Abstract
Aeromonas hydrophila causes disease in fish known as Motile Aeromonas Septicemia (MAS), also named as bacterial hemorrhagic septicemia. In this study, a pathogenic A. hydrophila strain was isolated from common carp Cyprinus carpio L., which were suffering from severe hemorrhagic septicemia. According to the phylogenetic analysis derived from 16S rDNA sequence, the isolate formed a single branch in the A. hydrophila group, named AhHN1. Artificial infection results indicated that AhHN1 showed strong pathogenicity in C. carpio and the LD50 was 1.38 × 106 CFU/fish, the clinical symptoms and pathological features of infected fish were similar to those observed in natural infections. The antimicrobial susceptibility testing revealed that AhHN1 resistance to more than 13 kinds of antimicrobial agents. However, the AhHN1 strain exhibited an extremely sensitivity to enrofloxacin, the in vitro activities of enrofloxacin were subsequently investigated and drug selection window (MSW) was 0.0016–0.0125 µg/ml. Pharmacokinetics data showed that plasma concentration of enrofloxacin was 0.0016, 0.0148 and 0.0282 µg/ml at 24 hr after orally administered with 2.5, 5 and 10 mg/kg enrofloxacin. Moreover, dosing once a day of 2.5, 5 and 10 mg/kg enrofloxacin, which the relative protection ratio (RPS) was amounted to 33.3, 66.7, and 83.3%, respectively. Therefore, 5 mg/kg enrofloxacin was considered to be the rational regimen for controlling AhHN1 infection in C. carpio in the countries where the use of enrofloxacin is permitted in aquaculture. The aim of this study was to establish a scientific medication regimen for the prevention and therapy of the mutidrug-resistant A. hydrophila infection.
Keywords: Aeromonas hydrophila, common carp (Cyprinus carpio L.), enrofloxacin, medication regimen
Common carp (Cyprinus carpio L.) belongs to Cyprinidae, is undoubtedly one of the most cultured fish all over the world and accounts for about 10% (over 3 million metric tons) of global annual freshwater aquaculture production [1]. However, frequent outbreaks of infectious diseases pose a serious threat to the further development of the rapidly expanding carp production industry, which not only caused profound economic losses to fishery production, also affected the quality and safety of aquatic products, as well as human health [10].
Aeromonas hydrophila is a Gram negative bacterium that widely distributed in aquatic environments [7] and an opportunistic pathogen for fish, reptiles, amphibians and humans, which is capable of causing severe hemorrhagic septicemia and skin ulceration in aquatic animals and diarrhea in mammals [16, 19]. It was known that A. hydrophila caused motile Aeromonas septicemia (MAS), also named as bacterial hemorrhagic septicemia. It is defined as worldwide distributed septicemic disease that affecting numerous species of freshwater and marine fishes, such as carp (C. carpio & Carassius auratus) [5, 8, 24], grass carp (Ctenopharynodon idellus) [13], channel catfish (Ictalurus punctatus) [19] and tilapia (Oreochromis niloticus) [17], leading to massive mortalities of wild and farmed fish.
Antimicrobial agents considered to be the most important factors influencing the emergence of resistance in bacterial pathogens. However, the inappropriate overuse of antimicrobial agents in the aquaculture industry has led to the development of bacteria resistance, thereby reducing drug efficacy and affect treatment outcomes for infectious diseases [2, 23]. Recently, A. hydrophila display a notable trend of resistance to commonly used antimicrobial agents, multidrug resistant A. hydrophila were isolated from different parts of the world [11, 21]. Moreover, the accumulation of antimicrobial agents in animals and water ponds can be potentially risky to human health and water environment [12]. Therefore, there should be a continuous and concerted effort to control and stimulate the proper use of antibiotics in aquaculture.
In this study, we observed a bacterial disease occurred in cultured common carp (C. carpio L.) and the bacterial pathogen was identified by morphological features and 16S rDNA analysis. Based on the antimicrobial susceptibility analysis, the activities of enrofloxacin and the pharmacokinetics parameters of enrofloxacin were investigated, a rational medication regimen to prevent drug resistant bacteria was proposed for controlling AhHN1 infection in C. carpio. This study will provide a scientific reference to countries where the use of enrofloxacin is permitted in aquaculture, and provide partial protection against infection with drug-resistant pathogens by drawing a specific dosage guideline of antimicrobial agents.
