Abstract
Background
We investigated the pregnancy outcomes in women who were diagnosed with gestational diabetes mellitus (GDM) by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria but not by the Carpenter-Coustan (CC) criteria.
Methods
A total of 8,735 Korean pregnant women were identified at two hospitals between 2014 and 2016. Among them, 2,038 women participated in the prospective cohort to investigate pregnancy outcomes. Diagnosis of GDM was made via two-step approach with 50-g glucose challenge test for screening followed by diagnostic 2-hour 75-g oral glucose tolerance test. Women were divided into three groups: non-GDM, GDM diagnosed exclusively by the IADPSG criteria, and GDM diagnosed by the CC criteria.
Results
The incidence of GDM was 2.1% according to the CC criteria, and 4.1% by the IADPSG criteria. Women diagnosed with GDM by the IADPSG criteria had a higher body mass index (22.0±3.1 kg/m2 vs. 21.0±2.8 kg/m2, P<0.001) and an increased risk of preeclampsia (odds ratio [OR], 6.90; 95% confidence interval [CI], 1.84 to 25.87; P=0.004) compared to non-GDM women. Compared to neonates of the non-GDM group, those of the IADPSG GDM group had an increased risk of being large for gestational age (OR, 2.39; 95% CI, 1.50 to 3.81; P<0.001), macrosomia (OR, 2.53; 95% CI, 1.26 to 5.10; P=0.009), and neonatal hypoglycemia (OR, 3.84; 95% CI, 1.01 to 14.74; P=0.049); they were also at an increased risk of requiring phototherapy (OR, 1.57; 95% CI, 1.07 to 2.31; P=0.022) compared to the non-GDM group.
Conclusion
The IADPSG criteria increased the incidence of GDM by nearly three-fold, and women diagnosed with GDM by the IADPSG criteria had an increased risk of adverse pregnancy outcomes in Korea.
Keywords: Diabetes, gestational; Glucose tolerance test; Infant, newborn; Pregnancy; Women
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with an onset or initial recognition during the present pregnancy [1,2]. GDM is a common medical complication of pregnancy and is associated with various perinatal morbidities, including preeclampsia, cesarean delivery, macrosomia, birth injury, and neonatal hypoglycemia [3,4,5,6]. The number of women diagnosed with GDM has increased in the past decades, and this statistic is attributable to increased obesity and age among pregnant women [7,8]. It is also well known that treatment of GDM reduces serious perinatal morbidities [9]. Thus, an effective diagnostic method as well as optimal medical and obstetric management is crucial to reduce the adverse pregnancy outcomes of GDM.
In 2010, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) proposed new diagnostic criteria for GDM based on the results of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study [4,10]. The IADPSG criteria adopted a one-step approach using a 75-g 2-hour oral glucose tolerance test (OGTT). Compared to the Carpenter-Coustan (CC) criteria [11], the diagnostic threshold for glucose values was lowered, and only one abnormal value was sufficient for a diagnosis of GDM based on the IADPSG criteria. The introduction of the IADPSG criteria is estimated to increase the incidence of GDM from 5% to 6% to 15% to 20% [12]. In addition, there are concerns regarding increased healthcare cost and the impact on medical infrastructure [13]. Currently, the World Health Organization, the International Diabetes Federation, and the American Diabetes Association have endorsed the IADPSG criteria, but the American Congress of Obstetricians and Gynecologists has not [1,14,15,16].
There is ongoing debate regarding the impact of adopting the IADPSG criteria. Specifically, it is not clear to what extent the incidence of GDM would increase in different populations with different healthcare systems. In addition, there are conflicting reports concerning the influence of the IADPSG criteria on maternal and neonatal outcomes [17,18,19]. Although several studies have evaluated the utility of the IADPSG criteria in the East Asian population [20,21], the IADPSG criteria have not been investigated in Koreans. In this prospective observational cohort study, we investigated (1) the influence of the IADPSG criteria on the incidence of GDM in the Korean population and (2) pregnancy outcomes in women who were diagnosed with GDM by the IADPSG criteria but not by the CC criteria.
