Abstract
The metacestode stage of Echinococcus granulosus and Echinococcus multilocularis cause cystic echinococcosis and alveolar echinococcosis, respectively, which result in severe medical and veterinary problems. In this study, as an exploration of novel treatment agents against echinococcosis, we demonstrated that ampelopsin (AMP), which is extracted from Ampelopsis grossedentata and has been clinically used for treatments of various types of diseases including cancers for a long time, exhibited profound in vitro effect against E. granulosus protoscoleces and E. multilocularis metacestodes. Furthermore, in vitro cytotoxicity assay also demonstrated that AMP at the effective dose against E. granulosus protoscoleces and E. multilocularis metacestodes did not show significant toxicity to human hepatocytes. These results suggest that AMP has the potential as an alternative agent against echinococcosis.
Keywords: ampelopsin, echinococcosis, efficacy, toxicity
Echinococcosis, caused by larval stage of the genus of Echinococcus (Cestoda, Plathelminthes), is a health-affecting, even life-threatening disease of humans and livestock. Mebendazole and albendazole (ABZ) are the few of the current authorized anti-helminth drugs for humans [9]. However, these drugs have been shown to act parasitostatically rather than parasitocidally for alveolar echinococcosis [1, 23]. Meanwhile, high recurrence rates after interruption of therapy [2] and the occurrence of side effects limit their application [5]. Thus, new drug candidates for echinococcosis are urgently needed.
The Chinese herb Ampelopsis grossedentata is widely distributed in southern region of China and used in traditional Chinese medicine for treatments of cold and pyretic fever, tinea corporis, furuncle, primary hypertension and jaundice hepatitis, with a long history of several hundred years [14]. Ampelopsin (AMP, (2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one), which is extracted from the stems and leaves of A. grossedentata, is a type of flavonoid. AMP is also called dihydromyricetin and has been reported to have various pharmacological activities, including anti-inflammatory [22], anti-oxidative [7], hepatoprotective activities [6, 18] and chemotaxis effect of immunocytes [28]. In addition, AMP has potent anti-cancer activities in various types of cancers [15, 20, 21, 29, 30]. E. granulosus and E. multilocularis metacestodes exhibit continual asexual proliferation, and especially E. multilocularis shows tumor-like infiltrative growth. Therefore, screening of the drugs inhibiting proliferation of cancer cells becomes one of the useful strategies for chemotherapeutical treatment of echinococcosis [8]. Nowadays, it has been reported that a number of antitumor drugs exhibit promising effects against E. multilocularis [10, 27]. To the best of our knowledge, no evidence has been reported for the effect of AMP on echinococcosis up to the present. Therefore, in this study, we evaluated the in vitro efficacy of AMP against E. granulosus and E. multilocularis.
