Abstract
Owned, free-roaming domestic cats are abundant in the Chilean countryside, having high probability of contact with wildlife and potentially participating as reservoirs of zoonotic pathogens. In the present study, 131 cats from two remote study areas (Valdivia and Chiloe Island) in southern Chile were analyzed for infection/exposure to eight pathogens. Serum samples from 112 cats were tested for antigens against feline leukemia virus (FeLV antigen-ELISA) and antibodies against feline immunodeficiency virus (FIV-ELISA) and canine distemper virus (CDV-serum neutralization), yielded occurrence of 8.9, 1.7 and 0.8% respectively. The presence of DNA of five vector-borne pathogens, piroplasmids, Ehrlichia spp., Anaplasma spp., Rickettsia spp. and Bartonella spp. was investigated in thirty cats. Overall observed occurrence was 6.6% (2/30) for both Anaplasma platys, and B. henselae, and 3.3% (1/30) for both Bartonella sp. and Theileria equi. Observed occurrence for all vector-borne pathogens in Valdivia area was significantly higher than in Chiloe Island (5/15 vs 0/15; P=0.04). Our results represent the first description of exposure to CDV and DNA detection of T. equi and A. platys in domestic cats in Chile. The results highlight the importance of performing pathogen screening in owned, free-roaming rural cats to evaluate their potential role as reservoirs of infection and vectors for disease transmission to wildlife.
Keywords: feline and canine virus, PCR, rural free-roaming domestic cat, serology, vector-borne pathogen
The domestic cat (Felis silvestris catus) is one of the most widely distributed terrestrial mammals [7, 23] due to their association with humans, and it has been listed among the 100 worst non-native invasive species in the world [36]. Owned, free-roaming domestic cats are abundant in rural Chile, where they are usually allowed to wander, sometimes around natural preserved areas. Their ability to roam increases their probability of interacting with other domestic animals and wildlife species, and their chances of contact with a range of pathogens. These owned free-roaming cats are rarely subjected to any prophylactic programs or receive any type of veterinary care, further increasing their probability of pathogen infection [44, 64]. This makes them a potential reservoir of pathogens of relevance for wildlife [21, 40, 42, 45].
Examples of pathogen spillover from a domestic species to a wild counterpart have been described elsewhere [14, 25, 45, 48, 57, 73]. Canine distemper virus (CDV) has caused several fatal epidemics in wild canids such as African wild dogs (Lycaon pictus) [73] and wild felids such as Serengeti lions (Panthera leo) [15, 57]; and more recently domestic dogs have been identified as the origin of CDV infection in Iberian wolves (Canis lupus signatus) [47] and jackals (Canis mesomelas) [25]. Likewise, possible cross-species transmission of feline leukemia virus (FeLV) from domestic cats has been recorded in guigna (Leopardus guigna) [45] and bobcats (Felis rufus) [65] and FeLV outbreaks have been reported in Florida panthers (Puma concolor coryi) [14] and Iberian lynxes (Lynx pardinus) causing high mortalities [40, 45]. Regarding feline immunodeficiency virus (FIV), it is considered endemic in some wild felid populations such as African lions (Panthera leo) and many of the South American felids such as pumas (Puma concolor), jaguars (Panthera once), ocelots (Leopardus pardalis), margays (Leopardus wiedii), Geoffroy’s cat (Leopardus geoffroyi), and oncilla (Leopardus tigrinus) [69]. However, high FIV prevalence have been described in some species of Asian wild felids like Palla’s cat (Otocolobus manul) or leopard cat (Prionailurus bengalensis) [75] and possible spillover from domestic cats to wild felids has been described in guignas from Chile [45] and the Tsushima cat (Felis bengalensis euptilura) from Japan [50]. Epidemics associated with vector-borne agents such as piroplasmids have also been recorded in wildlife. It was proposed that the death of one third of the Serengueti lion population was due to the co-occurrence of Babesia and CDV infection [49]. Piroplasmids are considered the second most commonly found parasites in the blood of mammals after trypanosomes [61], making them important vector-borne agents in those species [78]. In addition to conservation implications, owned, free-roaming domestic cats could be a reservoir of zoonotic agents due to their poor health care and free-ranging behavior. Bartonella henselae is currently the most commonly encountered Bartonella zoonosis [27]. Asymptomatic infection with Bartonella spp. is frequently reported in cats, which are therefore considered to be a major reservoir for human infection. Cats can also be infected by members of Anaplasmataceae, which are rickettsial organisms that infect human and animal leukocytes [19].
However, despite the possibility of pathogen spillover from domestic cats to wildlife, and their zoonotic implications, data on pathogens infecting rural, owned, free-roaming domestic cats is still scarce [23, 44, 45, 55, 62]. Previous serological and molecular surveys in Chile have reported the presence of different pathogens, although these studies were mainly focused on owned domestic cats from urban areas [6, 20, 30, 48, 71, 79].
Therefore, our goal was to determine the occurrence of important carnivore pathogens in owned, free-roaming domestic cats from remote isolate rural communities in southern Chile, assessing the exposure and/or infection with bacteria and viruses with different transmission modes. The free roaming behavior of these cats in rural areas increases the possibilities of contact with wildlife. Thus, the results of this study evaluate how owned, free-roaming domestic cats could act as reservoir of pathogens to wildlife.
MATERIALS AND METHODS
Sampling
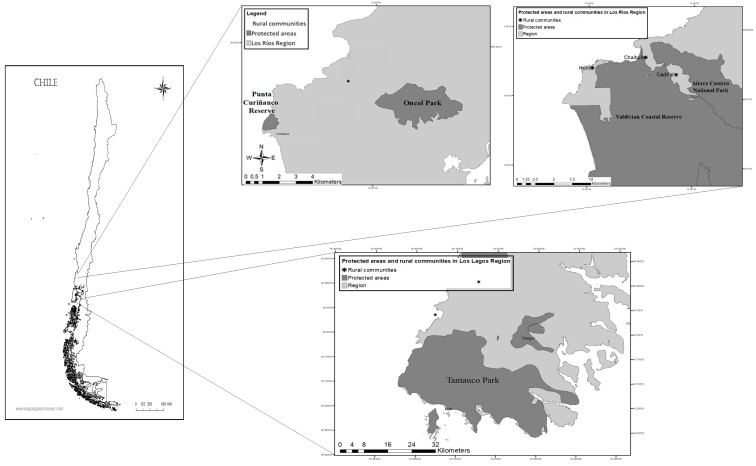
During 2015 and 2016 a cross-sectional study was conducted. A total of 131 owned, free-roaming cats were sampled in six rural communities adjacent to protected areas located in two different regions of southern Chile: four in the Valdivian coastal area (Los Ríos region, 39° S 73° W) and two on Chiloe Island (Los Lagos region, 43° S 73° W) (Fig. 1). These two regions were chosen since they contain remote and isolated rural communities, where prophylactic management and health care of domestic cats and dogs (Canis familiaris) is scarce or nonexistent [64].