METHODS
Study participants
This was a prospective observational cohort study involving women with singleton pregnancies at Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine and Seoul National University Bundang Hospital. Pregnant women who had their initial prenatal visit before 24 weeks of gestation and were scheduled to receive prenatal obstetric care and deliver at Cheil General Hospital & Women's Healthcare Center or Seoul National University Bundang Hospital were recruited between August 2014 and October 2016. Women were excluded if they had multiple pregnancies, they had overt or pregestational diabetes, their delivery was planned at another hospital, their last menstrual period was not definitive and an ultrasound evaluation was not performed between 6 and 24 weeks. Each of the participants gave written informed consent regarding this study. This study was approved by the Institutional Review Board (IRB) of Dankook University, Cheil General Hospital & Women's Healthcare Center (IRB number: CGH-IRB-2013-58) and the IRB of Seoul National University Bundang Hospital (IRB number: B-1309/220-001).
Diagnosis and treatment of GDM
Between 24 and 28 weeks of gestation, all women were screened via the 50-g glucose challenge test (GCT) regardless of fasting. Those who had glucose values above 140 mg/dL underwent diagnostic 75-g 2-hour OGTTs after 10 hours of fasting on a different day. GDM was diagnosed by the CC criteria (two or more abnormal values using the following blood glucose thresholds: fasting ≥95 mg/dL; 1-hour ≥180 mg/dL; and 2-hour ≥155 mg/dL). However, 3-hour glucose was not measured and was omitted from the CC criteria. Women diagnosed with GDM by the CC criteria were managed according to the standard clinical practice guidelines [22]. Treatment consisted of medical nutritional therapy, increased physical activity, and adequate weight management with the following glucose targets: fasting ≤95 mg/dL and 2-hour ≤120 mg/dL. If these goals were not met, insulin therapy was added. The diagnostic scheme and care of patients with GDM did not change during the study period. We also identified women who would be diagnosed with GDM by the IADPSG criteria (one or more abnormal values using the following glucose thresholds: fasting ≥92 mg/dL; 1-hour ≥180 mg/dL; and 2-hour ≥153 mg/dL) but not by CC criteria. These women were not managed as GDM patients and only had routine prenatal obstetric care. We stratified participants into nonoverlapping groups of (1) women with no GDM by either the IADPSG criteria or the CC criteria (no GDM group), (2) women exclusively diagnosed with GDM by the IADPSG criteria and not by the CC criteria (IADPSG GDM group), and (3) women diagnosed with GDM by the CC criteria (CC GDM group).
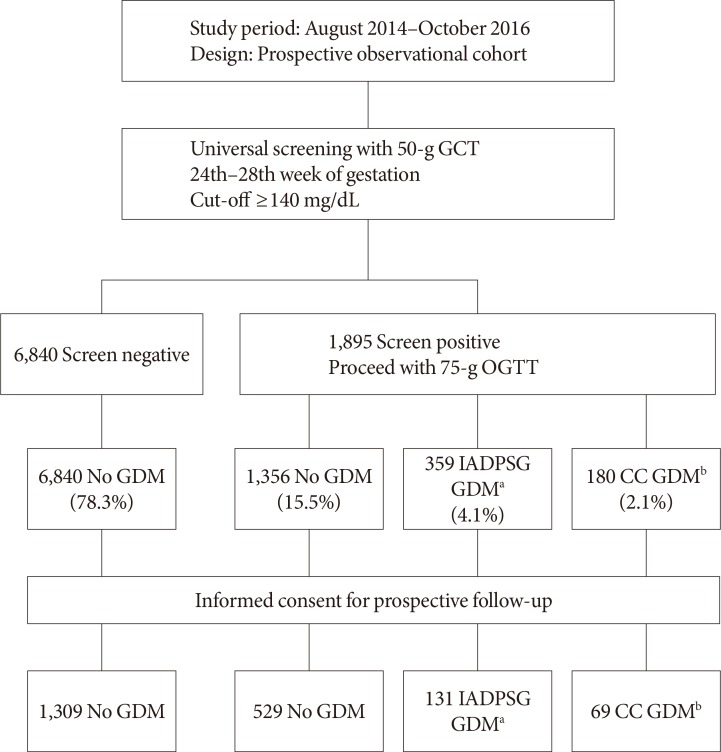
During the period between August 2014 and October 2016, 8,735 women with singleton pregnancies underwent a diagnostic test for GDM in our institutions (Fig. 1). Anonymized data on the 50-g GCT screening and the 75-g OGTT were used to estimate the incidence of GDM by either the IADPSG or CC criteria. For investigation of pregnancy outcomes, a total of 2,038 women agreed to participate in the prospective cohort study. The clinical characteristics of the prospective cohort participants are shown in Table 1.