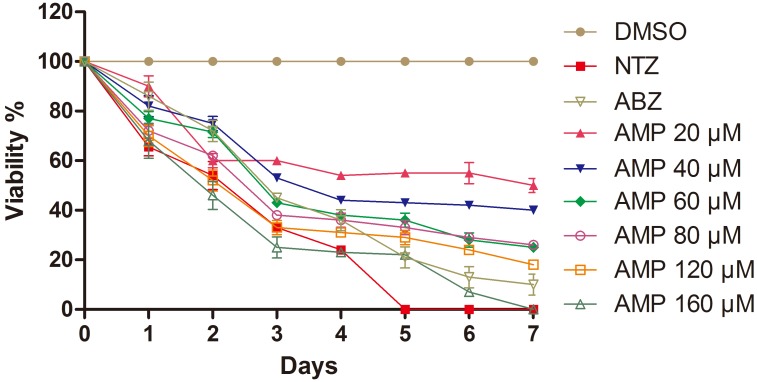
To evaluate the efficacy AMP against E. granulosus, protoscoleces were distributed to 24-well culture plates (100 protoscoleces/well). The same numbers of protoscoleces in culture medium containing 0.2% dimethyl sulphoxide (DMSO) alone, as well as 40 µM of nitazoxanide (NTZ) and ABZ was respectively used as normal and positive controls. Treatments were carried out at 37°C, 5% CO2. The protoscoleces were daily observed for consequent 7 days and viability was assessed by trypan blue exclusion test [26], in which, the dead protoscoleces were counted in triplicate for each concentration and the mean values of the viability were derived from three experiments. Until the end the experiment, almost all protoscoleces in the control group survived (Fig. 1) and maintained normal morphology (Fig. 2), while 40 µM AMP reduced the number of protoscoleces by 56% within a period of 4 days and killed 60% of protoscoleces after 7 days of treatment. As positive controls, NTZ and ABZ in the same concentration killed 100 and 92% of the protoscoleces after 5 and 7 days of treatments, respectively. Simultaneously, the treatment of 1 day by ABZ resulted in distortion, vesiculation of the protoscoleces, and collapse of suckers; after 3 and 7 days, protoscoleces were shrunken, with decrease of calcium precipitates. When the concentration of AMP increased to 160 µM, after 1 day of exposure, 14% protoscoleces were killed. Accordingly, the protoscoleces exhibited distortion, vesiculation and vacuolization in the scolex, and blebs on the tegument (Fig. 2) as well as a short and rapid tremor (data not shown), which normally remain motionless or move slowly and slightly. After 3 days treatment, 45% protoscoleces were killed and extensive damages on the protoscoleces occurred, such as severe distortion and contraction of the protoscoleces, collapse of suckers, and decrease of calcium precipitates. When the treatment time extended to 7 days, 100% of the protoscoleces became dead and showed complete destruction of the parenchyma. Clearly, the administration of AMP to protoscoleces showed profound dose- and time- dependent effects.
Fig. 1.
Viability of Echinococcus granulosus protoscoleces following in vitro treatment with dimethyl sulphoxide (DMSO), nitazoxanide (NTZ), albendazole (ABZ) and ampelopsin (AMP). NTZ and ABZ (positive controls) were added at a final concentration of 40 µM. Protoscoleces were incubated in the presence of AMP for up to 7 days, and the viability was measured by trypan blue exclusion test. The corresponding numbers of viable and nonviable protoscoleces were counted in three wells for each concentration. Mean values of viability were derived from three experiments.
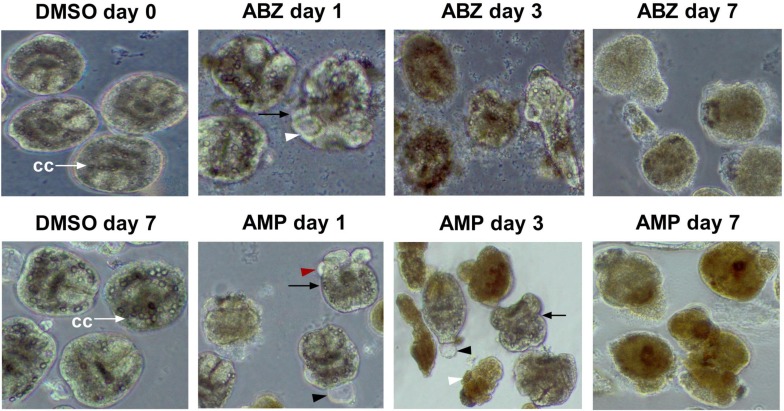
Fig. 2.