MATERIALS AND METHODS
Fish
Diseased common carp (C. carpio L.) were collected from a local fish farm. The typical disease signs were external haemorrhages, inflammation and ulcers. Healthy C. carpio L. were obtained from the breeding farm in Hebi city of Henan province, average weight 20 ± 5 g. Fish were acclimated in 250 l aerated water with pH 8.0 ± 0.2, dissolved oxygen 7 ± 0.5 mg/l, water hardness 20 ± 1 mg/l CaCO3, total ammonia 0.006 ± 0.001 mg/l, and nitrite 0.03 ± 0.01 mg/l. Temperature was maintained at 25 ± 2°C. Fish were fed twice daily for two weeks before experiments. All procedures and handling of fish were conducted in compliance with the guidelines of the Institutional Laboratory Animal Care and Use Committee, Henan Normal University.
Isolation and identification of bacteria
Gill, intestine, kidney and liver tissues of diseased C. carpio were excised and homogenized in 1 ml of 0.85% saline. The samples were plated on brain heart infusion (BHI) plates incubated at 28°C for 24 hr. The morphological features of bacterial colonies were observed on plates, dominant colonies were selected for bacteria identification. The purified isolates were amplified in BHI medium and DNA extraction was done with a DNA extraction kit (Sangon, Shanghai, China). Then, the bacteria were subjected to PCR with 16S ribosomal DNA primers (F: 5′-AGAGTTTGATCATGGCTCAG-3′ and R: 5′-CTACGGTTACCTTGTTACGAC-3′). The PCR reaction system contains 1 µl of template DNA, 1 µl each of 16S rDNA sense and anti-sense primers, 2.5 µl of dNTP, 2.5 µl 10X PCR Buffer (Mg2+ plus), 0.25 µl of Ex Taq DNA polymerase and RNase-Free Water in a final volume of 20 µl. PCR was carried out for 30 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 1 min with a final extension at 72°C for 10 min. The PCR products were analyzed on 1.5% agarose gel electrophoresis, purified and sequenced in Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China). The BLAST search was analysed at the National Center for Biotechnology Information website (NCBI, http://www.ncbi.nih.gov/BLAST/). Phylogenetic tree was constructed using the neighbor-joining method in the software MEGA 5.05.
Pathogenicity assays
Isolated A. hydrophila strain (AhHN1) was cultured in BHI medium for 18 hr at 28°C. An appropriate amount of bacterial culture was centrifuged at 12,000 rpm for 5 min, the pellet was washed twice with 0.85% saline and re-suspended in saline, the optical density at 600 nm (OD600) was adjusted to 0.6. LD50 dose used for challenge was determined by intraperitoneal injection into C. carpio at 25°C. In brief, healthy fish were randomly divided into five groups, each group contained 20 fish. Fish were challenged with 0.1 ml A. hydrophila suspension (5-fold dilution series of doses ranging from 2 × 106 to 2.5 × 108 CFU/ml) intraperitoneally, the control group were injected with an equal volume of saline. C. carpio started to show infectious symptoms after 12 hr and had a consistent mortality rate within 48 hr, no further death occurred. The infected fish showed external hemorrhages in fins, which is the typical symptom of A. hydrophila infection. Deaths were observed up to 7 days and the LD50 was calculated by Reed-Muench method [22].
MIC and MBC determination
For antimicrobial susceptibility testing, 14 antibacterial agents including enrofloxacin, Balofloxacin, florfenicol, gentamicin, kanamycin, streptomycin, amoxicillin, ampicillin, penicillin G, ceftriaxone, neomycin sulfate, sulfamethazine, sulfamethoxazole and sulfadiazine were chose. The minimum inhibitory concentration (MIC) for AhHN1 were determined using the broth microdilution protocol following VET04-A2 guideline of Clinical and Laboratory Standards Institute (CLSI) [15]. In brief, two-fold dilutions of antimicrobial agents were added to 96-well plates with adjusted bacterial concentration (about 1 × 105 CFU per well), negative control contained only inoculated BHI medium at 28°C for 24 hr. The MIC is the lowest concentration of antimicrobial agents that visually inhibits the growth of microorganisms. A. hydrophila ATCC 7966 was reference strain in parallel with AhHN1 for quality control (QC) purposes. The MICs were determined by the broth microdilution method wtih A. hydrophila ATCC 7966 and AhHN1 on three different days, each tray contained three biological replicates. Thus, each strain produced 9 MIC results for each antimicrobial agent, the mean MIC was calculated.
