Abstract
Liver-type fatty acid–binding protein (L-FABP) is a biomarker for the early detection of renal diseases in humans. It is secreted along with cytotoxic oxidation products from proximal tubular epithelial cells under conditions of ischemia and/or oxidative stress. This study examined urinary L-FABP excretion under renal ischemia in feline acute kidney injury (AKI) model. L-FABP excretion increased immediately after renal ischemia/reperfusion, despite the absence of obvious structural damage to the kidneys, in the two AKI model cats studied. L-FABP was detected in the renal tubular lumen immediately after renal ischemia/reperfusion in the two cats, but not in a sham surgery cat. These results suggested that high L-FABP excretion is a pathophysiological response associated with antioxidant defense in proximal tubules with renal ischemia and/or oxidative stress in a feline model.
Keywords: acute kidney injury, biomarker, cat model, Liver-type fatty acid–binding protein (L-FABP)
Acute kidney injury (AKI) is a critical disease associated with high morbidity and mortality, and may lead to the development of chronic kidney diseases (CKD) [1]. Renal ischemia is known as one of the main causes of AKI in cats as well as in humans [1, 6]. Ischemia leads to an imbalance in cellular metabolic supply and demand and causes tissue hypoxia and microvascular dysfunction [4]. The detection of early renal pathophysiological responses to renal ischemia would be useful for the clinical diagnosis of AKI in cats [6].
Urinary excretion of liver-type fatty acid binding protein (L-FABP) is a useful biomarker for the early detection of acute kidney injury (AKI) in humans [10, 15]. In humans, L-FABP is expressed in renal proximal tubular epithelial cells, in addition to the liver, pancreas, and small intestine [1, 12]. The functions of L-FABP include binding and transporting fatty acids to mitochondria or peroxisomes, where the fatty acids are β-oxidized; and participation in intracellular fatty acid homeostasis [13]. The urinary excretion of L-FABP due to ischemia and oxidative stress on renal tubules precedes the progression of renal damage [7, 8]. However, little is known about the pathophysiological role of L-FABP in cats with renal ischemia/reperfusion injury. The aim of this study was to examine changes in urinary excretion and renal localization of L-FABP after renal ischemia in the AKI cat model.
This study was performed in accordance with local animal ethics guidelines and was approved by the Animal Research Committee of Iwate University (accession number: A201432). Three healthy male cats (mixed breed) with body weights ranging from 4.1 to 6.5 kg and ages ranging from 2 to 4 years old were used for generating the AKI models. Prior to this study, all three cats were confirmed to be healthy on the basis of physical examination, along with estimation of complete blood count, biochemical profiles, and urinalysis. AKI models were generated as described in previous studies [11]. The three cats were randomly allocated to receive one of the following three treatments: sham surgery, 40 min of renal ischemia, and 50 min of renal ischemia. Two days before surgery, all cats underwent brief anesthesia for the surgical placement of an indwelling 18-gauge 15-cm catheter (SMAC Plus; Covidien, Shizuoka, Japan) in their right jugular vein.
Cats were subjected to a 12 hr period of fasting prior to surgery. Each cat was premedicated with butorphanol (0.4 mg/kg) and midazolam (0.3 mg/kg) administered via intravenous (IV) injections. Anesthesia was induced with propofol (4 mg/kg IV to effect) and maintained with isoflurane in 100% oxygen. Physiological body temperature was maintained with a hot air warming system. Temperature, pulse, respiration, blood pressure, and oxygen saturation (via pulse oximetry) were recorded every 10 min during anesthesia. A urethral catheter (3 Fr) was kept in place by a Chinese finger trap suture for intraoperative urine collection. The catheter was removed after the final intraoperative urine collection (at 2 hr after reperfusion). A balanced electrolyte solution (lactated Ringer’s solution, 10 ml/kg/hr) was administered during surgery. In all cats, lactated Ringer’s solution (200 ml/head/day) was administered by subcutaneous injection as a single dose every 24 hr after surgery, until the cat began eating and drinking. Antibiotic (cefazolin sodium, 22 mg/kg IV) was administered when anesthesia was induced and after 2 hr of anesthesia; and was continued twice daily for 5 days. Postoperative pain was controlled by IV injections of butorphanol (0.4 mg/kg, every 2 hr) for 24 hr as necessary.