Fig. 1.
Study area. Rural communities near protected areas of Valdivia, Los Ríos region, and Chiloe Island, Los Lagos region, in southern Chile.
Domestic cats were selected based on their non-vaccinated and non-neutered status as well as their owned, free-roaming behavior. All of them were mix-breed, domestic short-haired cats. EDTA-anticoagulated blood and serum samples collected through manual restraint, by cephalic or jugular venipuncture were preserved at −20°C until further processing. The collection of samples was performed with the informed consent of the owners and under considerations of animal welfare and ethical aspects under the approval of Animal Ethics committee of the Institute of Ecology and Biodiversity in Universidad de Chile, resolution of 20 November 2015.
Serum analysis
Serum samples from 112 cats (37 from the Valdivia area, 75 from Chiloe Island) were tested for FeLV, FIV and CDV. Serum samples from the remaining 19 cats were not available. Progressive FeLV infection was determined by detection of the p27 antigen using a commercial ELISA kit (sensitivity 94% and specificity 100%) (INgezim FeLV DAS; Ingenasa, Madrid, Spain), and antibodies against FIV were detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit INgezim FIV (sensitivity 99% and specificity 100%) (Ingenasa), following the manufacturer’s instructions. Due to the low volume of serum available, samples were pre-screened for CDV antibodies using an immunofluorescence assay (IFA). In brief, Vero cells (CCL-81) were infected with the Onderstepoort strain of CDV with a multiplicity of infection (MOI) of 0.1 or were mock-infected with Dulbecco’s modified Eagle medium (DMEM) to generate a negative control. After one hr of infection at 37°C the inoculum was removed, cells were washed once with phosphate-buffered saline (PBS) and DMEM supplemented with 2% (v/v). Heat-inactivated fetal bovine serum (FBS) was added for further cultivation at 37°C and 5% CO2 for 48 hr. Cells were then washed once with PBS, fixed with 80% (v/v) acetone at −20°C for 10 min and rinsed twice with PBS. To prevent non-specific binding, a blocking step using 5% (w/v) bovine serum albumin (BSA) in PBS for 30 min at 37°C was performed. Serum samples diluted 1:100 in 1% (w/v) BSA in PBS were added and incubated for two hr at 37°C. Cells were then washed two times with PBS and CDV-specific antibodies were visualized by adding a fluorochrome-labeled secondary antibody (anti cat-IgG-Alexafluor488; Jackson ImmunoResearch Laboratories, West Grove, PA, U.S.A.), diluted 1:500 in 1% BSA in PBS for one hr at 37°C. Finally, cells were washed twice with PBS and analyzed using a fluorescence microscope (IX70, Olympus, Hamburg, Germany) [26]. Titers higher than 1:16 were considered positive.
IFA test shows high sensitivity (97%) but low specificity (70%) in comparison to an ELISA test [26]. Consequently, IFA positive serum samples were further investigated using a serum neutralization test (SNT) with high specificity [52], an accepted gold standard for the diagnosis of CDV-antibodies as described by [34], thereby avoiding the possible cross-reaction between CDV antibodies and other morbilliviruses.
DNA extraction and PCR amplification
The DNA of 30 cats (15 from the Valdivia area, 15 from Chiloe Island) was extracted from 0.3 ml peripheral whole blood samples as described by [22]. The eukaryotic 18S RNA Pre-Developed TaqMan Assay Reagents (Applied Biosystems, Foster City, CA, U.S.A.) were used as internal reference for cat genomic DNA amplification, to ensure the quality of each sample for PCR amplification and that negative results corresponded to true negative samples rather than to a problem with DNA loading, sample degradation or PCR inhibition. Real-time PCR (qPCR) targeting Ehrlichia/Anaplasma spp., piroplasmids, Rickettsia spp. and Bartonella spp. was performed as previously described [13, 38]. The thermal cycling profile was 50°C 2 min and 95°C 10 min followed by 40 cycles at 95°C 15 sec and 60°C 1 min, using a QuantStudioTM 12K Flex Real-Time PCR System (Life Technologies, Carlsbad, CA, U.S.A.). Sterile water was used as a negative PCR control and positive controls were obtained from commercial slides coated with cells infected with the pathogens (MegaScreen® 118 FLUOEHRLICHIA c., MegaScreen® FLUOBABESIA canis, MegaScreen® FLUORICKETTSIA 119 ri., MegaScreen® BARTONELLA h. (Megacor, Hörbranz, Vorarlberg, Austria). Target genes amplified for each pathogen, primers used and sequencing of each positive qPCR product was conducted as previously described [53] (Table 1). Positive samples were sequenced with the BigDye Terminator Cycle Sequencing Ready Reaction Kit (AB, Life Technologies). The sequences obtained were compared with sequences from the GenBank database (www.ncbi.nlm.nih.gov/BLAST). Ultrapure water was employed as negative control in each PCR run and sequenced positive cat samples were used as positive controls.
Table 1. Primers used in pathogen detection, target genes amplified and size of the amplified product for each pathogen using real time PCR.
| Pathogen | Region amplified | Primer Forward (5′-3′) | Primer Reverse (5′-3′) | Final [primer] (µM) | PCR product (bp) |
|---|---|---|---|---|---|
| Ehrlichia/ Anaplasma spp. | 16S rRNA | GCAAGCYTAACACATGCAAGTCG | CTACTAGGTAGATTCCTAYGCATTACTCACC | 0.5 | 102a) |
| Piroplasmid | 18S rRNA | GACGATCAGATACCGTCGTAGTCC | CAGAACCCAAAGACTTTGATTTCTCTC | 0.3 | 114a) |
| Rickettsia spp. | ITS1 | GCTCGATTGRTTTACTTTGCTGTGAG | CATGCTATAACCACCAAGCTAGCAATAC | 0.5/0.3 | 300a) |
| Bartonella spp. | ITS1 | AGATGATGATCCCAAGCCTTCTG | CCTCCGACCTCACGCTTATCA | 0.3 | 180a) |
a) Targeted size could vary depending on the species.
