671, p = 0.009) and MCH+ (F(1,36) = 19.502, p = 0.000) cells and a significant interaction between sex and maternal ethanol treatment for CCL2+ (F(2,36) = 6.284, p = 0.044) and MCH+ (F(2,36) = 7.656, p = 0.002) cells. While simple main effect analyses revealed no difference between female and male control groups in their density of CCL2+ (p = 0.867 for Control and p = 0.893 for Untreated) and MCH+ (p = 0.863 for Control and p = 0.070 for Untreated) cells, ethanol-exposed females had a significantly higher density of both the CCL2+ (p = 0.000) and MCH+ (p = 0.000) cells in the LH compared to males (Fig. 2A). While ethanol compared to control groups increased the density of CCL2+ cells in both females (p = 0.000 for Control and p = 0.000 for Untreated) and males (p = 0.002 for Control and p = 0.000 for Untreated) and of MCH+ cells in both females (p = 0.000 for Control and p = 0.000 for Untreated) and males (p = 0.020 for Control and p = 0.000 for Untreated), direct comparisons (via paired t-test) between sexes showed this stimulatory effect of ethanol to be significantly greater in females than males for both CCL2+ (t(12) = 3.590, p = 0.004 compared to Control and t(12) = 6.821, p = 0.01 compared to Untreated) and MCH+ (t(12) = 4.606, p = 0.001 compared to Control and t(12) = 2.274 p = 0.007 compared to Untreated) cells. These analyses of cell density in the neonate at P2, consistent with the changes in gene expression in the embryo at E19, demonstrate that prenatal ethanol exposure stimulates CCL2+ and MCH+ neurons in the LH in a sexually dimorphic manner, with female embryos more responsive to ethanol than male embryos.
Figure 2:
Prenatal exposure to ethanol (2 g/kg/day, E10-E15) compared with Untreated and isocaloric Control groups affects CCL2+ and MCH+ neurons in the LH of neonatal offspring at P2 (n = 7/group/sex). A. Ethanol compared with control groups significantly increased the density of CCL2+ (top) and MCH+ (bottom) single-labeled neurons in both female and male offspring, with the effects in females significantly greater. B. This effect in female offspring is illustrated in representative immunostaining confocal images of CCL2+ (green) and MCH+ (red) single-labeled neurons. Scale bar, 100 μm. Data are mean ± SEM. * p < 0.05 versus control groups. # p < 0.05 versus male groups.
Prenatal ethanol increases density of CCL2+ neurons in postnatal offspring in a cell-type and sex-dependent manner
With our recent study in adolescent rats (Chang et al., 2018) showing maternal ethanol administration to stimulate two types of CCL2+ neurons in the LH, some of which are large and colocalize MCH and others that are small and do not colocalize MCH, we used single- and double-labeled IF to further examine these different CCL2+ neurons in postnatal offspring to determine if they exhibit MCH co-labeling and are differentially affected by prenatal ethanol exposure. While our analyses of female and male embryos at E19 and the neonatal P2 offspring showed all CCL2+ cells in the hypothalamus to be small and similar in size, we were able to detect in both female and male postnatal offspring at P7 the clearly differentiated large and small CCL2+ cells in the LH, almost all of which were neurons and co-labeled NeuN, as described in the Methods and Procedures. Analysis via two-way ANOVA of the total single-labeled CCL2+ cell population in P7 offspring revealed a significant main effect of prenatal ethanol exposure on their density (F(1,20) = 64.709, p = 0.000), a significant effect of sex (F(1,20) = 8.838, p = 0.008), and a significant interaction between sex and prenatal treatment (F(1,20) = 11.040, p = 0.003). While showing no difference between the female and male postnatal offspring in the control group (6.28 × 10−5 ± 3.00 × 10−6 vs 6.40 × 10−5 ± 1.01 × 10−6, p = 0.807, respectively) as shown above in the P2 neonates, a simple main effect analysis in the ethanol-exposed groups of the total population of CCL2+ cells showed a clear difference between the females and males (8.47 × 10−5 ± 1.06 × 10−6 vs 7.27 × 10−5 ± 1.94 × 10−6), with a direct comparison (via paired t-test) between the ethanol-induced increase in cell density revealing a significantly greater effect in females than males (p = 0.005).
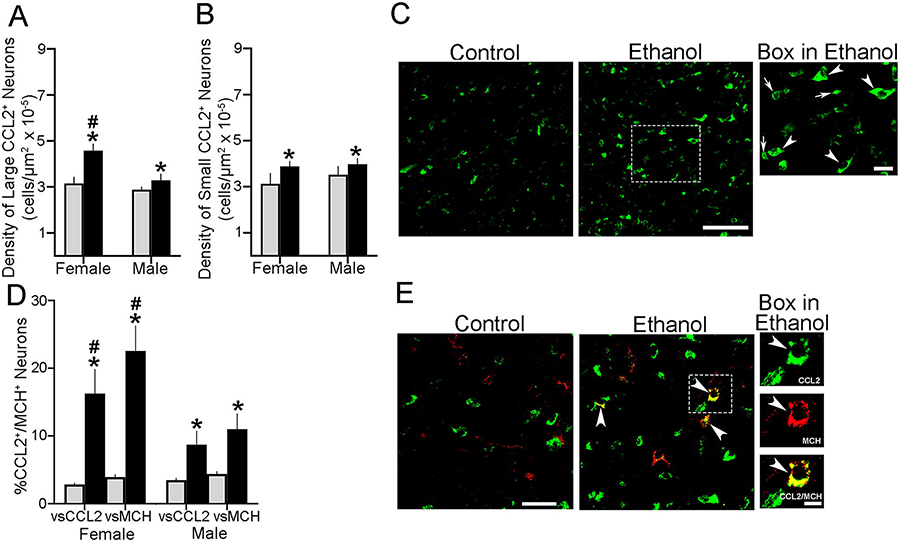
A similar analysis separately of the large, single-labeled CCL2+ cells via a two-way ANOVA revealed a significant main effect of ethanol on their density (F(1,20) = 74.041, p = 0.000), a significant effect of sex (F(1,20) = 53.467, p = 0.000), and a significant interaction between sex and maternal treatment (F(1,20) = 22.638, p = 0.000) (Fig. 3A), as illustrated for females in the photomicrographs of single-labeled large cells identified by large arrowheads (Fig. 3C). While simple main effect analyses showed no differences in the density of these large CCL2+ cells between the female and male Control groups (p = 0.086), their density as shown with the total CCL2+ cells was significantly higher in ethanol-exposed females (p = 0.000), with ethanol having a significantly greater effect in females (+45%) than males (+14%) when compared directly via a paired t-test (t(10) = −3.569, p = 0.016). Our separate analysis of the small, single-labeled CCL2+ cells in the LH of postnatal offspring yielded very different results (Fig. 3B). Analysis via a two-way ANOVA similarly revealed a significant main effect of prenatal ethanol exposure on their density (F(1,20) = 19.430, p = 0.000), as illustrated in the photomicrographs with small cells identified by a small arrow (Fig. 3C). However, it showed no effect of sex (F(1,20) = 3.062, p = 0.095) and no interaction between sex and prenatal exposure (F(1,20) = 0.000, p = 1.000). Also, a simple main effect analysis showed no difference between females and males in the density of these small CCL2+ cells in the Control (p = 0.117) and ethanol-exposed (p = 0.370) groups, with ethanol compared to Control having a small but significant stimulatory effect in both the female (+24%, p = 0.001) and male (+13%, p = 0.028) offspring.