A midline laparotomy was performed in all the cats. Both kidneys were inspected, and the renal arteries and veins were exposed. In cats receiving renal ischemia, a non-traumatic vascular clamp was applied across each renal artery and vein. Clamping of the bilateral renal vessels was confirmed by the visual inspection of stopping an arterial pulse distal to the clamp. A sample for renal biopsy was collected from the right kidney immediately after clamping of the vessels. The vascular clamps were left in place for 40 or 50 min. A second renal biopsy sample was taken from the left kidney just prior to the release of vascular clamps. This was followed by 60 min of reperfusion, after which the arterial clamps were reapplied briefly, and a third renal biopsy sample was taken from the left kidney. Renal hemostasis was ensured after the biopsies, and the abdomen was closed in the routine manner. The sham surgery cat had three renal biopsies taken at the same time points, when vascular clamping was applied for prevention of severe bleeding from a biopsy site (<3 min, unilateral). Urine was collected before the ischemia, and 60 and 120 min after reperfusion. The cats were kept under observation for 14 days post-surgery. Urine production, appetite, and mental attitude were monitored daily. The concentrations of blood urea nitrogen (BUN), serum creatinine (sCre), and urinary L-FABP in each cat were measured daily for the first 5 days after surgery, and then on days 7 and 14.
Urinary L-FABP concentration was measured using a two-step sandwich enzyme-linked immunosorbent assay (ELISA) (Feline L-FABP ELISA kit; CMIC Holdings, Tokyo, Japan). Documentation provided with the kit indicates that this ELISA method is sensitive within a L-FABP concentration range of 1.9–375 ng/ml. When the sample contained L-FABP concentration above the upper limit of detection of this kit (>375 ng/ml), it was measured after diluting the urine samples with standard diluent solution (0 ng/ml) included in this kit. Urinary L-FABP levels were normalized relative to urinary creatinine concentration to avoid variations caused by urinary volume and expressed as urinary L-FABP index. Urinary creatinine levels were measured by Folin’s method [2].
Paraffin sections of feline kidneys were prepared as described below. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol. Deparaffinized sections were incubated with 10% goat serum in PBS at room temperature for 1 hr and then with anti-human L-FABP monoclonal antibody (diluted 1:200, clone 2; CMIC Holdings) at room temperature for 1 hr. This was followed by treatment with HRP-labeled polymer-conjugated anti-mouse IgG antibody (EnVision+, peroxidase, rabbit; Dako, Glostrup, Denmark) for 30 min at room temperature. The sections were treated with diaminobenzidine to visualize the antigen-antibody reaction. Finally, the sections were counterstained with hematoxylin and observed under a light microscope.
In this study, we examined whether urinary excretion of L-FABP was increased in renal ischemic conditions in two AKI model cats and a control cat. Vascular clamping of bilateral renal vessels for 40 or 50 min were performed in the two AKI model cats. The control cat was subjected to a sham operation, in which only a midline laparotomy for 50 min was carried out without any vascular clamping of the renal arteries. All cats survived till 14 days post-surgery with no abnormalities, as confirmed by daily physical examinations.