Fig. 1. Study scheme and number of participants in each group. GCT, glucose challenge test; OGTT, oral glucose tolerance test; GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; CC, Carpenter-Coustan. aGDM diagnosed exclusively by the IADPSG criteria, bGDM diagnosed by the CC criteria.
Table 1. Maternal characteristics according to GDM diagnosis.
| Maternal characteristic | No GDM | IADPSG GDM | CC GDM | PANOVAa | Ppost hocb |
|---|---|---|---|---|---|
| Number | 1,838 | 131 | 69 | ||
| Age at 50-g GCT, yr | 34.3±3.9 | 34.7±3.8 | 35.6±3.6 | 0.011 | c |
| Prepregnancy BMI, kg/m2 | 21.0±2.8 | 22.0±3.1 | 23.5±4.2 | <0.001 | c,d,e |
| Prepregnancy overweight and obesity | <0.001 | ||||
| Normal weight (BMI <23 kg/m2) | 1,471 (81.2) | 91 (69.5) | 39 (56.5) | ||
| Overweight (BMI 23–24.9 kg/m2) | 190 (10.5) | 22 (16.8) | 11 (16.0) | ||
| Obesity (BMI ≥25 kg/m2) | 150 (8.3) | 18 (13.7) | 19 (27.5) | ||
| BMI at 50-g GCT, kg/m2 | 23.5±2.8 | 24.3±2.9 | 26.1±3.9 | <0.001 | c,d,e |
| BMI at delivery, kg/m2 | 26.0±3.1 | 26.6±3.2 | 27.2±4.1 | 0.001 | c |
| Pregnancy weight gain, kg | 13.2±4.5 | 12.0±4.7 | 9.7±4.7 | <0.001 | c,d,e |
| Systolic blood pressure, mm Hg | 107±12 | 107±11 | 109±13 | 0.329 | |
| Diastolic blood pressure, mm Hg | 60±9 | 59±8 | 61±10 | 0.145 | |
| Parity | 0.321 | ||||
| 0 | 1,167 (63.5) | 74 (56.5) | 47 (68.1) | ||
| 1 | 581 (31.6) | 49 (37.4) | 17 (24.6) | ||
| ≥2 | 901 (4.9) | 8 (6.1) | 5 (7.2) | ||
| 50-g GCT glucose, mg/dL | 123.7±23.7 | 157.2±13.9 | 173.3±20.0 | <0.001 | c,d,e |
| Gestational age at 75-g OGTT, wkf | 25.9±1.1 | 25.8±0.9 | 25.8±0.9 | 0.279 | |
| 75-g OGTT glucose, mg/dLf | |||||
| Fasting | 81.0±5.1 | 86.6±7.1 | 95.3±14.9 | <0.001 | c,d,e |
| 1-hr | 137.6±23.4 | 163.6±20.1 | 198.6±20.2 | <0.001 | c,d,e |
| 2-hr | 121.0±17.8 | 150.4±21.2 | 172.9±25.1 | <0.001 | c,d,e |
| Insulin treatment | 0 | 0 | 28 (40.6) |
Values are presented as mean±standard deviation or number (%).
GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; CC, Carpenter-Coustan; ANOVA, analysis of variance; GCT, glucose challenge test; BMI, body mass index; OGTT, oral glucose tolerance test.
aP for ANOVA in the case of a continuous variable and chi-square test in the case of a discrete variable, bPost hoc analysis by Tukey's method, cP<0.05 for no GDM vs. CC GDM, dP<0.05 for no GDM vs. IADPSG GDM, eP<0.05 for IDPSG GDM vs. CC GDM, f75-g OGTT was performed for only 729 GCT-positive women.
Adverse pregnancy outcomes
Maternal and neonatal outcomes were compared among the three groups. For maternal outcomes, we investigated preeclampsia, labor induction, primary cesarean delivery, and preterm delivery. Preeclampsia was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg on two or more occasions and proteinuria ≥1+ on a dipstick test or urine protein level ≥300 mg during a 24-hour period [23]. Preterm delivery was defined as delivery before 37 gestational weeks. For neonatal outcomes, we investigated large for gestational age (LGA) births, defined as birth weight > the 90th percentile; macrosomia, defined as birth weight >4,000 g regardless of the gestational age of the fetus [24]; small for gestational age births, defined as birth weight <10th percentile; shoulder dystocia or birth injury; neonatal hypoglycemia; requirement of phototherapy; and neonatal intensive care unit (NICU) admission. Birth weight percentile was estimated using the baby's sex, gestational age, and maternal parity [25]; neonatal hypoglycemia was defined as glucose values ≤30 mg/dL in the first 24 hours after birth or ≤45 mg/dL after the first 24 hours after birth [10]. NICU admission included any type of unit care that was more intensive than normal newborn care and lasted 24 hours. The overall adverse pregnancy outcomes included preeclampsia, labor induction, primary cesarean delivery, LGA, macrosomia, birth weight below the 10th percentile, shoulder dystocia or birth injury, neonatal hypoglycemia, phototherapy, and NICU admission.