Morphologies of Echinococcus granulosus protoscoleces after treatment with albendazole (ABZ) and ampelopsin (AMP). Protoscoleces were incubated in the presence of 40 µM ABZ or 160 µM AMP. Protoscoleces in dimethyl sulphoxide (DMSO) control showed normal morphology during the culture period. White arrow points towards calcareus corpuscles (cc). At day 1 after treatment with ABZ, protoscoleces exhibited distortion and vesiculation (black arrow), and collapse of suckers (white arrowhead); at day 3 and 7, most protoscoleces were shrunken, with decrease of calcium precipitates. At day 1 after treatment with AMP, the protoscoleces exhibited distortion and vesiculation (black arrow), vacuolization in the scolex (black arrowhead), and blebs on the tegument (red arrowhead); at day 3, the protoscoleces exhibited severe distortion and contraction of protoscoleces, and collapse of the sucker (white arrowhead); and at day 7, protoscoleces were darkening and demonstrated complete destruction of parenchyma.
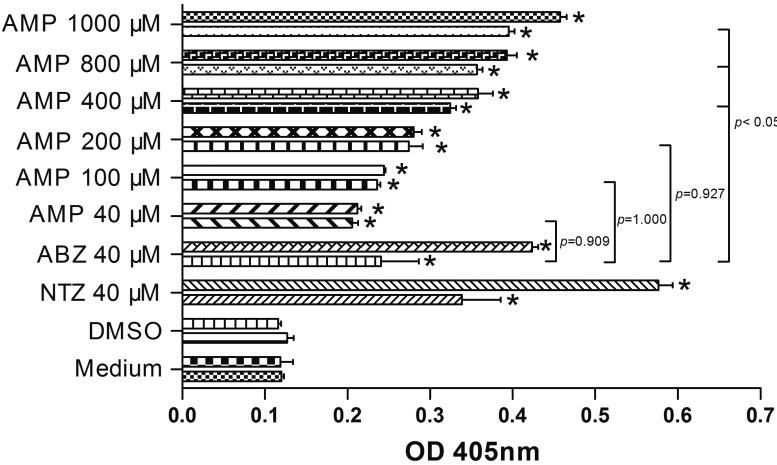
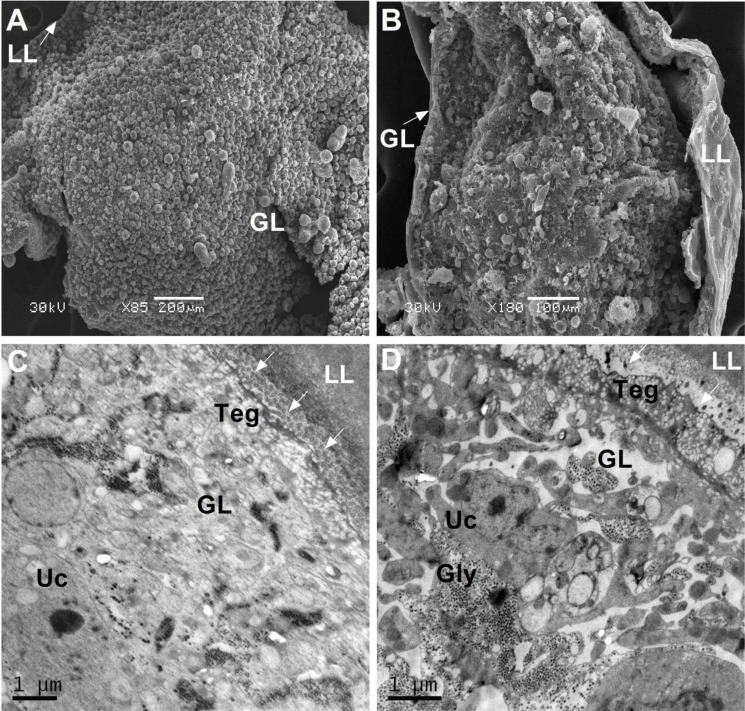
The evaluation of the efficacy of AMP against E. multilocularis metacestodes (Xinjiang isolate) was carried out as previously described [23]. The vesicles of metacestodes which reached expected diameters (2 to 4 mm) were distributed to separate culture with approximately 35 vesicles. At 36 and 120 hr of drug treatments, the culture supernatants were collected for measurements of E. multilocularis alkaline phosphatase (EmAP) activity [24]. A405 values were expressed as the mean ± standard deviation (SD).Variance was analyzed by one-way ANOVA using SPSS 19.0 software. The results at 120 hr demonstrated a continuous increase of alkaline phosphatase activity from metacestodes during treatment with each drug (Fig. 3). The effect of AMP against metacestodes was dose dependent. At 36 hr of treatment, AMP at 40 µM resulted in a relatively lower increase of EmAP activity than ABZ at the same concentration. While when AMP increased to 100 µM, its effect on EmAP activity became similar to ABZ. The results indicated significant increases of EmAP activity in 400, 800 and 1,000 µM AMP treated groups versus the ABZ group. Scanning electron microscopes (SEM) (Fig. 