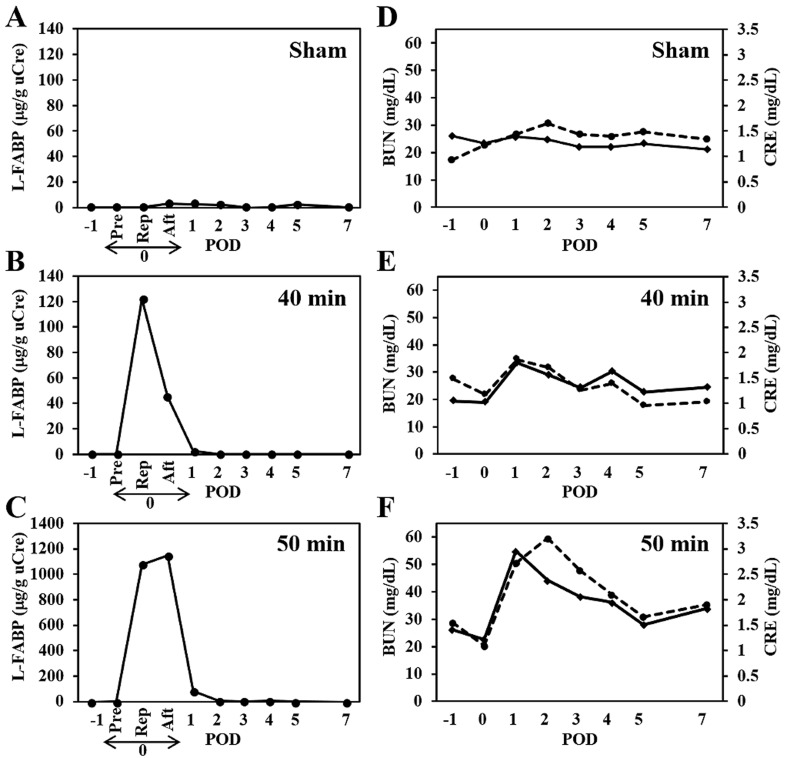
Figure 1A–C show temporal changes in urinary L-FABP levels in each cat. Urinary L-FABP excretions were normalized to urinary Cre (uCre) concentrations to correct for variations due to changes in urine volume. In the sham surgery cat, there was no marked variation in urinary L-FABP indexes before and after the operation (Fig. 1A). Urinary L-FABP was below the limit of detection by ELISA except for samples collected at 120 min (L-FABP index, 4.9 µg/g uCre) after laparotomy, and on post-operative day 1 (POD 1, 4.2 µg/g uCre) and 2 (3.8 µg/g uCre). In contrast, transiently high values of urinary L-FABP indexes were observed immediately after renal ischemia/reperfusion in the two AKI model cats. Maximum values of L-FABP indexes were observed in urine samples collected at 60 and 120 min time points after the 40 and 50 min renal ischemia/reperfusion, respectively. The maximum value of L-FABP index was about 10 times higher in the cat subjected to 50 min renal ischemia (1,148.9 µg/g uCre) compared to values in the cat with 40 min renal ischemia (122.1 µg/g uCre). Although urinary L-FABP indexes were markedly reduced on POD 1 in both cats, the cat with 50 min renal ischemia showed relatively high values (82.1 µg/g uCre) on POD 1 and normal values (<8 µg/g uCre) on POD 2 and beyond. These results suggest that the levels of cellular oxidation products increase in a time-dependent manner with renal ischemia, especially in cases subjected to more than 40 min of renal ischemia.
Fig. 1.
Temporal changes in urinary liver-type fatty acid binding protein (L-FABP), serum creatinine (sCre), and blood urea nitrogen (BUN) before and after renal ischemia/reperfusion in feline acute kidney injury (AKI) models. A–C. Urinary L-FABP index. D–F. sCre (line) and BUN (dotted line) values. To generate AKI models in cats, vascular clamping of the bilateral renal arteries for 40 or 50 min was performed in two cats. The remaining (control) cat was subjected to a sham operation, in which only a midline laparotomy was performed for 50 min without vascular clamping. Urine and blood samples were collected daily, starting from 1 day before (day −1) till 7 days (day 1 to 7) after the treatment. A and D, B and E, and C and F show the results for the sham surgery cat, and the AKI model cat with 40 and 50 min of renal ischemia, respectively. Day 0 indicates the day of surgery. Pre, Rep, and Aft indicate sampling points on day 0 immediately before clamping, and 60 and 120 min after the renal reperfusion, respectively. POD denotes post-operative day. Urine samples of the sham surgery cat were collected at the same time points as that for the AKI model cats.
Temporal changes in sCre and BUN were also examined in each cat before and after the operation. In the sham surgery cat, the postoperative sCre and BUN concentrations showed no variation and were almost within their normal reference ranges by POD 7 (Fig. 1D). In the AKI model with 40 min renal ischemia, sCre concentrations increased and peaked on POD 1 (1.8 mg/dl), and then decreased to the reference range (1.3 mg/dl) by POD 3 (Fig. 1E). BUN concentrations increased slightly and peaked on POD 1 (34.6 mg/dl) within the normal reference range. In the AKI model with 50 min renal ischemia, sCre concentrations increased and peaked on POD 1 (3.0 mg/dl; Fig. 1F), and then were recovered to the normal reference range by POD 14 (1.5 mg/dl; data not shown) (Fig. 1F). BUN concentrations changed in a manner similar to sCre concentrations and showed the maximum value (60 mg/dl) on POD 2.