Statistical analysis
Categorical values are reported as frequencies or percentages, and continuous variables are reported using the mean±standard deviation. Comparisons among the three groups were performed using analysis of variance (ANOVA) for continuous variables and chi-square test for categorical variables. A post hoc analysis of ANOVA was performed using Tukey's method. Multiple logistic regression was performed to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for dichotomous outcomes for both the IADPSG GDM group and the CC GDM group compared to the no GDM group. In model 1, no covariate was included for adjustment. In model 2, maternal age, parity, height, body mass index (BMI) at delivery, gestational age at delivery, and baby's sex were included for adjustment. All statistical analyses were performed using IBM SPSS Statistics version 23 (IBM Co., Armonk, NY, USA). A P<0.05 was considered statistically significant.
RESULTS
Incidence of GDM according to diagnostic criteria
During the study period, a total of 8,735 singleton pregnancies that were eligible according to the inclusion and exclusion criteria were identified. A total of 1,895 women (21.7%) had a glucose value ≥140 mg/dL in the 50-g GCT and were given a further diagnostic 75-g OGTT. Among those who participated in the 75-g OGTT, 1,356 women were not diagnosed with GDM, 359 women were diagnosed with GDM exclusively by the IADPSG criteria, and 180 women were diagnosed with GDM by the CC criteria (Fig. 1). The overall incidence of GDM based on the CC criteria was 2.1% (95% CI, 1.8 to 2.4), and an additional 4.1% (95% CI, 3.7 to 4.6) of the participants were diagnosed with GDM by the IADPSG criteria. When the IADPSG criteria were applied, the incidence of GDM increased nearly 3-fold, from 2.1% to 6.2% (95% CI, 5.7 to 6.7). Among the 131 women who participated in the prospective follow-up and were additionally diagnosed with GDM by the IADPSG criteria, 122 (93.1%) had one abnormal value, eight (6.1%) had two abnormal values, and one (0.8%) had three abnormal values during the 75-g OGTT. Out of these women, 42 (32.1%) had abnormal fasting glucose, 27 (20.6%) had abnormal 1-hour glucose, and 72 (55.0%) had abnormal 2-hour glucose values.
Maternal characteristics of GDM
Among the 8,375 pregnant women who were eligible, 2,038 (24.3%) participated in the prospective observational cohort to investigate pregnancy outcomes (Table 1). There were significant differences in age and BMI at the 50-g GCT, prepregnancy BMI, BMI at delivery, and weight gain during pregnancy among the three groups. In the post hoc analysis, women in the CC GDM group had higher prepregnancy BMI and BMI at the 50-g GCT and lower weight gain during pregnancy compared to women in the IADPSG GDM group (P<0.05). The prevalence of combined overweight and obesity was 18.8%, 30.5%, and 43.5% in the No GDM, IADPSG GDM, and CC GDM groups, respectively (P<0.001). There were also significant differences in glucose values of the 50-g GCT and 75-g OGTT (P<0.05). However, there was no significant difference in blood pressure, parity, or gestational age at the 75-g OGTT among the three groups. In the CC GDM group, 28 women (40.8%) received insulin treatment during pregnancy.
Association between GDM diagnostic criteria and pregnancy outcomes
Women in the CC GDM group were managed according to standard clinical practice guidelines. However, women in the IADPSG GDM group were not managed as GDM patients and had routine prenatal obstetric care. Regarding maternal outcomes (Table 2), there was a significant difference in the frequency of preeclampsia among the three groups (P<0.05). The frequency of labor induction, primary cesarean delivery, and preterm delivery did not differ among the three groups. Regarding neonatal outcomes (Table 2), we observed significant differences in gestational age at delivery, birth weight, LGA, macrosomia, neonatal hypoglycemia, and requirement for phototherapy (P<0.05).