For minimum bactericidal concentration (MBC) determination, 0.1 ml of culture solution from all wells in the MIC broth dilution assay were plated on BHI agar plates, were incubated for 18 hr at 28°C. The colonies on the agar was counted and the lowest concentration of reagents that could kill 99.9% of the bacteria was determined as the MBC. Three biological replicates for each antimicrobial agent were performed.
MPC and MSW determination
The determination of the mutation preventive concentration (MPC) was described previously [4]. Briefly, a single colony of AhHN1 was inoculated on BHI for overnight, 0.1 ml cultures contained about 3 × 109 CFU were plated on BHI agar plates containing 1–10 folds MIC enrofloxacin, and each drug concentration had three plates, incubated at 28°C overnight. To estimate the MPC of enrofloxacin, logarithms of bacterial numbers were plotted against enrofloxacin. The MPC was taken as the minimum drug that completely inhibited growth. According to the hypothesis of the mutant selection window (MSW) [26], the concentration range from the MIC to the MPC, within which it is proposed that resistant mutants are enriched or selected.
Plasma sample collection
To detect plasma concentrations of enrofloxacin, the fish were divided into three groups, enrofloxacin was administered orally at 2.5, 5, 10 mg/kg body weight, as described previously [27]. Following dosing, blood samples were collected periodically via a needle, and heparin-treated syringe at 0, 0.5, 1, 3, 6, 9, 12, 24, 48 and 72 hr after administration of enrofloxacin, 3 fish were sampled at each time point. Blood samples were centrifuged at 3,500 rpm for 15 min at 4°C and the plasma was then collected and stored at −20°C until analyzed.
Ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) for detection of enrofloxacin
Concentrations of enrofloxacin in plasma was measured on an Acquity UPLC system (Waters, Milford, MA, U.S.A.), and the separations were achieved using an Acquity UPLC BEH C18 column (1.7 µm particle size, 50 mm ×2.1 mm). Separations were performed using binary gradient mobile phases, consisting of acetonitrile (eluent A) and 0.1% formic acid in water (eluent B) at a flow rate of 0.3 ml/min. The separation was performed at 40°C, applying the following gradient program: 0–1 min, 5% A; 1–4 min, linear increase to 90% A; 4–6 min, 5% A. The samples were kept in an autosampler at 16°C. The mass spectrometry analyses were carried out using a Quattro Micro triple quadrupole mass spectrometer equipped with an electrospray ionization source (ESI) (Waters, Milford, MA, U.S.A.). The mass spectrometer was settled in the positive ion mode (ES+), with a capillary voltage of 0.5 kV, cone voltages of 30 V, collision energies of 19 eV, a source temperature of 120°C and a desolvation temperature of 500°C. The nitrogen gas flow was 800 l/hr and 50 l/hr for desolvation and cone, respectively. Data acquisition was performed using MassLynx V4.1 software with the Quanlynx program.
Enrofloxacin was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Stock standard solution of enrofloxacin was prepared by dissolving 10 mg compound in 10 ml of acetonitrile to obtain a final concentration of 1 mg/ml. Working standard solution was prepared in the range 0.001–10.00 µg/ml by diluting the stock standard solutions in 50:50 acetonitrile: water. The precursor and product ions of enrofloxacin were 360.2 and 316.1, the standard curves constructed by plotting the area of enrofloxacin against the working standard concentrations of enrofloxacin (0.001, 0.01, 0.1, 1, 5 and 10 µg/ml). The calibration curves were obtained and the sample concentration was calculated by comparing peak area with external calibration curve.
C. carpio infection and enrofloxacin therapy
Healthy C. carpio were randomly divided into four groups, 20 fish for each groups, each group of test fish were challenged with 0.1 ml 5 × 107 CFU/ml AhHN1. After 1 hr, three test groups including 2.5, 5 and 10 mg/kg of enrofloxacin were administered orally once a day for three consecutive days, respectively. The control group was administered with an equal volume of saline. After treatment with enrofloxacin, the mortality of the infected fish was observed every 12 hr for 7 days. The protective ability of the drug was calculated using the equation: drug protective rate=[1−(Mortality rate of drug administration group / Mortality rate of the control group)] × 100%.
RESULTS
Morphologic and molecular characteristics
The pathogenic bacteria isolated from the four tissues of the diseased fish, colonies on BHI plates were slightly yellow pigmented, circular, convex, smooth, and about 1 mm in diameter after incubation for 24 hr at 28°C (data not shown).