Statistical analyses
The R program [68] was used to perform statistical analyses. Fisher’s exact test was performed, and statistical significance was set at P<0.05 to compare pathogen occurrence between areas, ages and sexes.
RESULTS
Of the total 131 sampled cats, 58 were males and 73 were females; 115 were adults and 16 were juveniles. All animals were mix-bred, short-haired cats. Forty-six cats belonged to rural communities in the Valdivia area and 85 cats to rural communities in Chiloe Island. All cats were evaluated by a veterinarian: 116 cats were clinically healthy, and 15 cats presented different clinical signs of illness (gingivitis, conjunctivitis, paraplegia, ascites or ocular secretion) (Table 2).
Table 2. PCR results with correlated serological results and clinical signs information.
| Sample | Clinical signs | Location | Sex | Age | FIV-ELISA | FeLV-ELISA | CDV-IFT/SNT | Piro-plasmid | H. felis | Bartonella spp. | Ehrlichia/Anaplasma spp. | Rickettsia spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16046 | Asymptomatic | Chiloe Island | Female | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15221 | Asymptomatic | Valdivia | Female | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15225 | Asymptomatic | Valdivia | Female | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15226 | Asymptomatic | Valdivia | Female | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15007 | Asymptomatic | Chiloe Island | Male | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15231 | Asymptomatic | Valdivia | Male | Adult | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 15240 | Fever | Valdivia | Male | Adult | Negative | Negative | Negative | Negative | Negative | Bartonella sp. | Anaplasma platys | Negative |
| 16011 | Paraplegia | Chiloe Island | Female | Adult | Negative | Positive | Negative | Negative | Negative | Negative | Negative | Negative |
| 15004 | Gingivitis | Chiloe Island | Female | Adult | Negative | Positive | Negative | Negative | Negative | Negative | Negative | Negative |
| 15002 | Asymptomatic | Chiloe Island | Female | Adult | Positive | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| 16013 | Asymptomatic | Chiloe Island | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 16014 | Asymptomatic | Chiloe Island | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15029 | Asymptomatic | Chiloe Island | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15191 | Asymptomatic | Valdivia | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15207 | Asymptomatic | Valdivia | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15212 | Asymptomatic | Valdivia | Female | Juvenile | NE | NE | NE | Negative | Negative | B. henselae | Negative | Negative |
| 15223 | Asymptomatic | Valdivia | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15227 | Asymptomatic | Valdivia | Female | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 16031 | Asymptomatic | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 16037 | Asymptomatic | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15014 | Asymptomatic | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15022 | Asymptomatic | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15030 | Asymptomatic | Chiloe Island | Male | Juvenile | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15034 | Respiratory symptoms | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15038 | Asymptomatic | Chiloe Island | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15184 | Asymptomatic | Valdivia | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Anaplasma platys | Negative |
| 15195 | Ocular secretion | Valdivia | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15201 | Asymptomatic | Valdivia | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
| 15203 | Asymptomatic | Valdivia | Male | Adult | NE | NE | NE | Negative | Negative | Negative | Negative | Negative |
NE, not explored; FIV, feline immunodeficiency virus; FeLV, feline leukemia virus; CDV, canine distemper virus.
Serum analysis
Of the 112 cat serum samples tested, 10 individuals (8.9%, 95% CI=3.5–14.29) were FeLV-positive; two individuals (1.7% 95% CI=0.07–4.2) were FIV-positive and one individual was CDV-serum neutralization positive, as a reciprocal titer of 1:160 (0.8%, 95% CI=0.08–2.6) (Table 3). No coinfections or significant differences between ages, sex groups or areas were found related to positive status.
Table 3. Molecular and serological screening results of pathogens in owned, free-roaming domestic cats. Pathogen occurrence by study area and sex.
| Serology | Molecular analyses | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA | IFA/SNT | Piroplasmida | Bartonella spp. | Ehrlichia/Anaplasma | Rickettsia spp. | ||||||||||||||||||
| FeLV | FIV | CDV | |||||||||||||||||||||
| n/total | % | 95%CI | n | % | 95%CI | n/total | % | 95%CI | n/total | % | 95%CI | n/total | % | 95%CI | n/total | % | 95%CI | n/total | % | 95%CI | |||
| Area | |||||||||||||||||||||||
| Valdivia | 1/37 | 2.7 | 2.7–8.1 | 0/37 | 0.0 | 0.0 | 1/37 | 2.7 | 1.6–2.7 | 1/15 | 6.6 | 7.6–20.9 | 3/15 | 20.0 | 2.9–42.9 | 2/15 | 13.3 | 6.1–32.82 | 0/15 | 0.0 | 0.0 | 0.0 | |
| Chiloe | 9/75 | 12.0 | 3.7–32.7 | 2/75 | 2.6 | 1.0–6.3 | 0/75 | 0.0 | 0.0 | 0/15 | 0.0 | 0.0 | 0/15 | 0.0 | 0.0 | 0/15 | 0.0 | 0.0 | 0/15 | 0.0 | 0.0 | 0.0 | |
| Total | 10/112 | 8.9 | 3.5–14.2 | 2/112 | 1.7 | 0.7–4.2 | 1/112 | 0.8 | 0.08–2.6 | 1/30 | 3.3 | 3.4–10.15 | 3/30 | 10.0 | 1.3–21.39 | 2/30 | 6.6 | 2.8–16.14 | 0/30 | 0.0 | 0.0 | 0.0 | |
| Sex | |||||||||||||||||||||||
| Male | 4/47 | 8.5 | 0.2–16.7 | 0/47 | 0.0 | 0.0 | 1/47 | 2.1 | 2.1–6.4 | 0/14 | 0.0 | 0.0 | 1/14 | 7.1 | 8.2–22.5 | 2/14 | 14.2 | 6.6–35.2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Female | 6/65 | 9.23 | 2.0–16.4 | 2/65 | 3.0 | 1.2–7.3 | 0/65 | 0.0 | 0.0 | 1/16 | 6.2 | 7.7–19.5 | 2/16 | 12.5 | 5.7–30.7 | 0/16 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
IFA, immunofluorescence assay; SNT, serum neutralization test; C.I.,Confidence intervals (lower-upper); FIV, feline immunodeficiency virus; FeLV, feline leukemia virus; CDV, canine distemper virus.