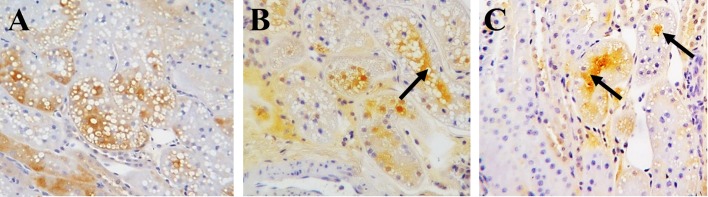
Figure 2 shows representative immunohistochemical images of L-FABP localization in kidneys collected immediately after vascular clamping of the renal vessels; and immediately before, and 60 min after renal ischemia/reperfusion by biopsies in the sham surgery cat and the renal ischemia cat models. L-FABP signal was observed in the cytoplasm of the proximal tubular epithelial cells during and after the operation in the sham surgery cat (Fig. 2A). In contrast, L-FABP signals were detected in the proximal tubular lumen immediately before (Fig. 2B) and 60 min after (Fig. 2C) renal ischemia/reperfusion in the AKI models. There were insignificant differences in L-FABP expression in the kidney of cats subjected to 40 and 50 min ischemia, as observed by microscopy.
Fig. 2.
Representative immunohistochemical images showing the localization of liver-type fatty acid binding protein (L-FABP) in paraffin-embedded renal sections from the sham surgery and acute kidney injury (AKI) model cats. A. Renal sections of the sham surgery cat immediately after clamping. B and C. Renal sections of the AKI model cats subjected to 50 min of renal ischemia immediately before renal reperfusion (B), and 60 min after renal reperfusion (C) by needle biopsy. Arrows indicate signals from L-FABP localized in the renal tubular lumen.
In the present study, we examined renal localization and urinary excretion of L-FABP in AKI model cats. A major finding is the increase in urinary L-FABP excretion immediately after renal ischemia/reperfusion with no obvious changes (as observed by light microscopy) in kidney tissues collected from AKI model cats. L-FABP was detected in the renal tubular lumen immediately after renal ischemia/reperfusion in the AKI model cats, but not in the sham surgery cat. L-FABP has a high affinity for long-chain fatty acid oxidation products and therefore, may be an effective endogenous antioxidant in proximal tubular cells. L-FABP binds cytotoxic oxidation products produced in the proximal tubular cells under conditions of ischemia and oxidative stress, and is then secreted from the cells along with the oxidation products [3, 14]. This is also supported by a previous report showing that L-FABP mildly inhibits tubulointerstitial damage by reducing tubulointerstitial inflammation in a nephropathy model in transgenic mice with human L-FABP expressed in the kidneys [9]. These results suggest that L-FABP excretion is increased in proximal renal tubules under conditions of ischemia and consequent oxidative stress in cats, but is not always associated with renal structural damage.
Urine L-FABP/Cre ratio might be inaccurate in AKI, where the glomerular filtration rate is significantly decreased. An increase in urinary excretion of N-acetyl-β-glucosaminidase (NAG) and γ-glutamyl transferase (GGT) in renal proximal tubular injury has been reported in dogs with gentamicin-induced nephrotoxicosis [5]. NAG/Cre and GGT/Cre ratios estimated in this study showed significant correlation with urinary NAG and GGT excretion, respectively, in spot urine samples collected till 24 hr. However, 24 hr urine collection in cats is quite difficult in the clinical setting. Therefore, in this study, urine L-FABP/Cre ratio was measured as an alternative to monitoring 24 hr urinary L-FABP excretion.
In conclusion, urinary L-FABP excretion increased immediately after renal ischemia/reperfusion without and obvious structural damage to kidneys in feline AKI model. Our study shows that urinary L-FABP could be useful as a potential biomarker for the early detection of initial pathophysiological responses in feline kidneys.