4A) and transmission electron microscopes (TEM) (Fig. 4C) demonstrated that the control metacestode vesicles had a typical structure: the outer acellular laminated layer was followed by a tegument and inner germinal layer. The tegument lied adjacent to the laminated layer, with distinct microtriches protruding well into the laminated layer. The germinal layer was composed of glycogen storage cells, undifferentiated cells and connective tissue. The metacestodes treated with AMP exhibited profound ultrastructural changes (Fig. 4B and 4D): the microtriches reduced dramatically and absent in most parts of tegument. The laminated layer had separated from the tegument. The germinal layer tissues showed a clear reduction in cell number, and in some areas, were completely destructed.
Fig. 3.
Optical density values measured for Echinococcus multilocularis alkaline phosphatase activity following in vitro treatment on metacestodes with dimethyl sulphoxide (DMSO), nitazoxanide (NTZ), albendazole (ABZ) and ampelopsin (AMP) at 36 and 120 hr. In each of drug treated groups, the upper column exhibited EmAP activity in culture supernatants of metacestodes treated with drugs in 120 hr, and the column below exhibited in 36 hr. *P<0.05 vs. medium and DMSO groups.
Fig. 4.
The morphology of Echinococcus multilocularis metacestode under the scanning electron microscopes (SEM) and transmission electron microscopes (TEM). SEM of control metacestodes after 120 hr in vitro culture in medium containing 0.2% dimethyl sulphoxide (DMSO) (A) showed that the germinal cells had an intact morphology. LL, laminated layer; GL, germinal layer. Metacestodes treated with 400 µM ampelopsin (AMP) in 120 hr (B) showed massive signs of destruction and most of the GL cells were detached from the LL. TEM of control metacestodes (C) showed an intact structure. Teg, tegument; Uc, undifferentiated cell; Gly, glycogen storage cell. White arrows indicated microtriches. Metacestodes treated with 400 µM AMP in 120 hr (D) showed profound ultrastructural changes: the microtriches reduced dramatically and absent in most parts of tegument. The LL had separated from the tegument. The GL tissues showed loose arrangement and in some areas, were completely destructed.