Molecular analysis
DNA of the following vector-borne pathogens was amplified in five individuals: (1) piroplasmids (n=1), (2) Ehrlichia/Anaplasma sp. (n=2), (3) Bartonella sp. (n=3). No Rickettsia sp. DNA was detected. These pathogens were identified through sequencing as: (1) Theileria equi (1/30, 3.3%, 95% CI=0.3–10.15), 100% identity to GenBank T. equi sequences in equids worldwide; (2) Anaplasma platys (2/30, 6.6%, 95% CI=2.8–16.14), 100% identity to GenBank A. platys sequences in dogs worldwide; (3) Bartonella sp. (1/30, 3.3%, 95% CI=3.4–10.15), 100% identity to GenBank Bartonella spp. sequences detected in fleas worldwide, and Bartonella henselae (2/30, 6.6%, 95% CI=2.8–16.14), 100% identity to GenBank Bartonella henselae sequences in lions from Namibia, and cats from Malaysia, Guatemala and Brazil. The new T. equi, A. platys, Bartonella sp. and B. henselae nucleotide sequences were submitted to the GenBank database under accession numbers MK774808, MK791257, MK791259 and MK791258 respectively. Four of the five vector-borne infected individuals were healthy and carried only one pathogen, whereas one cat was pyrexic at the time of sampling and coinfected with B. henselae and A. platys (Tables 2 and 3).
All PCR-positive individuals were from the Valdivia area. Overall vector-borne occurrence in Valdivia (5/15, 33.3%, 95% CI=6.3–60.3) was higher than on Chiloe Island (0/15, 0.0%, 95% CI=0–0) (P=0.04). Age and sex were not significantly related to the probability of infection from any of the vector-borne pathogens evaluated in this study.
DISCUSSION
FeLV and FIV infection in cats lead to immunosuppressive wasting syndromes. Both viruses are among the most common infectious diseases affecting domestic cats, and have a worldwide distribution [12, 31, 37, 56, 75]. Worldwide prevalences of FeLV and FIV are estimated between 2–18 and 1.2–43%, respectively [12, 31, 37, 56, 75], varying widely depending on lifestyle, gender, health condition and other variables [31, 37, 77]. In the current study, FeLV and FIV seroprevalences were relatively low compared to other studies conducted in large cities in Chile (FeLV=13.5% (Valdivia city), 23% (Concepción city); FIV=10.3% (Valdivia city), 4% (Concepción city)) [6, 71, 72] and South America [29, 66, 69, 70]. These differences could be attributed to the rural origin of the cats from this study, where cat abundance and aggregation is lower than in urban areas.
Canine distemper virus is one of the most important infectious disease of carnivores worldwide and has the second highest fatality rate of any infectious disease after rabies in domestic dogs [17]. Evidence of CDV infection has been reported in several families of terrestrial carnivores, including Canidae, Felidae, Hyaenidae, Mustelidae, Mephitidae, Procyonidae, Ursidae, Ailuridae and Viverridae [8]. In the last decades, CDV has occurred as large-scale epidemics in felids [5, 57]. Both in vivo and in vitro studies have demonstrated that domestic cats can be efficiently infected by CDV [39]. However, few reports of CDV in domestic cats have been described worldwide [39], with seroprevalences ranging between 4.5 and 23% [32, 43]. To the best of our knowledge, this is the first report of CDV exposure in a domestic cat in Chile.
Exposure to the vector-borne pathogens Bartonella spp. and R. felis has been evaluated in cats in large cities in Chile. Seroreactivity for Bartonella spp. was 86% in cats sampled in three large cities (Coquimbo, Santiago and Valdivia) [20], and 71% in Valdivia city [79]; while for R. felis, it was 72.7% in cats from Santiago city [30]. Only a few studies in South America have evaluated the molecular occurrence of Bartonella sp. in cats, mostly in Brazil [10, 16, 41, 67]. In Chile, Bartonella sp. (18.1%) was molecularly detected in cats from the city of Valdivia [48]. Occurrence of Bartonella sp. in the present study was lower than that reported in Valdivia city [48]. Different sample size, type of sampled populations (i.e. demographics, urban vs. rural populations), or vector distribution may explain the difference in pathogen occurrence [48]. B. henselae, detected in this study, is one of the three species associated with cat-scratch disease and other syndromes in humans [9, 11]. In Chile, mandatory reporting of this disease is not required. Nevertheless, more than 200 human cases of bartonellosis were diagnosed between 1997 and 2000 in Chile [20], emphasizing the zoonotic importance of this agent in the country.
We detected an occurrence of 13.3% for A. platys, an obligate intracellular gram-negative bacteria of the family Anaplasmataceae, described mainly in dogs from various regions of the world such as Brazil [60], Venezuela [28] and Japan [46], causing infectious canine cyclic thrombocytopenia [18]. The tick Rhipicephalus sanguineus sensu lato is suspected to be involved as its principal vector [2]. The presence of R. sanguineus in Chile has been described from Arica in the north (18°29′01ʺS) to Valdivia in the south (39°49′11ʺS) [1, 24, 35]. Although the mode of transmission of A. platys in cats is unknown, R. sanguineus has been described parasitizing cats [63]. Anaplasma-like bodies have been detected in cat platelets and molecular detection of A. platys has been reported in cats from Brazil, Thailand and North America [33, 54, 58, 59], however the clinical implication of A. platys in cats is poorly understood. To our knowledge, this is the first description of A. platys in domestic cats in Chile.
With reference to the molecular detection of piroplasmids, DNA detection of T. equi was recorded in one individual. According to the World Organization for Animal Health, Central and South America are considered among the endemic regions of Theileria equi [76]. The disease is readily identified in all regions of South America with the exception of the southernmost areas of Chile and Argentina [76]. T. equi is transmitted to equids by ticks of the genera Hyalomma and Rhipicephalus [3]. A sequence from a free-roaming domestic cat caught within a Brazilian zoo showed 94% identity with T. equi [4]. However, reports of T. equi in domestic cats are rare. To our knowledge, our study constitutes the first report of T. equi in Chile.