Table 2. Maternal and neonatal outcomes according to GDM diagnosis.
| Variable | No GDM | IADPSG GDM | CC GDM | P valuea |
|---|---|---|---|---|
| Number | 1,838 | 131 | 69 | |
| Maternal outcome | ||||
| Preeclampsia | 7 (0.4) | 4 (3.1) | 1 (1.4) | <0.001 |
| Labor induction | 149 (8.1) | 12 (9.2) | 6 (8.7) | 0.903 |
| Primary Cesarean delivery | 592 (32.2) | 37 (28.2) | 21 (30.4) | 0.620 |
| Preterm delivery | 94 (5.1) | 11 (8.4) | 5 (7.2) | 0.217 |
| Neonatal outcome | ||||
| Male sex | 933 (50.8) | 72 (55.0) | 38 (55.1) | 0.527 |
| Gestational age, wk | 39.3±1.4 | 39.1±1.4 | 39.0±1.3 | 0.035 |
| Birth weight, g | 3,253±419 | 3,362±495 | 3,319±545 | 0.010 |
| LGA | 178 (9.7) | 28 (21.4) | 14 (20.3) | <0.001 |
| Macrosomia (>4.0 kg) | 63 (3.4) | 11 (8.4) | 7 (10.1) | 0.001 |
| SGA | 187 (10.2) | 10 (7.6) | 7 (10.1) | 0.645 |
| Shoulder dystocia or birth injury | 9 (0.5) | 2 (1.5) | 0 | 0.244 |
| Neonatal hypoglycemia | 9 (0.5) | 3 (2.3) | 3 (4.3) | <0.001 |
| Phototherapy | 515 (28.1) | 51 (38.9) | 17 (24.6) | 0.023 |
| NICU admission | 181 (9.9) | 9 (6.9) | 9 (13.0) | 0.349 |
| Overall adverse pregnancy outcome | 1,149 (62.5) | 94 (71.8) | 48 (69.6) | 0.058 |
Values are presented as number (%) or mean±standard deviation.
GDM, gestational diabetes mellitus; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; CC, Carpenter-Coustan; LGA, large for gestational age; SGA, small for gestational age; NICU, neonatal intensive care unit.
aP for analysis of variance in the case of continuous variables and chi-square test in the case of discrete variables. The overall adverse pregnancy outcomes included preeclampsia, labor induction, primary cesarean delivery, birth weight above the 90th percentile, macrosomia, birth weight below the 10th percentile, shoulder dystocia or birth injury, neonatal hypoglycemia, phototherapy, and NICU admission.
In the multiple logistic regression analysis (Table 3), the IADPSG GDM group showed a significantly increased risk of preeclampsia, LGA, macrosomia, neonatal hypoglycemia, and requirement for phototherapy in both the unadjusted and adjusted models (P<0.05). The CC GDM group, in which management for glycemic control was provided, had an increased risk of LGA, macrosomia, and neonatal hypoglycemia compared to the no GDM group (P<0.05). The overall adverse pregnancy outcomes were more frequently observed in the IADPSG GDM group compared to the no GDM group, with an OR of 1.63 (95% CI, 1.08 to 2.47; P=0.021). However, there was no significant increase in overall adverse outcomes in the CC GDM group.
Table 3. Association between GDM diagnosis and pregnancy outcomes.