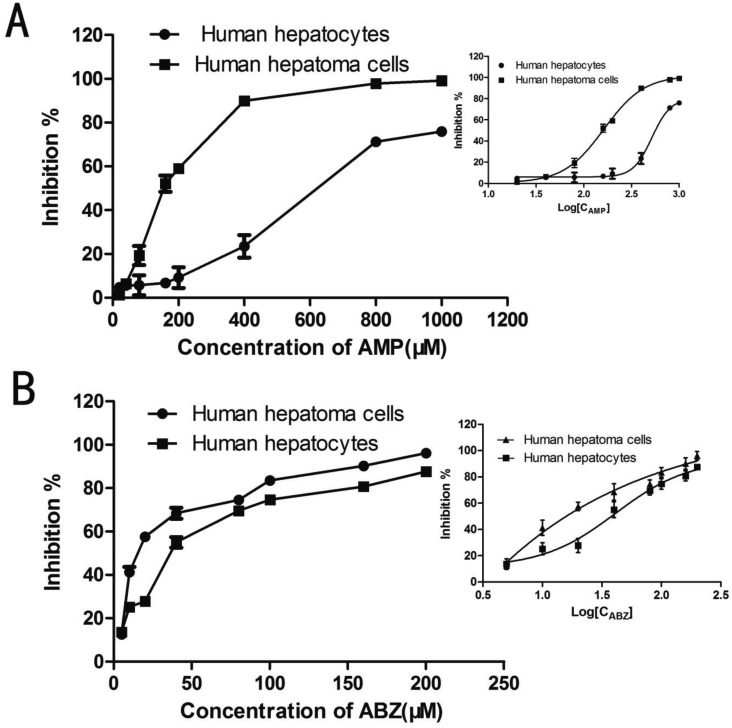
Furthermore, we evaluated the cytotoxicity of AMP in human hepatocytes and hepatoma cells. The cells were added onto 96-well culture plates at a cell density of 10,000 cells/well and incubated for 24 hr at 37°C, 5% CO2. Then, AMP was added to the cultures at concentrations of 20, 40, 80, 160, 200, 400, 800 and 1,000 µM. ABZ was added to the cultures at concentrations of 5, 10, 20, 40, 80, 100, 160 and 200 µM. As controls, the cells were performed with culture medium containing 0.2% DMSO. Viability was examined 48 hr later using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) reduction assay [25]. A490 values were read on an ELISA reader and the IC50 values were calculated in GraphPad Prism 5. The results exhibited that AMP had cytotoxicity in a concentration-dependent manner and the IC50 value for AMP in human hepatocytes was 517.8 µM, which was far higher than in hepatoma cells (147.1 µM). For ABZ, the IC50 value in human hepatocytes and hepatoma cells were 38.7 µM and 12.3 µM respectively. AMP at the effective dose against E. granulosus protoscoleces and E. multilocularis metacestodes did not show significant toxicity to human hepatocytes. Comparatively, ABZ exhibited significant cytotoxicity to human hepatocytes.
As a type of flavonoid extracted from A. grossedentata, ampelopsin has multiple functions in inflammation, oxidation, and cancer. Flavonoids and flavonoid-containing extracts possess in vitro and in vivo activities against a number of helminth parasites, such as Schistosoma mansoni [3, 11], Fasciolopsis buski [12], Haemonchus contortus [17], Brugia malayi [13] and Raillietina echinobothrida [4]. In this study, our results demonstrated that AMP exhibited significant activities against E. granulosus protoscoleces and E. multilocularis metacestodes in vitro. Considering the prominent difference of IC50 between AMP and ABZ, which was over a dozen times, AMP really showed a safer and more potent efficacy against Echinococcus. In E. granulosus protoscoleces, AMP exhibited an in vitro efficacious activity at short times (Fig. 1). In E. multilocularis metacestodes, SEM and TEM (Fig. 4) showed the ultrastructural damages of metacestodes, especially the germinal layer, which were closely correlated with the increase of EmAP activity (Fig. 3). AMP triggered the release of high levels of EmAP activity from metacestodes after 36 hr treatment. At 120 hr, the EmAP activities in culture supernatants were higher than that at 36 hr though the increases were insignificant. The results revealed that it was in the early stage of incubation that AMP had promptly produced significant anti-metacestodes effect. The characteristic of the quick-acting function of AMP was similar to other types of flavonoids, the effects of which on echinococcosis had been previously reported [19]. In addition, on ovarian cancer cell, AMP also exhibits in vitro anticancer activity at short times [20].
AMP has in vitro activities against E. granulosus and E. multilocularis, but its underlying mechanism of action is not clear and needed to be investigated in future studies. Some studies reported that AMP exhibits significant activity in inhibiting the proliferation of cancer cells via apoptosis induction associated with downregulation of Bcl-2 expression [20] and in suppressing tumor blood vessels by inhibiting the secretion of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [16]. In addition, other types of flavonoids exhibit the activity against helminth parasites, such as R. echinobothrida [4] and F. buski [12], by influencing the nitric oxide synthase (NOS) activity and nitric oxide (NO) production. So, it was highly suggestive that AMP might share the same mechanisms in anti-echinococcosis activities.