The occurrence of vector-borne pathogens was higher in rural Valdivia, the northern part of the study area, compared to rural Chiloe Island, in the south [13], analyzed different vector-borne agents infecting Darwin’s fox from Chiloe Island and found only one animal positive for Rickettsia sp. A wide range of factors (e.g., climatic differences, human and animal population dynamics) may affect the occurrence and spread of vector-borne pathogens [51]. The main factor that could explain the difference in vector borne occurrence between the two study areas is the distribution of R. sanguineous in Chile; Valdivia is the southern distribution limit of this tick, it is therefore absent from Chiloe Island [1, 24, 35]. Other drivers may lie behind the observed occurrence difference, such as different cat abundance or cat population migratory flows [74], which could be limited on Chiloe Island due to its intrinsic isolation, posing an obstacle for the arrival of pathogens. However, small sample size for vector-borne pathogen detection in this study suggests our results constitute only preliminary data, and therefore more analyses are needed.
The results of this study indicate that exposure to feline pathogens is lower when compared to previous urban domestic cat studies. However, due to their intrinsic behavior, rural domestic cats might transmit pathogens and parasites to sympatric threatened carnivores inhabiting nearby natural habitats. In addition, these cats can act as reservoirs of zoonotic agents. This study highlights the importance of performing pathogen screening in owned free-roaming rural cats in order to identify their pathogens. This should be the first step to establish its potential as reservoirs and the risk of disease transmission to wildlife, other domestic animals and humans, guiding the implementation of effective management and control measures in rural communities.
The present work represents a pilot study, being the first prospecting of these pathogens in owned-free, rural cats from isolate regions of Chile. This pilot study has limited capacity for sample analysis. However, the lines of evidence achieved on this first approach, will be expand in the future.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Acknowledgments
We gratefully acknowledge local inhabitants of rural communities for kindly giving us the opportunity to sample their domestic cats. Special thanks to Diego Peñaloza, Gonzalo Canto, Camila Núñez and Patricia Barría for their valuable support in sample collection. We thank Tantauco Park, especially Alan Bannister and Catherine Chirgwin. We are grateful to Sandra Díaz (Ministry of the Environment), CONAF Región de Los Ríos and the Valdivian Coastal Reserve for logistic support. Our work was funded by CONICYT FONDECYT Iniciación Nº 11150934 (CN), Morris Animal Foundation (MAF) Fellowship Training Award Nº D15ZO-413 (CN), National Geographic Society Conservation Trust Nº C309-15 (CN), Mohamed bin Zayed Species Conservation Fund Nº 152510351 (CN), CONICYT PIA APOYO CCTE Nº AFB170008 (CN, EP), Wild Felid Association (IS), Fondo Interno UNAB Nº DI-778-15/R (JM), Morris Animal Foundation Nº D16Z-825 (JM), CONICYT FONDECYT Regular Nº 1161593 (JM, CN).
REFERENCES
- 1.Abarca K., Gárate D., López J., Acosta-Jamett G.2016. Flea and ticks species from dogs in urban and rural areas in four districts in Chile. Arch. Med. Vet. 48: 247–253. doi: 10.4067/S0301-732X2016000200017 [DOI] [Google Scholar]
- 2.Abarca K., López J., Perret C., Guerrero J., Godoy P., Veloz A., Valiente-Echeverría F., León U., Gutjahr C., Azócar T.2007. Anaplasma platys in dogs, Chile. Emerg. Infect. Dis. 13: 1392–1395. doi: 10.3201/eid1309.070021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allsopp M. T. E. P., Lewis B. D., Penzhorn B. L.2007. Molecular evidence for transplacental transmission of Theileria equi from carrier mares to their apparently healthy foals. Vet. Parasitol. 148: 130–136. doi: 10.1016/j.vetpar.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 4.André M. R., Baccarim Denardi N. C., Marques de Sousa K. C., Gonçalves L. R., Henrique P. C., Grosse Rossi Ontivero C. R., Lima Gonzalez I. H., Cabral Nery C. V., Fernandes Chagas C. R., Monticelli C., Alexandre de Santis A. C., Machado R. Z.2014. Arthropod-borne pathogens circulating in free-roaming domestic cats in a zoo environment in Brazil. Ticks Tick Borne Dis. 5: 545–551. doi: 10.1016/j.ttbdis.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 5.Appel M. J., Yates R. A., Foley G. L., Bernstein J. J., Santinelli S., Spelman L. H., Miller L. D., Arp L. H., Anderson M., Barr M., et al. 1994. Canine distemper epizootic in lions, tigers, and leopards in North America. J. Vet. Diagn. Invest. 6: 277–288. doi: 10.1177/104063879400600301 [DOI] [PubMed] [Google Scholar]
- 6.Azócar-Aedo L., Monti G.2015. Artículo Original: Virus de la Leucemia y de la Inmunodeficiencia felina: determinación de la prevalencia y del conocimiento de los propietarios en la ciudad de Valdivia, Chile. Revista Hospitales Veterinarios. 7: 78–84. [Google Scholar]
- 7.Baker P. J., Soulsbury C. D., Iossa G., Harris S.2010. Domestic cat (Felis catus) and domestic dog (Canis familiaris). pp. 157–172. In: Urban Carnivores. Ecology, Conflict and Conservation (Gehrt, S. D, Riley, S. P. D. and Cypher, B. L. eds.), John Hopkins University Press, Baltimore. [Google Scholar]
- 8.Beineke A., Baumgärtner W., Wohlsein P.2015. Cross-species transmission of canine distemper virus-an update. One Health 1: 49–59. doi: 10.1016/j.onehlt.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulouis H. J., Chang C. C., Henn J. B., Kasten R. W., Chomel B. B.2005. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet. Res. 36: 383–410. doi: 10.1051/vetres:2005009 [DOI] [PubMed] [Google Scholar]
- 10.Braga M. S., Diniz P. P. V., André M. R., Bortoli C. P., Machado R. Z.2012. Molecular characterisation of Bartonella species in cats from São Luís, state of Maranhão, north-eastern Brazil. Mem. Inst. Oswaldo Cruz 107: 772–777. doi: 10.1590/S0074-02762012000600011 [DOI] [PubMed] [Google Scholar]
- 11.Breitschwerdt E. B., Maggi R. G., Chomel B. B., Lappin M. R.2010. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J. Vet. Emerg. Crit. Care (San Antonio) 20: 8–30. doi: 10.1111/j.1476-4431.2009.00496.x [DOI] [PubMed] [Google Scholar]