Figure 3:
Prenatal exposure to ethanol (2 g/kg/day, E10-E15) compared with Control group increased the density of both large and small CCL2+ neurons in the LH of postnatal offspring in P7 offspring. A. Ethanol compared with Control (n=7/group) significantly increased the density of large CCL2+ single-labeled neurons in both female and male offspring, with the effect on the large CCL2+ neurons significantly larger in females. B. Ethanol compared with Control (n = 7/group) significantly increased the density of small CCL2+ single-labeled neurons in both female and male offspring, with no significant effect of sex. C. The stimulatory effect of ethanol compared to control is shown in representative 20x confocal images of CCL2+ single-labeled neurons in female offspring. These are more clearly illustrated at 40x by the few large (arrowhead) and small (arrow) CCL2+ neurons in the box from the Ethanol group. D. Prenatal ethanol compared with Control (n = 6/group) increased the density also of large CCL2+/MCH+ double-labeled neurons relative to the large single-labeled CCL2+ and MCH+ neurons, with this effect significantly stronger in females. E. This effect of ethanol is illustrated in 20x confocal images showing CCL2+/MCH+ double-labeled neurons, with those in the box illustrated to the right at 40x, with a CCL2+ (green) or MCH+ (red) single-labeled neuron exhibiting CCL2+/MCH+ double-labeling (yellow). Scale bar, 100 μm. Data are mean ± SEM. *p < 0.05 versus control group. #, p < 0.05 versus males.
Further analysis of CCL2+ cells that co-labeled MCH in the LH showed in postnatal offspring that this occurred only in the large CCL2+ cells and not the small CCL2+ cells, as described in adolescent offspring (Chang et al., 2018). Analysis of these large CCL2+/MCH+ double-labeled cells via a two-way ANOVA revealed a significant main effect of maternal ethanol treatment on their density relative to the large single-labeled CCL2+ (F(1,20) = 120.55, p = 0.000) and MCH+ (F(1,20) = 193.63, p = 0.000) cells (Fig. 3D), reflecting a significant increase after ethanol exposure as illustrated in the confocal images (Fig. 3E). It also revealed a significant main effect of sex on the density of these double-labeled cells relative to the single-labeled CCL2+ (F(1,20) = 16.633, p = 0.001) and MCH+ (F(1,20) = 37.510, p = 0.000) cells and a significant sex x maternal treatment interaction on their density relative to single-labeled CCL2+ (F(1,20) = 23.401, p = 0.000) and MCH+ (F(1,20) = 43.683, p = 0.000) cells. As with the single-labeled cells, simple main effect analyses of these large CCL2+/MCH+ double-labeled cells in the Control group revealed no differences between the females and males in their density relative to single-labeled CCL2+ (p = 0.597) and MCH+ (p = 0.736) cells. In the ethanol-exposed offspring, however, these analyses revealed clear sex differences, with the females compared to males having a significantly higher density of the CCL2+/MCH+ cells relative to both CCL2+ (p = 0.000) and MCH+ (p = 0.000) single-labeled cells (Fig. 3D). Direct comparisons between the females and males (via paired t-test) of ethanol’s stimulatory effect on CCL2+/MCH+ cell density vs Control showed this to be significantly greater in females, relative to single-labeled CCL2+ (t(10) = 4.583, p = 0.002) and MCH+ (t(10) = 6.360, p = 0.000) cells. Thus, while prenatal ethanol exposure increased the density of both large and small CCL2+ cells in the female and male postnatal offspring, only the large CCL2+ neurons that colocalize MCH were affected in a sex-related manner, with ethanol increasing their density more strongly in females than males.
Maternal administration of CCL2 antibody blocks the ethanol-induced increase in MCH+ neurons in the embryo
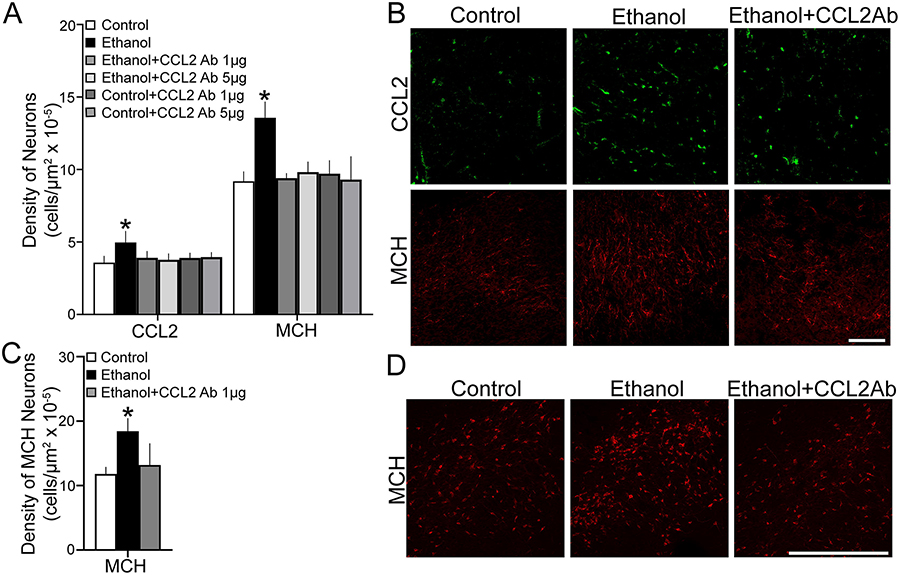
This experiment tested with maternal administration of a CCL2 antibody (Ab) whether neutralizing of endogenous CCL2 affects the stimulatory effect of maternal ethanol administration in E19 embryos on the development of MCH+ neurons in the LH. We examined female embryos from dams in the following six groups: Isocaloric Control + Vehicle (Control); Ethanol + Vehicle (Ethanol); Ethanol + 1 μg CCL2 Ab; Ethanol + 5 μg CCL2 Ab; Control + 1 μg CCL2 Ab; and Control + 5 μg CCL2 Ab. Maternal injection (i.p.) of the CCL2 antibody during the period of ethanol exposure produced in the embryo significant changes in the density of CCL2+ neurons and also of MCH+ neurons in the LH. A one-way ANOVA revealed a significant main effect of maternal treatment on the density of CCL2+ cells (F(5,36) = 6.69, p = 0.000) (Fig. 4A), as illustrated in the photomicrographs (Fig. 4B). The post-hoc analysis showed that maternal administration of ethanol compared to Control group significantly increased the density of CCL2+ cells (+39%, p = 0.000). This stimulatory effect was reversed by maternal administration of the CCL2 antibody which significantly decreased the density of CCL2+ cells in both the Ethanol + 1 μg CCL2 Ab and Ethanol + 5 μg CCL2 Ab groups compared to the Ethanol group (p = 0.000 and p = 0.000, respectively), reduced to the same level as in the Control + 1 μg CCL2 (p = 0.460) and Control + 5 μg CCL2 (p = 0.229) groups, with no difference between the two doses of antibodies (p = 0.636). These changes in the density of CCL2+ cells in