As can be seen in Fig. 5, in vitro cytotoxicity assays demonstrated that human hepatoma cells were much more sensitive than human hepatocytes to AMP. AMP has been shown to possess not only effective hepatoprotective activities, but also potent activities on treatment of various types of cancers including hepatocellular carcinoma based on many studies. Therefore, it is well known that AMP is not harmful to human normal cells [20, 29, 30]. It is worth noting that AMP at the effective dose against E. granulosus protoscoleces and E. multilocularis metacestodes did not show significant toxicity to human hepatocytes. However, in human hepatocytes, ABZ exhibited significant cytotoxicity. According to the suggested mechanisms, it is easy to understand the remarkable difference of cytotoxicity between AMP and ABZ, where AMP exhibited much less cytotoxicity to human hepatocytes versus hepatoma cells: ABZ plays role by inhibiting polymerization of tubulin that takes place in all organisms and cells including both human hepatocytes and hepatoma cells, while AMP probably plays its role by complete different ways such as NOS activity and NO production, which does not equally act on both the cells. Meanwhile, it is well-known that, the proliferation of Echinococcus metacestodes is mainly in the liver of humans and other hosts, leads to severe destruction in liver. Therefore, as a natural compound extracted from A. grossedentata, the relatively low toxic effects and hepatoprotective properties of AMP show obvious advantages for the treatment of echinococcosis.
Fig. 5.
Cytotoxicity of ampelopsin (AMP) and albendazole (ABZ) on human hepatocytes and hepatoma cells. Cells were grown to confluence and incubated with different concentrations of AMP and ABZ for 48 hr. Assessments were made by MTT reduction assay. Values were presented as percentages relative to the values for dimethyl sulphoxide (DMSO) control group. The fitting curve of cell inhibitory rate and the IC50 values were calculated in GraphPad Prism 5.
In conclusion, the present study demonstrates that AMP exhibits profound activities against E. granulosus protoscoleces and E. multilocularis metacestodes in vitro with relatively low toxicity and is worth further in vivo experiment as a new strategy in treating echinococcosis.
Acknowledgments
This research was supported by NSFC (grant No.81171632), the Fundamental Research Funds for the Central Universities (Protocol code lzujbky-2018-90).
REFERENCES
- 1.Ammann R. W., Ilitsch N., Marincek B., Freiburghaus A. U., Swiss Echinococcosis Study Group.1994. Effect of chemotherapy on the larval mass and the long-term course of alveolar echinococcosis. Hepatology 19: 735–742. doi: 10.1002/hep.1840190328 [DOI] [PubMed] [Google Scholar]
- 2.Ammann R. W., Hirsbrunner R., Cotting J., Steiger U., Jacquier P., Eckert J.1990. Recurrence rate after discontinuation of long-term mebendazole therapy in alveolar echinococcosis (preliminary results). Am. J. Trop. Med. Hyg. 43: 506–515. doi: 10.4269/ajtmh.1990.43.506 [DOI] [PubMed] [Google Scholar]
- 3.Braguine C. G., Bertanha C. S., Gonçalves U. O., Magalhães L. G., Rodrigues V., Melleiro Gimenez V. M., Groppo M., Silva M. L., Cunha W. R., Januário A. H., Pauletti P. M.2012. Schistosomicidal evaluation of flavonoids from two species of Styrax against Schistosoma mansoni adult worms. Pharm. Biol. 50: 925–929. doi: 10.3109/13880209.2011.649857 [DOI] [PubMed] [Google Scholar]