CONFLICTS OF INTEREST
Tsuyoshi Oikawa, Keiichi Ohata, and Takeshi Sugaya are senior scientists at CMIC Holdings Co., Ltd. (Tokyo, Japan), a company that produced the high sensitivity L-FABP ELISA kits for L-FABP analysis. No other potential conflicts of interest relevant to this article exist.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Japan Society for the Promotion of Science (JSPS) (grant 25350559, 16K01404, 19K12838).
REFERENCES
- 1.Bonventre J. V., Yang L.2011. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121: 4210–4221. doi: 10.1172/JCI45161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delanghe J. R., Speeckaert M. M.2011. Creatinine determination according to Jaffe-what does it stand for? NDT Plus 4: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ek-Von Mentzer B. A., Zhang F., Hamilton J. A.2001. Binding of 13-HODE and 15-HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J. Biol. Chem. 276: 15575–15580. doi: 10.1074/jbc.M011623200 [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig H. K., Eckle T.2011. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 17: 1391–1401. doi: 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grauer G. F., Greco D. S., Behrend E. N., Mani I., Fettman M. J., Allen T. A.1995. Estimation of quantitative enzymuria in dogs with gentamicin-induced nephrotoxicosis using urine enzyme/creatinine ratios from spot urine samples. J. Vet. Intern. Med. 9: 324–327. doi: 10.1111/j.1939-1676.1995.tb01091.x [DOI] [PubMed] [Google Scholar]
- 6.Guess S. C., Grauer G. F.2017. Acute kidney injury. pp.246–253. In: BSAVA Manual of Canine and Feline Nephrology and Urology, 3rd ed. (Elliot, J., Grauer, G. F. and Westropp, J. L. eds.), British Small Animal Veterinary Association, Gloucester. [Google Scholar]
- 7.Kamijo-Ikemori A., Sugaya T., Obama A., Hiroi J., Miura H., Watanabe M., Kumai T., Ohtani-Kaneko R., Hirata K., Kimura K.2006. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am. J. Pathol. 169: 1107–1117. doi: 10.2353/ajpath.2006.060131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamijo A., Kimura K., Sugaya T., Yamanouchi M., Hikawa A., Hirano N., Hirata Y., Goto A., Omata M.2004. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J. Lab. Clin. Med. 143: 23–30. doi: 10.1016/j.lab.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Kamijo A., Sugaya T., Hikawa A., Okada M., Okumura F., Yamanouchi M., Honda A., Okabe M., Fujino T., Hirata Y., Omata M., Kaneko R., Fujii H., Fukamizu A., Kimura K.2004. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am. J. Pathol. 165: 1243–1255. doi: 10.1016/S0002-9440(10)63384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portilla D., Dent C., Sugaya T., Nagothu K. K., Kundi I., Moore P., Noiri E., Devarajan P.2008. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 73: 465–472. doi: 10.1038/sj.ki.5002721 [DOI] [PubMed] [Google Scholar]
- 11.Schmiedt C. W., Nelson S. A., Brainard B. M., Brown C. A., Vandenplas M., Hurley D. J.2012. Bilateral renal ischemia as a model of acute kidney injury in cats. Res. Vet. Sci. 93: 950–959. doi: 10.1016/j.rvsc.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 12.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B.2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U.S.A. 101: 6062–6067. doi: 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veerkamp J. H., Peeters R. A., Maatman R. G.1991. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim. Biophys. Acta 1081: 1–24. doi: 10.1016/0005-2760(91)90244-C [DOI] [PubMed] [Google Scholar]
- 14.Wang G., Gong Y., Anderson J., Sun D., Minuk G., Roberts M. S., Burczynski F. J.2005. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology 42: 871–879. doi: 10.1002/hep.20857 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T., Noiri E., Ono Y., Doi K., Negishi K., Kamijo A., Kimura K., Fujita T., Kinukawa T., Taniguchi H., Nakamura K., Goto M., Shinozaki N., Ohshima S., Sugaya T.2007. Renal L-type fatty acid--binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 18: 2894–2902. doi: 10.1681/ASN.2007010097 [DOI] [PubMed] [Google Scholar]