the LH of the embryos was accompanied by significant alterations in the density of MCH+ cells, as revealed by a one-way ANOVA (F(5,36) = 20.489 p = 0.000) (Fig. 4A) and illustrated in the photomicrographs (Fig. 4B). Post-hoc analyses of the ethanol-exposed compared to Control offspring revealed a significant increase in the density of MCH+ cells (+48%, p = 0.000). There was a reversal of this effect after administration of the CCL2 antibody, as demonstrated by a significant decrease in the density of MCH+ cells in the Ethanol + 1 μg CCL2 Ab and Ethanol + 5 μg CCL2 Ab group compared to the Ethanol groups (p = 0.000 and p = 0.000 respectively), again reduced to the same levels as in the Control + 1 μg CCL2 Ab (p = 0.231) and Control + 5 μg CCL2 Ab (p = 0.690) groups. The CCL2 antibody had little impact of its own on the density of CCL2+ and MCH+ neurons in the Control group, as revealed by no differences between the Control + 1 μg CCL2 Ab and Control + 5 μg CCL2 Ab compared to Control group for CCL2+ (p = 0.163 and p = 0.235, respectively) and MCH+ (p = 0.812 and p = 0.321, respectively) cells.
Figure 4:
Maternal administration of anti-CCL2 neutralizing antibody (1 and 5 μg/rat/day, i.p.), during the period of prenatal ethanol exposure (2g/kg/day, E10-E15), prevented the stimulatory effect of ethanol on CCL2+ and MCH+ single-labeled neurons of female embryos and postnatal offspring. A. Ethanol treatment compared to Control increased the density of CCL2+ and MCH+ neurons in E19 embryos (n = 7/group), while anti-CCL2 neutralizing antibody (Ab) treatment blocked this effect of ethanol, similarly at both doses. B. This effect is illustrated in representative confocal images of CCL2+ and MCH+ single-labeled neurons from Control, Ethanol and Ethanol + CCL2 Ab treated embryos. C. Ethanol treatment compared to Control increased the density of MCH+ neurons in P15 offspring (n = 7/group), whereas anti-CCL2 neutralizing Ab treatment blocked this effect of ethanol. D. This effect is illustrated in representative images of MCH+ single-labeled neurons in P15 offspring. Scale bar, 100 μm. Data are mean ± SEM. *p < 0.05 versus Control and versus Ethanol + CCL2 Ab.
To determine if this effect of the CCL2 antibody on MCH+ cells persists postnatally, we tested 3 additional groups of offspring, Control, Ethanol and Ethanol + 1 μg CCL2 Ab, and analyzed the offspring at P15. Analysis of the data via a one-way ANOVA revealed a significant main effect of maternal treatment on the MCH+ cells (F(2,18) = 13.46, p = 0.000) (Fig. 4C), as illustrated in the photomicrographs (Fig. 4D). Post-hoc analyses showed that ethanol administration compared to Control group significantly increased the density of MCH+ cells (+56%, p = 0.000), and this ethanol-induced effect was blocked by maternal administration of the CCL2 antibody, with the density of MCH+ cells significantly decreased in the Ethanol + CCL2 Ab compared to Ethanol group (p = 0.000) and reduced to the same level as in the Control group (p = 0.307). These results demonstrate that administration of a CCL2 antibody during ethanol exposure, which reduces endogenous CCL2 in the embryo, blocks the stimulatory effect of ethanol on CCL2+ and MCH+ neurons in the LH, as shown in both the embryo and postnatal offspring.
Maternal administration of a CCR2 antagonist blocks ethanol’s stimulatory effect on CCL2, CCR2 and MCH mRNA
Building on our recent report that prenatal administration of the CCR2 antagonist INCB3344 blocks the stimulatory effect of prenatal ethanol on CCL2/MCH neurons in adolescent rats (Chang et al., 2018), this experiment by administering the CCR2 receptor antagonist INCB3344 during the period of ethanol exposure (E10-E15) tested in the embryo a possible involvement of the endogenous CCR2 receptor in mediating ethanol’s stimulatory effect on MCH along with CCL2 and CCR2 expression in the LH. We examined female embryos at E19 from dams in the following four groups: Isocaloric Control + Vehicle (Control); Ethanol + Vehicle (Ethanol); Ethanol + INCB3344; and Control + INCB3344. A one-way ANOVA revealed a significant main effect of treatment on the expression of CCL2 (F(3,27) = 10.004, p = 0.000), CCR2 (F(3,27) = 66.515, p = 0.000), and MCH (F(3,27) = 25.054, p = 0.000) (Table 3). Post-hoc analyses showed that maternal administration of ethanol compared to Control significantly increased the expression of CCL2 (+75%, p = 0.000), CCR2 (+125%, p = 0.000) and MCH (+98%, p = 0.000), and maternal administration of the CCR2 antagonist blocked these effects, as indicated by a significant decrease in mRNA levels of CCL2 (p = 0.001), CCR2 (p = 0.000), and MCH (p = 0.000) in the Ethanol + INCB3344 compared to Ethanol group, reduced to the same level as those in the Control group (p = 0.604, p = 0.524 and p = 0.378, respectively). There was no effect of the CCR2 antagonist alone (Table 3), with no differences detected between the Control + INCB3344 and the Control group in their expression of CCL2 (p = 0.604), CCR2 (p = 0.524), and MCH (p = 0.193). These results with pharmacological antagonism of the CCR2 receptor during in utero ethanol exposure show that the integrity of endogenous CCL2 to CCR2 signaling in the embryo is essential for the stimulatory effects of prenatal ethanol exposure on mRNA expression of MCH in addition to CCL2 and CCR2 in the LH.