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Preeclampsia | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 8.17 (2.36–28.3) | 0.001a | 6.90 (1.84–25.87) | 0.004a |
| CC GDM | 3.82 (0.46–31.44) | 0.213 | 2.47 (0.277–22.11) | 0.419 |
| Labor induction | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 1.14 (0.62–2.12) | 0.671 | 1.31 (0.68–2.50) | 0.420 |
| CC GDM | 1.08 (0.46–2.54) | 0.861 | 1.62 (0.66–3.97) | 0.289 |
| Primary cesarean delivery | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 0.83 (0.56–1.23) | 0.348 | 0.80 (0.50–1.26) | 0.330 |
| CC GDM | 0.92 (0.55–1.55) | 0.757 | 0.60 (0.33–1.08) | 0.087 |
| Preterm deliveryb | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 1.70 (0.89–3.26) | 0.110 | 1.86 (0.96–3.59) | 0.065 |
| CC GDM | 1.45 (0.57–3.68) | 0.436 | 1.58 (0.62–4.08) | 0.341 |
| LGA | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 2.54 (1.62–3.96) | <0.001a | 2.39 (1.50–3.81) | <0.001a |
| CC GDM | 2.37 (1.29–4.36) | 0.005a | 2.07 (1.08–3.94) | 0.028a |
| Macrosomia (>4.0 kg) | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 2.58 (1.33–5.03) | 0.005a | 2.53 (1.26–5.10) | 0.009a |
| CC GDM | 3.18 (1.40–7.23) | 0.006a | 3.34 (1.39–8.00) | 0.007a |
| SGA | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 0.73 (0.38–1.42) | 0.351 | 0.75 (0.38–1.47) | 0.396 |
| CC GDM | 0.99 (0.45–2.21) | 0.997 | 0.94 (0.42–2.13) | 0.879 |
| Shoulder dystocia or birth injury | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 3.14 (0.67–14.69) | 0.146 | 3.59 (0.75–17.18) | 0.109 |
| CC GDM | NA | NA | NA | NA |
| Neonatal hypoglycemia | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 4.75 (1.27–17.76) | 0.021a | 3.84 (1.01–14.74) | 0.049a |
| CC GDM | 9.21 (2.44–34.82) | 0.001a | 4.98 (1.17–21.31) | 0.030a |
| Phototherapy | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 1.63 (1.13–2.35) | 0.009a | 1.57 (1.07–2.31) | 0.022a |
| CC GDM | 0.84 (0.48–1.46) | 0.530 | 0.63 (0.36–1.13) | 0.122 |
| NICU admission | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 0.68 (0.34–1.35) | 0.266 | 0.67 (0.30–1.24) | 0.169 |
| CC GDM | 1.37 (0.67–2.81) | 0.388 | 1.15 (0.55–2.41) | 0.702 |
| Overall adverse outcomes | ||||
| No GDM | 1.00 | 1.00 | ||
| IADPSG GDM | 1.52 (1.03–2.25) | 0.035a | 1.63 (1.08–2.47) | 0.021a |
| CC GDM | 1.17 (0.90–1.52) | 0.236 | 1.05 (0.80–1.39) | 0.711 |
Model 1, unadjusted; Model 2, adjusted for maternal age, parity, height, body mass index at delivery, gestational age at delivery, and baby's sex; GDM, gestational diabetes mellitus; OR, odds ratio; CI, confidence interval; IADPSG, International Association of the Diabetes and Pregnancy Study Groups; CC, Carpenter-Coustan; LGA, large for gestational age; SGA, small for gestational age; NA, not applicable; NICU, neonatal intensive care unit.
aP<0.05, bGestational age at delivery was not adjusted for preterm delivery in model 2.
DISCUSSION
In this prospective observational study, we estimated that the incidence of GDM would increase by nearly 3-fold, from 2.1% using the CC criteria to 6.2% using the IADPSG criteria. There were significant differences in maternal characteristics regarding prepregnancy BMI and BMI at delivery among the three groups, with women in the CC GDM group having the highest BMI. Compared to the no GDM group, women in the IADPSG GDM group had an increased risk of preeclampsia, and neonates had an increased risk of LGA, macrosomia, and neonatal hypoglycemia. To the best of our knowledge, this is the first prospective cohort to investigate the impact of the IADPSG criteria on the incidence of GDM and pregnancy outcomes in Korea. The results of this study provide important data to discuss whether the IADPSG criteria should be applied more widely in Korea.
The incidence of GDM depends not only on the diagnostic method but also on the ethnicity of the study population, and prevalence of obesity and diabetes. Therefore, it would be important to investigate how the IADPSG criteria would influence the incidence of GDM in different ethnic populations and different healthcare systems. According to the study design, GDM incidence might have been underestimated using both the IADPSG criteria and the CC criteria in this study. It is expected that the incidence of GDM would increase by two to three-fold if the IADPSG criteria are used [26]. In the original HAPO study, the incidence of GDM based on the IADPSG criteria was estimated to be 17.8% [12]. In a study comparing the IADPSG criteria and CC criteria in a Chinese population, it was shown that the incidence of GDM would increase to 19.9% from 7.98% [20]. In Japan, the adoption of the IADPSG criteria also increased the incidence of GDM to 6.6% compared to 2.4% based on the Japan Society of Obstetrics and Gynecology criteria [21]. The three-fold increase in GDM incidence observed in our study is in accordance with other study results in the Asian population. It is expected that the increase in GDM incidence would have a substantial impact on medical costs, resource allocation, and medicalization of pregnancy [13]. Further investigations are warranted regarding the cost effectiveness of the IADPSG criteria in terms of reducing overall adverse pregnancy outcomes in this population.