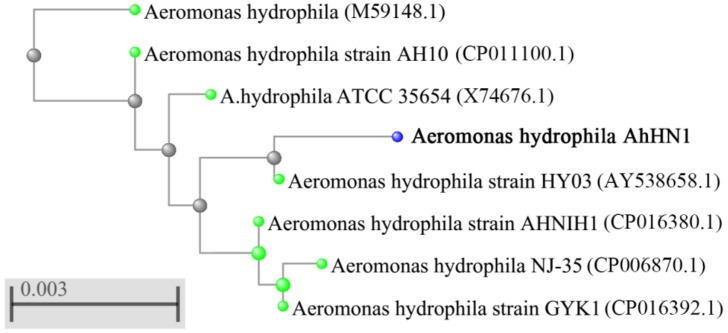
For molecular characterization, a phylogenetic tree was constructed based on BLAST search between isolated strain and other homologous sequences. From Fig. 1, the 16S rDNA sequence of isolated strain showed above 99% similarity with various species of A. hydrophila. Combined with the morphologic and molecular characteristics, the isolated strain was identified as A. hydrophila, named AhHN1.
Fig. 1.
Phylogenetictree of isolated strain based on 16S rDNA nucleotide sequences. The scale bar represents 0.003 substitutions per site. The tree was generated using neighbour-joining method by the MEGA software and displayed the high similarities between isolated strain and Aeromonas hydrophila group.
Pathogenicity for C. carpio
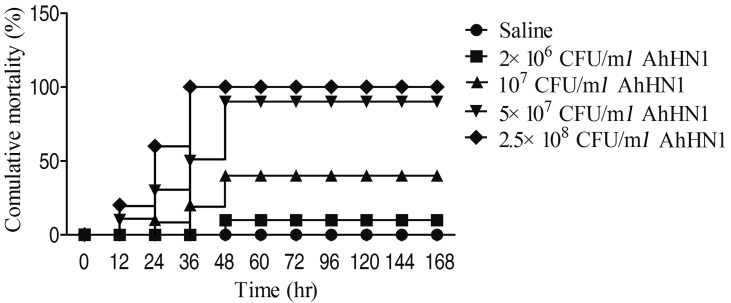
The pathogenicity of AhHN1 strain was confirmed in common carp C. carpio by artificial infection. After challenged with AhHN1 strain, the infected fish showed external hemorrhages in fins or intestine and death occurred at 12 hr in high dose groups (5 × 107 CFU/ml and 2.5 × 108 CFU/ml groups), the symptoms were similar to those observed in natural infections. The 48-hr mortality rate was 10 to 100% in four infection groups, respectively. However, there was no death or no sign of disease in fish infected with saline (Fig. 2). According to the cumulative method, the LD50 of AhHN1 was 1.38 × 106 CFU/fish after experimental infection to C. carpio.
Fig. 2.
The cumulative mortality rates of Cyprinus carpio after challenged with AhHN1 strain.
Antimicrobial susceptibility
The susceptibility pattern of A. hydrophila AhHN1 to 14 antibacterial agents was measured using microdilution method and A. hydrophila ATCC 7966 was used in this study as a control strain. The MIC and MBC results showed that AhHN1 was resistant to balofloxacin, florfenicol, gentamicin, kanamycin, streptomycin, ceftriaxone and neomycin sulfate, compared with control strain. However, A.hdrophila including AhHN1 and control strain, was highly resistance to amoxicillin, ampicillin, penicillin G, sulfamethazine, sulfamethoxazole and sulfadiazine (MIC ≥64 µg/ml), we speculated that A. hdrophila can harbour the corresponding antibiotic resistance genes. However, AhHN1 showed extremely sensitive to enrofloxacin in all tested antibacterial agents, the MIC and MBC was 0.0016 µg/ml and 0.0031 µg/ml, respectively (Table 1). These results indicated that AhHN1 is a typical multidrug resistant bacteria and enrofloxacin maybe an effective antibacterial agent for the prevention and treatment of this infectious disease.