Maternal administration of CCR2 antagonist blocks ethanol’s stimulatory effect on density of CCL2 and MCH neurons
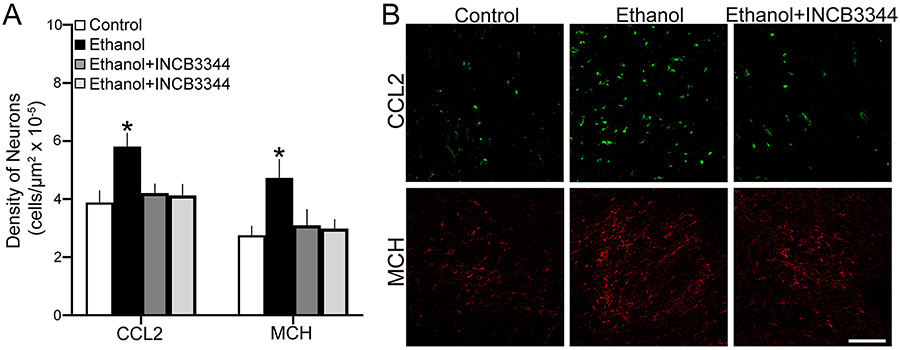
This experiment tested in embryos at E19 the effect of maternal administration of the CCR2 receptor antagonist INCB3344 during the period of ethanol exposure on the density of CCL2+ and MCH+ neurons in the LH. As with the analysis of gene expression, four groups of female embryos were examined: Control, Ethanol, Ethanol + INCB3344 and Control + INCB3344. Analysis of the data via one-way ANOVA revealed a significant main effect of treatment on the density of CCL2+ (F(3,27) = 33.24, p = 0.000) and MCH+ (F(3,27) = 24.18, p = 0.000) cells in the LH. Post-hoc analyses showed that maternal ethanol administration compared to Control significantly increased the density of CCL2+ (+50%, p = 0.000) and MCH+ (+72%, p = 0.000) cells (Fig. 5A), as illustrated in the photomicrographs (Fig. 5B), and that these stimulatory effects of ethanol were blocked by maternal administration of the CCR2 antagonist, as indicated by a significant decrease in the Ethanol + INCB3344 compared to Ethanol group in the density of CCL2+ (p = 0.000) and MCH+ (p = 0.000) cells that returned their density to the same level as in the Control group (p = 0.156 and p = 0.238, respectively). There were no differences between the Control + INCB3344 and the Control group in the density of CCL2+ (p = 0.270) and MCH+ (p = 0.383) cells, showing that the antagonist had no effect of its own on these LH cells under control conditions. These results in the embryo demonstrate that the integrity of CCR2 receptors, similar to the activity of endogenous CCL2, is essential for the stimulatory effects of prenatal ethanol exposure on the density of both CCL2+ and MCH+ neurons as well as their gene expression in the LH
Figure 5:
Maternal administration of CCR2 receptor antagonist, INCB3344 (1 mg/kg/day, i.p.), during the period of prenatal ethanol exposure (2 g/kg/day, E10-E15), prevented the stimulatory effect of ethanol compared to control on CCL2+ and MCH+ single-labeled neurons in the LH of female E19 embryos (n = 7/group). A. Ethanol treatment compared to Control increased the density of CCL2+ and MCH+ neurons, while INCB3344 treatment blocked this effect of ethanol. B. This effect in the embryo is illustrated in representative confocal images of CCL2+ and MCH+ single-labeled neurons from Control, Ethanol, and Ethanol + INCB3344 treated offspring. Scale bar, 100 μm. Data are mean ± SEM. *p < 0.05 versus Control and versus Ethanol + INCB3344.
DISCUSSION
The present study demonstrates that prenatal ethanol exposure at a moderate dose (2 g/kg/day) from E10-E15 during the period of peak neurogenesis has strong effects on neuronal systems in the LH of the embryo and neonate, similar to that described during adolescence. The major findings listed in Table 4 demonstrate that: 1) prenatal ethanol stimulates the CCL2/CCR2 neuroimmune system, the expression and density of MCH neurons, and the co-localization of CCL2 with MCH in neurons of the LH; 2) these stimulatory effects of prenatal ethanol on CCL2 and MCH neurons are blocked by maternal administration of an anti-CCL2 neutralizing antibody and a CCR2 receptor antagonist during the period of ethanol exposure; 3) these effects of ethanol are sexually dimorphic, with female embryos consistently responding more strongly than male embryos and CCL2 mRNA in male embryos unresponsive to ethanol; and 4) CCL2 neurons in the LH, while small and undifferentiated in the embryo, become differentiated shortly after birth into two distinct subtypes, large CCL2 neurons that are concentrated in the LH and colocalize MCH and are stimulated by ethanol in a sexually dimorphic manner, and small CCL2 neurons that are stimulated by ethanol but show no colocalization with MCH and no sex difference in their response to ethanol. Together, these findings demonstrate marked effects of prenatal ethanol exposure on neuronal development in the LH of the embryo, which involve a close relationship between the CCL2/CCR2 chemokine system and MCH neurons, are consistently stronger in female embryos, and are long lasting, with the ethanol-induced effects in the embryo and neonate very similar to those described in adolescent offspring (Chang et al., 2018).
With much of the literature on prenatal challenges focusing on adolescent and adult rodent models, there is little information on the immediate or short-term changes in the neuroimmune system of the developing embryo, which ultimately form the basis of later disturbances in the brain and behavior and, if identified, may be targeted with treatment to ameliorate such disturbances. While there are no studies of CCR2 in the embryo, the limited evidence with measurements of CCL2 in the rat have revealed this chemokine in the hypothalamus at E15 (Rosin and Kurrasch, 2018) and the hippocampus and cortex during the early postnatal period (Pousset, 1994). The present report provides evidence for CCL2 and CCR2 mRNA in E19 embryos and also for CCL2 cells in the neonate, which similar to that described in adolescent rats (Chang et al., 2018) are particularly dense in the LH and are found after prenatal ethanol exposure at a moderate dose to be almost exclusively neurons co-labeling NeuN, in contrast to the increased CCL2 and other chemokines detected in microglia and astrocytes when stimulated by higher concentrations of ethanol and inflammatory agents (Zou and Crews, 2010; Kane et al., 2014). In addition, we demonstrate that prenatal ethanol exposure markedly increases the expression and density of these CCL2 neurons, consistent with ethanol’s effect on CCL2 gene expression in the hippocampus and cortex of the rat embryo (Terasaki and Schwarz, 2016) and on CCR2 in the LH of adolescent rats (Chang et al., 2015; Chang et al., 2018). These changes in the embryonic LH are evident up to at least 21 days after maternal administration of ethanol is stopped and at a dose of ethanol that has no impact on the CCL2/chemokine balance in the amniotic environment (Chang et al., 2018). Being similar to those reported in adolescent offspring, it is clear that these effects occur at an early age in the embryo, indicating that local alterations in neuronal CCL2 and CCR2 arise immediately or shortly after ethanol exposure, and that they are robust enough to persist at least into adolescence.