Women who were diagnosed with GDM by the IADPSG criteria were characterized by a mild degree of metabolic deterioration. Women in the IADPSG GDM group had elevated glucose concentrations compared to the no GDM group but not as high as those of the CC GDM group. Compared to the CC GDM group, women in the IADPSG GDM group were less obese as estimated by lower prepregnancy BMI and lower BMI at the time of the 50-g GCT. The mean BMI of the IADPSG GDM group was only 22.0 kg/m2. Nevertheless, it should be noted that there was a linear increase in the prevalence of combined overweight/obesity in the no GDM (18.8%), IADPSG GDM (30.5%), and CC GDM (43.5%) groups. It is well known that obesity is a major risk factor for GDM [27]. A recent study showed that prepregnancy overweight and obesity account for a significant proportion of LGA infants [28]. From these findings, it would be acceptable to state that a small increase in prepregnancy BMI or being overweight/obese is a significant risk factor for GDM and related adverse pregnancy outcomes in our study population.
Women diagnosed with GDM by the IADPSG criteria were at increased risk of several adverse pregnancy outcomes, including preeclampsia, LGA, macrosomia, neonatal hypoglycemia, and requirement of phototherapy. When the IADPSG criteria were applied to the HAPO study, the frequency of LGA increased by approximately two-fold [4]. Consistently, our results showed that those in the IADPSG GDM group had a 2.39-fold and 2.53-fold increased risk for LGA and macrosomia, respectively. Although clinical practice for glycemic control was provided according to the guidelines, there was still an increased risk of macrosomia in the CC GDM group. This suggests that further efforts are required to reduce adverse pregnancy outcomes in GDM women. Other outcomes, including preeclampsia, birth injury, and neonatal hypoglycemia, developed in only 12, 11, and 15 subjects among 2,038 participants, respectively, and the incidences were below 1%. Among these infrequent adverse outcomes, rates of preeclampsia and neonatal hypoglycemia were significantly higher in the IADPSG GDM group. However, the estimated ORs might have been overestimated due to the small number of outcomes. We also estimated overall adverse outcomes by including 11 adverse pregnancy outcomes and found that women in the IADPSG GDM group had a 1.63-fold increased risk. Interestingly, the risk of overall adverse outcomes in the CC GDM group was not significantly different from that in the No GDM group. It could be speculated that treatment of GDM by medical nutritional therapy and insulin might have reduced several adverse pregnancy outcomes, such as preeclampsia and requirement of phototherapy.
There were several limitations regarding our study. First, the IADPSG criteria were not applied as a one-step approach. Instead, 50-g GCT was used for universal screening, and only those who had glucose levels above 140 mg/dL were selected for the 75-g OGTT. Therefore, we might have missed a number of women who would have been additionally diagnosed with GDM by the IADPSG criteria. Second, regarding the CC GDM criteria, a 75-g OGTT was used instead of a 100-g OGTT, and the 3-hour glucose level was omitted. It is also likely that the incidence of GDM in the CC GDM group is underestimated. Third, we were not able to collect data on cord C-peptide level or neonate body fat percentage. These two outcomes were used to determine the diagnostic threshold of the IADPSG criteria [4]. Nevertheless, we included clinical outcomes that are associated with these measures, such as neonatal hypoglycemia, shoulder dystocia, and birth injury.
Currently, the Korean Diabetes Association endorses using both the IADPSG criteria and the CC criteria for the diagnosis of GDM. However, the IADPSG criteria are not widely used in Korea. There are certain concerns regarding the adoption of the IADPSG criteria. It is still controversial whether using the IADPSG criteria would be cost effective [18,29], and this depends on various factors, including the healthcare system and the total cost to treat GDM. Furthermore, it is not clear whether treatment of these mild GDM patients will result in improved pregnancy outcomes. Previous clinical trials have shown that interventions were effective in reducing adverse outcomes in mild GDM patients [9,30]. Nevertheless, further investigations are required to validate this finding using the same IADPSG criteria. In summary, our study confirms that the IADPSG criteria would increase the incidence of GDM by nearly three-fold, and women diagnosed with GDM based on the IADPSG criteria would be at increased risk of overall adverse pregnancy outcomes, including preeclampsia, LGA, macrosomia, neonatal hypoglycemia, and requirement of phototherapy. Further investigation on the cost effectiveness and pregnancy outcomes in treating women diagnosed by the IADPSG criteria in Korea will be critical for deciding whether to accept the IADPSG criteria more widely.