Table 1. Antimicrobial susceptibility patterns of AhHN1 and Aeromonas hydrophila ATCC 7966.
| Antimicrobial agents | MIC (µg/ml) | >MBC (µg/ml) | ||
|---|---|---|---|---|
| >Aeromonas hydrophila ATCC 7966 |
>AhHN1 | >Aeromonas hydrophila ATCC 7966 |
>AhHN1 | |
| Enrofloxacin | 0.25 | 0.0016 | 0.5 | 0.0031 |
| Balofloxacin | 1.56 | 6.25 | 1.56 | 6.25 |
| Florfenicol | 0.5 | 8 | 2 | 32 |
| Gentamicin | 0.5 | 4 | 1 | 8 |
| Kanamycin | 0.5 | 4 | 1 | 8 |
| Streptomycin | 0.25 | 64 | 0.5 | 128 |
| Amoxicillin | 256 | 512 | >512 | >512 |
| Ampicillin | 64 | 64 | >512 | >512 |
| Penicillin G | 256 | 256 | >512 | >512 |
| Ceftriaxone | 0.156 | 5 | 0.312 | 10 |
| Neomycin sulfate | 0.125 | 2 | 0.5 | 8 |
| Sulfamethazine | 256 | 256 | >512 | >512 |
| Sulfamethoxazole | 128 | 128 | >512 | >512 |
| Sulfadiazine | 128 | 128 | >512 | >512 |
MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration.
Activities of enrofloxacin on AhHN1 in vitro
The in vitro activities of enrofloxacin were subsequently investigated. Based on the MIC and MBC determination, we detected the MPC and MSW of enrofloxacin to AhHN1. MPC values should be considered in drawing dosing strategies since traditional MIC-based dosing level might give rise to treatment failure due to the selection of drug-resistant mutant. The MPC of enrofloxacin against AhHN1 was determined as 8 MIC (0.0125 µg/ml). The MSW of enrofloxacin on isolate AhHN1was determined as 0.0016–0.0125 µg/ml, which reflected the difference between the measured MIC and MPC values. Existence of the MSW allowed us to predict that the likelihood for resistance selection or prevention based on achievable and therapeutic drug concentration.
Pharmacokinetics of enrofloxacin in common carp
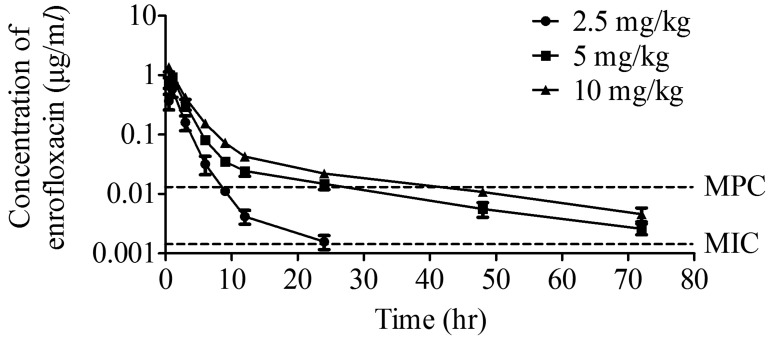
UPLC-MS presents a simple, rapid, and reliable analytical method for detection and quantification of enrofloxacin. The plasma concentration-time curves for enrofloxacin was shown in Fig. 3. The maximum concentration (Cmax) was 0.5824 and 0.9326 µg/ml at 1 hr after orally administered of 2.5 and 5 mg/kg enrofloxacin, respectively. High dose (10 mg/kg) reached the peak level with 1.3620 µg/ml in a shorter time (0.5 hr). Twenty-four hr post drug delivery, plasma concentration of enrofloxacin was 0.0016, 0.0148 and 0.0282 µg/ml for the three doses, respectively. For 5 and 10 mg/kg group, the plasma concentration of enrofloxacin was above the MPC value till 24 hr, while in 2.5 mg/kg group, the plasma concentration of enrofloxacin fell inside the MSW from 9–24 hr. However, enrofloxacin levels of 2.5 mg/kg group at 48 and 96 hr treatment were below the limit of detection (<0.0015 µg/ml).
Fig. 3.
Plasma enrofloxacin-time curves in plasma of Cyprinus carpio at different administration doses.