- 12.Burling A. N., Levy J. K., Scott H. M., Crandall M. M., Tucker S. J., Wood E. G., Foster J. D.2017. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. J. Am. Vet. Med. Assoc. 251: 187–194. doi: 10.2460/javma.251.2.187 [DOI] [PubMed] [Google Scholar]
- 13.Cabello J., Altet L., Napolitano C., Sastre N., Hidalgo E., Dávila J. A., Millán J.2013. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): high prevalence and diversity of hemotrophic mycoplasmas. Vet. Microbiol. 167: 448–454. doi: 10.1016/j.vetmic.2013.09.034 [DOI] [PubMed] [Google Scholar]
- 14.Chiu E. S., Kraberger S., Cunningham M., Cusack L., Roelke M., VandeWoude S.2019. Multiple introductions of domestic cat Feline leukemia virus in endangered Florida panthers. Emerg. Infect. Dis. 25: 92–101. doi: 10.3201/eid2501.181347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleaveland S., Appel M. G. J., Chalmers W. S. K., Chillingworth C., Kaare M., Dye C.2000. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Vet. Microbiol. 72: 217–227. doi: 10.1016/S0378-1135(99)00207-2 [DOI] [PubMed] [Google Scholar]
- 16.Crissiuma A., Favacho A., Gershony L., Mendes-de-Almeida F., Gomes R., Mares-Guia A., Rozental T., Barreira J., Lemos E., Labarthe N.2011. Prevalence of Bartonella species DNA and antibodies in cats (Felis catus) submitted to a spay/neuter program in Rio de Janeiro, Brazil. J. Feline Med. Surg. 13: 149–151. doi: 10.1016/j.jfms.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deem S. L., Spelman L. H., Yates R. A., Montali R. J.2000. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 31: 441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 18.Dumler J. S., Barbet A. F., Bekker C. P., Dasch G. A., Palmer G. H., Ray S. C., Rikihisa Y., Rurangirwa F. R.2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51: 2145–2165. doi: 10.1099/00207713-51-6-2145 [DOI] [PubMed] [Google Scholar]
- 19.Dvorak G., Spickler A. R., Roth J. A.2008. Handbook for Zoonotic Diseases of Companion Animals. Center for Food Security and Public Health, Iowa State University, College of Veterinary Medicine, Ames. [Google Scholar]
- 20.Ferrés M., Abarca K., Godoy P., García P., Palavecino E., Méndez G., Valdés A., Ernst S., Thibaut J., Koberg J., Chanqueo L., Vial P. A. C.2005. [Presence of Bartonella henselae in cats: natural reservoir quantification and human exposition risk of this zoonoses in Chile]. Rev. Med. Chil. 133: 1465–1471 (in Spanish). doi: 10.4067/S0034-98872005001200008 [DOI] [PubMed] [Google Scholar]
- 21.Foley J. E., Swift P., Fleer K. A., Torres S., Girard Y. A., Johnson C. K.2013. Risk factors for exposure to feline pathogens in California mountain lions (Puma concolor). J. Wildl. Dis. 49: 279–293. doi: 10.7589/2012-08-206 [DOI] [PubMed] [Google Scholar]
- 22.Francino O., Altet L., Sánchez-Robert E., Rodriguez A., Solano-Gallego L., Alberola J., Ferrer L., Sánchez A., Roura X.2006. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet. Parasitol. 137: 214–221. doi: 10.1016/j.vetpar.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Gehrt S. D., Wilson E. C., Brown J. L., Anchor C.2013. Population ecology of free-roaming cats and interference competition by coyotes in urban parks. PLoS One 8: e75718. doi: 10.1371/journal.pone.0075718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Acuña D., Guglielmone A. A.2005. Ticks (acari: ixodoidea: argasidae, ixodidae) of Chile. Exp. Appl. Acarol. 35: 147–163. doi: 10.1007/s10493-004-1988-2 [DOI] [PubMed] [Google Scholar]
- 25.Gowtage-Sequeira S., Banyard A. C., Barrett T., Buczkowski H., Funk S. M., Cleaveland S.2009. Epidemiology, pathology, and genetic analysis of a canine distemper epidemic in Namibia. J. Wildl. Dis. 45: 1008–1020. doi: 10.7589/0090-3558-45.4.1008 [DOI] [PubMed] [Google Scholar]
- 26.Gray L. K., Crawford P. C., Levy J. K., Dubovi E. J.2012. Comparison of two assays for detection of antibodies against canine parvovirus and canine distemper virus in dogs admitted to a Florida animal shelter. J. Am. Vet. Med. Assoc. 240: 1084–1087. doi: 10.2460/javma.240.9.1084 [DOI] [PubMed] [Google Scholar]
- 27.Guptill-Yoran L.2006. Bartonellosis. pp. 511–523. In: Infectious Diseases of the Dog and Cat (Greens, C. E. ed.), Saunders Elsevier, St. Louis. [Google Scholar]
- 28.Huang H., Unver A., Perez M. J., Orellana N. G., Rikihisa Y.2005. Prevalence and molecular analysis of Anaplasma platys in dogs in Lara, Venezuela. Braz. J. Microbiol. 36: 211–216. doi: 10.1590/S1517-83822005000300002 [DOI] [Google Scholar]
- 29.Jk A. R., Hagiwara M. K., Lucas S. R. R.1997. Estudo clínico da síndrome de imunodeficiência adquirida em gatos domésticos de São Paulo. Braz. J. Vet. Res. Anim. Sci. 34: 152–155. doi: 10.11606/issn.2318-3659.v34i3p152-155 [DOI] [Google Scholar]
- 30.Labruna M. B., Ogrzewalska M., Moraes-Filho J., Lepe P., Gallegos J. L., López J.2007. Rickettsia fells in Chile. Emerg. Infect. Dis. 13: 1794–1795. doi: 10.3201/eid1311.070782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy J. K., Scott H. M., Lachtara J. L., Crawford P. C.2006. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J. Am. Vet. Med. Assoc. 228: 371–376. doi: 10.2460/javma.228.3.371 [DOI] [PubMed] [Google Scholar]
- 32.Lickey A. L., Kennedy M., Patton S., Ramsay E. C.2005. Serologic survey of domestic felids in the Petén region of Guatemala. J. Zoo Wildl. Med. 36: 121–123. doi: 10.1638/03-059 [DOI] [PubMed] [Google Scholar]
- 33.Lima M. L. F., Soares P. T., Ramos C. A. N., Araújo F. R., Ramos R. A. N., Souza I. I. F., Faustino M. A. G., Alves L. C. A.2010. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol. 41: 381–385. doi: 10.1590/S1517-83822010000200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litster A. L., Pressler B., Volpe A., Dubovi E.2012. Accuracy of a point-of-care ELISA test kit for predicting the presence of protective canine parvovirus and canine distemper virus antibody concentrations in dogs. Vet. J. 193: 363–366. doi: 10.1016/j.tvjl.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 35.López J., Pinto V., Rojas J., Weitzel T., Abarca K.2015. Detección y posterior diseminación de Rhipicephalus sanguineus en Chile. Biomedica 26: 151–152. [Google Scholar]
- 36.Lowe S., Browne M., Boudjelas S., De Poorter M.2008. 100 of the world’s worst invasive alien species. Global Invasive Species Program. Available at: http://www.gisp.org/ publications/brochures/100worst.pdf.