In addition to the CCL2/CCR2 system, our results demonstrate in the LH that prenatal ethanol exposure also increases mRNA expression of MCH in the embryo and the density of MCH neurons in the neonate. While there are few studies of MCH in the embryo, those that exist indicate that MCH neurogenesis peaks around E12-E13, with the vast majority of neurons concentrated in the LH (Brischoux et al., 2001; Chometton et al., 2014). This developmental stage is precisely the same period in our paradigm when ethanol is administered in utero, indicating that its effects on the developing MCH neurons also arise very early and can persist into adolescence as previously shown (Chang et al., 2018). There is evidence that the effect on MCH neurons may vary with the intensity of the stimulation produced by prenatal exposure to ethanol and also to stress and inflammatory factors. Whereas a high level of prenatal stimulation is shown to either decrease (Le Thuc et al., 2016) or have no impact on MCH expression and neuronal density (Chometton et al., 2014), we find here and in other reports (Chang et al., 2015; Chang et al., 2018) that low-to-moderate levels of ethanol during gestation stimulate neurogenesis and persistently increase MCH expression and cell density in the offspring, an effect also seen with ethanol exposure in adult rats (Morganstern et al., 2010). These dose-dependent effects of ethanol on MCH neurons may reflect varying degrees of oxidative stress produced by ethanol at different doses (Montoliu et al., 1995), with low stress levels stimulating MCH neurons and high stress levels inhibiting or damaging MCH neurons.
With prenatal ethanol exposure found to stimulate MCH neurons in the embryo concurrently with an increase in the CCL2/CCR2 signaling cascade, the effects of ethanol on MCH may involve the actions of this chemokine system. This is supported by the close, anatomical relationship of CCL2 or CCR2 with MCH neurons, as indicated by their co-localization in the LH where these neuroimmune and neuropeptide systems are particularly dense (Banisadr et al., 2005b; Banisadr et al., 2005c; Chometton et al., 2016; Chang et al., 2018) and by our finding here in the neonate showing prenatal ethanol to markedly increase the percentage of MCH neurons that colocalize CCL2, similar to results described in adolescent offspring (Chang et al., 2018). It is noteworthy that the percentage of CCL2/MCH co-labeled neurons detected in the neonate after ethanol exposure greatly increases with age, from ~20% up to ~80% in females, in the absence of any further ethanol exposure. This suggests that the changes initiated by ethanol exposure in the embryo are dynamic and after initial priming continue to grow over time to reach the high level of colocalization during adolescence.
In addition to this anatomical relationship between CCL2 and MCH in the LH, our findings also suggest a functional relationship that ascribes a mechanistic role to endogenous CCL2 in mediating ethanol’s effects on the development of MCH neurons. Maternal administration of CCL2 during pregnancy (E10-E15), similar to maternal ethanol administration at lowto-moderate doses, increases endogenous CCL2 in addition to CCR2 in the LH consistent with a positive feedback loop shown for CCL2 in monocytes and mesenchymal progenitor cells (Sakai et al., 2006; Harris et al., 2013), with our recent study (Chang et al., 2018) suggesting that CCR2 which colocalizes with CCL2 is involved in these stimulatory effects on CCL2 neurons. Maternal CCL2 administration also stimulates the development of MCH neurons and their expression and density in adolescent offspring (Chang et al., 2015), with high-grade inflammation having an opposite effect on MCH (Le Thuc et al., 2016). Further, CCL2 in vitro stimulates neurogenesis and increases the expression and density of hypothalamic neurons containing the orexigenic peptide, enkephalin (Poon et al., 2014). On a mechanistic level, neuronal CCL2 can induce Ca2+ mobilization that stimulates neurotransmitters and neuropeptides (Banisadr et al., 2005a; van Gassen et al., 2005) and can enhance spontaneous excitatory currents in neurons (Sun et al., 2006; Gao et al., 2009; Takeda et al., 2018). The importance of CCL2/CCR2 signaling in the development of MCH neurons is further substantiated by our evidence that maternal administration of an anti-CCL2 neutralizing antibody during the period of ethanol exposure blocks ethanol’s stimulatory effect on both MCH and CCL2 neurons in the LH of the neonate as well as postnatal offspring. Whereas there are no prior studies that have used this or any other chemokine antibody to manipulate brain development in utero, its efficacy in neutralizing cognate CCL2 molecules has been confirmed in numerous in vivo and in vitro reports in human and adult rodent models (Loberg et al., 2007; Guo et al., 2011; Zhu et al., 2011; Zhang et al., 2018), and a human anti-CCL2 neutralizing antibody CNTO888 that is effective in blocking endogenous CCL2 is shown to have therapeutic potential in inhibiting CCL2/CCR2 mediated primary tumor growth in epithelial cells (Huang et al., 2007; Loberg et al., 2007). With the placenta known to have specific receptor-mediating mechanisms to actively transport immunoglobulins (Saji et al., 1999), the effects of this peripherally administered CCL2 antibody found to be opposite to those of peripherally administered CCL2 itself may reflect their actions directly on the fetal brain. With early stimulation by ethanol of neuronal CCL2 found here to fundamentally alter the patterning of MCH neurons in the embryonic LH, such treatment which neutralizes endogenous CCL2 molecules could help to rescue offspring from the effects of in utero ethanol exposure on the brain and behavior.
Our findings in the embryo also demonstrate the importance of CCR2, the receptor for CCL2, in mediating the stimulatory effects of prenatal ethanol exposure on the development of MCH neurons and their colocalization with CCR2 as well as CCL2. Antagonists of this receptor are effective in blocking the actions of CCL2 signaling, including its stimulatory effects on the differentiation of neural precursor cells as well as on inflammatory factors, stress, and microglial activation (Turbic et al., 2011; Shahlaei et al., 2013; Belkouch et al., 2014; Ren et al., 2017). Maternal administration of the CCR2 receptor antagonist INCB3344, during the period of ethanol exposure, is shown here to block the stimulatory effect of ethanol on MCH neurons in the LH of the embryo, consistent with its antagonism of ethanol’s behavioral as well as neural effects demonstrated in adolescent offspring (Chang et al., 2018). This evidence with selective blocking of CCL2’s main receptor supports the importance of CCR2 in mediating ethanol’s effect on this neuropeptide, revealing therapeutic potential for a CCR2 receptor antagonist in preventing the detrimental effects of moderate ethanol administration on neuronal development.