Medication regimen of enrofloxacin
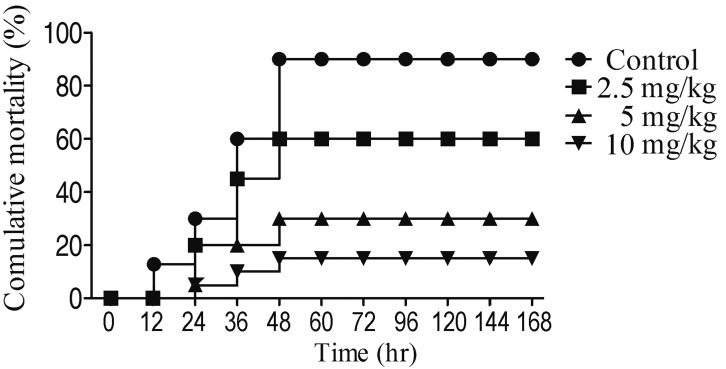
In order to study the efficacy of enrofloxacin, oral dose of 2.5, 5 and 10 mg/kg enrofloxacin was chose in this study. Healthy C. carpio were infected with 5 × 107 CFU/ml AhHN1, then treatment with different dose of enrofloxacin, the results showed that the relative protection rates of 2.5, 5 and 10 mg/kg of enrofloxacin were 33.3, 66.7, and 83.3%, respectively (Fig. 4). Compared with control, the death time of fish in the administration group was postponed at least 12 hr. Combined with pharmacokinetics of enrofloxacin, dosing once a day of 5 mg/kg was determined to be the rational regimen for controlling AhHN1 infection and preventing mutant selection in common carp.
Fig. 4.
The cumulative mortality rates of Cyprinus carpio after treatment with different dose of enrofloxacin.
DISCUSSION
In the past decades, the genus Aeromonas has received great attention in fish and human [7]. The taxonomy of Aeromonas has been extended since the development of biochemical and molecular techniques, these species can be accurately identified. In this study, a A. hydrophila strain was isolated from the diseased common carp C. carpio. In fact, A. hydrophila is also commonly occurred in species inpond and river waters [18]. The pathogenicity was confirmed in C. carpio by artificial infection and similar symptoms were observed, which verified that the isolated A. hydrophila AhHN1 strain showed highly pathogenicity to C. carpio.
Reports of growing bacterial resistance to drugs in aquaculture were revealed [22], and which thus are necessary for antimicrobial susceptibility testing to guide clinical medicine. In this study, the isolates of AhHN1 showed serious drug resistant to 3 kinds of penicillins, established MICs for these drug were over 64 µg/ml. These discovery attributed to the fact that Aeromonads spp. produce different β-lactamases, which confer resistance to a broad spectrum of β-lactam antibiotics [3]. Several studies demonstrated that all investigated A. hydrophila strains were resistant to ampicillin [6, 21]. Furthermore, AhHN1 also showed highly resistance to sulfonamides, balofloxacin, florfenicol, gentamicin, kanamycin, streptomycin, ceftriaxoneand neomycin sulfate. These results indicated that the isolated strain is a typical multidrug resistant A. hydrophila. However, among all tested antimicrobial agents, AhHN1 only showed sensitivity to enrofloxacin, whether the isolated strain is sensitive to other antibiotics, such as erythromycin and oxytetracycline, requires further verification. Furthermore, enrofloxacin can be used for treatment and prevention of bacterial disease caused by AhHN1. Enrofloxacin, as the first of the fluoroquinolones approved for use in animals, was approved in the late 1980s. Martinez et al. reported that the newer quinolones such as enrofloxacin are among the most important antimicrobial agents for treatment of severe and invasive infections in human and animals [14]. However, enrofloxacin are strictly banned in some countries such as Japan, Canada, Scotland, Vietnam, etc and allowed for use in Chinese and Thailand aquaculture. Thus, the use of fluoroquinolones in aquaculture should, therefore, always be carefully considered and controlled by the food and drug national authority. The aim of this study was to establish a scientific medication regimen to reduce the use of enrofloxacin and prevent drug resistant bacteria in the countries where the use of enrofloxacin is permitted in aquaculture environment.
In general, the plasma concentration was the key factor for drug effectivity, AUC24/MIC >100 and Cmax/MIC >8 predicted a clinical outcome of enrofloxacin [9]. Based on the UPLC-MS results, Cmax/MIC=364, 583 and 850 at 2.5, 5 and 10 mg/kg of enrofloxacin group, respectively. These data were similar with PK parameters of enrofloxacin in grass carp C. idellus [25]. Furthermore, to determine the enrofloxacin therapy efficacy in vivo, three dosage of enrofloxacin treatment significantly improved the survival rate of carp, especially 5 and 10 mg/kg groups. However, the recommended dose of enrofloxacin for Atlantic salmon, rainbow trout, sea bass and sea bream is 10 mg/kg [20]. According to the MSW hypothesis, resistant mutants are selected or enriched at antibiotic concentrations above the MIC but below the MPC. This assumption can be used to estimate the optimal dose, because many traditional dosing regimens may constitute misuse of antimicrobial agents. To determine a rational therapeutic guideline, treatments should maximize the time during which enrofloxacin concentrations above the MPC. In our study, plasma concentration of enrofloxacin above MPC was observed at 9, 24 and 24 hr for the three doses, respectively. At the dose of 2.5 mg/kg of enrofloxacin, the plasma concentration of enrofloxacin fell inside the MSW from 9–24 hr, that resistant mutants may enriched selectively. Thus, a dosage of 5 mg/kg was a safe dose, might achieve effective therapeutic result for controlling bacterial disease in C. carpio, and also reduce the occurrence probability of enrofloxacin-resistant.