- 37.Luria B. J., Levy J. K., Lappin M. R., Breitschwerdt E. B., Legendre A. M., Hernandez J. A., Gorman S. P., Lee I. T.2004. Prevalence of infectious diseases in feral cats in Northern Florida. J. Feline Med. Surg. 6: 287–296. doi: 10.1016/j.jfms.2003.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-Díaz V. L., Silvestre-Ferreira A. C., Vilhena H., Pastor J., Francino O., Altet L.2013. Prevalence and co-infection of haemotropic mycoplasmas in Portuguese cats by real-time polymerase chain reaction. J. Feline Med. Surg. 15: 879–885. doi: 10.1177/1098612X13480985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Gutierrez M., Ruiz-Saenz J.2016. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Vet. Res. 12: 78. doi: 10.1186/s12917-016-0702-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meli M. L., Cattori V., Martínez F., López G., Vargas A., Simón M. A., Zorrilla I., Muñoz A., Palomares F., López-Bao J. V., Pastor J., Tandon R., Willi B., Hofmann-Lehmann R., Lutz H.2009. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS One 4: e4744. doi: 10.1371/journal.pone.0004744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miceli N. G., Gavioli F. A., Gonçalves L. R., André M. R., Sousa V. R. F., Sousa K. C. M., Machado R. Z.2013. Molecular detection of feline arthropod-borne pathogens in cats in Cuiabá, state of Mato Grosso, central-western region of Brazil. Rev. Bras. Parasitol. Vet. 22: 385–390. doi: 10.1590/S1984-29612013000300011 [DOI] [PubMed] [Google Scholar]
- 42.Millán J., Cabezón O., Pabón M., Dubey J. P., Almería S.2009. Seroprevalence of Toxoplasma gondii and Neospora caninum in feral cats (Felis silvestris catus) in Majorca, Balearic Islands, Spain. Vet. Parasitol. 165: 323–326. doi: 10.1016/j.vetpar.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 43.Millán J., Candela M. G., Palomares F., Cubero M. J., Rodríguez A., Barral M., de la Fuente J., Almería S., León-Vizcaíno L.2009. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet. J. 182: 114–124. doi: 10.1016/j.tvjl.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millán J., Casanova J. C.2009. High prevalence of helminth parasites in feral cats in Majorca Island (Spain). Parasitol. Res. 106: 183–188. doi: 10.1007/s00436-009-1647-y [DOI] [PubMed] [Google Scholar]
- 45.Mora M., Napolitano C., Ortega R., Poulin E., Pizarro-Lucero J.2015. Feline immunodeficiency virus and feline leukemia virus infection in free-ranging guignas (Leopardus guigna) and sympatric domestic cats in human perturbed landscapes on Chiloé Island, Chile. J. Wildl. Dis. 51: 199–208. doi: 10.7589/2014-04-114 [DOI] [PubMed] [Google Scholar]
- 46.Motoi Y., Satoh H., Inokuma H., Kiyuuna T., Muramatsu Y., Ueno H., Morita C.2001. First detection of Ehrlichia platys in dogs and ticks in Okinawa, Japan. Microbiol. Immunol. 45: 89–91. doi: 10.1111/j.1348-0421.2001.tb01263.x [DOI] [PubMed] [Google Scholar]
- 47.Müller A., Silva E., Santos N., Thompson G.2011. Domestic dog origin of canine distemper virus in free-ranging wolves in Portugal as revealed by hemagglutinin gene characterization. J. Wildl. Dis. 47: 725–729. doi: 10.7589/0090-3558-47.3.725 [DOI] [PubMed] [Google Scholar]
- 48.Müller A., Walker R., Bittencourt P., Machado R. Z., Benevenute J. L., DO Amaral R. B., Gonçalves L. R., André M. R.2017. Prevalence, hematological findings and genetic diversity of Bartonella spp. in domestic cats from Valdivia, Southern Chile. Parasitology 144: 773–782. doi: 10.1017/S003118201600247X [DOI] [PubMed] [Google Scholar]
- 49.Munson L., Terio K. A., Kock R., Mlengeya T., Roelke M. E., Dubovi E., Summers B., Sinclair A. R., Packer C.2008. Climate extremes promote fatal co-infections during canine distemper epidemics in African lions. PLoS One 3: e2545. doi: 10.1371/journal.pone.0002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura Y., Goto Y., Yoneda K., Endo Y., Mizuno T., Hamachi M., Maruyama H., Kinoshita H., Koga S., Komori M., Fushuku S., Ushinohama K., Akuzawa M., Watari T., Hasegawa A., Tsujimoto H.1999. Interspecies transmission of feline immunodeficiency virus from the domestic cat to the Tsushima cat (Felis bengalensis euptilura) in the wild. J. Virol. 73: 7916–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otranto D., Dantas-Torres F., Breitschwerdt E. B.2009. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. 25: 157–163. doi: 10.1016/j.pt.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 52.Park E. S., Suzuki M., Kimura M., Mizutani H., Saito R., Kubota N., Hasuike Y., Okajima J., Kasai H., Sato Y., Nakajima N., Maruyama K., Imaoka K., Morikawa S.2016. Epidemiological and pathological study of feline morbillivirus infection in domestic cats in Japan. BMC Vet. Res. 12: 228. doi: 10.1186/s12917-016-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pennisi M. G., Persichetti M. F., Serrano L., Altet L., Reale S., Gulotta L., Solano-Gallego L.2015. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit. Vectors 8: 512. doi: 10.1186/s13071-015-1128-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qurollo B. A., Balakrishnan N., Cannon C. Z., Maggi R. G., Breitschwerdt E. B.2014. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J. Feline Med. Surg. 16: 713–720. doi: 10.1177/1098612X13519632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rampersad J. N., Watkins J. D., Samlal M. S., Deonanan R., Ramsubeik S., Ammons D. R.2005. A nested-PCR with an Internal Amplification Control for the detection and differentiation of Bartonella henselae and B. clarridgeiae: an examination of cats in Trinidad. BMC Infect. Dis. 5: 63. doi: 10.1186/1471-2334-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravi M., Wobeser G. A., Taylor S. M., Jackson M. L.2010. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: Prevalence, disease associations, and survival analysis. Can. Vet. J. 51: 271–276. [PMC free article] [PubMed] [Google Scholar]