Interestingly, the stimulatory effects of ethanol on fetal programming of both the CCL2/CCR2 system and MCH neurons in the LH are found to be sexually dimorphic, with female embryos exhibiting consistently stronger responses than male embryos. While not seen under basal conditions as indicated here and in another report showing no effect of sex on CCL2 gene expression in rat hippocampus and cortex at any age (Schwarz et al., 2012), this sexual dimorphism becomes evident after prenatal exposure to ethanol, indicating that it is challenge based. Our findings here in the embryo illustrated in Figs. 2 and 3, revealing similar effects as reported in adolescent offspring (Chang et al., 2018), demonstrate that the sexual dimorphism of ethanol’s actions occurs very early in development, around the time when sexual differentiation starts (Vito and Fox, 1981) and well before the onset of puberty (Sengupta, 2013; Greco et al., 2015; Gillette et al., 2017), and it is remarkably robust and long lasting, continuing well into adolescence. Whereas ethanol’s stimulatory effects on MCH neurons and most effects on the CCL2/CCR2 system in the LH are evident in both female and male embryos, they are consistently and significantly stronger in females. Notably, measurements of CCL2 mRNA which show a strong stimulatory effect of ethanol in female embryos reveal no response in male embryos. This marked sex difference, consistent with evidence that prenatal ethanol increases CCL2 expression in the cortex of female embryos but reduces it in male embryos (Terasaki and Schwarz, 2016), may reflect a reduced CCL2 signaling and Ca2+ modulation in males during neurogenesis (Bizzarri et al., 1995; van Gassen et al., 2005). While the molecular mechanism underlying such sex differences remains to be determined, this evidence focuses our attention specifically on neuronal CCL2 during the embryonic period of peak neurogenesis as a possible source and/or mediator of the persistent sexual dimorphism in response to in utero ethanol exposure. Further investigations of this chemokine and its possible priming effects may help us to understand the general finding that females and males are differentially affected by adolescent and adult exposure to ethanol, with females more vulnerable to its effects and exhibiting a greater increase in inflammatory mediators along with more neuroinflammatory damage and neurodevelopmental dysfunction (Hommer et al., 2001; Squeglia et al., 2012; Alfonso-Loeches et al., 2013; Pascual et al., 2017).
Further insight into these neuronal sex differences is provided by our analysis of the subtypes of CCL2 neurons in the LH. In adolescent rats, we recently described two distinct subtypes of CCL2 neurons that colabel NeuN and are stimulated by ethanol, showing large CCL2 neurons to be densely concentrated in the LH and to co-express MCH and small CCL2 neurons to be scattered throughout the hypothalamus and surround MCH neurons rather than colocalize MCH (Chang et al., 2018). In the present study, we found the CCL2 cells in the embryonic LH to be generally small and similar in size but to develop further postnatally into these two distinct subtypes of CCL2 neurons. While prenatal ethanol exposure significantly increased the density of both CCL2 neuronal subtypes in female and male offspring at 7 days of age, we discovered that its effect on the large CCL2 neurons many of which co-label MCH is sexually dimorphic, significantly stronger in females than males, while its effect on the small CCL2 neurons is not. To our knowledge, this is the first evidence showing that cells in the brain with many similar characteristics respond differently in relation to sex depending on their size, a phenomenon similarly described in an earlier study of large and small cells in the pineal body (Bastianelli and Pochet, 1993).
This effect of ethanol on hypothalamic neuronal programming, involving a marked increase in the density of MCH neurons in the embryo that colocalize CCL2, is likely to have behavioral consequences. Persisting into adolescence, this effect occurs predominantly in the LH (Chang et al., 2018), where the CCL2, CCR2 and MCH neurons are most concentrated (Brischoux et al., 2001; Banisadr et al., 2005b; Banisadr et al., 2005c; Chang et al., 2015). The LH is known to be involved in arousal and active during exploratory behavior and to have a major role in substance abuse (Barson and Leibowitz, 2017; James et al., 2017). There is considerable evidence that prenatal ethanol exposure increases ethanol consumption and anxiety in adolescent offspring, as demonstrated by our lab (Chang et al., 2015) and others (Carneiro et al., 2005; March et al., 2009; Fabio et al., 2015). Moreover, there are numerous studies positively linking CCL2 and CCR2 to excess ethanol drinking (Blednov et al., 2005; June et al., 2015; Valenta and Gonzales, 2016; Chang et al., 2018) and anxiety (Pascual et al., 2014; Sawicki et al., 2015) and also MCH to increased ethanol consumption (Duncan et al., 2005; Cippitelli et al., 2010; Morganstern et al., 2010) and anxiety (Gonzalez et al., 1996; Borowsky et al., 2002; Smith et al., 2006), with MCH injection into the LH found to stimulate drinking behavior in adult rats (Morganstern et al., 2010). Our recent evidence in adolescent rats, showing that ethanol drinking is stimulated by maternal administration of CCL2 and prenatal ethanol’s stimulatory effect on this behavior is blocked by a CCR2 antagonist (Chang et al., 2018), indicates that CCL2/CCR2 signaling is a major factor in the ethanol-induced increase in such MCH-mediated behaviors, having long-term positive feedback or priming effects. Thus, the fetal programming induced by ethanol of this CCL2/CCR2 chemokine system, as it relates to and colocalizes with MCH neurons in the LH, may contribute to the behavioral disturbances during adolescence, a time when substance abuse strongly predicts later adult abuse and dependence (Anthony and Petronis, 1995).
In conclusion, with most studies of this nature examining adolescent or adult animals and administering higher ethanol doses for longer periods, the present study provides much needed, new information about the origins and early developmental mechanisms by which low-to-moderate prenatal ethanol exposure for a few days affects developing neurons and later behavior. These lower doses and short duration of exposure more accurately reflect common maternal ethanol consumption and the most prevalent and mild forms of fetal alcohol spectrum disorders (Astley, 2010; Brady et al., 2012; Chang et al., 2015). From our rat model studies, it is clear that low-to-moderate amounts of maternal ethanol exposure produces marked changes in neuroimmune function and neuronal organization in the LH of the developing embryo which persist and manifest as behavioral disturbances in adolescence (Chang et al., 2018). Our results revealing the stimulated CCL2 and MCH neurons, together with the greater observed sensitivity in females, are the first to demonstrate a potential functional pathway during embryonic development that can explain the origins of long-term prenatal ethanol-induced changes in neuronal populations of the LH and behavioral outcomes during adolescence that disproportionately impact females (Foster et al., 2015).