In conclusion, the drug parameters (MIC, MBC, MPC and MSW) of A. hydrophila AhHN1, isolated from common carp C. carpio were characterized. Our approach for combining antimicrobial susceptibility with drug parameters (including MPC and MSW), provided a more scientific and effective strategy to face the challenge of drug-resistant bacteria by drawing a specific dosage guideline of antimicrobial agents.
Acknowledgments
This work was sponsored by the Foundation of Henan Educational Committee (182102110236, 182102110326, 182102110328) and the China Postdoctoral Science Foundation (2017M622351). We would like to thank the University Research Facility in Life Sciences (ULS) of the Hong Kong Polytechnic University for access to mass spectrometry.
REFERENCES
- 1.Bostock J., McAndrew B., Richards R., Jauncey K., Telfer T., Lorenzen K., Little D., Ross L., Handisyde N., Gatward I., Corner R.2010. Aquaculture: global status and trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 2897–2912. doi: 10.1098/rstb.2010.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabello F. C., Godfrey H. P., Buschmann A. H., Dölz H. J.2016. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 16: e127–e133. doi: 10.1016/S1473-3099(16)00100-6 [DOI] [PubMed] [Google Scholar]
- 3.Chen P. L., Ko W. C., Wu C. J.2012. Complexity of β-lactamases among clinical Aeromonas isolates and its clinical implications. J. Microbiol. Immunol. Infect. 45: 398–403. doi: 10.1016/j.jmii.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 4.Firsov A. A., Vostrov S. N., Lubenko I. Y., Drlica K., Portnoy Y. A., Zinner S. H.2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 47: 1604–1613. doi: 10.1128/AAC.47.5.1604-1613.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harikrishnan R., Rani M. N., Balasundaram C.2003. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 221: 41–50. doi: 10.1016/S0044-8486(03)00023-1 [DOI] [Google Scholar]
- 6.Hatha M., Vivekanandhan A. A., Joice G. J., Christol Antibiotic resistance pattern of motile aeromonads from farm raised fresh water fish. Int. J. Food Microbiol. 98: 131–134. doi: 10.1016/j.ijfoodmicro.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 7.Janda J. M., Abbott S. L.2010. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23: 35–73. doi: 10.1128/CMR.00039-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong X., Qiao D., Zhao X., Wang L., Zhang J., Liu D., Zhang H.2017. The molecular characterizations of Cu/ZnSOD and MnSOD and its responses of mRNA expression and enzyme activity to Aeromonas hydrophila or lipopolysaccharide challenge in Qihe crucian carp Carassius auratus. Fish Shellfish Immunol. 67: 429–440. doi: 10.1016/j.fsi.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 9.Labreche M. J., Frei C. R.2012. Declining susceptibilities of gram-negative bacteria to the fluoroquinolones: effects on pharmacokinetics, pharmacodynamics, and clinical outcomes. Am. J. Health Syst. Pharm. 69: 1863–1870. doi: 10.2146/ajhp110464 [DOI] [PubMed] [Google Scholar]
- 10.Lafferty K. D., Harvell C. D., Conrad J. M., Friedman C. S., Kent M. L., Kuris A. M., Powell E. N., Rondeau D., Saksida S. M.2015. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 7: 471–496. doi: 10.1146/annurev-marine-010814-015646 [DOI] [PubMed] [Google Scholar]
- 11.Li H., Lin X. M., Wang S. Y., Peng X. X.2007. Identification and antibody-therapeutic targeting of chloramphenicol-resistant outer membrane proteins in Escherichia coli. J. Proteome Res. 6: 3628–3636. doi: 10.1021/pr070307y [DOI] [PubMed] [Google Scholar]
- 12.Liu X., Steele J. C., Meng X. Z.2017. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 223: 161–169. doi: 10.1016/j.envpol.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Mao W. F., Wang Y. P., Wang W. B., Bo W., Feng J. X., Zhu Z. Y.2004. Enhanced resistance to Aeromonas hydrophila infection and enhanced phagocytic activities in human lactoferrin-transgenic grass carp (Ctenopharyngodon idellus). Aquaculture 242: 93–103. doi: 10.1016/j.aquaculture.2004.07.020 [DOI] [Google Scholar]