- 4.Chetia M., Das R.2018. Effect of (-)-epicatechin, a flavonoid on the NO and NOS activity of Raillietina echinobothrida. Acta Trop. 178: 311–317. doi: 10.1016/j.actatropica.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Davis A., Pawlowski Z. S., Dixon H.1986. Multicentre clinical trials of benzimidazolecarbamates in human echinococcosis. Bull. World Health Organ. 64: 383–388 (in Chinese). [PMC free article] [PubMed] [Google Scholar]
- 6.Hase K., Ohsugi M., Xiong Q., Basnet P., Kadota S., Namba T.1997. Hepatoprotective effect of Hovenia dulcis THUNB. on experimental liver injuries induced by carbon tetrachloride or D-galactosamine/lipopolysaccharide. Biol. Pharm. Bull. 20: 381–385. doi: 10.1248/bpb.20.381 [DOI] [PubMed] [Google Scholar]
- 7.He G., Du F., Yang W., Pei G., Zhu Y.2003. [Effects of tengcha flavonoids on scavenging oxygen free radicals and inhibiting lipid-peroxidation]. Zhong Yao Cai 26: 338–340. [PubMed] [Google Scholar]
- 8.Hemphill A., Müller J.2009. Alveolar and cystic echinococcosis: towards novel chemotherapeutical treatment options. J. Helminthol. 83: 99–111. doi: 10.1017/S0022149X0928936X [DOI] [PubMed] [Google Scholar]
- 9.Hemphill A., Spicher M., Stadelmann B., Mueller J., Naguleswaran A., Gottstein B., Walker M.2007. Innovative chemotherapeutical treatment options for alveolar and cystic echinococcosis. Parasitology 134: 1657–1670. doi: 10.1017/S0031182007003198 [DOI] [PubMed] [Google Scholar]
- 10.Hemphill A., Stadelmann B., Rufener R., Spiliotis M., Boubaker G., Müller J., Müller N., Gorgas D., Gottstein B.2014. Treatment of echinococcosis: albendazole and mebendazole-what else? Parasite 21: 70. doi: 10.1051/parasite/2014073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jatsa H. B., Kenfack C. M., Simo D. N., Feussom N. G., Nkondo E. T., Tchuem Tchuente L. A., Tsague C. D., Dongo E., Kamtchouing P.2015. Schistosomicidal, hepatoprotective and antioxidant activities of the methanolic fraction from Clerodendrum umbellatum Poir leaves aqueous extract in Schistosoma mansoni infection in mice. BMC Complement. Altern. Med. 15: 248. doi: 10.1186/s12906-015-0788-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kar P. K., Tandon V., Saha N.2002. Anthelmintic efficacy of Flemingia vestita: genistein-induced effect on the activity of nitric oxide synthase and nitric oxide in the trematode parasite, Fasciolopsis buski. Parasitol. Int. 51: 249–257. doi: 10.1016/S1383-5769(02)00032-6 [DOI] [PubMed] [Google Scholar]
- 13.Lakshmi V., Joseph S. K., Srivastava S., Verma S. K., Sahoo M. K., Dube V., Mishra S. K., Murthy P. K.2010. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop. 116: 127–133. doi: 10.1016/j.actatropica.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Zhou T.1999. A Pharmacognostical study of tengcha, bigdentate ampelopsis (Ampelopsis grossedentata). Chin. Tradit. Herbal Drugs 30: 459–463. [Google Scholar]
- 15.Liu T., Liu P., Ding F., Yu N., Li S., Wang S., Zhang X., Sun X., Chen Y., Wang F., Zhao Y., Li B.2015. Ampelopsin reduces the migration and invasion of ovarian cancer cells via inhibition of epithelial-to-mesenchymal transition. Oncol. Rep. 33: 861–867. doi: 10.3892/or.2014.3672 [DOI] [PubMed] [Google Scholar]
- 16.Luo G. Q., Zeng S., Liu D. Y.2006. [Inhibitory effects of ampelopsin on angiogenesis]. Zhong Yao Cai 29: 146–150 (in Chinese). [PubMed] [Google Scholar]