Highlights:
Prenatal ethanol exposure stimulates CCL2, MCH and their colocalization in LH of the embryo and neonate
These effects of prenatal ethanol exposure are sexually dimorphic, stronger in female than male embryos
Endogenous CCL2 and CCR2 receptors are required for prenatal ethanol’s stimulatory effect on MCH neurons
These effects in the embryo are long lasting, similar to those described in adolescent offspring that affect behavior.
ACKNOWLEDGEMENTS
This research was supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA024798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to extend gratitude to The Rockefeller University’s Bio-Imaging Resource Center for the use of their equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrahao KP, Salinas AG, Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96:1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Suzuki S, Matsuoka H, Fushimi S, Ono J, Ohta KI, Hirai Y, Miki T, Koshimizu H (2018) Corticotropin-releasing hormone-binding protein is up-regulated by brain-derived neurotrophic factor and is secreted in an activity-dependent manner in rat cerebral cortical neurons. Journal of neurochemistry. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W (2006) In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry 63:1009–1016. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C (2013) Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311:27–34. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1995) Atlas of Prenatal Rat Brain Development. Boca Raton: CRC Press. [Google Scholar]
- Anthony JC, Petronis KR (1995) Early-onset drug use and risk of later drug problems. Drug and alcohol dependence 40:9–15. [DOI] [PubMed] [Google Scholar]
- Astley SJ (2010) Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique 17:e132–164. [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP (2003) A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry 60:377–385. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM (2005a) Chemokines and brain functions. Curr Drug Targets Inflamm Allergy 4:387–399. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostene W, Parsadaniantz SM (2005b) Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol 489:275–292. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S (2005c) Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol 492:178–192. [DOI] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF (2017) Orexin/Hypocretin System: Role in Food and Drug Overconsumption. Int Rev Neurobiol 136:199–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF (2013) Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcohol Clin Exp Res 37 Suppl 1:E141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, Leibowitz SF (2009) Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol 43:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianelli E, Pochet R (1993) Sexual dimorphism among calbindin-D28K immunoreactive cells in the rat pineal body. Histochemistry 100:449–455. [DOI] [PubMed] [Google Scholar]
- Belkouch M, Dansereau MA, Tetreault P, Biet M, Beaudet N, Dumaine R, Chraibi A, Melik-Parsadaniantz S, Sarret P (2014) Functional up-regulation of Nav1.8 sodium channel in Abeta afferent fibers subjected to chronic peripheral inflammation. Journal of neuroinflammation 11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ (2013) Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: Modeling adolescent and adult binge-like drinking. Alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE (1992) The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319:218–245. [DOI] [PubMed] [Google Scholar]
- Bizzarri C, Bertini R, Bossu P, Sozzani S, Mantovani A, Van Damme J, Tagliabue A, Boraschi D (1995) Single-cell analysis of macrophage chemotactic protein-1-regulated cytosolic Ca2+ increase in human adherent monocytes. Blood 86:2388–2394. [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA (2005) Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural brain research 165:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C (2002) Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med 8:825–830. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM, Caldwell KK (2012) A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res 36:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Reyes KC, Roberts AJ, Gruol DL (2018) Altered hippocampal synaptic function in transgenic mice with increased astrocyte expression of CCL2 after withdrawal from chronic alcohol. Neuropharmacology 135:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA (2008) Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 33:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Fellmann D, Risold PY (2001) Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. The European journal of neuroscience 13:1733–1744. [DOI] [PubMed] [Google Scholar]
- Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS (2005) Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol. Neurotoxicol Teratol 27:585–592. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2013) Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci 33:13600–13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Leibowitz SF (2015) Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience 310:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF (2008) Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Liang SC, Barson JR, Leibowitz SF (2012) Prenatal ethanol exposure stimulates neurogenesis in hypothalamic and limbic peptide systems: possible mechanism for offspring ethanol overconsumption. Neuroscience 222:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Halkina V, Edelstien J, Ramirez E, Leibowitz SF (2018) Hypothalamic CCL2/CCR2 Chemokine System: Role in Sexually Dimorphic Effects of Maternal Ethanol Exposure on Melanin-Concentrating Hormone and Behavior in Adolescent Offspring. J Neurosci 38:9072–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chometton S, Croizier S, Fellmann D, Risold PY (2016) The MCH neuron population as a model for the development and evolution of the lateral and dorsal hypothalamus. J Chem Neuroanat 75:28–31. [DOI] [PubMed] [Google Scholar]
- Chometton S, Franchi-Bernard G, Houdayer C, Mariot A, Poncet F, Fellmann D, Risold PY (2014) Melanin-concentrating hormone expression in the rat hypothalamus is not affected in an experiment of prenatal alcohol exposure. Brain research bulletin 107:102–109. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G (2007) Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev 31:181–191. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M (2010) Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology (Berl) 211:367–375. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr. (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology 122:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC (2014) Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiology of learning and memory 115:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ (2015) Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC (2005) Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res 29:958–964. [DOI] [PubMed] [Google Scholar]
- Fabio MC, Macchione AF, Nizhnikov ME, Pautassi RM (2015) Prenatal ethanol increases ethanol intake throughout adolescence, alters ethanol-mediated aversive learning, and affects mu but not delta or kappa opioid receptor mRNA expression. The European journal of neuroscience 41:1569–1579. [DOI] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M (2015) Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med 45:3047–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR (2009) JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 29:4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC (2017) Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav 87:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Olvera-Cortes ME, Perez-Vega MI, Evans S, Feria-Velasco A (2006) Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal CA1 field of juvenile rats: a stereology and Golgi study. Neuroscience research 56:400–408. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Vaziri S, Wilson CA (1996) Behavioral effects of alpha-MSH and MCH after central administration in the female rat. Peptides 17:171–177. [DOI] [PubMed] [Google Scholar]
- Greco T, Hovda DA, Prins ML (2015) Adolescent TBI-induced hypopituitarism causes sexual dysfunction in adult male rats. Dev Neurobiol 75:193–202. [DOI] [PubMed] [Google Scholar]