- 14.Martinez M., McDermott P., Walker R.2006. Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet. J. 172: 10–28. doi: 10.1016/j.tvjl.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 15.Miller R., Carson J., Dalsgaard I., Gaunt P., Gieseker C., Hawke J., Wu C.2014. Methods for broth dilution susceptibility testing of bacteria isolated from aquatic animals; approved guideline. Book Chapter 2: 20–25. [Google Scholar]
- 16.Nielsen M. E., Høi L., Schmidt A. S., Qian D., Shimada T., Shen J. Y., Larsen J. L.2001. Is Aeromonas hydrophila the dominant motile Aeromonas species that causes disease outbreaks in aquaculture production in the Zhejiang Province of China? Dis. Aquat. Organ. 46: 23–29. doi: 10.3354/dao046023 [DOI] [PubMed] [Google Scholar]
- 17.Pachanawan A., Phumkhachorn P., Rattanachaikunsopon P.2008. Potential of Psidium guajava supplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus). J. Biosci. Bioeng. 106: 419–424. doi: 10.1263/jbb.106.419 [DOI] [PubMed] [Google Scholar]
- 18.Pang M., Jiang J., Xie X., Wu Y., Dong Y., Kwok A. H. Y., Zhang W., Yao H., Lu C., Leung F. C., Liu Y.2015. Novel insights into the pathogenicity of epidemic Aeromonas hydrophila ST251 clones from comparative genomics. Sci. Rep. 5: 9833. doi: 10.1038/srep09833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peatman E., Mohammed H., Kirby A., Shoemaker C. A., Yildirim-Aksoy M., Beck B. H.2018. Mechanisms of pathogen virulence and host susceptibility in virulent Aeromonas hydrophila infections of channel catfish (Ictalurus punctatus). Aquaculture 482: 1–8. doi: 10.1016/j.aquaculture.2017.09.019 [DOI] [Google Scholar]
- 20.Rigos G., Troisi G. M.2005. Antibacterial agents in mediterranean finfish farming: A synopsis of drug pharmacokinetics in important euryhaline fish species and possible environmental implications. Rev. Fish Biol. Fish. 15: 53–73. doi: 10.1007/s11160-005-7850-8 [DOI] [Google Scholar]
- 21.Stratev D., Odeyemi O. A.2016. Antimicrobial resistance of Aeromonas hydrophila isolated from different food sources: A mini-review. J. Infect. Public Health 9: 535–544. doi: 10.1016/j.jiph.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 22.Thakur A. K., Fezio W. L.1981. A computer program for estimating LD50 and its confidence limits using modified Behrens-Reed-Muench cumulant method. Drug Chem. Toxicol. 4: 297–305. doi: 10.3109/01480548109018136 [DOI] [PubMed] [Google Scholar]
- 23.Van Boeckel T. P., Brower C., Gilbert M., Grenfell B. T., Levin S. A., Robinson T. P., Teillant A., Laxminarayan R.2015. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U.S.A. 112: 5649–5654. doi: 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M., Zhao X., Kong X., Wang L., Jiao D., Zhang H.2016. Molecular characterization and expressing analysis of the c-type and g-type lysozymes in Qihe crucian carp Carassius auratus. Fish Shellfish Immunol. 52: 210–220. doi: 10.1016/j.fsi.2016.03.040 [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Wang H., Yang X., Lu L.2013. Integrated pharmacokinetics/pharmacodynamics parameters-based dosing guidelines of enrofloxacin in grass carp Ctenopharyngodon idella to minimize selection of drug resistance. BMC Vet. Res. 9: 126. doi: 10.1186/1746-6148-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X., Drlica K.2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33Suppl 3: S147–S156. doi: 10.1086/321841 [DOI] [PubMed] [Google Scholar]
- 27.Zhao X. L., Han Y., Ren S. T., Ma Y. M., Li H., Peng X. X.2015. L-proline increases survival of tilapias infected by Streptococcus agalactiae in higher water temperature. Fish Shellfish Immunol. 44: 33–42. doi: 10.1016/j.fsi.2015.01.025 [DOI] [PubMed] [Google Scholar]