- 57.Roelke-Parker M. E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S. J., Pospischil A., Hofmann-Lehmann R., Lutz H., Mwamengele G. L., Mgasa M. N., Machange G. A., Summers B. A., Appel M. J.1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379: 441–445. doi: 10.1038/379441a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salakij C., Lertwatcharasarakul P., Salakij J., Nunklang K., Rattanakunuprakarn J.2012. Molecular characterization of Anaplasma platys in a domestic cat from Thailand. Comp. Clin. Pathol. 21: 345–348. doi: 10.1007/s00580-011-1378-1 [DOI] [Google Scholar]
- 59.Santarém V. A., Laposy C. B., Farias M. R.2005. Anaplasma platys (Ehrlichia platys)-like inclusion bodies in platelets of a cat. Colloq. Agrar. 1: 60–66. doi: 10.5747/ca.2005.v01.n2.a015 [DOI] [Google Scholar]
- 60.Santos F., Coppede J. S., Pereira A. L., Oliveira L. P., Roberto P. G., Benedetti R. B., Zucoloto L. B., Lucas F., Sobreira L., Marins M.2009. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto, Brazil. Vet. J. 179: 145–148. doi: 10.1016/j.tvjl.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 61.Schnittger L., Rodriguez A. E., Florin-Christensen M., Morrison D. A.2012. Babesia: a world emerging. Infect. Genet. Evol. 12: 1788–1809. doi: 10.1016/j.meegid.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 62.Serrano E., Millán J.2014. What is the price of neglecting parasite groups when assessing the cost of co-infection? Epidemiol. Infect. 142: 1533–1540. doi: 10.1017/S0950268813002100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw S. E., Birtles R. J., Day M. J.2001. Arthropod-transmitted infectious diseases of cats. J. Feline Med. Surg. 3: 193–209. doi: 10.1053/jfms.2001.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva-Rodríguez E. A., Sieving K. E.2011. Influence of care of domestic carnivores on their predation on vertebrates. Conserv. Biol. 25: 808–815. doi: 10.1111/j.1523-1739.2011.01690.x [DOI] [PubMed] [Google Scholar]
- 65.Sleeman J. M., Keane J. M., Johnson J. S., Brown R. J., Woude S. V.2001. Feline leukemia virus in a captive bobcat. J. Wildl. Dis. 37: 194–200. doi: 10.7589/0090-3558-37.1.194 [DOI] [PubMed] [Google Scholar]
- 66.Souza H. J. M., Teixeira C. H. R., Graça R. F. S.2002. Epidemiological study of feline leukaemia virus and feline immunodeficiency virus infections in domestic cats in the city of Rio de Janeiro. Clín. Vet. 36: 14–21. [Google Scholar]
- 67.Staggemeier R., Venker C. A., Klein D. H., Petry M., Spilki F. R., Cantarelli V. V.2010. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats in the south of Brazil: a molecular study. Mem. Inst. Oswaldo Cruz 105: 873–878. doi: 10.1590/S0074-02762010000700006 [DOI] [PubMed] [Google Scholar]
- 68.R Core Team2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 69.Teixeira B. M., Hagiwara M. K., Cruz J. C. M., Hosie M. J.2012. Feline immunodeficiency virus in South America. Viruses 4: 383–396. doi: 10.3390/v4030383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tohya Y., Castellano M. C., Norimine J., Etcheverrigaray M. E.1994. Anticuerpos contra el virus de la inmunodeficiencia felina: Primera comprobación en Argentina. Rev. Med. Vet. 75: 242–246. [Google Scholar]
- 71.Troncoso I.2012. [Leukemia virus in domestic cats: Seroprevalence in 60 cases]. Hospitales Veterinarios 5: 14–19(in Spanish). [Google Scholar]
- 72.Troncoso I., Rojas R., Fischer C., Venegas N.2013. Inmunodeficiencia viral en felinos domésticos: Seroprevalencia de 50 casos. Hospitales Veterinarios. 5: 14–19. [Google Scholar]
- 73.van de Bildt M. W. G., Kuiken T., Visee A. M., Lema S., Fitzjohn T. R., Osterhaus A. D. M. E.2002. Distemper outbreak and its effect on African wild dog conservation. Emerg. Infect. Dis. 8: 211–213. doi: 10.3201/eid0802.010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villatoro F. J., Sepúlveda M. A., Stowhas P., Silva-Rodríguez E. A.2016. Urban dogs in rural areas: Human-mediated movement defines dog populations in southern Chile. Prev. Vet. Med. 135: 59–66. doi: 10.1016/j.prevetmed.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 75.Westman M. E., Paul A., Malik R., McDonagh P., Ward M. P., Hall E., Norris J. M.2016. Seroprevalence of feline immunodeficiency virus and feline leukaemia virus in Australia: risk factors for infection and geographical influences (2011–2013). JFMS Open Rep 2: 2055116916646388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wise L. N., Kappmeyer L. S., Mealey R. H., Knowles D. P.2013. Review of equine piroplasmosis. J. Vet. Intern. Med. 27: 1334–1346. doi: 10.1111/jvim.12168 [DOI] [PubMed] [Google Scholar]
- 77.Witt C. J., Moench T. R., Gittelsohn A. M., Bishop B. D., Childs J. E.1989. Epidemiologic observations on feline immunodeficiency virus and Toxoplasma gondii coinfection in cats in Baltimore, Md. J. Am. Vet. Med. Assoc. 194: 229–233. [PubMed] [Google Scholar]
- 78.Yabsley M. J., Shock B. C.2012. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2: 18–31. doi: 10.1016/j.ijppaw.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaror L., Ernst S., Navarrete M., Ballesteros A., Boroscheck D., Ferres M., Thibaut J.2002. [Serologic detection of Bartonella henselae in cats in the city of Valdivia, Chile]. Arch. Med. Vet. 34: 103–110 (in Spanish). doi: 10.4067/S0301-732X2002000100011 [DOI] [Google Scholar]