- 17.Morais-Costa F., Bastos G. A., Soares A. C., Costa E. G., Vasconcelos V. O., Oliveira N. J., Braga F. C., Duarte E. R., Lima W. S.2016. In vitro and in vivo action of Piptadenia viridiflora (Kunth) Benth against Haemonchus contortus in sheep. Vet. Parasitol. 223: 43–49. doi: 10.1016/j.vetpar.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Murakami T., Miyakoshi M., Araho D., Mizutani K., Kambara T., Ikeda T., Chou W. H., Inukai M., Takenaka A., Igarashi K.2004. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. Biofactors 21: 175–178. doi: 10.1002/biof.552210136 [DOI] [PubMed] [Google Scholar]
- 19.Naguleswaran A., Spicher M., Vonlaufen N., Ortega-Mora L. M., Torgerson P., Gottstein B., Hemphill A.2006. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. Agents Chemother. 50: 3770–3778. doi: 10.1128/AAC.00578-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni F., Gong Y., Li L., Abdolmaleky H. M., Zhou J. R.2012. Flavonoid ampelopsin inhibits the growth and metastasis of prostate cancer in vitro and in mice. PLoS One 7: e38802. doi: 10.1371/journal.pone.0038802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi S., Kou X., Lv J., Qi Z., Yan L.2015. Ampelopsin induces apoptosis in HepG2 human hepatoma cell line through extrinsic and intrinsic pathways: Involvement of P38 and ERK. Environ. Toxicol. Pharmacol. 40: 847–854. doi: 10.1016/j.etap.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 22.Qi S., Xin Y., Guo Y., Diao Y., Kou X., Luo L., Yin Z.2012. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int. Immunopharmacol. 12: 278–287. doi: 10.1016/j.intimp.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Reuter S., Merkle M., Brehm K., Kern P., Manfras B.2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 47: 620–625. doi: 10.1128/AAC.47.2.620-625.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stettler M., Siles-Lucas M., Sarciron E., Lawton P., Gottstein B., Hemphill A.2001. Echinococcus multilocularis alkaline phosphatase as a marker for metacestode damage induced by in vitro drug treatment with albendazole sulfoxide and albendazole sulfone. Antimicrob. Agents Chemother. 45: 2256–2262. doi: 10.1128/AAC.45.8.2256-2262.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K.1986. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 93: 157–165. doi: 10.1016/0022-1759(86)90183-3 [DOI] [PubMed] [Google Scholar]
- 26.Walker M., Rossignol J. F., Torgerson P., Hemphill A.2004. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J. Antimicrob. Chemother. 54: 609–616. doi: 10.1093/jac/dkh386 [DOI] [PubMed] [Google Scholar]
- 27.Yuan M., Song X., Lv W., Xin Q., Wang L., Gao Q., Zhang G., Liao W., Lian S., Jing T.2019. Effect of anacardic acid against echinococcosis through inhibition of VEGF-induced angiogenesis. Vet. Res. (Faisalabad) 50: 3. doi: 10.1186/s13567-019-0621-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng S., Luo G. Q., Liu D. Y.2006. [The chemotaxis effect of ampelopsin on the immunocytes]. Zhong Yao Cai 29: 260–262 (in Chinese). [PubMed] [Google Scholar]
- 29.Zhang B., Dong S., Cen X., Wang X., Liu X., Zhang H., Zhao X., Wu Y.2012. Ampelopsin sodium exhibits antitumor effects against bladder carcinoma in orthotopic xenograft models. Anticancer Drugs 23: 590–596. doi: 10.1097/CAD.0b013e32835019f9 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y., Shu F., Liang X., Chang H., Shi L., Peng X., Zhu J., Mi M.2014. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS One 9: e89021. doi: 10.1371/journal.pone.0089021 [DOI] [PMC free article] [PubMed] [Google Scholar]