- Guo S, Meng S, Chen B, Liu J, Gao L, Wu Y (2011) C-reactive protein can influence the proliferation, apoptosis, and monocyte chemotactic protein-1 production of human umbilical vein endothelial cells. DNA and cell biology 30:157–162. [DOI] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Park MA, Breese GR (2017) Age-related differences in anxiety-like behavior and amygdalar CCL2 responsiveness to stress following alcohol withdrawal in male Wistar rats. Psychopharmacology (Berl) 234:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Q, Seto J, O’Brien K, Lee PS, Kondo C, Heard BJ, Hart DA, Krawetz RJ (2013) Monocyte chemotactic protein-1 inhibits chondrogenesis of synovial mesenchymal progenitor cells: an in vitro study. Stem Cells 31:2253–2265. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology 210:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R (2001) Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158:198–204. [DOI] [PubMed] [Google Scholar]
- Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH (2007) CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer letters 252:86–92. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2017) A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L (2015) CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Drew PD (2016) Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J Leukoc Biol 100:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD (2014) Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol Clin Exp Res 38:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thuc O, Cansell C, Bourourou M, Denis RG, Stobbe K, Devaux N, Guyon A, Cazareth J, Heurteaux C, Rostene W, Luquet S, Blondeau N, Nahon JL, Rovere C (2016) Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep 17:1738–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer research 67:9417–9424. [DOI] [PubMed] [Google Scholar]
- Malone SM, McGue M, Iacono WG (2010) Mothers’ maximum drinks ever consumed in 24 hours predicts mental health problems in adolescent offspring. Journal of child psychology and psychiatry, and allied disciplines 51:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March SM, Abate P, Spear NE, Molina JC (2009) Fetal exposure to moderate ethanol doses: heightened operant responsiveness elicited by ethanol-related reinforcers. Alcohol Clin Exp Res 33:1981–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoliu C, Sancho-Tello M, Azorin I, Burgal M, Valles S, Renau-Piqueras J, Guerri C (1995) Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. Journal of neurochemistry 65:2561–2570. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, Leibowitz SF (2010) Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiology & behavior 101:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Pla A, Minarro J, Guerri C (2014) Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol and alcoholism 49:187–192. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CM, Guerri C (2015) Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology 89:352–359. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F, Laso FJ, Guerri C (2017) Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol 22:1829–1841. [DOI] [PubMed] [Google Scholar]
- Poon K, Leibowitz SF (2016) Consumption of Substances of Abuse during Pregnancy Increases Consumption in Offspring: Possible Underlying Mechanisms. Front Nutr 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Ho HT, Barson JR, Leibowitz SF (2014) Stimulatory role of the chemokine CCL2 in the migration and peptide expression of embryonic hypothalamic neurons. Journal of neurochemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousset F (1994) Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Brain Res Dev Brain Res 81:143–146. [DOI] [PubMed] [Google Scholar]
- Qiang M, Wang MW, Elberger AJ (2002) Second trimester prenatal alcohol exposure alters development of rat corpus callosum. Neurotoxicol Teratol 24:719–732. [DOI] [PubMed] [Google Scholar]
- Ren Z, Wang X, Yang F, Xu M, Frank JA, Wang H, Wang S, Ke ZJ, Luo J (2017) Ethanol-induced damage to the developing spinal cord: The involvement of CCR2 signaling. Biochim Biophys Acta 1863:2746–2761. [DOI] [PubMed] [Google Scholar]
- Roberto M, Patel RR, Bajo M (2017) Ethanol and Cytokines in the Central Nervous System. Handbook of experimental pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin JM, Kurrasch DM (2018) In utero electroporation induces cell death and alters embryonic microglia morphology and expression signatures in the developing hypothalamus. Journal of neuroinflammation 15:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji F, Samejima Y, Kamiura S, Koyama M (1999) Dynamics of immunoglobulins at the feto-maternal interface. Reviews of reproduction 4:81–89. [DOI] [PubMed] [Google Scholar]
- Sakai N, Wada T, Furuichi K, Shimizu K, Kokubo S, Hara A, Yamahana J, Okumura T, Matsushima K, Yokoyama H (2006) MCP-1/CCR2-dependent loop for fibrogenesis in human peripheral CD14positive monocytes. Journal of leukocyte biology 79:555–563. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, McKim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM, Godbout JP, Sheridan JF (2015) Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience 302:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. Journal of neurochemistry 120:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P (2013) The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med 4:624–630. [PMC free article] [PubMed] [Google Scholar]
- Shahlaei M, Fassihi A, Papaleo E, Pourfarzam M (2013) Molecular dynamics simulation of chemokine receptors in lipid bilayer: a case study on C-C chemokine receptor type 2. Chemical biology & drug design 82:534–545. [DOI] [PubMed] [Google Scholar]
- Sherwood N, Timiras P. (1970) A Stereotaxic Atlas of the Developing Ear Brain: University of California Press. [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG, Gehlert DR (2006) Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology 31:1135–1145. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF (2012) Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 220:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH (2006) MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. Journal of neurophysiology 96:2189–2199. [DOI] [PubMed] [Google Scholar]
- Takeda M, Nasu M, Kanazawa T, Takahashi M, Shimazu Y (2018) Chemokine ligand 2/chemokine receptor 2 signaling in the trigeminal ganglia contributes to inflammatory hyperalgesia in rats. Neuroscience research 128:25–32. [DOI] [PubMed] [Google Scholar]
- Terasaki LS, Schwarz JM (2016) Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J Neuroimmune Pharmacol 11:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB (2009) CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 13:263–272. [DOI] [PubMed] [Google Scholar]
- Turbic A, Leong SY, Turnley AM (2011) Chemokines and inflammatory mediators interact to regulate adult murine neural precursor cell proliferation, survival and differentiation. PLoS One 6:e25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta JP, Gonzales RA (2016) Chronic Intracerebroventricular Infusion of Monocyte Chemoattractant Protein-1 Leads to a Persistent Increase in Sweetened Ethanol Consumption During Operant Self-Administration But Does Not Influence Sucrose Consumption in Long-Evans Rats. Alcohol Clin Exp Res 40:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL (2005) The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. The European journal of neuroscience 21:2949–2957. [DOI] [PubMed] [Google Scholar]
- Vito CC, Fox TO (1981) Androgen and estrogen receptors in embryonic and neonatal rat brain. Brain research 254:97–110. [DOI] [PubMed] [Google Scholar]
- West NR et al. (2017) Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med 23:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang S, Qi Y, Chen L, Frank JA, Yang XH, Zhang Z, Shi X, Luo J (2016) Role of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cells. Molecular carcinogenesis 55:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Glendinning JI (2009) Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proceedings of the National Academy of Sciences of the United States of America 106:5359–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma P, Guan Q, Meng L, Su L, Wang L, Yuan B (2018) Effect of chemokine CC ligand 2 (CCL2) on alphasynucleininduced microglia proliferation and neuronal apoptosis. Molecular medicine reports 18:4213–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Fujita M, Snyder LA, Okada H (2011) Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. Journal of neuro-oncology 104:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F (2010) Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcoholism: Clinical and Experimental Research 34:777–789. [DOI] [PubMed] [Google Scholar]