ACKNOWLEDGMENTS
This research was supported by a grant from Seoul National University Bundang Hospital funded by SK Telecom (06-2013-098) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HI15C3131).
Footnotes
CONFLICTS OF INTEREST: Funding agency, SK Telecom did not involve in not only selection of project but also research work. No other potential conflict of interest relevant to this article was reported.
- Conception or design: S.H.K., M.Y.K., H.C.J.
- Acquisition, analysis, or interpretation of data: M.H.K., S.H.K., J.S.H., H.R.C., S.H.C., M.Y.K., H.C.J.
- Drafting the work or revising: M.H.K., S.H.K., H.C.J.
- Final approval of the manuscript: M.H.K., S.H.K., S.H.K., J.S.H., H.R.C., S.H.C., M.Y.K., H.C.J.
References
- 1.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–B167. [PubMed] [Google Scholar]
- 3.Jang HC, Cho NH, Min YK, Han IK, Jung KB, Metzger BE. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM. Diabetes Care. 1997;20:1582–1588. doi: 10.2337/diacare.20.10.1582. [DOI] [PubMed] [Google Scholar]
- 4.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettitt DJ, Knowler WC, Baird HR, Bennett PH. Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care. 1980;3:458–464. doi: 10.2337/diacare.3.3.458. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara A, Weiss NS, Hedderson MM, Quesenberry CP, Jr, Selby JV, Ergas IJ, Peng T, Escobar GJ, Pettitt DJ, Sacks DA. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia. 2007;50:298–306. doi: 10.1007/s00125-006-0517-8. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 8.Kim C. Gestational diabetes mellitus in Korean women: similarities and differences from other racial/ethnic groups. Diabetes Metab J. 2014;38:1–12. doi: 10.4093/dmj.2014.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 10.HAPO Study, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 12.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, Persson B, Trimble ER HAPO Study Cooperative Research Group. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, Minkoff HL, Poindexter B, Prosser LA, Sawaya GF, Scott JR, Silver RM, Smith L, Thomas A, Tita AT. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 14.World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 15.IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23:579–593. doi: 10.1111/j.1464-5491.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Practice Bulletins: Obstetrics. Obstetrics Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 17.Feldman RK, Tieu RS, Yasumura L. Gestational diabetes screening: the International Association of the Diabetes and Pregnancy Study Groups compared with Carpenter-Coustan screening. Obstet Gynecol. 2016;127:10–17. doi: 10.1097/AOG.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 18.Duran A, Saenz S, Torrejon MJ, Bordiu E, Del Valle L, Galindo M, Perez N, Herraiz MA, Izquierdo N, Rubio MA, Runkle I, Perez-Ferre N, Cusihuallpa I, Jimenez S, Garcia de, Fernandez MD, Montanez C, Familiar C, Calle-Pascual AL. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care. 2014;37:2442–2450. doi: 10.2337/dc14-0179. [DOI] [PubMed] [Google Scholar]
- 19.Waters TP, Dyer AR, Scholtens DM, Dooley SL, Herer E, Lowe LP, Oats JJ, Persson B, Sacks DA, Metzger BE, Catalano PM HAPO Cooperative Study Research Group. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care. 2016;39:2204–2210. doi: 10.2337/dc16-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol. 2014;34:100–104. doi: 10.1038/jp.2013.143. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa M, Yamada T, Yamada T, Akaishi R, Nishida R, Cho K, Minakami H. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract. 2010;90:339–342. doi: 10.1016/j.diabres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 24.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins: Obstetrics. Practice bulletin no. 173: fetal macrosomia. Obstet Gynecol. 2016;128:e195–e209. doi: 10.1097/AOG.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ. Birth weight for gestational age patterns by sex, plurality, and parity in Korean population. Korean J Pediatr. 2007;50:732–739. [Google Scholar]
- 26.Brown FM, Wyckoff J. Application of one-step IADPSG versus two-step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes. Curr Diab Rep. 2017;17:85. doi: 10.1007/s11892-017-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedderson MM, Williams MA, Holt VL, Weiss NS, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2008;198:409.e1–409.e7. doi: 10.1016/j.ajog.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black MH, Sacks DA, Xiang AH, Lawrence JM. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care. 2013;36:56–62. doi: 10.2337/dc12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes in a tertiary care hospital: implications of applying the IADPSG criteria. Arch Gynecol Obstet. 2012;286:373–378. doi: 10.1007/s00404-012-2324-4. [DOI] [PubMed] [Google Scholar]
- 30.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]